Abstract
Purpose
This study aims to evaluate the anti-inflammatory and antioxidant effects of N. gaditana on streptozotocin (STZ)-induced diabetes mellitus in Wistar rats.
Methods
Diabetes was induced in male Wistar rats by single intraperitoneal injection of STZ (45 mg/kg). Male rats were fed on control diet supplemented or not with N. gaditana (10%) for a period of 2 months. At the end of the experiment, biochemical parameters and oxidant/antioxidant markers in liver and pancreas tissues, as well as mitochondria isolated from liver of rats, were determined.
Results
It was notice that levels of glucose, glycated hemoglobin (HbA1c), lipid profile, kidney functions and liver enzymes in addition to markers of the inflammatory reactions interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) increased significantly (P < 0.05) in diabetic rats. Moreover, undesirable alterations of oxidative stress markers of tissue and mitochondria isolated from the liver were noted in these rats. N. gaditana supplementation was shown effective in lowering the levels of glucose, HbA1c and improving the renal and hepatic function and also in attenuating the oxidative stress and inflammation in diabetic rats.
Conclusion
N. gaditana possesses antioxidant properties that might have beneficial effect in treatment of diabetes.
Keywords: Nannochloropsis gaditana, Diabetes, Mitochondria, Oxidative stress, Inflammation
Introduction
Diabetes mellitus is a group of metabolic disorders which are caused par absolute or relative deficiencies of insulin secretion that is associated with elevated levels of blood glucose and also with disturbances in the metabolism of carbohydrates, lipids, and proteins [1]. Diabetes affects individuals’ functional capacities and quality of life, leading to significant morbidity and premature mortality [2].
Streptozotocin (STZ) is a natural diabetic agent that is used to generate diabetic animal models by damaging or destroying the pancreatic β cells; this leads to the cessation of insulin production [3]. On the other hand, STZ has drawn much attention as a potential source of oxidative stress that plays a pivotal role in the development of diabetes and its complications [4, 5].
During diabetes, inflammation can be triggered by increased ROS (reactive oxygen species), which activates several inflammatory signaling cascades. In addition, the condition of diabetes can increase the availability of free fatty acids due to the process of lipolysis. The increase of free fatty acids will activate the immune system for releasing cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) [6].
Nowadays, microalgae constitute a major field of investigation for uncovering new molecules with biological activities likely to further enhance metabolic treatment responses. Microalgae are photosynthetic organisms that convert solar energy to biomass. It is well known that microalgae constitute a source of bioactive compounds with potential applications in functional food supplements as well as in nutraceutical, cosmetic, and pharmaceutical products [7]. Nutritional studies have also revealed that the incorporation of a microalgal biomass and/or its bioactive compounds in foods may reduce the risk of multiple diseases [8].
The pharmacological properties of microalgae exhibit antioxidant, antiviral, and anticancer activity [9]. Microalgae also provide benefits for the treatment of diabetes and its hypoglycemic effect was shown both in alloxan and STZ-induced diabetic rats [10, 11].
Nannochloropsis gaditana is a microalga that belongs to the class of Eustigmatophyceae. This marine alga is very rich in lipids and pigments, and is acknowledged for its natural antioxidant potential, and carotenoids pigments, including astaxanthin, β-carotene, canthaxanthin, neoxanthin, violaxanthin, zeaxanthin, and other components [12, 13]. N. gaditana is also recognised as a good potential source of EPA (20: 5ω3), and also an important polyunsaturated fatty acid that helps to prevent many diseases [14]. Previous reports have revealed the antioxidant and anticancer activities of N. gaditana or its extracts in vitro and in vivo [15, 16].
The present study was undertaken in an attempt to explore the anti-inflammatory and antioxidant properties of the microalgae N. gaditana in STZ-induced diabetic rats.
Materials and methods
Plant material
The microalgae Nannochloropsis gaditana used in our experimental protocol originated from Mediterranean Sea; it came from closed microalgae photo bioreactors (Sidi Bel-Abbes, a northwestern Algerian town). After cultivation, the microalgae biomass was harvested and lyophilized. The resulting lyophilisate was then analyzed in order to determine the physico-chemical composition of N. gaditana, as shown in Table 1.
Table 1.
Composition of the microalgae N. gaditana
| Components | Amount |
|---|---|
| Water content | 42.9 g/100 g |
| Dry mater content | 57 g/100 g |
| Ash content/DM | 5.74 g/100 g |
| Protein content/DM | 28 g/100 g |
| Fat content/DM | 18.4 g/100 g |
| Cellulose content/DM | 386 mg/100 g |
| Carbohydrate content/DM | 45 g/100 g |
| Calcium content/DM | 4.41 g/100 g |
| Manganese content/DM | 420 mg/100 g |
| Zinc content/DM | 10 mg/100 g |
| Copper content/DM | 5 mg /100 g |
| Iron content/DM | 17 mg/100 g |
| Beta carotene content/DM | 7.85 mg/100 g |
| Linoleic Acid content | 11 mg/100 g |
| Linolenic Acid content | 17 mg/100 g |
| Oleic Acid content | 61 mg/100 g |
DM dry material
Determination of N. gaditana composition
Water and dry mater contents were determined in accordance with the AOAC guidelines [17]. In addition, the crude ash content was estimated by incineration in a muffle furnace at 500–600 °C (Heraeus Instruments) according to the AOAC recommendations. The operation was considered as complete when the residues turned white after cooling [18]. Moreover, the total carbohydrates were found using the phenol-sulfuric acid method [19]. The total lipid content of N. gaditana was extracted using the Soxhlet extraction method. A quantity of 150 ml of pure n-hexane was used to extract the lipid content, during 6 h, at the rate of 10 refluxes per hour, to achieve maximum extraction efficiency. The amount of lipid extracted was then measured after removing the solvent by means of a rotary vacuum evaporator (Heidolph instruments) for the purpose of evaporating the n-hexane, at 35 °C for 60 min. Afterwards, the lipid content was evaluated [20]. The crude protein content in the samples was determined by the Kjeldahl method; it was calculated using a nitrogen conversion factor of 6.25. The inorganic constituents of the biomass, i.e. calcium, manganese, zinc, copper and iron, were determined by means of a VARIAN AA20 type air-acetylene flame atomic adsorption spectrophotometer. Carotenoids were extracted using the method of Sass-Kiss et al. [21]. The quantity of 20 ml of the mixture of hexane, acetone and ethanol (2: 1: 1) was added to 5 g of the sample under study. Afterwards, the upper phase was recovered after stirring for 30 min. Then, 10 ml of hexane were added for a second extraction. The two phases were then mixed for the purpose of determining the total carotenoids content by spectrophotometry at 450 nm. Carotenoid concentrations were estimated by reference to the calibration curve that uses ß-carotene.
Animals and experimental design
Adult 2 month-old male Wistar rats, weighing between 150 and 200 g, provided by Pasteur’s institute (Algeria), were used in this study. The animals were maintained under standard laboratory conditions, i.e. (12 h light-dark cycle), temperature of 25 °C, and relative humidity of (60 ± 5%). They had free access to food (control commercial diet for rats ONAB, Algeria) and water. All aspects of the experiments were conducted according to the guidelines provided by the ethical committee of the experimental animal care at Tlemcen.
Diabetes was induced in rats by STZ. A single dose of STZ (45 mg/kg body weight) dissolved in 0.1 M citrate buffer (pH 4.5), was administered to each animal by intraperitoneal injection. Three days after STZ injection, the blood glucose level was checked using a glucose meter. Rats with blood glucose level above 120 mg/dL were considered diabetic and were included in the study.
The selected animals were divided into four experimental groups. The first group (control, C, n = 10) included normal rats fed a control diet (ONAB); the second group (control microalgae, CM, n = 10) contained normal rats fed a control diet enriched with 10% microalgae N. gaditana; the third one (diabetic group, D, n = 10) involved diabetic rats fed only with control diet; and the fourth and last one (diabetic microalgae, DM, n = 10) comprised diabetic rats fed a control diet enriched with 10% microalgae N. gaditana. The microalgae N. gaditana dosage used in this study was performed according to the protocol of Markovits et al. [22].
Blood and tissue samples
After eight weeks of the experiment, the rats were anesthetized with intraperitoneal injection of 10% chloral (0.3 ml per 100 g of body weight), after 12 h of fasting. Blood samples were collected from the abdominal aorta in order to determine various biochemical estimations.
Samples of liver and pancreatic tissues were removed, washed with ice-cold saline, quickly blotted and weighed. An aliquot of each sample was homogenized by means of an Ultra-Turrax homogenizer (Bioblock Scientific, Illkirch, France) in 10 volumes of ice-cold 10 mmol/l phosphate-buffered saline (pH 7.4) containing 1.15% KCl. The homogenate was subjected to 6000 g centrifugation at 4 °C for 15 min. The supernatant fractions from the samples were collected and used for the determination of redox status markers.
Another portion of the liver tissue was collected and used for isolation of mitochondria.
Liver mitochondria isolation
Liver mitochondria were isolated as previously described by Frezza et al. [23], with some modifications. A piece of liver with a mass of 10 g was crushed in a pot containing 30 ml of TSE (Tris-Sucrose-EGTA, with 250 mM of Sucrose, 50 mM of Tris, 5 mM of EGTA, pH 7.2), in order to allow the release of mitochondria. Afterwards, the homogenate was subjected to a 1770 rpm centrifugation for a period of 10 min. The resulting supernatant was collected and subjected to 9600 rpm centrifugation for 10 min. The mitochondrial nerve obtained was resuspended in 13 ml of TSE. This suspension was once again subjected to a 9600 rpm centrifugation for 10 min. Afterwards, the mitochondrial nerve was put again in 15 ml of TS buffer (Tris-Sucrose, with 250 mM of Sucrose, 50 mM of Tris, pH 7) and was then centrifugated at 9600 rpm for 10 min. The final pellet was divided into two fractions; the first one was placed into 200 μl of TS buffer in order to get a mitochondrial suspension, and the second was added to the hypotonic solution (25 mM of KH2PO4, 5 mM of Mgcl2, pH 7.2) in order to examine the mitochondrial antioxidant enzymes.
Determination of biochemical parameters
Serum glucose, uric acid, urea, creatinine concentrations were determined using colorimetric enzymatic assays (kits from BioAssay Systems, Hayward, CA). Glycated hemoglobin (HbA1c) was determined in whole blood using a kit from Pointe Scientific, Inc., United States. Liquid chromatography method. Serum triglycerides, cholesterol were measured using colorimetric enzymatic kits (Sigma, St. Louis, MO). Serum alanine aminotransferase EC 2.6.1.2 (ALT), aspartate aminotransferase EC 2.6.1.1 (AST), alkaline phosphatase EC 3.1.3.1 (ALP), lactate dehydrogenase EC 1.1.1.27 (LDH) were determined using colorimetric enzymatic kits (Spinreact, Girona, Spain). Serum levels of tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) were estimated using enzyme-linked immunosorbent assay (ELISA) kits (Genzyme corp, Cambridge, MA, USA).
Determination of markers of the oxidant/antioxidant status
The catalase (CAT) activity was measured by spectrophotometric analysis of the decomposition rate of hydrogen peroxide [24]. The reduced glutathione (GSH) was assayed using Ellman’s method [25]. The superoxide dismutase (SOD) activity was assessed following the NADPH oxidation procedure [26]. The activity of glutathione S-transferases (GST) was estimated by investigating its action on the compound 1-chloro-2,4-dinitrobenzene (CDNB), in the presence of glutathione as a co-substrate [27]. The level of malondialdehyde (MDA), which is commonly known as a marker of lipid peroxidation, was determined by considering the reaction of MDA with thiobarbituric acid [28]. Carbonyl proteins (markers of protein oxidation) were assayed by the 2,4-dinitrophenyl hydrazine (DNPH) reaction [29].
Statistical analysis
The results obtained were expressed as mean ± SD (Standart deviation).The results were tested for normal distribution using the Shapiro–Wilk test. Data not normally distributed were logarithmically transformed. Significant differences among the groups were analyzed statistically by a one-way analysis of variance (ANOVA). When significant changes were observed in ANOVA tests, Fisher least significant difference tests were applied to locate the source of significant difference. The significance level was set at P < 0.05. These calculations were performed using Statistica version 4.1 (Statsoft, Tulsa, OK).
Results
Effects of N. gaditana on body weight, food and energy intake
The results of this study showed a significant decrease in the body weight of STZ-induced diabetic rats as compared with normal rats. The group of diabetic rats showed a significant increase in food intake and energy intake as compared to control rats. However, supplementation of the diet of diabetic rats with N. gaditana at 10% caused a body weight increase with recovery from these disorders (Table 2).
Table 2.
Effects of N. gaditana on body weight, food and energy intake
| Parameters | Control rats | Diabetic rats | P (ANOVA) | ||
|---|---|---|---|---|---|
| C | CM | D | DM | ||
| Body weight (g) | 225 ± 6.21a | 220.82 ± 5.11a | 198.17 ± 4.37c | 210.27 ± 3.21b | 0.04 |
| Food intake (g/day/rat) | 22.41 ± 0.22c | 23.11 ± 0.92c | 54.51 ± 0.34a | 38.34 ± 0.12b | 0.03 |
| Energy intake (kcal/day/rat) | 125.50 ± 10.59c | 128.27 ± 8.48c | 206.18 ± 5.63a | 150.08 ± 16.80b | 0.001 |
C normal rats fed a control diet, CM normal rats fed a control diet enriched with microalgae at 10%, D diabetic rats fed only with control diet, DM diabetic rats fed a control diet enriched with microalgae at 10%
Values are presented as means ± SD (Standart deviation). Values with different superscript letters (a, b, c, d) are significantly different (ANOVA)
Effects of N. gaditana on biochemical markers
The effects of N. gaditana on the levels of glucose, HbA1c, triglycerides, cholesterol and markers of renal and hepatic function are shown in Table 3. In diabetic rats, there was a significant increase in the levels of glucose, HbA1c, triglycerides, cholesterol, ALP, AST, ALP and LDH, uric acid, urea and creatinine comparatively to the levels found in control rats. However, treatment of diabetic rats with N. gaditana significantly decreased levels of these parameters.
Table 3.
Effects of N. gaditana on biochemical parameters
| Parameters | Control rats | Diabetic rats | P (ANOVA) | ||
|---|---|---|---|---|---|
| C | CM | D | DM | ||
| Glucose (g/L) | 1.17 ± 0.01c | 1.20 ± 0.04c | 4.41 ± 0.08a | 2.44 ± 0.02b | 0.010 |
| HbA1c (%) | 4.40 ± 0.14c | 4.10 ± 0.2c | 10.70 ± 0.87a | 9.60 ± 0.7b | 0.004 |
| Triglycerides (g/L) | 1.15 ± 0.01c | 1.10 ± 0.01c | 2.28 ± 0.04a | 1.94 ± 0.03b | 0.006 |
| Cholesterol (g/L) | 1.53 ± 0.01c | 1.44 ± 0.01c | 2.43 ± 0.04a | 2.02 ± 0.03b | 0.004 |
| ALT (IU/L) | 42.45 ± 1.47c | 40.71 ± 1.47c | 52.94 ± 0.88a | 48.51 ± 1.91b | 0.010 |
| AST (IU/L) | 36.86 ± 4.00c | 30.8 ± 4.00c | 50.51 ± 0.71a | 41.94 ± 0.03b | 0.007 |
| ALP (IU/L) | 83.35 ± 4.15b | 81.14 ± 4.15b | 90.33 ± 3.83a | 84.16 ± 3.48b | 0.006 |
| LDH (IU/L) | 323.41 ± 159.42c | 314.38 ± 166.70c | 655.85 ± 191.06a | 462.13 ± 286.72b | 0.033 |
| Uric acid (mg/dL) | 35.38 ± 0.93c | 39.18 ± 1.64b | 72.76 ± 1.82a | 39.44 ± 2.73b | 0.010 |
| Urea (mg/dL) | 36.62 ± 1.95c | 36.15 ± 3.24c | 41.26 ± 2.72a | 38.45 ± 1.83b | 0.010 |
| Creatinine (mg/dL) | 65.90 ± 5.55c | 64.54 ± 2.07c | 90.26 ± 3.29a | 87.73 ± 3.25b | 0.010 |
Values are presented as means ± SD (Standart deviation). Values with different superscript letters (a, b, c, d) are significantly different (ANOVA). HbA1c glycated hemoglobin, ALT alanine aminotransferase, AST aspartate aminotransferase, ALP alkaline phosphatase, LDH lactate dehydrogenase
C normal rats fed a control diet, CM normal rats fed a control diet enriched with microalgae at 10%, D diabetic rats fed only with control diet, DM diabetic rats fed a control diet enriched with microalgae at 10%
Effects of N. gaditana on levels of pro-inflammatory cytokines
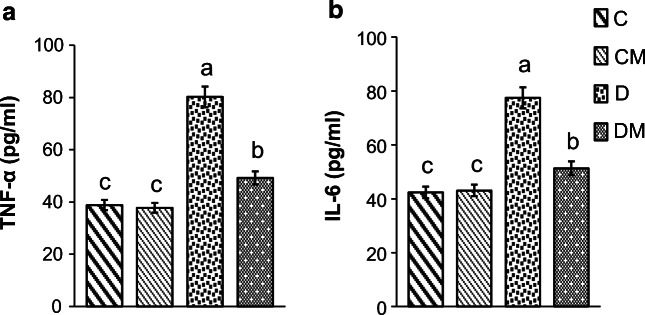
Serum levels of TNF-α and IL-6 were significantly increased in the diabetic rats as compared with the control rats. However, diabetic rats fed a diet supplemented with N. gaditana presented significantly lower levels of TNF-α and IL-6 as compared to diabetic rats fed a control diet (Fig. 1a, b).
Fig. 1.
Effects of N. gaditana on TNF-α (a) and IL-6 (b) levels. TNF-α tumor necrosis factor-alpha, IL-6 interleukin-6, C normal rats fed a control diet, CM normal rats fed a control diet enriched with microalgae at 10%, D diabetic rats fed only with control diet, DM diabetic rats fed a control diet enriched with microalgae at 10%. Values are presented as means ± SD (Standart deviation). Values with different superscript letters (a, b, c, d) are significantly different (ANOVA)
Effects of N. gaditana on oxidative stress markers
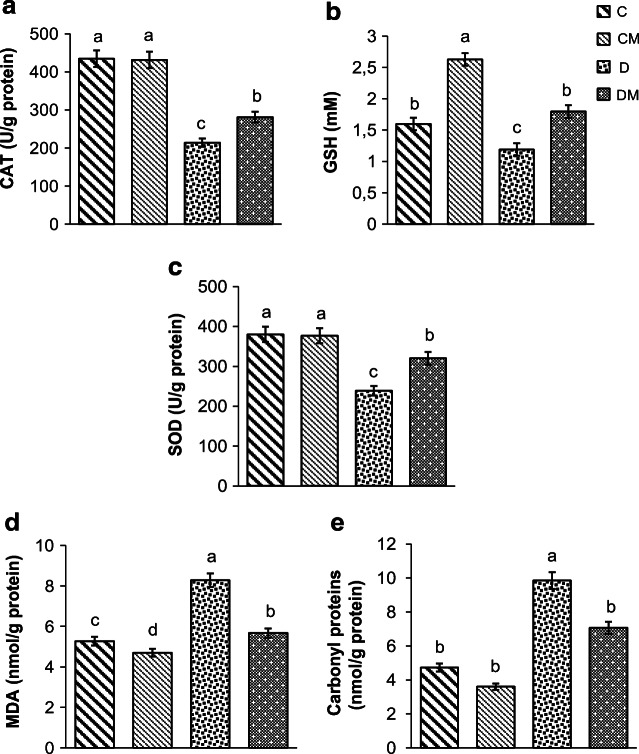
As shown in Fig. 2, the activities of catalase (CAT), reduced glutathione (GSH) and superoxide dismutase (SOD) decreased significantly in the hepatic mitochondria of diabetic rats as compared to control rats; however, when these antioxidant enzymes were measured after dietary supplementation with N. gaditana, contradictory results were obtained. In addition, N. gaditana was able to reduce malondialdehyde (MDA) and carbonyl proteins levels in liver mitochondria of diabetic rats (Fig. 2a-e).
Fig. 2.
Effects of N. gaditana on CAT (a), GSH (b), SOD (c), MDA (d) and carbonyl proteins (e) levels in the liver mitochondria. CAT catalase, GSH reduced glutathione, SOD superoxide dismutase, MDA malondialdehyde, C normal rats fed a control diet, CM normal rats fed a control diet enriched with microalgae at 10%, D diabetic rats fed only with control diet, DM diabetic rats fed a control diet enriched with microalgae at 10%. Values are presented as means ± SD (Standart deviation). Values with different superscript letters (a, b, c, d) are significantly different (ANOVA)
The levels of MDA and carbonyl proteins in liver tissues were significantly increased in diabetic rats by comparison with control rats. However, diabetic animals fed a diet supplemented with N. gaditana presented significantly lower levels of MDA and carbonyl proteins. On the other hand, N. gaditana induced a substantial rise in the activities of CAT, GSH, SOD and GST in the liver tissues of diabetic rats (Table 4).
Table 4.
Effects of N. gaditana on oxidant/antioxidant markers in liver tissues
| Parameters | Control rats | Diabetic rats | P (ANOVA) | ||
|---|---|---|---|---|---|
| C | CM | D | DM | ||
| MDA (μmol/g) | 1.30 ± 0.50c | 1.32 ± 0.30c | 3.30 ± 0.94a | 2.15 ± 0.56b | 0.001 |
| Carbonyl proteins (μmol/mg) | 2.21 ± 0.56c | 2.27 ± 0.50c | 3.51 ± 0.44a | 2.88 ± 0.56b | 0.001 |
| CAT (U/g) | 68.56 ± 4.26a | 65.15 ± 9.20a | 46.53 ± 8.48c | 57.26 ± 3.51b | 0.001 |
| GSH (μmol/g) | 5.00 ± 0.60a | 4.98 ± 1.33a | 2.56 ± 0.22c | 3.56 ± 0.28b | 0.001 |
| SOD (U/g) | 3.44 ± 0.73a | 3.48 ± 0.99a | 1.61 ± 0.04c | 2.37 ± 0.14b | 0.000 |
| GST (nmol/g) | 6.18 ± 0.48a | 5.84 ± 0.94a | 4.36 ± 0.52c | 4.98 ± 1.10b | 0.004 |
Values are presented as means ± SD (Standart deviation). Values with different superscript letters (a, b, c, d) are significantly different (ANOVA). MDA malondialdehyde, CAT catalase, GSH reduced glutathione, SOD superoxide dismutase, GST glutathione S-transferase
C normal rats fed a control diet, CM normal rats fed a control diet enriched with microalgae at 10%, D diabetic rats fed only with control diet, DM diabetic rats fed a control diet enriched with microalgae at 10%
It is worth recalling that diabetic animals fed a diet rich in microalgae N. gaditana showed considerably lower contents in MDA and carbonyl proteins with substantially elevated activities of CAT, GSH and SOD in the pancreas, as depicted in Table 5.
Table 5.
Effects of N. gaditana on oxidant/antioxidant markers in pancreatic tissues
| Parameters | Control rats | Diabetic rats | P (ANOVA) | ||
|---|---|---|---|---|---|
| C | CM | D | DM | ||
| MDA (μmol/g) | 1.47 ± 0.60c | 1.66 ± 0.50c | 3.67 ± 0.83a | 2.03 ± 0.59b | 0.005 |
| Carbonyl proteins (μmol/mg) | 2.54 ± 0.25c | 2.60 ± 0.41c | 3.07 ± 0.71a | 2.98 ± 0.14b | 0.001 |
| CAT (U/g) | 62.59 ± 5.55a | 60.02 ± 4.35a | 30.07 ± 4.00c | 53.31 ± 8.82b | 0.001 |
| GSH (μmol/g) | 5.40 ± 1.19a | 5.92 ± 0.26a | 4.18 ± 0.35c | 5.03 ± 0.19b | 0.002 |
| SOD (U/g) | 1.63 ± 0.02a | 1.66 ± 0.01a | 1.31 ± 0.13c | 1.45 ± 0.27b | 0.002 |
Values are presented as means ± SD (Standart deviation). Values with different superscript letters (a, b, c, d) are significantly different (ANOVA). MDA malondialdehyde, CAT catalase, GSH reduced glutathione, SOD superoxide dismutase
C normal rats fed a control diet, CM normal rats fed a control diet enriched with microalgae at 10%, D diabetic rats fed only with control diet, DM diabetic rats fed a control diet enriched with microalgae at 10%
Discussion
Diabetes mellitus is a metabolic disorder that is characterized by hyperglycemia due to an absolute or relative deficiency in insulin secretion. Therefore, the present study investigated the anti-inflammatory and antioxidant properties of the microalgae N. gaditana and its preventive effects on STZ-induced diabetes and its complications in Wistar rats. The intraperitoneal administration of STZ to normal rats effectively induces diabetes, which is reflected by glycosuria, polyphagia and body weight loss, as previously observed by Akbarzadeh et al. [30]. The current study found out that when STZ-induced diabetic rats are fed a diet supplemented with N. gaditana, the above listed diabetic complications are reversed. The body weight increase could probably be attributed to potentiation of insulin secretion by beta cells of the pancreas. Moreover, it has been observed that the incorporation of N. gaditana into a diet has remarkable beneficial effects on body weight [31].
In our study, diabetic rats fed a diet supplemented with N. gaditana presented significantly lower levels of glucose and glycated hemoglobin, suggesting their antidiabetic effect. Previous studies have tested the antidiabetic activity of microalgae and their anti-glycation properties [32].
It is interesting to note that the most common lipid abnormalities in diabetes are hypertriglyceridemia and hypercholesterolemia [33]. Treatment of diabetic rats with N. gaditana markedly reduced cholesterol and triglycerides levels. This study indicated that N. gaditana possesses interesting antihyperlipidemic properties. Previous studies reported that the alga Nannochloropsis has remarkable hypocholesterolemic effects on rats [34]. It was also revealed that N. gaditana has the capacity to improve the lipid metabolism [31].
Furthermore, it has been shown that in general serum enzyme activities (ALT, AST, ALP and LDH) increase in diabetic group; this is mainly attributed to the leakage of these enzymes from the liver cytosol into the bloodstream, which may cause liver dysfunction or damage of liver cells. The results obtained were found consistent with those reported by Nithiya et al. [35]. Therefore, treatment of diabetic rats with N. gaditana caused reduction in the activity of these enzymes, which indicates the hepatoprotective effect of N. gaditana in diabetic rats.
Moreover, this study allowed discovering that a diet supplemented with microalgae N. gaditana provided good protection against STZ-induced renal dysfunction in diabetic rats because this alga has great potential to normalize the contents of serum uric acid, urea and creatinine in rats with diabetes. Similar results have been reported about diabetic rats fed a diet supplemented with Nannochloropsis oculata [36].
In our study, the serum concentrations of TNF-α and IL-6 were significantly increased in diabetic rats, which is in good agreement with the findings of Mesbahzadeh et al. [37] and Samarghandian et al. [38]. In contrast, N. gaditana supplementation was shown effective in lowering the levels of TNF-α and IL-6 in diabetic rats, this suggests that N. gaditana supplementation could play a preventive role against inflammation. Moreover, the anti-inflammatory potential of N. gaditana could be due to various valuable pigments contained in this microalga such as the carotenoids [39].
It is well known that mitochondria are complex organelles capable of generating intracellular reactive oxygen species [40]. When mitochondrial ROS production exceeds the cellular antioxidant capacity, the increase in ROS levels can lead to oxidative stress [41]. This phenomenon has been shown to play a major role in the development of STZ-induced diabetes mellitus and mitochondria may be the main target of this toxicity [42].
The present work made it possible to show that the concentrations of antioxidant enzymes (CAT, GSH, SOD) increased remarkably in liver mitochondria of diabetic rats fed with N. gaditana in comparison with diabetic animals fed an ordinary diet. Indeed, N. gaditana reduce contents of carbonyl proteins and MDA in liver mitochondria of diabetic rats. Thus, observed results indicate that N. gaditana may improve the antioxidant property in mitochondria, and attenuate the mitochondrial oxidative damage in the liver.
Regarding the oxidant/antioxidant status of liver and pancreas tissues, our results indicate that microalgae N. gaditana can help to alleviated oxidative stress in diabetic rats by reducing oxidant markers, such as MDA and carbonyl proteins, and increasing antioxidant defense. These results support the fact that N. gaditana has the potential to enhance the antioxidant activities and protect tissues from lipid and protein oxidation during diabetes. This elevation in antioxidants enzymes may be due to the neutralization of reactive oxygen species. To further support our findings, it has been reported that N. gaditana has the ability to scavenge free radicals and inhibit lipid peroxidation [43]. Moreover, supplementation with N. gaditana reduced the oxidative stress and enhanced antioxidant status in diabetic rats [16].
Conclusion
The present study revealed that N. gaditana attenuates hyperglycemia and hyperlipidemia and improves the renal and hepatic functions in STZ-induced diabetic rats. In addition, N. gaditana could also attenuate oxidative stress and inflammation and prevent complications associated with diabetes.
Acknowledgments
The authors would like to express their deepest gratitude to Professor Gomis Catalac (a marine biologist from Spain), and the principal technician from Marine Biotechnology (HASNAOUI group) for his technical support.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Khan AN, Khan RA, Ahmad M, Mushtaq N. Role of antioxidant in oxidative stress and diabetes mellitus. J Pharmacogn Phytochem. 2015;3(6):217–220. [Google Scholar]
- 2.Moien ABK, Muhammed JH, Jeffrey KK, Romona DG, Halla M, Juma AK. Epidemiology of type 2 diabetes – global burden of disease and forecasted trends. Epidemiol Glob Health. 2020;10(1):107–111. doi: 10.2991/jegh.k.191028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goud BJ, Dwarakanath V. Chikka swamy BK. Streptozotocin - a diabetogenic agent in animal models. Int J Pharm Pharm Res. 2015;3(1):253–269. [Google Scholar]
- 4.Ozra T-M, Bagher L, Mohammad A. Targeting metabolic disorders by natural products. J Diabetes Metab Disord. 2015;14:57. doi: 10.1186/s40200-015-0184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabatabaei-Malazy O, Larijani B, Abdollahi M. A novel management of diabetes by means of strong antioxidants’ combination. J Med Hypotheses Ideas. 2013;7(1):25–30. doi: 10.1016/j.jmhi.2012.12.002. [DOI] [Google Scholar]
- 6.Mardiah, Zakariaa FR, Prangdimurtia E, Damanikc R. Anti-inflammatory of purple roselle extract in diabetic rats induced by streptozotocin. Procedia Food Sci. 2015;3:182–189. doi: 10.1016/j.profoo.2015.01.020. [DOI] [Google Scholar]
- 7.Priyadarshani I, Rath B. Commercial and industrial applications of micro algae- a review. J Algal Biomass Utln. 2012;3(4):89–100. [Google Scholar]
- 8.Matos J, Cardoso C, Bandarra NM, Afonso C. Microalgae as healthy ingredients for functional food: a review. Food Funct. 2017;8(8):2672–2685. doi: 10.1039/C7FO00409E. [DOI] [PubMed] [Google Scholar]
- 9.Kherraf A, Tehami W, Boufeldja W, Yahla I, Dra GA, Mansour IFZ, Benali M. Determination of the nutritional and functional metabolites of Nannochloropsis gaditana produced in Algeria and evaluation of its antioxidant activity. Der Pharma Chem. 2017;9(14):8–13. [Google Scholar]
- 10.Aissaoui O, Amiali M, Bouzid N, Belkacemi K, Bitam A. Effect of Spirulina platensis ingestion on the abnormal biochemical and oxidative stress parameters in the pancreas and liver of alloxan-induced diabetic rats. Pharm Biol. 2017;55(1):1304–1312. doi: 10.1080/13880209.2017.1300820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nasirian F, Sarir H, Moradi-kor N. Antihyperglycemic and antihyperlipidemic activities of Nannochloropsis oculata microalgae in streptozotocin-induced diabetic rats. BioMol Concepts. 2019;10:37–43. doi: 10.1515/bmc-2019-0004. [DOI] [PubMed] [Google Scholar]
- 12.Millao S, Uquiche E. Antioxidant activity of supercritical extracts from Nannochloropsis gaditana: correlation with its content of carotenoids and tocopherols. J Supercrit Fluids. 2016;111:143–150. doi: 10.1016/j.supflu.2016.02.002. [DOI] [Google Scholar]
- 13.Medina C, Rubilar M, Shene C, Torres S, Verdugo M. Protein fractions with techno-functional and antioxidant properties from Nannochloropsis gaditana microalgal biomass. J Biobased Mater Bio. 2015;9(4):417–425. doi: 10.1166/jbmb.2015.1534. [DOI] [Google Scholar]
- 14.Abirami S, Murugesan S, Sivamurugan V, Narender SS. Screening and optimization of culture conditions of Nannochloropsis gaditana for omega 3 fatty acid production. J App Biol Biotech. 2017;5(3):13–17. [Google Scholar]
- 15.Mekdade L, Baba Hamed MB, El-Kebir FZ, Abi Ayad SM. Evaluation of antioxidant and antiproliferative activities of Nannochloropsis gaditana extracts. RJPBCS. 2016;7(3):904–913. [Google Scholar]
- 16.Nacer W, Baba Ahmed FZ, Merzouk H, Benyagoub O, Bouanane S. Antihyperlipidemic and antioxidant effects of the microalgae Nannochloropsis gaditana in streptozotocin-induced diabetic rats. Rev Agrobiol. 2019;9(2):1474–1483. doi: 10.1007/s40200-020-00681-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.AOAC . Official methods of analysis. 15th ed. Association of Official Analytical Chemists: Arlington; 1990. [Google Scholar]
- 18.Audigie CL, Dupont G. Principes des méthodes d’analyses biochimiques. 1ère ed; 1982. pp. 566–567.
- 19.Fox JD, Robyt JF. Miniaturization of three carbohydrate analyses using a microsample plate reader. Anal Biochem. 1991;195:93–96. doi: 10.1016/0003-2697(91)90300-I. [DOI] [PubMed] [Google Scholar]
- 20.Sukarni S, Hamidi N, Yanuhar U, Wardana ING. Potential and properties of marine microalgae Nannochloropsis oculata as biomass fuel feedstock. Int J Energy Environ Eng. 2014;5:279–290. doi: 10.1007/s40095-014-0138-9. [DOI] [Google Scholar]
- 21.Sass-Kiss A, Kiss J, Milotay P, Kerek MM, Toth-Markus M. Differences in anthocyanin and carotenoid content of fruits and vegetables. Food Res Int. 2005;38:1023–1029. doi: 10.1016/j.foodres.2005.03.014. [DOI] [Google Scholar]
- 22.Markovits A, Conjeros R, Lopez L, Lutz M. Evaluation of marine microalgae Nannochloropsis sp. as a potential dietary supplement. Chemical, nutritional and short term toxicological evaluation in rats. Nutr Res. 1992;12:1273–1284. doi: 10.1016/S0271-5317(05)80784-5. [DOI] [Google Scholar]
- 23.Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc. 2007;2(2):287–295. doi: 10.1038/nprot.2006.478. [DOI] [PubMed] [Google Scholar]
- 24.Abei H. Catalase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. Academic Press: New York; 1974. pp. 673–684. [Google Scholar]
- 25.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 26.Elstner EF, Youngman RJ, Obwad W. Superoxide dismutase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. Weinheim: Verlag Chemie; 1983. pp. 293–302. [Google Scholar]
- 27.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249(22):7130–7139. doi: 10.1016/S0021-9258(19)42083-8. [DOI] [PubMed] [Google Scholar]
- 28.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxydes in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 29.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-H. [DOI] [PubMed] [Google Scholar]
- 30.Akbarzadeh A, Norouzian D, Mehrabi MR, Jamshidi S, Farhangi A, Allah Verdi A, et al. Induction of diabetes by streptozotocin in rats. Indian J Clin Biochem. 2007;22(2):60–64. doi: 10.1007/BF02913315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bendimerad-Benmokhtar S, Bouanane S, Merzouk H, Baba Ahmed FZ, Bendaoud A. Effects of Nannochloropsis fed on serum and tissue lipids metabolism in obese offspring of overfed dams. Curr Nutr Food Sci. 2018;14:1–15. doi: 10.2174/157340131401180115121643. [DOI] [Google Scholar]
- 32.Lauritano C, Ianora A. Marine organisms with anti-diabetes properties. Mar Drugs. 2016;14:220. doi: 10.3390/md14120220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dixit AK, Dey R, Suresh A, Chaudhuri S, Panda AK, Mitra A, et al. The prevalence of dyslipidemia in patients with diabetes mellitus of ayurveda Hospital. J Diabetes Metab Disord. 2014. 10.1186/2251-6581-13-58. [DOI] [PMC free article] [PubMed]
- 34.Werman MJ, Sukenik A, Mokady S. Effects of the marine unicellular alga Nannochloropsis sp. to reduce the plasma and liver cholesterol levels in male rats fed on diets with cholesterol. Biosci Biotechnol Biochem. 2003;67(10):2266–2268. doi: 10.1271/bbb.67.2266. [DOI] [PubMed] [Google Scholar]
- 35.Nithiya T, Udayakumar R. Hepato and renal protective effect of phloretin on streptozotocin induced diabetic rats. J Biomed Pharm Sci. 2018;1:105. [Google Scholar]
- 36.Aboulthana WM, El-Feky AM, Ibrahim NE, Sahu RK, El-Sayed AEB. Evaluation of the pancreatoprotective effect of Nannochloropsis oculata extract against streptozotocin-induced diabetes in rats. J App Pharm Sci. 2018;8(6):46–58. doi: 10.7324/JAPS.2018.8607. [DOI] [Google Scholar]
- 37.Mesbahzadeh B, Rajaei SA, Tarahomi P, Seyedinia SA, Rahmani M, Rezamohamadi F, Kakar MA, Moradi-Kor N. Beneficial effects of spirogyra Neglecta extract on antioxidant and anti-inflammatory factors in streptozotocin-induced diabetic rats. Biomol Concepts. 2018;9(1):184–189. doi: 10.1515/bmc-2018-0015. [DOI] [PubMed] [Google Scholar]
- 38.Samarghandian S, Borji A, Farkhondeh T. Attenuation of oxidative stress and inflammation by Portulaca oleracea in streptozotocin-induced diabetic rats. J Evid Based Complement Altern Med. 2017;22(4):562–566. doi: 10.1177/2156587217692491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montero-Lobato Z, Vázquez M, Navarro F, Fuentes JL, Bermejo E, Garbayo I, et al. Chemically-induced production of anti-inflammatory molecules in microalgae. Mar Drugs. 2018. 10.3390/md16120478. [DOI] [PMC free article] [PubMed]
- 40.Vakifahmetoglu-Norberg H, Ouchida AT, Norberg E. The role of mitochondria in metabolism and cell death. Biochem Biophys Res Commun. 2017;482(3):426–431. doi: 10.1016/j.bbrc.2016.11.088. [DOI] [PubMed] [Google Scholar]
- 41.Liemburg-Apers DC, Willems PH, Koopman WJ, Grefte S. Interactions between mitochondrial reactive oxygen species and cellular glucose metabolism. Arch Toxicol. 2015;89(8):1209–1226. doi: 10.1007/s00204-015-1520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jang YY, Song JH, Shin YK, Han ES, Lee CS. Protective effect of boldine on oxidative mitochondrial damage in streptozotocin-induced diabetic rats. Pharmacol Res. 2000;42(4):361–371. doi: 10.1006/phrs.2000.0705. [DOI] [PubMed] [Google Scholar]
- 43.Bendaoud A, Baba Ahmed FZ, Merzouk H, Bouanane S, Bendimerad S. Effects of dietary microalgae Nannochloropsis gaditana on serum and redox status in obese rats subjected to a high fat diet. Phytothérapie. 2018;17:177–187. doi: 10.3166/phyto-2018-0019. [DOI] [Google Scholar]