Abstract
Introduction
To compare the effects of ipragliflozin, a sodium-glucose transporter 2 inhibitor, with those of metformin on visceral fat (as well as muscles and bones) in Japanese elderly patients with type 2 diabetes (T2D), we conducted a sub-analysis of a prospective, multicenter, blinded-endpoint randomized-controlled study.
Methods
In total, 103 patients with T2D (body mass index ≥ 22 kg/m2; glycated hemoglobin, 7–10%) and being treated with sitagliptin (a dipeptidyl peptidase-4 inhibitor) were included and randomized to receive ipragliflozin or metformin. The primary outcome was the change in visceral fat area measured using computed tomography 24 weeks following treatment. The secondary outcomes included changes in subcutaneous and total fat area, muscle volume, bone density measured using computed tomography, handgrip strength, bone markers, plasma glucose, insulin, homeostasis model assessment (HOMA)2-beta, HOMA2-R, glycated hemoglobin, lipid panel, uric acid, blood pressure, adiponectin, and high-sensitivity C-reactive protein. All patients aged 65–74 years were selected for sub-analysis.
Results
The sub-analysis included 15 and 14 patients in the ipragliflozin and metformin groups, respectively. The patients’ backgrounds were well balanced. Visceral fat area reduction was greater in the ipragliflozin group than in the metformin group (− 10.58% vs. − 6.93%; P = 0.034). There were significant differences in the changes in bone absorption markers, uric acid, and total cholesterol levels between the groups.
Conclusion
Ipragliflozin significantly reduced the visceral fat area compared with metformin when added to sitagliptin in elderly patients with T2D. Long-term and large-scale studies are required to elucidate whether ipragliflozin is suitable for elderly patients.
Trial Registration
The study was registered at https://www.umin.ac.jp/ctr/ (UMIN-ID: UMIN 000015170).
Electronic supplementary material
The online version of this article (10.1007/s13300-020-00949-0) contains supplementary material, which is available to authorized users.
Keywords: Bone, DPP-4 inhibitor, Elderly, Ipragliflozin, Metformin, Muscle, SGLT2 inhibitor, Sitagliptin, Type 2 diabetes, Visceral fat
Key Summary Points
| Why carry out this study? |
| In our previous randomized control study on Japanese patients with type 2 diabetes being treated with sitagliptin, we found that the mean percentage reduction in the visceral fat area was significantly greater in the ipragliflozin group than in the metformin group. |
| Although it is known that elderly patients are at a higher risk for sarcopenia and osteoporosis than non-elderly patients, information regarding the effects of sodium-glucose transporter 2 inhibitors on elderly patients remains inadequate. |
| We conducted a sub-analysis of our previous randomized control study to evaluate whether the sodium-glucose transporter 2 inhibitor ipragliflozin and metformin reduce visceral fat and control glucose in elderly patients with T2D and how these treatments affect muscle mass and bone density. |
| What was learned from the study? |
| Ipragliflozin significantly reduced the visceral fat area compared with metformin when added to sitagliptin in elderly patients with type 2 diabetes. |
| Ipragliflozin significantly reduced the uric acid concentration compared with metformin when added to sitagliptin in elderly patients with type 2 diabetes. |
| Long-term and large-scale studies are required to elucidate whether ipragliflozin is suitable for elderly patients. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.13078832.
Introduction
In Japan, 2 million patients, accounting for more than two-thirds of patients with type 2 diabetes (T2D), are aged ≥ 65 years, and approximately 60% of male and 30% of female patients are obese [1]. Although the use of dipeptidyl peptidase-4 (DPP-4) inhibitors as first-line therapy is not recommended by international guidelines, they are often used as first-line treatment for elderly patients with T2D in Japan [2], because they have few side effects such as hypoglycemia [3]. However, in a subset of patients, blood glucose levels are insufficiently controlled by DPP-4 inhibitors; thus, multiple drug treatment is required.
Previous studies have shown that the accumulation of visceral fat is significantly associated with metabolic abnormalities [4]. We had previously conducted a randomized-controlled study [5] to evaluate the efficacy of ipragliflozin, a novel sodium-glucose transporter 2 (SGLT2) inhibitor, compared with that of metformin, for visceral fat reduction and glycemic control in Japanese patients with T2D being treated with sitagliptin. Our results showed that the mean percentage reduction in visceral fat area was significantly greater in the ipragliflozin group than in the metformin group. As the percentage of visceral fat to body weight generally increases with age, elderly patients are at a higher risk of associated metabolic abnormalities than young patients [6].
Moreover, elderly patients have a higher risk for sarcopenia and osteoporosis [7] and are at a higher risk of experiencing side effects related to the administered drugs than non-elderly patients [8]. However, information regarding the effects of SGLT2 inhibitors on elderly patients remains inadequate.
To study whether the SGLT2 inhibitors ipragliflozin and metformin reduce visceral fat and control glucose in elderly patients with T2D and how these treatments affect muscle mass and bone density, we conducted a sub-analysis of a previous randomized control study involving elderly patients aged 65–74 years.
Methods
This is a sub-analysis of a prospective, multicenter, open-label, blinded-endpoint randomized-controlled study involving 103 patients with T2D aged 20–74 years. The study design has been described previously [9]. The protocol for this research was approved by the relevant constituted ethics committees of the institutions, and it conforms to the provisions of the Declaration of Helsinki. The participants provided written informed consent and were enrolled between September 2014 and September 2016. The inclusion criteria were as follows: patients with T2D receiving sitagliptin for > 12 weeks; body mass index (BMI) ≥ 22 kg/m2; estimated glomerular filtration rate > 50.0 ml/min/1.73 m2; glycated hemoglobin (HbA1c) ≥ 7% and < 10%. All patients who satisfied these eligibility criteria were randomized (1:1) to receive either ipragliflozin or metformin. Patients in the ipragliflozin group received 50 mg of oral ipragliflozin daily. Patients in the metformin group were initially administered 500 mg of metformin daily, which was increased to 1000 mg of metformin daily after 2–4 weeks; however, the lower dose was continuously administered to patients who could not tolerate the higher dose. The primary outcome was any change in visceral fat area between the groups, as measured using computed tomography (CT) before study drug administration and after 24 weeks. CT images were then centrally evaluated by two radiologists who were blinded to the personal clinical information of the patients and treatment assignment. The secondary outcomes included changes in total and subcutaneous fat area, muscle volume, bone density measured by CT, body weight, BMI, waist circumference, handgrip strength, the bone formation marker bone alkali phosphatase (BAP), the bone absorption marker tartrate-resistant acid phosphatase-5b (TRACP-5b), fasting plasma glucose, insulin, homeostasis model assessment (HOMA)2-beta, HOMA2-R, HbA1c, total cholesterol, low-density lipoprotein cholesterol (LDL-C), fasting triglycerides, high-density lipoprotein cholesterol (HDL-C), non-HDL-C, uric acid levels, blood pressure, adiponectin, and high-sensitivity C-reactive protein (hs-CRP). The visceral adiposity index (VAI) [10] was evaluated based on waist circumference, BMI, and triglyceride and HDL-C levels. Treatment safety was assessed by recording all adverse events that were observed during the study. Hypoglycemia was evaluated based on symptom reports. Patients aged ≥ 65 years were selected for sub-analysis.
The primary and secondary outcomes in elderly and non-elderly patients (< 65 years) were also compared for reference.
Statistical Methodology
Outcomes were analyzed according to the data sets and expressed as the mean (standard deviation [SD] or 95% confidence interval [95% CI]) or median (95% CI), as appropriate. For the primary outcome, the least-square mean difference in any change in the visceral fat area in 24 weeks between the groups and its 95% CIs were estimated using analysis of covariance adjusted for baseline waist circumference, HbA1c, and baseline visceral fat area. The allocation factors were defined as possible confounders and previously listed in the statistical analysis plan, based on the ICH harmonized tripartite guideline statical principals for clinical trials. The power calculated as follows: the difference in the primary endpoint between the groups was − 17.5, SD ± 19, the significance level was 5% on both sides, and the number of patients was 29 (15 in the ipragliflozin group and 14 in the metformin group). For secondary outcomes, treatment group results were compared using Student’s t test or the Wilcoxon rank-sum test and Hodges-Lehmann estimator for continuous variables or using Pearson’s chi-square test or Fisher’s exact test for categorical outcomes, as appropriate. All P values were two sided, and results with P values < 0.05 were considered statistically significant. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
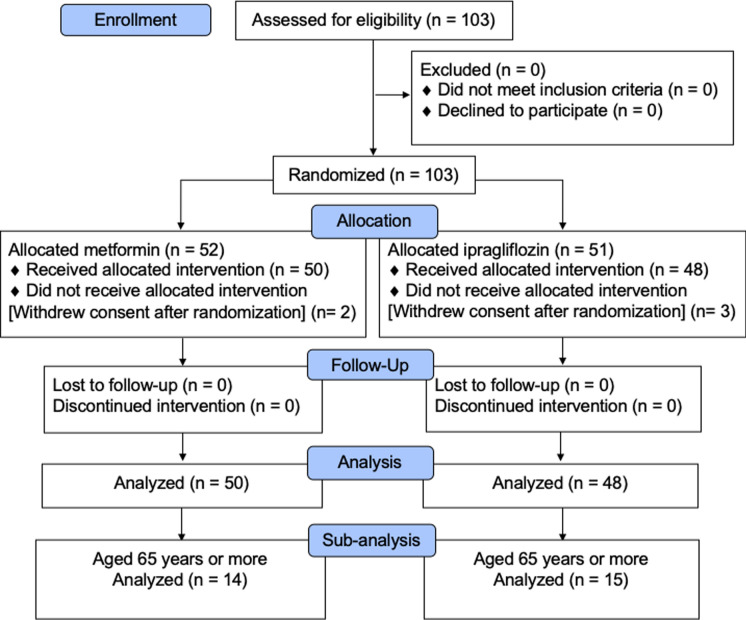
The enrollment process for the study is described in Fig. 1. The sub-analysis was performed with 15 elderly patients in the ipragliflozin group and 14 elderly patients in the metformin group. In this sub-analysis, 15 females and 14 males with a mean age of 69.0 ± 2.6 years and mean HbA1c 8.0 ± 0.8% were included. Patient characteristics were well balanced between the groups except for the percentage of blood pressure-lowering medicine use (Table 1). The characteristics of all patients in the two age groups (elderly/non-elderly) are shown in Table S1. The ratio of the visceral fat area to body weight was significantly greater in the elderly than in the non-elderly (mean ± SD, 2.3 ± 0.7 vs. 1.9 ± 0.7; P = 0.015).
Fig. 1.
CONSORT diagram
Table 1.
Baseline clinical characteristics
| Ipragliflozin, n = 15 | Metformin, n = 14 | P value | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age (years) | 68.7 ± 2.4 | 69.4 ± 2.9 | 0.483 |
| Male, n (%) | 7 (46.7) | 7 (50.0) | 0.858 |
| Body weight (kg) | 67.6 ± 10.6 | 70.1 ± 9.2 | 0.507 |
| BMI (kg/m2) | 26.8 ± 3.8 | 27.1 ± 3.9 | 0.842 |
| Waist circumference (cm) | 92.5 ± 9.6 | 93.2 ± 8.9 | 0.837 |
| Diabetes duration (years) | 8.0 ± 5.1 | 7.5 ± 6.2 | 0.847 |
| HbA1c (%) | 7.9 ± 0.7 | 8.0 ± 0.9 | 0.744 |
| Fasting plasma glucose (mg/dl) | 171.9 ± 38.6 | 166.5 ± 30.5 | 0.688 |
| Fasting insulin (μU/ml) | 13.0 ± 12.7 | 10.0 ± 4.4 | 0.872 |
| HOMA2-beta | 39.2 ± 22.3 | 34.8 ± 13.9 | 0.890 |
| HOMA2-R | 2.0 ± 2.0 | 1.5 ± 0.7 | 0.818 |
| Total cholesterol (mg/dl) | 187.5 ± 33.0 | 183.4 ± 34.8 | 0.753 |
| Triglyceride (mg/dl) | 126.8 ± 37.9 | 129.6 ± 69.8 | 0.629 |
| LDL-cholesterol (mg/dl) | 110.5 ± 27.4 | 102.7 ± 23.8 | 0.429 |
| HDL-cholesterol (mg/dl) | 50.0 ± 12.4 | 51.6 ± 7.2 | 0.867 |
| Non-HDL-cholesterol (mg/dl) | 136.5 ± 26.5 | 132.5 ± 32.3 | 0.721 |
| SBP (mmHg) | 139.5 ± 15.4 | 136.1 ± 18.2 | 0.591 |
| DBP (mmHg) | 80.9 ± 7.7 | 74.6 ± 9.8 | 0.065 |
| Uric acid (mg/dl) | 4.8 ± 1.2 | 4.8 ± 1.2 | 0.965 |
| Adiponectin (μg/ml) | 8.7 ± 5.8 | 8.1 ± 3.8 | 0.731 |
| hs-CRP (mg/dl) | 0.14 ± 0.13 | 0.15 ± 0.11 | 0.854 |
| Visceral fat area (cm2) | 163.2 ± 66.3 | 158.9 ± 55.0 | 0.856 |
| Subcutaneous fat area (cm2) | 169.5 ± 65.2 | 186.3 ± 66.4 | 0.512 |
| Total fat area (cm2) | 332.7 ± 106.2 | 345.3 ± 96.0 | 0.749 |
| Handgrip strength (kg) | 28.7 ± 9.9 | 28.1 ± 11.6 | 0.882 |
| BAP (µg/l) | 15.6 ± 5.6 | 13.7 ± 4.0 | 0.490 |
| TRACP-5b (mU/dl) | 350.6 ± 130.2 | 376.5 ± 112.1 | 0.534 |
| Bone density in fourth lumbar vertebra (HU) | 219.1 ± 49.8 | 194.6 ± 62.8 | 0.252 |
| Abdominal muscle area (cm2) | 236.8 ± 61.5 | 233.3 ± 63.4 | 0.884 |
| Visceral fat area/body weight (cm2/kg) | 2.41 ± 0.73 | 2.25 ± 0.68 | 0.566 |
| Visceral adiposity index | 2.4 ± 1.2 | 2.3 ± 1.4 | 0.863 |
| Smoking, n (%) | 6 (40) | 5 (35.7) | 0.812 |
| Complications | |||
| Hypertension, n (%) | 14 (93.3) | 9 (64.3) | 0.054 |
| Dyslipidemia, n (%) | 11 (73.3) | 12 (85.7) | 0.411 |
| Hyperuricemia, n (%) | 0 (0.0) | 0 (0.0) | NA |
| Diabetic retinopathy, n (%) | 1 (6.7) | 1 (7.1) | 0.629 |
| Diabetic nephropathy, n (%) | 7 (46.7) | 7 (50.0) | 0.617 |
| Diabetic neuropathy, n (%) | 2 (13.3) | 3 (21.4) | 0.629 |
| Medication | |||
| Blood pressure lowering, n (%) | 14 (93.3) | 8 (57.1) | 0.023 |
| Lipid lowering, n (%) | 9 (60.0) | 8 (57.1) | 0.876 |
Data are presented as the mean ± SD or n (%) unless otherwise indicated
SD standard deviation, BMI body mass index, HbA1c glycated hemoglobin, HOMA2 homeostasis model assessment 2, LDL-cholesterol low-density lipoprotein cholesterol, HDL-cholesterol high-density lipoprotein cholesterol, SBP systolic blood pressure, DBP diastolic blood pressure, hs-CRP high-sensitivity C-reactive protein, BAP bone alkali phosphatase, TRACP-5b tartrate-resistant acid phosphatase-5, NA not available
In the metformin group, none of the patients could tolerate an increased dose so they continued with the 500 mg metformin daily dose. Therefore, the average dose of metformin was 500 mg.
Body Composition
The primary and secondary outcomes are shown in Fig. 2 and Table 2. The percentage reduction in visceral fat area in the ipragliflozin group was significantly higher than that in the metformin group [mean (95% CI), − 10.58 (− 28.12, 6.95) % vs. 6.93 (− 10.66, 24.51) %; P = 0.034] (Fig. 2). Moreover, the reduction in the ratio of visceral fat area to body weight was significantly greater in the ipragliflozin group than in the metformin group (mean ± SD, − 15.10 ± 17.76% vs. 1.30 ± 15.37%; P = 0.019) (Table 2). Although not statistically significant, the reductions in total fat area (− 9.07 ± 14.04% vs. 4.10 ± 19.22%; P = 0.058) and waist circumference (− 2.03 ± 3.71% vs. 1.02 ± 5.04%; P = 0.073) were greater in the ipragliflozin group than in the metformin group (Fig. 2). However, there was no significant difference in subcutaneous fat area, body weight, BMI, and VAI between the groups (Table 2). Moreover, in the ipragliflozin group, the reduction in the ratio of the visceral fat area to body weight in the elderly tended to be greater than that in the non-elderly [− 15.10 (− 26.38, − 3.81) % vs. − 3.31 (− 10.04, 3.41) %; P = 0.066] (Table S2).
Fig. 2.
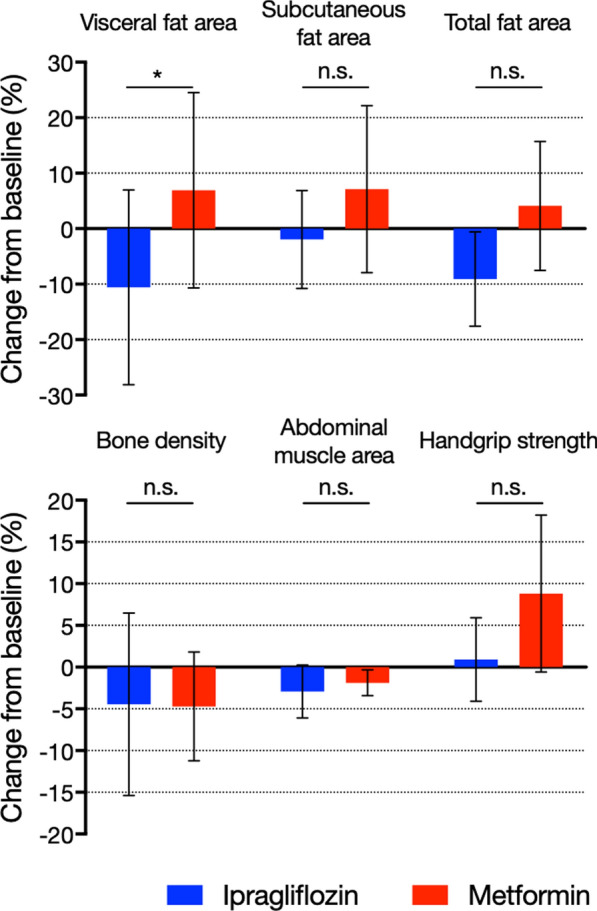
Changes in visceral fat area, subcutaneous fat area, total fat area (upper), bone density, abdominal muscle area, and handgrip strength (lower) from baseline following 24 weeks of treatment. Colored columns show mean values, whereas black bars show 95% confidence intervals. *P < 0.05. n.s. not significant
Table 2.
Primary and secondary endpoints at 24 weeks
| Ipragliflozin, n = 15 | Metformin, n = 14 | Difference between groups (%) | 95% CI | P value | ||
|---|---|---|---|---|---|---|
| Change from baseline (%) | Change from baseline (%) | |||||
| Visceral fat area (adjusted) | − 10.58 | 6.93 | − 17.51 | − 33.51 | − 1.52 | 0.034 |
| Visceral fat area (crude) | − 17.82 | − 0.26 | − 17.56 | − 33.02 | − 2.11 | 0.028 |
| Subcutaneous fat area | − 1.95 | 7.13 | − 9.08 | − 25.62 | 7.45 | 0.268 |
| Total fat area | − 9.07 | 4.10 | − 13.17 | − 26.79 | 0.46 | 0.058 |
| Body weight | − 2.99 | − 1.16 | − 1.83 | − 4.31 | 0.65 | 0.141 |
| BMI | − 2.99 | − 1.16 | − 1.83 | − 4.31 | 0.65 | 0.141 |
| Waist circumference | − 2.03 | 1.02 | − 3.05 | − 6.41 | 0.30 | 0.073 |
| Bone density | − 4.45 | − 4.71 | 0.26 | − 12.38 | 12.90 | 0.967 |
| Abdominal muscle area | − 2.92 | − 1.87 | − 1.04 | − 4.50 | 2.41 | 0.540 |
| Visceral fat area/body weight | − 15.10 | 1.30 | − 16.39 | − 29.80 | − 2.99 | 0.019 |
| Visceral adiposity index | − 10.70 | − 8.14 | − 2.57 | − 33.74 | 28.61 | 0.867 |
| Handgrip strength | 0.91 | 8.83 | − 7.92 | − 18.05 | 2.21 | 0.120 |
| BAP* | − 0.77 | − 7.74 | 12.03 | − 6.14 | 28.62 | 0.182 |
| TRACP-5b* | 7.56 | − 10.41 | 17.97 | 2.73 | 33.71 | 0.027 |
| HbA1c | − 6.48 | − 12.89 | 6.41 | − 0.31 | 13.13 | 0.061 |
| Fasting blood glucose | − 11.76 | − 14.38 | 2.62 | − 11.19 | 16.43 | 0.700 |
| Insulin level change* | − 20.51 | − 1.96 | − 6.80 | − 38.55 | 19.15 | 0.747 |
| HOMA2-beta (%)* | 9.61 | 16.01 | − 7.64 | − 44.30 | 24.65 | 0.608 |
| HOMA2-R (%)* | − 21.97 | − 8.19 | − 11.56 | − 44.22 | 17.65 | 0.510 |
| Total cholesterol | 1.11 | − 8.46 | 9.57 | 2.39 | 16.74 | 0.011 |
| LDL-C | − 0.27 | − 9.86 | 9.59 | − 2.11 | 21.28 | 0.104 |
| HDL-C | 8.49 | 4.80 | 3.70 | − 4.96 | 12.35 | 0.389 |
| Triglyceride* | − 9.29 | − 7.89 | − 1.86 | − 28.98 | 28.54 | 0.890 |
| Non-HDL-C | − 1.61 | − 12.86 | 11.25 | − 0.55 | 23.05 | 0.061 |
| Uric acid | − 8.83 | 6.10 | − 14.93 | − 27.33 | − 2.54 | 0.020 |
| SBP | − 0.57 | − 2.14 | 1.57 | − 8.07 | 11.20 | 0.741 |
| DBP | − 1.06 | 1.36 | − 2.42 | − 12.61 | 7.77 | 0.630 |
| Adiponectin | 6.56 | 4.99 | 1.56 | − 9.55 | 12.67 | 0.775 |
| hs-CRP* | − 21.12 | − 11.76 | − 10.11 | − 64.55 | 31.70 | 0.519 |
The difference between groups corresponds to the difference between the ipragliflozin and metformin groups. Negative values indicate the superiority of ipragliflozin over metformin
The least-square mean difference in any change in the visceral fat area in 24 weeks between the two groups and its 95% confidence intervals (CIs) were estimated using analysis of covariance adjusted for baseline waist circumference, HbA1c, and baseline visceral fat area
BAP bone alkali phosphatase, HbA1c glycated hemoglobin, HOMA2 homeostasis model assessment 2, TRACP-5b tartrate-resistant acid phosphatase-5, hs-CRP high-sensitivity C-reactive protein, SBP systolic blood pressure, DBP diastolic blood pressure, LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol
*Median and Hodges-Lehmann estimator
In addition, there were no significant differences between the ipragliflozin and metformin groups regarding changes in abdominal muscle area (− 2.92 ± 5.51% vs. − 1.87 ± 2.58%; P = 0.540), bone density (− 4.45 ± 19.74% vs. − 4.71 ± 10.78%; P = 0.967), and handgrip strength (0.91 ± 8.66% vs. 8.83 ± 16.29%; P = 0.120) (Table 2; Fig. 2). However, handgrip strength in the elderly was significantly higher than that in the non-elderly (8.83 ± 16.29% vs. 0.33 ± 11.22%; P = 0.043) following metformin treatment (Table S3).
Although there was no difference in the observed changes in BAP level between the treatment groups, the change in TRACP-5b level in the ipragliflozin group was significantly greater than that in the metformin group [median (95% CI), 7.56 (− 5.23, 34.75) % vs. − 10.41 (− 18.72, − 0.83) %; P = 0.027] (Table 2).
Glycemic Control
Both drug regimens reduced HbA1c and fasting plasma glucose levels relative to the baseline value. Although HbA1c levels tended to be lower in the metformin group than in the ipragliflozin group, the differences in the changes in fasting plasma glucose, fasting insulin levels, HOMA2-beta, and HOMA2-R at 24 weeks between the groups were not statistically significant (Table 2).
Additional Secondary Outcomes (Blood Lipid Panel, Uric Acid, Blood Pressure, Adiponectin, and hs-CRP)
Although the reduction percentage in the total cholesterol level in the metformin group was significantly larger than that in the ipragliflozin group (1.11 ± 6.55% vs. − 8.46 ± 11.58%; P = 0.011), there was no significant difference between the groups regarding changes in LDL-C, HDL-C, and triglyceride levels (Table 2). Changes in non-HDL-C tended to be lower in the metformin group than in the ipragliflozin group. The uric acid level was lower in the ipragliflozin group than in the metformin group (− 8.83 ± 14.52% vs. 6.10 ± 17.93; P = 0.020). The changes in blood pressure, adiponectin, and hs-CRP were similar between the groups (Table 2).
Adverse Events
The metformin group showed a significantly higher incidence of gastrointestinal disturbances than the ipragliflozin group [13.3 (1.7, 40.5) % vs. 64.3 (35.1, 87.2) %; P = 0.008]. There were no severe adverse events in either group.
Discussion
The aim of this sub-analysis was to compare the effects of ipragliflozin and the antihyperglycemic agent metformin on visceral fat and several other outcomes in young-elderly Japanese patients (65–74 years) diagnosed with T2D that was being insufficiently controlled with sitagliptin.
In this study, the SGLT2 inhibitor ipragliflozin reduced visceral fat, did not significantly decrease muscle mass and bone density, and was associated with a lower risk of gastrointestinal disturbance compared with metformin. At 24 weeks, the HbA1c and total cholesterol levels were lower in the metformin group than in the ipragliflozin group. Uric acid levels were lower and TRACP-5b levels were higher in the ipragliflozin group than in the metformin group. There were no severe adverse events in either group. Although several outcomes in this sub-analysis are the same as those in the analysis of all participants, the point to be noted is that ipragliflozin and metformin likely have the same effects in elderly patients and non-elderly patients. Moreover, the uric acid reduction effect was reported for the first time.
In the age group of 65–74 years included in this sub-analysis, the prevention of diabetes-associated complications, such as cardiovascular disease, sarcopenia, frailty, and osteoporosis, is critical to ensure healthy and active life. In elderly individuals, high visceral fat accumulation rather than high BMI is associated with a higher risk of death [11]. Indeed, BMI generally decreases and visceral fat increases with age, resulting in various metabolic disorders. These changes lead to an increased risk of cardiovascular disease [12, 13]. In contrast, the decrease in muscle mass associated with age can lead to sarcopenia and sarcopenic obesity. Although abdominal fat accumulation and loss of muscle mass are common changes associated with aging, elderly patients with sarcopenic obesity are generally more inactive and are at a higher risk of falls, fractures, and death [14, 15]. Therefore, it is important to reduce visceral fat while maintaining muscle mass and bone volume.
The reduction in visceral fat per kilogram of body weight following ipragliflozin treatment was great in the elderly. The visceral fat is the main depot of white adipose tissue for energy storage, whereas the subcutaneous fat is necessary to maintain body temperature and protect organs from external shocks. Reportedly, visceral fat has smaller adipocytes [16] and higher basal lipolysis rates than subcutaneous fat [17]. Therefore, reductions in visceral fat are more rapidly [18, 19] and easily accomplished than reductions in subcutaneous fat [20]. The elderly have more visceral fat and less subcutaneous fat per kilogram of body weight than the non-elderly. Therefore, visceral fat might be used as an energy supplement at first. As a result, the reduction in visceral fat per kilogram of body weight was assumed to be greater in the elderly. In this study, although the visceral fat was reduced, subcutaneous fat was not. Most of the fat in the whole body is subcutaneous fat [21, 22], and even if the visceral fat reduces, the effect on the total fat is small. Therefore, the total fat was not significantly reduced.
SGLT2 inhibitors may be an effective treatment strategy to prevent various metabolic disorders in the age group of 65–74 years, especially in elderly patients whose visceral fat is high. Uric acid levels were significantly decreased in the ipragliflozin group, as previously reported [23], because ipragliflozin promotes uric acid excretion in the proximal tubule. Although the total cholesterol level was reduced to a greater extent in the metformin group than in the ipragliflozin group, changes in other lipid and glucose profiles, blood pressure, adiponectin, and hs-CRP were not significantly different between the groups. A similar result was reported in a previous study, in which, although dapagliflozin therapy improved glucose control and reduced body weight, it exerted no significant effect on HDL-C levels and HDL functionality [24]. However, the number of patients in this sub-analysis might not have been large enough to show small differences between the groups.
Reportedly, the observed prevalence of sarcopenia in patients with T2D aged ≥ 65 years is as high as 18.7% in Japan, and this proportion increases with age [25], making it a significant concern among elderly patients with T2D. In general, weight loss may reduce muscle mass as well as fat. In this study, there was no significant difference in the changes in abdominal muscle area between the groups. SGLT2 inhibitors have been shown to increase skeletal muscle glucose uptake [26] and decrease insulin resistance in skeletal muscle [27]. However, although ipragliflozin has been reported to reduce skeletal muscle mass [28, 29], dapagliflozin was not found to have the same effect [30]. Therefore, additional longitudinal studies are warranted to clarify the effects of ipragliflozin on muscle mass and provide a full safety profile.
In the metformin group, handgrip strength was increased by 8.8%. Metformin has previously been reported to affect the mitochondria and activate adenosine monophosphate-activated protein (AMP) kinase. In muscle, AMP kinase activation promotes glucose transporter type 4 translocation to the cell membrane to increase glucose uptake [31–33]. Thus, metformin may enhance handgrip strength via these mechanisms. Reportedly, aging impairs interleukin-15 signaling via aging-induced defective AMP kinase activation in muscle [34, 35]. Therefore, the metformin effect regarding AMP kinase may be remarkable in the elderly. Moreover, the administration of 1500 mg of metformin has been reported to improve walking speed in pre-frail patients aged ≥ 60 years without diabetes [36].
Although the decrease in fasting plasma glucose levels was similar in both groups, HbA1c levels were reduced to a greater extent in the metformin group than in the ipragliflozin group. This may be because ipragliflozin treatment increased the hemoglobin concentration 0.92 mg/dl compared with metformin treatment. HbA1c in the ipragliflozin group may have been affected by hemoglobin concentration increase. Overall, metformin may be able to prevent muscle weakness in elderly patients with T2D who are at risk of sarcopenia or are frail.
Although concerns have been raised over fractures during treatment with the SGLT2 inhibitor canagliflozin [37], it has been reported that metformin may have osteoanabolic effects. Reductions in body weight and adipose tissue can modulate bone turnover [38]. In this sub-analysis, the changes in bone density between the groups were not different, which might be attributed to several reasons. For example, no patients with previous bone fractures or osteoporosis were included in this study; moreover, the study duration was short and the sample size was small. An increase in the bone absorption marker TRACP-5b was observed in the ipragliflozin group in a previous study [39], as observed in elderly patients in this study. Reportedly, TRACP-5b demonstrates significant negative correlation with bone mineral density [40]. Thus, a longer study duration might have revealed the effects of ipragliflozin on bone.
In a Japanese clinical trial regarding the up-titration of metformin [41], the rate of gastrointestinal disturbances in the elderly (68.3%) was similar to that obtained in our study (64.3%). This indicates that the results and observed side effects obtained in this study may be extended to the larger population of elderly patients in Japan.
This study has several limitations. First, although this was an open-label trial, evaluators of the primary outcomes were blinded to the group allocation and clinical information. Second, the study population was small and limited to Japanese patients. The power was 0.7; thus, the power for the primary endpoint was satisfied. However, studies with a larger sample size and various ethnic backgrounds are warranted to confirm our results. Third, the study duration was limited to only 24 weeks. Fourth, the dose of metformin was low; however, based on an administrative claims database linked to health check-up data in Japan, 72.9% of patients in whom metformin is initiated are prescribed 500 mg or less daily; only 2.0% are prescribed a daily dose > 1000 mg [42]. Reportedly, the treatment of 1000 mg metformin cannot reduce visceral fat [43]. Even the treatment of 1500 mg metformin cannot reduce visceral fat more than another SGLT2 inhibitor, luseogliflozin [44]. Finally, this study has limited applicability to patients aged > 75 years, who were not included in this study and for whom active measures against undernutrition and frailty are essential.
Conclusions
Ipragliflozin significantly reduced the visceral fat area in elderly patients with T2D when added to a DPP-4 inhibitor compared with metformin co-treatment. Long-term and large-scale studies are required to elucidate whether ipragliflozin is suitable for elderly patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all additional staff as well as the patients who participated in the present study.
Funding
This study was funded by Astellas Pharma Inc. (Tokyo, Japan). The funding source had no role in the study design, execution, analysis, data interpretation, decision to publish, or manuscript preparation. The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Authorship Contributions
All authors made significant contributions to the study. KY designed the original concept. MK wrote the manuscript. MK and KI managed the project. RI, ST, KS, YB, KI, SI, TI, SO, KK, MT, and KY reviewed and edited the manuscript. KI, MK, TI, KK, and MT wrote the protocol. MK, KI, RI, KS, HY, YB, SI, KI, TI, SO, KK, and YM recruited patients and conducted physical examinations, including obtaining blood samples. TH and RS evaluated CT images. ST performed statistical analyses. All authors read the final manuscript and provided approval for the publication of the manuscript. KY is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Prior Presentation
This manuscript is based on work that has been previously presented at the 54th Annual Meeting of the European Association for the Study of Diabetes, 4 October 2018, Berlin.
Disclosures
Koutaro Yokote received research grants from Astellas Pharma Inc. and MSD K.K. (Tokyo, Japan). Koutaro Yokote also received lecture fees from Astellas Pharma Inc. and Sumitomo Dainippon Pharma (Tokyo, Japan). Masaya Koshizaka, Ko Ishikawa, Ryoichi Ishibashi, Sho Takahashi, Kenichi Sakamoto, Hidetaka Yokoh, Yusuke Baba, Shintaro Ide, Kana Ide, Takahiro Ishikawa, Shunichiro Onishi, Kazuki Kobayashi, Minoru Takemoto, Takuro Horikoshi, Ryota Shimofusa, and Yoshiro Maezawa have nothing to disclose.
Compliance with Ethics Guidelines
This study was approved by the ethics committees of Chiba University Hospital (ID no. G26009) as a main center, Asahi General Hospital (ID number: 2014091602), National Hospital Organization Chiba Medical Center, Seirei Sakura Citizen Hospital, Chiba Rosai Hospital (ID number: 26-21), Toho University Sakura Medical Center (ID number: 2014-077), Tokyo Women’s Medical University Yachiyo Medical Center (ID number: 150303), Chiba Aoba Municipal Hospital, Kimitsu Chuo Hospital, Funabashi Central Hospital (ID number: H27-1), and Chiba Kaihin Municipal Hospital. This study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments. All subjects provided informed consent to participate in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Ministry of Health, Labor and Welfare. The situation on diabetes. In: Heisei 28 Survey on National Health and Nutrition. Tokyo, Japan: Ministry of Health, Labor and Welfare; 2017. https://www.mhlw.go.jp/bunya/kenkou/eiyou/dl/h28-houkoku-03.pdf Accessed 11 Mar 2020.
- 2.Seino Y, Kuwata H, Yabe D. Incretin-based drugs for type 2 diabetes: focus on east Asian perspectives. J Diabetes Investig. 2016;7:102–109. doi: 10.1111/jdi.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishikawa T, Koshizaka M, Maezawa Y, et al. Continuous glucose monitoring reveals hypoglycemia risk in elderly patients with type 2 diabetes mellitus. J Diabetes Investig. 2018;9:69–74. doi: 10.1111/jdi.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okauchi Y, Nishizawa H, Funahashi T, et al. Reduction of visceral fat is associated with decrease in the number of metabolic risk factors in Japanese men. Diabetes Care. 2007;30:2392–2394. doi: 10.2337/dc07-0218. [DOI] [PubMed] [Google Scholar]
- 5.Koshizaka M, Ishikawa K, Ishibashi R, et al. Comparing the effects of ipragliflozin versus metformin on visceral fat reduction and metabolic dysfunction in Japanese patients with type 2 diabetes treated with sitagliptin: a prospective, multicentre, open-label, blinded-endpoint, randomized controlled study (PRIME-V study) Diabetes Obes Metab. 2019;21:1990–1995. doi: 10.1111/dom.13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Silva ST, Niriella MA, Ediriweera DS, et al. Incidence and risk factors for metabolic syndrome among urban, adult Sri Lankans: a prospective, 7-year community cohort, follow-up study. Diabetol Metab Syndr. 2019;11:66. doi: 10.1186/s13098-019-0461-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen BR, Andersen HE, Haddock B, et al. Prevalence of muscle dysfunction concomitant with osteoporosis in a home-dwelling Danish population aged 65–93 years—the Copenhagen Sarcopenia Study. Exp Gerontol. 2020;138:110974. doi: 10.1016/j.exger.2020.110974. [DOI] [PubMed] [Google Scholar]
- 8.Altuntaş Y. Approach toward diabetes treatment in the elderly. Sisli Etfal Hastan Tip Bul. 2019;53:96–102. doi: 10.14744/SEMB.2019.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koshizaka M, Ishikawa K, Ishikawa T, et al. Efficacy and safety of ipragliflozin and metformin for visceral fat reduction in patients with type 2 diabetes receiving treatment with dipeptidyl peptidase-4 inhibitors in Japan: a study protocol for a prospective, multicentre, blinded-endpoint phase IV randomised controlled trial (PRIME-V study) BMJ Open. 2017;7:e015766. doi: 10.1136/bmjopen-2016-015766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amato MC, Giordano C, Galia M, et al. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33:920–922. doi: 10.2337/dc09-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiuge-Shimizu A, Kishida K, Funahashi T, et al. Absolute value of visceral fat area measured on computed tomography scans and obesity-related cardiovascular risk factors in large-scale Japanese general population (the VACATION-J study) Ann Med. 2012;44:82–92. doi: 10.3109/07853890.2010.526138. [DOI] [PubMed] [Google Scholar]
- 12.Cabrera MA, Gebara OC, Diament J, et al. Metabolic syndrome, abdominal obesity, and cardiovascular risk in elderly women. Int J Cardiol. 2007;114:224–229. doi: 10.1016/j.ijcard.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Butler J, Rodondi N, Zhu Y, et al. Metabolic syndrome and the risk of cardiovascular disease in older adults. J Am Coll Cardiol. 2006;47:1595–1602. doi: 10.1016/j.jacc.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 14.Scott D, Chandrasekara SD, Laslett LL, et al. Associations of sarcopenic obesity and dynapenic obesity with bone mineral density and incident fractures over 5–10 years in community-dwelling older adults. Calcif Tissue Int. 2016;99:30–42. doi: 10.1007/s00223-016-0123-9. [DOI] [PubMed] [Google Scholar]
- 15.Hirani V, Naganathan V, Blyth F, et al. Longitudinal associations between body composition, sarcopenic obesity and outcomes of frailty, disability, institutionalization and mortality in community-dwelling older men: The Concord Health and Ageing in Men Project. Age Ageing. 2017;46:413–420. doi: 10.1093/ageing/afw214. [DOI] [PubMed] [Google Scholar]
- 16.Fang L, Guo F, Zhou L, et al. The cell size and distribution of adipocytes from subcutaneous and visceral fat is associated with type 2 diabetes mellitus in humans. Adipocyte. 2015;4:273–279. doi: 10.1080/21623945.2015.1034920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arner P. Not all fat is alike. Lancet. 1998;351:1301–1302. doi: 10.1016/S0140-6736(05)79052-8. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Bujo H, Takahashi K, et al. Visceral fat: higher responsiveness of fat mass and gene expression to calorie restriction than subcutaneous fat. Exp Biol Med (Maywood) 2003;228:1118–1123. doi: 10.1177/153537020322801004. [DOI] [PubMed] [Google Scholar]
- 19.Ross R, Rissanen J, Pedwell H, et al. Influence of diet and exercise on skeletal muscle and visceral adipose tissue in men. J Appl Physiol. 1996;81:2445–2455. doi: 10.1152/jappl.1996.81.6.2445. [DOI] [PubMed] [Google Scholar]
- 20.Ryo M, Kishida K, Nakamura T, et al. Short-term intervention reduces bioelectrical impedance analysis-measured visceral fat in type 2 diabetes mellitus. Diabetes Res Clin Pract. 2014;103:e27–e29. doi: 10.1016/j.diabres.2013.12.046. [DOI] [PubMed] [Google Scholar]
- 21.van Hout MJP, Dekkers IA, Westenberg JJM, et al. The impact of visceral and general obesity on vascular and left ventricular function and geometry: a cross-sectional magnetic resonance imaging study of the UK Biobank. Eur Heart J Cardiovasc Imaging. 2020;2:273–281. doi: 10.1093/ehjci/jez279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abate N, Garg A, Peshock RM, et al. Relationships of generalized and regional adiposity to insulin sensitivity in men. J Clin Invest. 1995;96:88–98. doi: 10.1172/JCI118083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fralick M, Chen SK, Patorno E, et al. Assessing the risk for gout with sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes: a population-based cohort study. Ann Intern Med. 2020;172:186–194. doi: 10.7326/M19-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fadini GP, Bonora BM, Zatti G, et al. Effects of the SGLT2 inhibitor dapagliflozin on HDL cholesterol, particle size, and cholesterol efflux capacity in patients with type 2 diabetes: a randomized placebo-controlled trial. Cardiovasc Diabetol. 2017;16:42. doi: 10.1186/s12933-017-0529-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukuoka Y, Narita T, Fujita H, et al. Importance of physical evaluation using skeletal muscle mass index and body fat percentage to prevent sarcopenia in elderly Japanese diabetes patients. J Diabetes Investig. 2019;10:322–330. doi: 10.1111/jdi.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Brien TP, Jenkins EC, Estes SK, et al. Correcting postprandial hyperglycemia in Zucker diabetic fatty rats with an SGLT2 inhibitor restores glucose effectiveness in the liver and reduces insulin resistance in skeletal muscle. Diabetes. 2017;66:1172–1184. doi: 10.2337/db16-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joannides CN, Mangiafico SP, Waters MF, et al. Dapagliflozin improves insulin resistance and glucose intolerance in a novel transgenic rat model of chronic glucose overproduction and glucose toxicity. Diabetes Obes Metab. 2017;19:1135–1146. doi: 10.1111/dom.12923. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto C, Miyoshi H, Ono K, et al. Ipragliflozin effectively reduced visceral fat in Japanese patients with type 2 diabetes under adequate diet therapy. Endocr J. 2016;63:589–596. doi: 10.1507/endocrj.EJ15-0749. [DOI] [PubMed] [Google Scholar]
- 29.Tsurutani Y, Nakai K, Inoue K, et al. Comparative study of the effects of ipragliflozin and sitagliptin on multiple metabolic variables in Japanese patients with type 2 diabetes: a multicentre, randomized, prospective, open-label, active-controlled study. Diab Obes Metab. 2018;20:2675–2679. doi: 10.1111/dom.13421. [DOI] [PubMed] [Google Scholar]
- 30.Sugiyama S, Jinnouchi H, Kurinami N, et al. Dapagliflozin reduces fat mass without affecting muscle mass in type 2 diabetes. J Atheroscler Thromb. 2018;25:467–476. doi: 10.5551/jat.40873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kristensen JM, Treebak JT, Schjerling P, et al. Two weeks of metformin treatment induces AMPK-dependent enhancement of insulin-stimulated glucose uptake in mouse soleus muscle. Am J Physiol Endocrinol Metab. 2014;306:E1099–E1109. doi: 10.1152/ajpendo.00417.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pernicova I, Korbonits M. Metformin–mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10:143–156. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]
- 33.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelke C, Dziewas R, Minnerup J, et al. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine. 2019;49:381–388. doi: 10.1016/j.ebiom.2019.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crane JD, MacNeil LG, Lally JS, et al. Exercise-stimulated interleukin-15 is controlled by AMPK and regulates skin metabolism and aging. Aging Cell. 2015;14:625–634. doi: 10.1111/acel.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laksmi PW, Setiati S, Tamin TZ, et al. Effect of metformin on handgrip strength, gait speed, myostatin serum level, and health-related quality of life: a double blind randomized controlled trial among non-diabetic pre-frail elderly patients. Acta Med Indones. 2017;49:118–127. [PubMed] [Google Scholar]
- 37.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 38.Uusi-Rasi K, Sievanen H, Kannus P, et al. Influence of weight reduction on muscle performance and bone mass, structure and metabolism in obese premenopausal women. J Musculoskelet Neuronal Interact. 2009;9:72–80. [PubMed] [Google Scholar]
- 39.Koshizaka M, Ishikawa K, Ishibashi R, et al. Effects of ipragliflozin versus metformin in combination with sitagliptin on bone and muscle in Japanese patients with type 2 diabetes mellitus: sub-analysis of a prospective randomized controlled study (PRIME-V study) J Diabetes Investig. 2020 doi: 10.1111/jdi.13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshii I, Chijiwa T, Sawada N. Screening osteoporotic femoral neck without measuring bone mineral density with the use of tartrate resistant acid phosphatase-5b and serum-creatinine-to-cystatin C ratio in Japanese postmenopausal women. J Orthop Sci. 2020;25:671–676. doi: 10.1016/j.jos.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Odawara M, Kawamori R, Tajima N, et al. Long-term treatment study of global standard dose metformin in Japanese patients with type 2 diabetes mellitus. Diabetol Int. 2017;8:286–295. doi: 10.1007/s13340-017-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kameda T, Kumamaru H, Nishimura S, et al. Use of oral antidiabetic drugs in Japanese working-age patients with type 2 diabetes mellitus: dosing pattern for metformin initiators. Curr Med Res Opin. 2020;36:749–756. doi: 10.1080/03007995.2020.1729710. [DOI] [PubMed] [Google Scholar]
- 43.Dhaliwal R, Shepherd JA, El Ghormli L, et al. Changes in visceral and subcutaneous fat in youth with type 2 diabetes in the TODAY Study. Diabetes Care. 2019;42:1549–1559. doi: 10.2337/dc18-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shibuya T, Fushimi N, Kawai M, et al. Luseogliflozin improves liver fat deposition compared to metformin in type 2 diabetes patients with non-alcoholic fatty liver disease: a prospective randomized controlled pilot study. Diabetes Obes Metab. 2018;20:438–442. doi: 10.1111/dom.13061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.