Abstract
Introduction
HIV infected persons are twofold likely to experience a heart attack, stroke, and other forms of Cardiometabolic Syndrome (CMetS).
Methods
Electronic searches of databases (MEDLINE and Google Scholar) were queried for articles written in English from 2000 to 2019.
Results
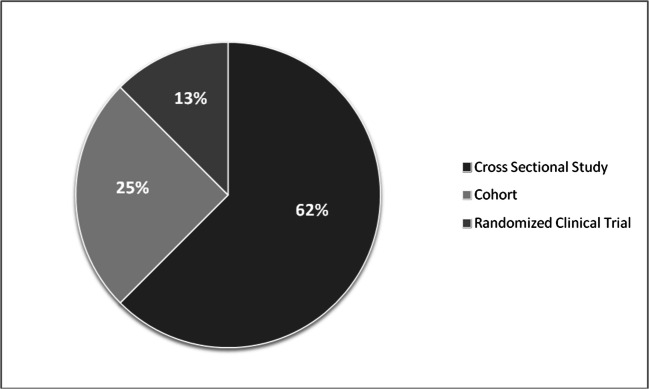
In this review (16 publications), a total of 14,002 participants from 8 countries were included. Two continents contributed to 62.5% of the CMetS studies while 38.1% from Latin America and 24.4% from North America. The studies were conducted in 113 different centers, with an average study length of 2.8 years. The majority of the study designs were cross-sectional (62%) followed by a cohort study (25%) and clinical trials (12.5%). The mean age of the population enrolled was 41.9 years and 54.6% of the participants were males. The overall prevalence of CMetS using the National Cholesterol Education Adult Treatment Panel definition was 20.6%. Only 31.3% of the studies were reported using the International Diabetes Federation definition. Smoking and high blood pressure were reported as a risk factor in 62.5% of the studies, while diabetes (31.3%), family history of CMetS (25%), and cardiac vascular and cancer diseases were reported in 12.5% of the studies. The average duration of stay with HIV after confirmation was 5.23 + 1.4 (years + SD) and the median duration on HAART was 4.5 + 2.3 (years + SD).
Conclusions
CMetS was a common problem among HIV infected persons. Several RFs can contribute to the development of CMetS with smoking and hypertension highly interrelated.
PROSPERO-number
CRD42018107187.
Keywords: Prevalence, Cardiometabolic Syndrome (CMetS), Human Immunodeficiency Virus (HIV), HIV comorbidities, Highly Active Antiretroviral Therapy (HAART)
Introduction
The Human Immunodeficiency Virus (HIV) associated morbidity and mortality have declined significantly since the introduction of Highly Active Antiretroviral Therapy (HAART) [1] and consequently, HAART extended the life expectancy of persons infected with HIV [2, 3]. As the life expectancy of HIV-infected patients increased, lifestyle-related co-morbidities such as Cardiovascular Diseases (CVDs), Diabetes Mellitus (DM) and hyperlipidemia began to emerge as a problematic issue and challenging the treatment of HIV [4]. People Living with HIV/ AIDS (PLWHA) exhibit multiple known Risk Factors (RFs) for Cardiometabolic Syndrome (CMetS) [2, 3]. The likelihood to experience heart attacks, strokes, and other forms of CVDs, is two-fold as compared to people who do not have the virus, even when HIV infection is well-controlled with HAART [5, 6].
CMetS is a constellation of metabolic dysfunctions including insulin resistance, impaired glucose tolerance, atherogenic dyslipidemia (high serum Triglycerides (TGs), and low High-Density Lipoprotein (HDL) levels), hypertension, intra-abdominal adiposity, and high blood sugar [7]. Additionally, diseases involving the cardiac system such as myocarditis, dilated cardiomyopathy, pericardial effusion, endocarditis, malignant neoplasms, etc., can also be included after confirmed by measuring the cardiac biomarkers in the laboratory and using the imaging modalities (echo and ECG) [8–10]. Hence, the term metabolic syndrome (MetS) can be used interchangeably with CMetS considering the overlap of the problems [11, 12]. There are five widely used definition tools for CMetS/MetS. These are the International Diabetes Federation (the IDF-2005), the Modified National Cholesterol Education Programme Adult Treatment Panel III (NCEP_ATP III-2005), the European Group for the Study of Insulin Resistance (EGIR-1999), the World Health Organization (the WHO-1998) and the American Heart Association/National Heart, Lung, and Blood Institute (AHA/ NHLBI) definitions [13–15]. Each definition tool has a unique purpose. For example, there are no absolute requirements for measurement of NCEP-ATP III definition, whereas in case of the WHO definition measuring insulin-like growth factor (IGF)/ impaired glucose tolerance (IGT)/ presence of type two diabetes mellitus (T2DM) or evidence of insulin resistance (IR) is an absolute requirement. In the case of EGIR, hyperinsulinemia I must measure, and in case of the IDF, the waist grid measurement is an absolute requirement and also microalbuminuria included [13, 14]. In all of the definitions fulfilling three out of the five requirements (any of three out of the five in case of NCEP and two of any plus one absolute requirement for the WHO, the EGIR, and the IDF) defines the criteria of CMetS. The five requirements are hypertension, hyperglycemia, dyslipidemia type one (uses the measurement of TG) and dyslipidemia type two (use measurement of HDL), and central obesity [16–19].
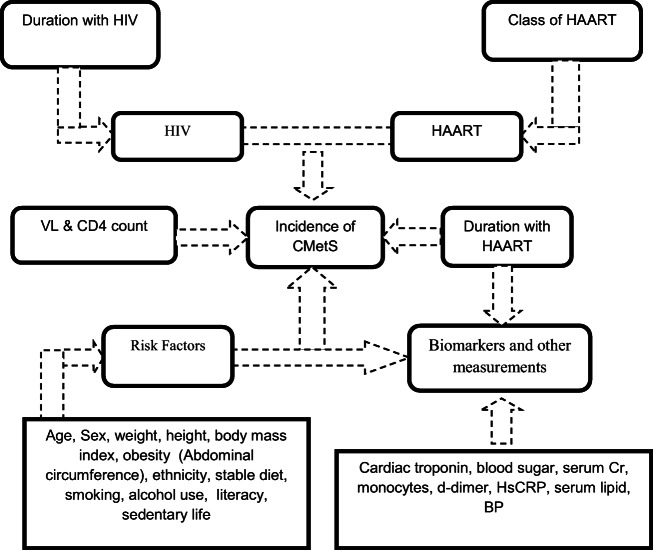
There are RFs believed to be contributed a lot for the development of CMetS in HIV infected persons, particularly with the advent of HAART [9, 20]. Proposed hypotheses include increased lipogenesis (independent of HAART) [21–24], increased monocytes [25], inflammation, abnormal blood clotting, tissue factors (TFs) [26], interleukin VI (IL-VI) & D-dimer [27, 28]. Of specific concern are the fact that the use of Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) and HIV protease inhibitors (PIs) has been reported to be associated with an increment in total cholesterol (TC) and TGs plasma levels irrespective of CD4 cell counts and HIV viral load (VL) [2, 29]. A report from the Multicenter AIDS Cohort Study (MACS) showed that HIV infection was associated with alteration in TC, HDL, and LDL [30]. Hence, our review study aimed to determine the prevalence of CMetS and associated RFs among PLWHA from articles published in English from 2000 to 2019 and fulfilling the criteria used for this purpose. The interaction of the different variables in the development and progression of CMetS has been illustrated in the following conceptual framework (Fig. 1).
Fig. 1.
Conceptual framework depicting the interaction of HIV, HAART medication and RFs to produce CMetS in HIV infected persons
Methods
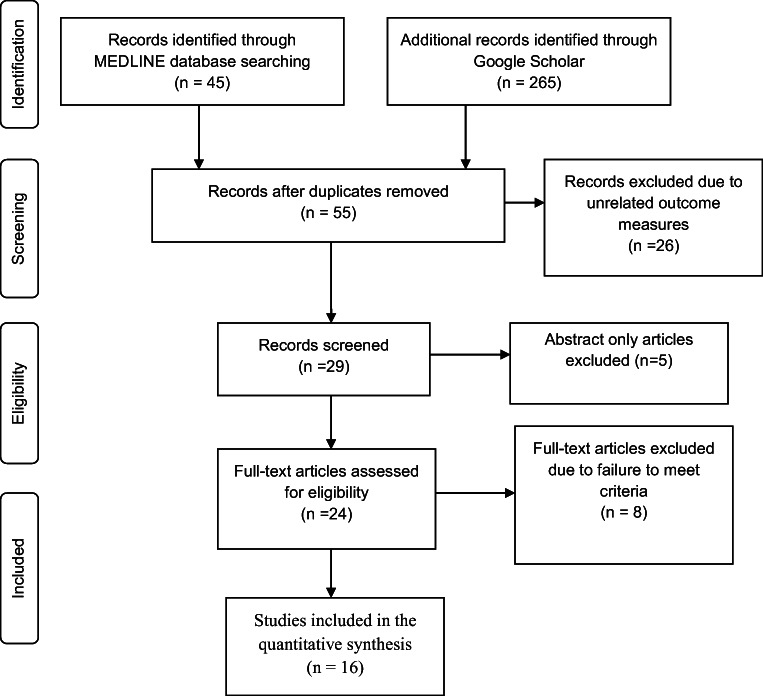
All methods for this systematic review were outlined through a Prospectively Registered Protocol (PROSPERO), which is found online with URL (https://www.crd.york.ac.uk/PROSPERO/#myprospero) and ID (CRD42018107187) and using the Preferred Reporting Items for Systematic Review (PRISMA) guidelines. The detail of the PRISMA has been shown in the following figure (Fig. 2).
Fig. 2.
Flow diagram of the study selection
Eligibility criteria
Eligible studies were randomized controlled trials and non-randomized studies (observational, cohort, and case-control) and cross-sectional studies that have investigated CMetS in HIV-infected persons.
Search strategy
Electronic searches of databases (MEDLINE and Google Scholar) were queried for articles written in English from 2000 to 2019. The following key search terms were used: “Risk”[Mesh] AND “Metabolic Syndrome”[Mesh] AND “HIV Infections/complications”[Mesh] AND “HIV Infections/diet therapy”[Mesh] OR “HIV Infections/drug therapy”[Mesh].
Inclusion criteria
Participants 18 years old and above (adults), all English language articles, and all eligible articles published in the last 20 years. Only published articles with abstract and/or full text were reviewed and assessed for inclusion in the study. Those meeting the following inclusion criteria were used for review: having described data on the relevant cardiometabolic risks in comparable HIV-infected populations, or comparable to the class of HAART medications, and included adult (aged 18 years or over). Studies were assessed for eligibility and, when sufficient information was not available from the title and/or abstract, the full- text was used and where the full-text was not available online, the authors were contacted via email.
Exclusion criteria
Studies not meeting both eligibility criteria were not included in the final review. Case reports, and case series were excluded. Duplicate articles and the number of authors fewer than three, as well as the volume of the article less than 5, were automatically excluded (Fig. 3).
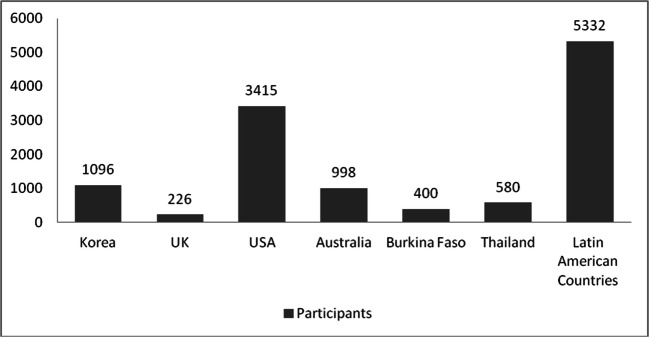
Fig. 3.
Sample size of the study participants from a different geographic location
Review questions
How prevalent is CMetS among HIV- infected persons?
What were the risk factors contributing to the development of CMetS among HIV-infected persons?
Outcome
Prevalence of CMetS in HIV infected persons
Risk factors contributing to CMetS in HIV infected persons
Risk of bias (quality) assessment
The risk of bias was assessed using the Cochrane risk of bias tool. The principal researcher’s background was tracked against the study area and the minimum number of researchers involved. The minimum number of researchers expected to be involved in the study would be three. The quality of the work of the researchers was checked against the volume of the publication (volume 5 or more) and published in international journals indexed to the PubMed database and also using a review of the article by our research team in case of any discrepancies.
Strategy for data synthesis
Only aggregate data were summarized and used for data analysis and interpretation of results. Quantitative and descriptive analysis was used to present the result. SPSS 21 was used to manage the quantitative data, to present results, and to do a descriptive analysis of the results. Our research team was composed of 2 professors and 1 assistant professor.
Search results
The search results have been illustrated in Fig. 1.
Analysis of subgroups or subsets
Subgroup analysis was not done due to the use of articles with different study designs.
Definition of terms
Cardiovascular diseases
Pathological conditions involving the cardiovascular system including heart; blood vessels; or pericardium [31].
HIV infections
Includes the spectrum of human immunodeficiency virus infections that range from asymptomatic seropositivity, thru AIDS-related complex (ARC), to acquired Immunodeficiency Syndrome (AIDS) [32].
Cardiometabolic Syndrome (CMetS)
Since its introduction in 1998, various diagnostic criteria for CMetS have been proposed [19]; the more widely used definitions for CMetS are from International Diabetes Federation (IDF) and the American Heart Association/National Heart, Lung, and Blood Institute (AHA/ NHLBI) [33]. In HIV-infected patients, there was an 85% agreement inpatient classification based on these two definitions [34]. More recently, the IDF and AHA/NHLBI agreed upon a common definition, which was used in our study [19, 35]. The other definition is using the National Cholesterol Education Program (NECP) Adult Treatment Panel III (ATP III) criteria [36], based on this CMetS was defined as the presence of three or more of the following components: i) waist circumference > 88 cm in women or > 102 cm in men; ii) blood pressure ≥ 130 mmHg systolic or ≥ 85 mmHg diastolic or use of antihypertensive medications; iii) triglycerides ≥150 mg/dL or use of lipid-lowering medications (niacin, fenofibrate, and gemfibrozil); iv) fasting blood glucose ≥100 mg/dL, physician-diagnosed diabetes or use of diabetic medications; v) high-density lipoprotein cholesterol (HDL) <50 mg/dL in women or < 40 mg/dL in men. The use of a lipid-lowering agent such as the use of a statin, fibrate, or niacin and antihypertensive medications can be used to define the criteria in the absence of laboratory evidence [37, 38].
Obesity
defined as a body mass index (BMI; calculated as the weight in kilograms divided by the square of height in meters) ≥30. HAART was defined as the use of 2 nucleosides (NRTIs) and a non-nucleoside reverse-transcriptase inhibitor (NNRTI), 2 NRTIs and a PI, or an NNRTI and a PI.
Risk factors
The probability that an event will occur. It encompasses a variety of measures of the probability of a generally unfavorable outcome. A group of six commonly accepted cardiometabolic risks were selected a priori for inclusion in this analysis: These are:- (i). Central Obesity: BMI > 30 kg/M2; (Waist girth >94CM for males and > 88 cm for females; or Waist/Hip ratio > 0.9; (ii). Hypertension: SBP > 140 and DBP > 90 [DM/CKD/HF BP > 130/85]; (iii). Impaired glucose handling: IR = FPG 100–125 mg/dL; DM = FPG >126 mg/dL or Random BSL >200 mg/dL; (iv). Dislipidemia: TG > 150 mg/dl (1.7 mmol/L); HDL-C 35 mg/dL (0.9 mmol/L); (v). Microalbuninuria: Urinary albumin excretion of 30–300 mg/day; (vi). Framingham risk >10% or cardiac/prothrombotic: Cardiac troponin T (cTnT) > 4.0 ng/ml; Positive echocardiography or d-dimer >500 ng/mL. Additional RFs used were older age, active cigarette smoking, family history, sedentary life, duration with HIV, type of HAART medication and substance use [39–41].
Definition of terms
Cardiovascular diseases:
Pathological conditions involving the cardiovascular system including the heart; the blood vessels; or the pericardium.
HIV Infections: Includes the spectrum of human immunodeficiency virus infections that range from asymptomatic seropositivity, thru AIDS-related complex (ARC), to acquired Immunodeficiency Syndrome (AIDS). The year introduced 1990.
Cardiometabolic Syndrome (CMetS):
Since its introduction in 1998, various diagnostic criteria for CMetS have been proposed (12); the more widely used definitions for CMetS are from International Diabetes Federation (IDF) and the American Heart Association/National Heart, Lung, and Blood Institute (AHA/ NHLBI). In HIV-infected patients, there was 85% agreement inpatient classification based on these two definitions (33). More recently, the IDF and AHA/NHLBI agreed upon a common definition, which was used in our study (12). CMetS is a constellation of metabolic dysfunction including insulin resistance, impaired glucose tolerance, atherogenic dyslipidemia [high Serum Triglycerides and low High-Density Lipoprotein levels], hypertension, intra-abdominal adiposity, high blood sugar, plus diseases involving the cardiac system like myocarditis, dilated cardiomyopathy, pericardial effusion, endocarditis, malignant neoplasms, etc. Based on the Adult Treatment Panel III (ATP III) criteria [42], CMetS was defined as the presence of three or more of the following components: 1) waist circumference > 88 cm in women or > 102 cm in men; 2) blood pressure ≥ 130 mm Hg systolic or ≥ 85 mm Hg diastolic or use of antihypertensive medications; 3) triglycerides ≥150 mg/dL or use of lipid-lowering medications (niacin, fenofibrate, and gemfibrozil); 4) fasting blood glucose ≥100 mg/dL, the physician diagnosed diabetes or use of diabetic medications; 5) high-density lipoprotein cholesterol (HDL) <50 mg/dL in women or < 40 mg/dL in men. The use of a lipid-lowering agent such as the use of a statin, fibrate, or niacin and antihypertensive medications can be used to define the criteria in the absence of laboratory evidence. Obesity was defined as a body mass index (BMI; calculated as the weight in kilograms divided by the square of height in meters) ≥30. HAART was defined as the use of 2 nucleosides (NRTIs) and a non-nucleoside reverse-transcriptase inhibitor (NNRTI), 2 NRTIs and a PI, or an NNRTI and a PI
Risk factors:
The probability that an event will occur. It encompasses a variety of measures of the probability of a generally unfavorable outcome. A group of six commonly accepted cardiometabolic risks was selected as a priori for inclusion in this analysis: These are:- (1). Central Obesity: BMI > 30 kg/M2; (Waist girth >94CM for males and > 88 cm for females; or Waist/Hip ratio > 0.9; (ii). Hypertension: SBP > 140 and DBP > 90 [DM/CKD/HF BP>130/85]; (iii). Impaired glucose handling: IR=FPG 100–125 mg/dL; DM=FPG >126 mg/dL or Random BSL >200 mg/dL; (iv). Dislipidemia: TG>150mg/dl (1.7mmol/L); HDL-C 35 mg/dL (0.9mmol/L); (v). Microalbuninuria: Urinary albumin excretion of 30-300 mg/day; (vi). Framingham risk >10% or cardiac/prothrombotic: Cardiac troponin T (cTnT) > 4.0 ng/ml; Positive echocardiography or d-dimer > 500 ng/mL. Additional RFs used were older age, active cigarette smoking, family history, sedentary life, duration with HIV, type of HAART medication and substance use [39-41].
Results
Demographic data
In this review (16 publications), a total of 14,002 participants from 8 countries were included. Two continents contributed to 62.5% of the CMetS studies in HIV- infected persons: Latin America mainly Argentina, Brazil, Venezuela, Colombia, Peru, Ecuador, and Chile had enrolled 38.1% of the population, while from North America, USA enrolled 24.4% participants (Table 1 & Fig. 3). The studies were done in around 113 different centers with an average study length of 2.8 years. The majority of the study design was cross-sectional (62%) followed by Cohort study (25%) and RCTs (12.5%). There was a shortage of articles reports from both Europe and Africa (Figs. 3 and 4).
Table 1.
Demographic data
| No | Country of study | Study design | Sample size | Number of sites | Study length in a year | Mean age | Percentage of male | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Korea | CSS | 1096 | 19 | 7 | 46 | 93 | [4] |
| 2 | UK | Cohort | 226 | 1 | 1 | 47 | 54 | [43] |
| 3 | Brazil | CSS | 87 | 1 | 1 | 37 | 76 | [44] |
| 4 | USA | Cohort | 567 | 1 | 4 | 43 | 47 | [45] |
| 5 | Brazil | CSS | 319 | 7 | 2 | 41 | 61 | [46] |
| 6 | USA | RCT | 2247 | 3 | 6 | 46 | 79 | [47] |
| 7 | Brazil | CSS | 819 | 1 | 1 | 41 | 55 | [48] |
| 8 | Australia | CSS | 788 | 32 | 3 | 45 | 18 | [49] |
| 9 | Burkina Faso | CSS | 400 | 1 | 1 | 41 | 38 | [50] |
| 10 | Thailand | CSS | 580 | 1 | 1 | 38 | 46 | [51] |
| 11 | Brazil | Cohort | 539 | 1 | 10 | 40 | 45 | [52] |
| 12 | Latin America* | Cohort | 4010 | 61 | 1 | 42 | 74 | [53] |
| 13 | Brazil | CSS | 273 | 1 | 2 | 48 | 55 | [54] |
| 14 | USA | CSS | 601 | 1 | 1 | 39 | 52 | [55] |
| 15 | Brazil | CSS | 1240 | 1 | 2 | 37 | 32 | [56] |
| 16 | Australia | RCT | 210 | 1 | 2 | 39 | 48 | [57] |
| Average | 875 | 8.3 | 2.8 | 41.9 | 54.6 | |||
Latin America* Countries (Argentina, Brazil, Venezuela, Colombia, Peru, Ecuador, and Chile); CSS = Cross-sectional study; RCT = Randomized Clinical Trial
Fig. 4.
Type of study designs used by the reviewed articles
The mean age of the population was 41.9 years and 54.6% of the participants were males. The study area, study design, and sample size distribution are shown in Table 1.
Clinical outcome
The overall prevalence of CMetS using the NCEP ATP III criteria was 20.6%. The average time after the first diagnosis with HIV among the participants was 5.23 + 1.4 (years + SD) and the median duration on HAART was 4.5 + 2.3 years. Around 72.2% of the study participants were on HAART medications and there were 20.6 HAART naïve participants in the studies at baseline. The NNRTIs were the most prevalent (61.9%) while only 2.5% were receiving INSTI (Table 2). 2NRTIs + NNRT regimen was the predominant combination (46.3%) followed by 2NRTI + PI (33.6%). The median CD4 cell count at the baseline was 328 cells/mm3 (Table 2).
Table 2.
Clinical data prevalence in association with CMetS
| Description | Mean | Median | Std. Deviation |
|---|---|---|---|
| CMetS using the NCEP ATP III, percent | 20.6 | 19.0 | 10.5 |
| CMetS using the IDF, percent | 13.5 | 13.5 | 2.9 |
| Average time since HIV was diagnosed, Year | 5.23 | 5.00 | 1.373 |
| Duration on HAART, Year | 4.46 | 3.80 | 2.302 |
| HAART use, percent | 72.19 | 70.00 | 9.425 |
| PI use, percent | 34.64 | 31.15 | 11.833 |
| NNRTIs use, percent | 61.88 | 63.70 | 11.733 |
| INSTI use, percent | 2.50 | 2.50 | 1.211 |
| NRTIs use, percent | 18.31 | 18.00 | 5.095 |
| 2NRTIs + NNRT, percent | 46.313 | 45.500 | 5.3006 |
| 2NRTI + PI, percent | 33.625 | 34.000 | 3.9306 |
| median CD4 cell count at the baseline, cells/mm3 | 328.063 | 344.000 | 116.5467 |
| 2NRTIs + NRT, percent | 22.188 | 22.000 | 9.8130 |
| 2NRTIs + INSTI, percent | 1.072 | 0.900 | 0.5348 |
| 3NNRTIs, percent | 0.325 | 0.200 | 0.2910 |
| BMI > 25 kg/m2 (overweight), percent | 21.500 | 21.000 | 2.4495 |
| BMI > 30 kg/m2 (obesity), percent | 12.688 | 12.500 | 2.7741 |
National Cholesterol Education Program-Adult Treatment Panel III (NCEP ATP III); IDF = National diabetes federation; PI = protease inhibitors; NNRTIs = Non-nucleoside reverse transcriptase inhibitors; NRTIs = nucleotide reverse transcriptase inhibitors; INSTI = integrase inhibitors
In 69% of the reported studies, CMetS was defined using the NCEP ATP III criteria. Only 31.3% were reported using the IDF criteria. A family history of cardiac and metabolic diseases was reported by 25%, while smoking was identified as a risk factor in 62.5% of the reports. The prevalence of DM, high BP, cardiac diseases, and cancer were associated with CMetS in 31.3%, 62.5%, 12.5%, and 12.5% of the studies respectively. Lipodystrophy was reported in 18.8% and concerning dyslipidemia low HDL-c and high TG was reported in 87.5% and 81.3% of the studies (Table 3).
Table 3.
Clinical report prevalence in association with CMetS
| Descriptions | Count (Percent) |
|---|---|
| Report on CMetS using the NCEP ATP III definition | 11(68.8) |
| Report of CMetS using the IDF definition | 5(31.3) |
| Report of Framingham cardiac risk score (10–19%) | 5(31.3) |
| Report of Framingham cardiac risk score (>20%) | 2(12.5) |
| FH of CMetS | 4(25.0) |
| High BP | 10(62.5) |
| DM | 5(31.3) |
| Current smoking | 10(62.5) |
| Cardiac disease | 2(12.5) |
| Cancer | 2(12.5) |
| Alcohol consumption | 4(25.0) |
| Baseline CD4 count report | 10(62.5) |
| Baseline VL report | 4(25.0) |
| LD report | 3(18.8) |
| Sedentary life | 3(18.8) |
| High TC | 7(43.8) |
| High LDL-c | 7(43.8) |
| Low HDL-c | 14 (87.5) |
| High TG | 13 (81.3) |
| High blood glucose | 4(25.0) |
| BMI > 25 kg/m2 (overweight & obesity) report | 10(62.5) |
National Cholesterol Education Program-Adult Treatment Panel III (NCEP ATP III); LD = Lipodystrophy; FH=Family History; CMetS = cardiometabolic Syndrome; DM = Diabetes Mellitus; BP = Blood Pressure; HAART = Highly Active Antiretroviral treatment; VL = viral load; High BP = BP ≥ 130/85 mmHg; Sedentary Life = <30 min active hours per day; High TC = Total cholesterol > 200 mg/dL; High LDL = Low density lipoprotein > 160 mg/dL; Low HDL = High density lipoprotein < 40 mg/dL, High TG = Triglycerides ≥ 150 mg/dL; High blood glucose = Fasting blood glucose > 100 mg/dL; Body Mass Index (BMI)
Discussion
This review aimed to assess the prevalence of CMetS, its risk factors, and co-morbidities in HIV infected persons using a systematically designed review study. Our finding revealed that the prevalence of CMetS in HIV infected persons was 20.6% by the NCEP-ATP III and 13.5% by IDF criteria. This was in agreement with the investigation done by Samaras & colleagues, where the prevalence was 18% by the ATP III and 14% by the IDF criteria. However, a bit higher figure was reported by Obirikorang & colleagues, where prevalence was 48.3% by ATP III and 42.3% by IDF criteria in HIV infected persons [58].
The European AIDS Clinical Society (EACS) guidelines on “the prevention and management of metabolic diseases in HIV” have strongly addressed that the risk of contracting CMetS is age-related [59]. In our aggregated data male participant was the predominant figure with 54.6% and the mean age was 42 year. This was a slightly higher figure than a survey done by Barbaro & colleagues in Italy, where the median age was 35 years (range, 22–50 years), [60]. Similarly, Mashinya and colleagues from South Africa reported that males were 2.94 times (p < 0.05) more likely to have metabolic disorders than females [61]. In another study conducted by Philip & colleagues from the U.S.A, 96.5% of patients who developed CMetS were male [9]. However, the issue of CMetS with gender has been reported differently among researchers. For example, Berhane and colleagues from Jimma (South West-Ethiopia) reported that female sex was independently associated with the prevalence of CMetS among PLWHA on HAART [62]. While Max and colleagues from Brazil reported that there was no significant association of CMetS by age and sex among the research participants [63].
Sedentary behavior has been defined as any activity with an energy expenditure of 1.0–1.5 metabolic equivalent units (METs). One MET is equivalent to the amount of oxygen consumed while sitting at rest and is equal to 3.5 ml O2 per kg body weight x min [64]. A sedentary lifestyle such as watching television, playing video games, surfing the internet/using a computer, doing homework/revisions, attending extra classes (not within regular school hours), reading, and sitting while playing for more than 4 h per day can contribute to the incidence of CMetS [65–67]. In this review, only 18.8% of the studies reported that sedentary life was associated with CMetS. However, measuring a person’s level of physical activity and measuring sedentary behaviors was a difficult task [68]. Guimarães and colleagues reported that a sedentary lifestyle was observed in 81% of HIV infected individuals. That was 4 times higher than our report [69]. Després JP reported that a sedentary lifestyle had contributed to the emergence of new drivers of CVD risks such as obesity and type 2 diabetes mellitus [70].
Cigarette smoking was the most important modifiable CV risk factor among HIV-infected patients (report from 62.5% of the studies). HIV-infected patients may have a higher prevalence of traditional CVD risk if they are smoking cigarettes than the general population [71]. Grinspoon and colleagues reported that smoking conferred a greater than twofold risk of Myocardial Infarction (MI). Hence, cessation of smoking is more likely to reduce CV risk than either the choice of antiretroviral therapy or the use of any lipid-lowering therapy [66].
Lipodystrophy (LD) was reported in 18.8% of our studies, whereas dyslipidemia such as low HDL-c and high TG was reported in 87.5% and 81.3% of the studies. Almost 63% of our studies reported that the combined overweight and obesity were responsible for greater than 33% of the metabolic alterations. This was in agreement with the study done by Max and colleagues, where the most frequent lipid abnormality in their research was Low HDL (53.5%), followed by high TG (36.1%). Ngatchou & colleague from Cameroon reported that fasting TGs and the atherogenic dyslipidemia ratio were significantly higher in HIV-infected persons than in controls [24]. According to Grinspoon and colleagues, such abnormalities in body composition had been responsible for 40 to 50% of ambulatory HIV-infected persons [66]. Similarly, Barbaro and colleagues reported that LD and metabolic alterations were associated with obesity in 62% of participants [60]. Dillon & colleagues further showed that HIV infection was associated with higher BMI [72] and many patients treated for HIV infection exhibit body composition changes, including peripheral fat atrophy and visceral lipodystrophy [73]. This is because people with HIV have an increased risk of developing a high waist circumference in both women and men [74].
In our review, the moderate to high risk of 10-year CVD risk using the Framingham equation was 31.3 and 12.5%, respectively. Mashinya and colleagues reported similarly that, a moderate to high 5-year CVD risk versus 10 year CVD risk was 31.1% and 6.7% respectively using the Framingham equation [61]. Barbaro & colleague reported that the annual incidence of Myocardial Infarction (MI) was 5.1/1000 (P < 0.001) (25). Philip & colleagues reported, 29 (4%) of the enrolled HIV infected participants developed acute MI in their investigation [9].
Our review revealed that 62% of the participants encountered high BP. Dillon & colleagues showed that HIV infection was associated with higher systolic blood pressure (SBP) and diastolic blood pressure (DBP), [72]. Manner and colleagues reported that low CD4 cell count <50 cells/μL was associated with sustained hypertension as a complication of CVD [75]. The prevalence of DM was found to be 31.3% in our investigation. Ngatchou & colleague reported that the prevalence of impaired fasting glucose and diabetes was higher in HIV-infected persons than in controls (47% versus 27%, and 26% versus 1%, respectively; both P < 0.01) [24].
The median CD4 cell count at the baseline of our study was 328 cells/mm3 as obtained from 62.5% of the studies. However, the evidence for an association between viral replication, CD4 cells count and CVD events in large epidemiologic studies appeared even more robust than the link with immunosuppression [76]. One report by Kaplan and colleagues in the USA measured the impact of HIV infection on carotid artery stiffness (cardiovascular complication) and among HIV-infected women, adjusted for age, HIV medications, and vascular risk factors, and higher CD4 was significantly associated with decreased carotid artery dispensability [77]. Carter and colleagues reported that CMetS was associated with lower CD4 cell counts and higher HIV viral load [78]. Gutierrez and Balasubramanyam also reported that low CD4 count (<200 cells/μl) by itself was a risk factor for developing LD and dysglycemia [79]. Jotwani and colleagues found HIV-related factors such as high VL and low CD4 count increased the risk of end-stage renal disease as it was found to be increased by diabetes, hypertension, CVD, and hepatitis C co-infection [80].
Duration with HAART had also been a key determinant of CVD risk [81]. In our investigation, the mean duration with HAART medication was 4.5 + 2.3 STD. Manner and colleagues found that duration with HAART was associated with sustained hypertension as a complication of CVD, and the highest proportion of hypertensive patients were observed in those who had prolonged HAART duration [75]. Mashinya and colleagues revealed that people on ART for less than 60 months were less likely to have a high TC/HDL-C ratio than people on ART for more than 60 months [61].
The use of HAART has significantly modified the course of HIV disease, lengthened survival, and improved the quality of life of HIV- infected persons. In contrast to this, recent data have raised concerns that HAART, HIV/AIDS itself, opportunistic infections (OIs), neoplasias, drugs used for OIs, and a combination of traditional vascular risk factors might have increased the risk for CMetS among PLWHA [60, 82, 83]. The common traditional vascular risk factors include aging, metabolic changes, smoking, high cholesterol, markers of innate immune activation, endothelial cell dysfunction, dilated cardiomyopathy, hyperinsulinemia, and thrombosis [83–85]. The emergence of such chronic disease complications in controlled HIV disease has then challenged the landscape of HIV clinical care [28, 86]. Some reports revealed HIV by itself can increase the relative risk of CVD by 61%, and the risk will be doubled for those taking HAART medications [87, 88]. HAART untreated patients had more likely to have low HDL and treated patients had high TGs level (39). Grinspoon and Deeks reported that compared with people without HIV infection, patients with the infection who are treated with HAART have an increased risk for several CMet complications [66, 89].
Currently, there are more than 25 HAART medications from six therapeutic classes for the management of HIV infection [89]. The most frequently prescribed antiretroviral regimen may vary from areas to area based on the guiding protocols, patients’ condition, and availability of HAART medication. In our review, around 72.2% of the participants were on HAART medications and there were 20.6 HAART naïve participants at the baseline. The NNRTIs were the most prevalent (61.9%) while only 2.5% were receiving INSTI (Table 2). 2NRTIs + NNRT regimen was the predominant combination (46.3%) followed by 2NRTI + PI (33.6%). In a study done by Max and colleague the most frequently prescribed HAART medication was the combination of two NRTI with one NNRTI in 50.4% of the research participants, followed by the combination of two NRTI with one PI in 42.5%. The use of three distinct classes of antiretroviral drugs (NRTI, NNRTI, and PI) was recorded for 4.4% of patients while the majority of patients (64.6%) were on three drugs; 25.7% were on four drugs and 8.0% on five or more drugs. The most commonly prescribed drugs were lamivudine (92.9%), zidovudine (66.4%), and efavirenz (49.6%), [63]. However, only some HAART medications have been reported to have a significant association with the incidence of CMetS in PLWHA. A systematic review done by P. Wilson showed that PLWHA on HAART is approximately as twice as likely to develop CMetS compared to non-HIV infected persons [88]. The risk differed between classes of ART and specific anti-HIV drugs [87, 88]. According to the study done by Sterne and colleague, CV risks were increased in a majority of patients initiated with HAART [90] and patients on a PI regimen had a 5.2-fold higher risk of dyslipidemia, even after adjusting for sex, age, and duration of HIV infection [63]. Barbaro & colleague reported that the annual incidence of MI was high in PI-positive wing than PI negative wing (P < 0.001). Additionally, PI-positive wing was more prone to develop lipodystrophy and metabolic alterations (62% of patients) compared to PI negative wing (4% of patients), [60]. Carter and colleagues reported that the PI exposed group had a significantly lower CD4 cell count and higher HIV viral load than the unexposed group [78]. Multiple reports from diverse longitudinally studied patient groups indicate links between PI uses in class or specific PI drug and increased risk for MI. Among the specific PI’s which have been identified to be associated with increased risks of MI were ritonavir, indinavir, and the fixed-dose combination of lopinavir/ritonavir [30]. However, our review study did not allow as seeing the impact of each class and individual drugs on the prevalence of the CMetS because of the difference in design and targeted outcome of the studies.
Dillon & colleagues reported the use of HAART medications can increase LDL and HDL and decrease HbA1 [72]. The use of non-nucleoside reverse transcriptase inhibitors (NNRTI), particularly efavirenz, has been demonstrated to give rise to pro-atherogenic serum lipid profiles. Early treatment (level of CD4 count up on initiating HAART) might reduce the negative effect of HIV on overall CV risk but may produce increased drug-related complications [30]. According to the study done by Barbaro & colleague patients were categorized based on the HAART regimen they received as 2 NRTIs + PIs (PI-positive wing) or 1 NNRTIs +2 NRTIs (PI negative wing) and underwent laboratory testing every 4 months. The cumulative annual incidence of Coronary Artery Disease (CAD)-related events was 9.8/1000 in PI-positive wing and 0.8/1000 in PI negative wing (P < 0.001) [60]. This was because PIs may induce lipoatrophy by inhibiting sterol regulatory enhancer-binding protein 1 (SREBP1)–mediated activation of the heterodimer consisting of adipocyte retinoid X receptor and peroxisome proliferator-activated receptor (PPAR) or related transcription factors such as PPARγ coactivator [66].
Conclusions
CMetS is a common problem among HIV infected persons. Different risk factors can contribute to the development of CMetS in HIV infected persons. The result of many of the published articles revealed that age, sex, weight, sedentary lifestyle, waist circumference, smoking, HIV itself, HAART medications, other co-morbidities like diabetes, hypertension, etc. could contribute to the incidence, pathogenesis, and progression of CMetS in HIV infected persons. From these, smoking and hypertension were highly interrelated with CMetS progression. Hence, identifying these RFs and monitoring their incidence could bring better HAART outcomes in HIV infected individuals. The findings of this review will be vital for researchers working in HIV and CMetS theme and also for quality service delivery and good treatment outcomes in clinical setups.
Limitations of the study
Research outputs inaccessible freely from database systems were not included in this study. This study is not a fully specific design study. Hence, comparing all outcomes among the different study designs was not possible.
Acknowledgements
We would like to thank Muhimbili University Health and Allied Sciences (MUHAS), Addis Ababa University (AAU), and the German Academic Exchanges Services (DAAD).
Authors’ contributions
MA, OM, and EE made substantial contributions to the conception and design of the study, analyzed and interpreted the data, drafted the manuscript, revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Funding information
The fund for conducting the review was sponsored by DAAD.
Compliance with ethical standards
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Minyahil Woldu, Omary Minzi and Ephrem Engidawork contributed equally to this work.
Contributor Information
Minyahil Woldu, Email: minyahil.alebachew@aau.edu.et, http://www.muhas.ac.tz, http://www.aau.edu.et.
Omary Minzi, Email: minziobejayesu@gmail.com, http://www.muhas.ac.tz.
Ephrem Engidawork, Email: ephrem.engidawork@aau.edu.et, http://www.aau.edu.et.
References
- 1.Paik J, II, Kotler DP. The prevalence and pathogenesis of diabetes mellitus in treated HIV-infection. Best Pract Res Clin Endocrinol Metab. 2011;25:469–478. doi: 10.1016/j.beem.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Friis-Møller NWR, Reiss P, Thiébaut R, Kirk O, d'Arminio Monforte A, Pradier C, Morfeldt L, Mateu S, Law M, El-Sadr W, De Wit S, Sabin CA, Phillips AN. Lundgren JD; DAD study group. Cardiovascular disease risk factors in HIV patients--association with antiretroviral therapy. AIDS. 2003;17(8):1179–1193. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 3.Cockerham LSR, Zolopa A, Rimland D, Lewis CE, Bacchetti P, Grunfeld C, Shlipak M, Tien PC. Association of HIV infection, demographic, and cardiovascular risk factors with all-cause mortality in the recent HAART era. J Acquir Immune Defic Syndr. 2010;53:102–106. doi: 10.1097/QAI.0b013e3181b79d22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oh DH, Ahn JY, et al. Metabolic complications among Korean patients with HIV infection: the Korea HIV/AIDS cohort study. J Korean Med Sci. 2017;32(8):1268–1274. doi: 10.3346/jkms.2017.32.8.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbaro G, Di Lorenzo G, Grisorio B, Barbarini G, Baum M, Shor-Posner G, et al. Dilated cardiomyopathy in HIV-infected patients. N Engl J Med. 1999;1999(340):732–735. [Google Scholar]
- 6.Steven G. Deeks, Sharon R Lewin, Diane V Havlir. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382(9903):1525–1533. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Todowede OO, Mianda SZ, Sartorius B. Prevalence of metabolic syndrome among HIV-positive and HIV-negative populations in sub-Saharan Africa-a systematic review and meta-analysis. Syst Rev. 2019;8(1):4. doi: 10.1186/s13643-018-0927-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srivastava AK. Challenges in the treatment of cardiometabolic syndrome. Indian J Pharm. 2012;44(2):155–156. doi: 10.4103/0253-7613.93579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varriale P, Saravi G, Eliezer Hernandez A, Carbon F. Acute myocardial infarction in patients infected with human immunodeficiency virus. Am Heart J. 2004;147(1):55–59. doi: 10.1016/j.ahj.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Willard S. The nurse practitioner’s role in managing dyslipidemia and other cardiovascular risk factors in HIV-infected patients: impact of antiretroviral therapy. JANAC. 2006;17(1):7–17. [DOI] [PubMed]
- 11.Papakonstantinou E, Lambadiari V, Dimitriadis G, Zampelas A. Metabolic syndrome, and cardiometabolic risk factors. Curr Vasc Pharmacol. 2013;11(6):858–879. doi: 10.2174/15701611113116660176. [DOI] [PubMed] [Google Scholar]
- 12.Naidu S, Ponnampalvanar S, Kamaruzzaman SB, Kamarulzaman A. Prevalence of metabolic syndrome among people living with HIV in developing countries: a systematic review. AIDS Patient Care STDs. 2017;31(1):1–13. doi: 10.1089/apc.2016.0140. [DOI] [PubMed] [Google Scholar]
- 13.Huang PL. A comprehensive definition of metabolic syndrome. Dis Model Mech. 2009;2(5–6):231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moy FM, Bulgiba A. The modified NCEP ATP III criteria may be better than the IDF criteria in diagnosing Metabolic Syndrome among Malays in Kuala Lumpur. BMC Public Health. 2010;10:678. doi: 10.1186/1471-2458-10-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Curr Opin Cardiol. 2006;21(1):1–6. doi: 10.1097/01.hco.0000200416.65370.a0. [DOI] [PubMed] [Google Scholar]
- 16.Saltzgiver S, Nielson A, Costello H, Baker A, Chan J, Aguilar D. Dietary determinants of metabolic syndrome parameters differ by gender in college students. Nutrients. 2019;11(12):2892. doi: 10.3390/nu11122892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miao W, Shen Q. Advance in metabolic syndrome research [J]. Academic Journal of Second Military Medical University. 2004;25(3):1–12.
- 18.Sasya M, Devi K, Babu JK, Rayappan B, Bosco J, Krishnan UM. Metabolic syndrome—an emerging constellation of risk factors: electrochemical detection strategies. Sensors. 2020;20(1):103. doi: 10.3390/s20010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sa-Caputo DC, Paineiras-Domingos L, Oliveira R, Neves MF, Brandão A, Marin PJ, et al. Acute effects of whole-body vibration on the pain level, flexibility, and cardiovascular responses in individuals with metabolic syndrome. Dose-Response. 2018;16(4):1559325818802139. doi: 10.1177/1559325818802139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen KA, Peer N, Mills EJ, Kengne AP. A meta-analysis of the metabolic syndrome prevalence in the global HIV-infected population. PLoS One. 2016;11(3):e0150970. doi: 10.1371/journal.pone.0150970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Judith S, Currier JDL, Carr A, Klein D, Sabin CA, Sax PE, Schouten JT, Smieja M. Epidemiological evidence for cardiovascular disease in HIV-infected patients and relationship to highly active antiretroviral therapy. Circulation. 2008;118:29–35. doi: 10.1161/CIRCULATIONAHA.107.189624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vernon LT, Babineau DC, Demko CA, Lederman MM, Wang X, Toossi Z, et al. A prospective cohort study of periodontal disease measures and cardiovascular disease markers in HIV-infected adults. AIDS Res Hum Retrovir. 2011;27(11):1157–1166. doi: 10.1089/AID.2010.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melzi S, Carenzi L, Cossu MV, Passerini S, Capetti A, Rizzardini G. Lipid metabolism and cardiovascular risk in HIV-1 infection and HAART: present and future problems. Cholesterol. 2010;2010:271504. doi: 10.1155/2010/271504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ngatchou W, Lemogoum D, Ndobo P, Yagnigni E, Tiogou E, Nga E, et al. Increased burden and severity of the metabolic syndrome and arterial stiffness in treatment-naive HIV+ patients from Cameroon. Vasc Health Risk Manag. 2013;9:509. doi: 10.2147/VHRM.S42350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayosi BM. Contemporary trends in the epidemiology and management of cardiomyopathy and pericarditis in sub-Saharan Africa. Heart. 2007;93(10):1176–1183. doi: 10.1136/hrt.2007.127746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, et al. Contemporary definitions and classification of the cardiomyopathies. Circulation. 2006;113(14):1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 27.Triant VA. Cardiovascular disease and HIV infection. Curr HIV/AIDS Rep. 2013;10(3):199–206. doi: 10.1007/s11904-013-0168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbaro G. Cardiovascular manifestations of HIV infection. Circulation. 2002;106(11):1420–1425. doi: 10.1161/01.cir.0000031704.78200.59. [DOI] [PubMed] [Google Scholar]
- 29.Herskowitz A. Cardiomyopathy and other symptomatic heart diseases associated with HIV infection. Curr Opin Cardiol. 1996;11(3):325–331. doi: 10.1097/00001573-199605000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Palella FJ, Phair JP. Cardiovascular disease in HIV infection. Curr Opin HIV AIDS. 2011;6(4):266–271. doi: 10.1097/COH.0b013e328347876c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lorell B, Grossman W. Profiles in constrictive pericarditis, restrictive cardiomyopathy, and cardiac tamponade. In: Febige La, editor. Cardiac catheterization and angiography Third edition. Philadelphia: Harvard Medical School; 1986. p. 427–45.
- 32.Grant I, Atkinson JH, Hesselink JR, Kennedy CJ, Richman DD, Spector SA, et al. Evidence for early central nervous system involvement in the acquired immunodeficiency syndrome (AIDS) and other human immunodeficiency viruses (HIV) infections: studies with neuropsychologic testing and magnetic resonance imaging. Ann Intern Med. 1987;107(6):828–836. doi: 10.7326/0003-4819-107-6-828. [DOI] [PubMed] [Google Scholar]
- 33.Reis AS, Paineiras-Domingos LL, Moreira-Marconi E, Moura-Fernandes MC, Quinart H, Boyer FC, et al. Body composition in metabolic syndrome: proposal of a protocol for a randomized clinical trial evaluating the effect of whole-body vibration exercise. Braz J Health Biomed Sci. 2019;18(103):33–40. [Google Scholar]
- 34.Hofer J, Niebauer J. Cardiovascular risk factors: lipids and lifestyle changes. Arch Med Sci. 2007;3(4A):S69. [Google Scholar]
- 35.Lim S, Eckel RH. Pharmacological treatment and therapeutic perspectives of metabolic syndrome. Rev Endocr Metab Disord. 2014;15(4):329–341. doi: 10.1007/s11154-014-9298-4. [DOI] [PubMed] [Google Scholar]
- 36.Takeuchi H, Saitoh S, Takagi S, Ohnishi H, Ohhata J, Isobe T, et al. Metabolic syndrome and cardiac disease in Japanese men: applicability of the concept of the metabolic syndrome defined by the National Cholesterol Education Program–Adult Treatment Panel III to Japanese men—the Tanno and Sobetsu study. Hypertens Res. 2005;28(3):203–208. doi: 10.1291/hypres.28.203. [DOI] [PubMed] [Google Scholar]
- 37.Grundy SM. Metabolic syndrome scientific statement by the American heart association and the national heart, lung, and blood institute. Am Heart Assoc. 2005. p. 2243–4. [DOI] [PubMed]
- 38.Skilton MR, Moulin P, Sérusclat A, Nony P, Bonnet F. A comparison of the NCEP-ATP III, IDF and AHA/NHLBI metabolic syndrome definitions with relation to early carotid atherosclerosis in subjects with hypercholesterolemia or at risk of CVD: evidence for sex-specific differences. Atherosclerosis. 2007;190(2):416–422. doi: 10.1016/j.atherosclerosis.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 39.Tarp J. Epidemiological relationships between physical activity, fitness, and adiposity with cardiometabolic risk factors in youth. Department of Sports Science and Clinical Biomechanics. Denmark: University of Southern Denmark; 2018.
- 40.Henry-Okafor QO. Effect of obesity on the traditional and emerging cardiovascular disease risk factors in African American women. Nursing. Memphis: University of Tennessee Health Science Center; 2009. p. 93.
- 41.Torrance T. Sleep, Shiftwork adaptation, autonomic dysfunction, and metabolic syndrome. Epidemiology and Biostatistics. Columbia: University of South Carolina; 2018. p. 170.
- 42.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 43.Rider OJ, Asaad M, Ntusi N, Wainwright E, Clutton G, Hancock G, et al. HIV is an independent predictor of aortic stiffness. Journal of cardiovascular magnetic resonance: official journal of the Society for Cardiovascular Magnetic. Resonance. 2014;16:57. doi: 10.1186/s12968-014-0057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raposo MA, Armiliato GNA, Guimaraes NS, Caram CA, Silveira RDS, Tupinambas U. Metabolic disorders and cardiovascular risk in people living with HIV/AIDS without the use of antiretroviral therapy. Rev Soc Bras Med Trop. 2017;50(5):598–606. doi: 10.1590/0037-8682-0258-2017. [DOI] [PubMed] [Google Scholar]
- 45.Jarrett OD, Wanke CA, Ruthazer R, Bica I, Isaac R, Knox TA. Metabolic syndrome predicts all-cause mortality in persons with human immunodeficiency virus. AIDS Patient Care STDs. 2013;27(5):266–271. doi: 10.1089/apc.2012.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silva EF, Bassichetto KC, Lewi DS. Lipid profile, cardiovascular risk factors, and metabolic syndrome in a group of AIDS patients. Arq Bras Cardiol. 2009;93(2):113–118. doi: 10.1590/s0066-782x2009000800008. [DOI] [PubMed] [Google Scholar]
- 47.Krishnan S, Schouten JT, Atkinson B, Brown T, Wohl D, McComsey GA, et al. Metabolic syndrome before and after initiation of antiretroviral therapy in treatment-naive HIV-infected individuals. J Acquir Immune Defic Syndr. 2012;61(3):381–389. doi: 10.1097/QAI.0b013e3182690e3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Signorini DJHP, Monteiro MCM, Andrade MdFCd, Signorini DH, Eyer-Silva WdA. What should we know about metabolic syndrome and lipodystrophy in AIDS? Rev Assoc Med Bras. 2012;58(1):70–5. [PubMed]
- 49.Samaras K, Wand H, Law M, Emery S, Cooper D, Carr A. Prevalence of metabolic syndrome in HIV-infected patients receiving highly active antiretroviral therapy using International Diabetes Foundation and Adult Treatment Panel III criteria: associations with insulin resistance, disturbed body fat compartmentalization, elevated C-reactive protein, and hypoadiponectinemia. Diabetes Care. 2007;30(1):113–119. doi: 10.2337/dc06-1075. [DOI] [PubMed] [Google Scholar]
- 50.Sawadogo A, Sanou S, Hema A, Kamboule BE, Kabore NF, Sore I, et al. Metabolic syndrome and cardiovascular risk patients under antiretrovirals in a day hospital at Bobo-Dioulasso (Burkina Faso) Bulletin de la Societe de pathologie exotique (1990) 2014;107(3):151–158. doi: 10.1007/s13149-014-0371-8. [DOI] [PubMed] [Google Scholar]
- 51.Jantarapakde J, Phanuphak N, Chaturawit C, Pengnonyang S, Mathajittiphan P, Takamtha P, et al. Prevalence of metabolic syndrome among antiretroviral-naive and antiretroviral-experienced HIV-1 infected Thai adults. AIDS Patient Care STDs. 2014;28(7):331–340. doi: 10.1089/apc.2013.0294. [DOI] [PubMed] [Google Scholar]
- 52.Dias RFG, Bento LO, Tavares C, Ranes Filho H, Silva M, Moraes LC, et al. Epidemiological and clinical profile of HIV-infected patients from Southwestern Goias state, Brazil. Rev Inst Med Trop Sao Paulo. 2018;60:e34. doi: 10.1590/s1678-9946201860034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cahn P, Leite O, Rosales A, Cabello R, Alvarez CA, Seas C, et al. Metabolic profile and cardiovascular risk factors among Latin American HIV-infected patients receiving HAART. Braz J Infect Dis: an official publication of the Brazilian Society of Infectious Diseases. 2010;14(2):158–166. doi: 10.1590/s1413-86702010000200008. [DOI] [PubMed] [Google Scholar]
- 54.Akl LD, Valadares ALR, Moraes MJ, Pinto-Neto AM, Lagrutta B, Costa-Paiva L. Metabolic syndrome in HIV-infected middle-aged women on antiretroviral therapy: prevalence and associated factors. J Korean Med Sci. 2017;21(3):263–9. 10.1016/j.bjid.2017.02.003. [DOI] [PMC free article] [PubMed]
- 55.Mondy K, Overton ET, Grubb J, Tong S, Seyfried W, Powderly W, et al. Metabolic syndrome in HIV-infected patients from an urban, midwestern US outpatient population. Clin Infect Dis. 2007;44(5):726–734. doi: 10.1086/511679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alencastro PR, Fuchs SC, Wolff FH, Ikeda ML, Brandão AB, Barcellos NT. Independent predictors of metabolic syndrome in HIV-infected patients. AIDS Patient Care STDs. 2011;25(11):627–634. doi: 10.1089/apc.2010.0360. [DOI] [PubMed] [Google Scholar]
- 57.Martin A, Moore CL, Mallon PW, Hoy JF, Emery S, Belloso WH, et al. HIV lipodystrophy in participants randomized to lopinavir/ritonavir (LPV/r) +2-3 nucleoside/nucleotide reverse transcriptase inhibitors (N(t)RTI) or LPV/r + raltegravir as second-line antiretroviral therapy. PLoS One. 2013;8(10):e77138. doi: 10.1371/journal.pone.0077138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Obirikorang C, Quaye L, Osei-Yeboah J, Odame EA, Asare I. Prevalence of metabolic syndrome among HIV-infected patients in Ghana: A cross-sectional study. Niger Med J: Journal of the Nigeria Medical Association. 2016;57(2):86–90. doi: 10.4103/0300-1652.182082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lundgren JD, Battegay M, Behrens G, De Wit S, Guaraldi G, Katlama C, et al. European AIDS clinical society (EACS) guidelines on the prevention and management of metabolic diseases in HIV. HIV Medicine. 2008;9(2):72–81. doi: 10.1111/j.1468-1293.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- 60.Barbaro G, Di Lorenzo G, Cirelli A, Grisorio B, Lucchini A, Hazra C, et al. An open-label, prospective, observational study of the incidence of coronary artery disease in patients with HIV infection receiving highly active antiretroviral therapy. Clin Ther. 2003;25(9):2405–2418. doi: 10.1016/s0149-2918(03)80283-7. [DOI] [PubMed] [Google Scholar]
- 61.Mashinya F, Alberts M, Colebunders R. Assessment of cardiovascular risk factors in people with HIV infection treated with ART in rural South Africa: a cross-sectional study. AIDS Res Ther. 2015;12(1):42. doi: 10.1186/s12981-015-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berhane T, Yami A, Alemseged F, Yemane T, Hamza L, Kassim M, et al. Prevalence of lipodystrophy and metabolic syndrome among HIV positive individuals on Highly Active Anti-Retroviral treatment in Jimma, South West Ethiopia. Pan Afr Med J. 2012;13(1)1–14. [PMC free article] [PubMed]
- 63.Nery MW, Maria C, Martelli T, Turchi MD. Dyslipidemia in AIDS patients on highly active antiretroviral therapy. Braz J Infect Dis. 2011;15(2):151–155. [PubMed] [Google Scholar]
- 64.Jette M, Sidney K, Blumchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol. 1990;13(8):555–565. doi: 10.1002/clc.4960130809. [DOI] [PubMed] [Google Scholar]
- 65.Pitsavos C, Panagiotakos D, Weinem M, Stefanadis C. Diet, exercise and the metabolic syndrome. Rev Diabet Stud. 2006;3(3):118. doi: 10.1900/RDS.2006.3.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352(1):48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 67.Lee ST, Wong JE, Nik Shanita S, Ismail MN, Deurenberg P, Poh BK. Daily physical activity and screen time, but not other sedentary activities, are associated with measures of obesity during childhood. Int J Environ Res Public Health. 2015;12(1):146–161. doi: 10.3390/ijerph120100146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ford ES, Kohl HW, Mokdad AH, Ajani UA. Sedentary behavior, physical activity, and metabolic syndrome among US adults. Obesity. 2005;13(3):608–614. doi: 10.1038/oby.2005.65. [DOI] [PubMed] [Google Scholar]
- 69.Guimarães MMM, Greco DB, de Figueiredo SM, Fóscolo RB, de Oliveira AR, de Campos Machado LJ. High-sensitivity C-reactive protein levels in HIV-infected patients treated or not with antiretroviral drugs and their correlation with factors related to cardiovascular risk and HIV infection. Atherosclerosis. 2008;201(2):434–439. doi: 10.1016/j.atherosclerosis.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 70.Després J-P. Body fat distribution and risk of cardiovascular disease. Circulation. 2012;126(10):1301–1313. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 71.Iloeje U, Yuan Y, L'italien G, Mauskopf J, Holmberg S, Moorman A, et al. Protease inhibitor exposure and increased risk of cardiovascular disease in HIV-infected patients. HIV Medicine. 2005;6(1):37–44. doi: 10.1111/j.1468-1293.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 72.Dillon DG, Gurdasani D, Riha J, Ekoru K, Asiki G, Mayanja BN, et al. Association of HIV and ART with cardiometabolic traits in sub-Saharan Africa: a systematic review and meta-analysis. Int J Epidemiol. 2013;42(6):1754–1771. doi: 10.1093/ije/dyt198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stanley TL, Grinspoon SK. Body composition and metabolic changes in HIV-infected patients. J Infect Dis. 2012;205(suppl_3):S383–SS90. doi: 10.1093/infdis/jis205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clark SJ, Gómez-Olivé FX, Houle B, Thorogood M, Klipstein-Grobusch K, Angotti N, et al. Cardiometabolic disease risk and HIV status in rural South Africa: establishing a baseline. BMC Public Health. 2015;15(1):135. doi: 10.1186/s12889-015-1467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Manner IW, Trøseid M, Oektedalen O, Baekken M, Os I. Low nadir CD4 cell count predicts sustained hypertension in HIV-infected individuals. J Clin Hypertens. 2013;15(2):101–106. doi: 10.1111/jch.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Longenecker CT, Triant VA. Initiation of antiretroviral therapy at high CD4 counts: does it reduce the risk of cardiovascular disease? Curr Opin HIV AIDS. 2014;9(1):54–62. doi: 10.1097/COH.0000000000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T cell activation predicts carotid artery stiffness among HIV-infected women. Atherosclerosis. 2011;217(1):207–213. doi: 10.1016/j.atherosclerosis.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carter V, Hoy J, Bailey M, Colman P, Nyulasi I, Mijch A. The prevalence of lipodystrophy in an ambulant HIV-infected population: it all depends on the definition. HIV Medicine. 2001;2(3):174–180. doi: 10.1046/j.1468-1293.2001.00073.x. [DOI] [PubMed] [Google Scholar]
- 79.Gutierrez AD, Balasubramanyam A. Dysregulation of glucose metabolism in HIV patients: epidemiology, mechanisms, and management. Endocrine. 2012;41(1):1–10. doi: 10.1007/s12020-011-9565-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jotwani V, Li Y, Grunfeld C, Choi AI, Shlipak MG. Risk factors for ESRD in HIV-infected individuals: traditional and HIV-related factors. Am J Kidney Dis. 2012;59(5):628–635. doi: 10.1053/j.ajkd.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aboud M, Elgalib A, Pomeroy L, Panayiotakopoulos G, Skopelitis E, Kulasegaram R, et al. Cardiovascular risk evaluation and antiretroviral therapy effects in an HIV cohort: implications for clinical management: the CREATE 1 study. Int J Clin Pract. 2010;64(9):1252–1259. doi: 10.1111/j.1742-1241.2010.02424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Odhiambo C, Davis J, Omolo B. Risk for cardiovascular disease in blacks with HIV/AIDS in America: a systematic review and meta-analysis. J Health Dispar Res Pract. 2017;10(2):8. [Google Scholar]
- 83.Sudano I, Spieker LE, Noll G, Corti R, Weber R, Lüscher TF. Cardiovascular disease in HIV infection. Am Heart J. 2006;151(6):1147–1155. doi: 10.1016/j.ahj.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 84.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Després J-P, Lamarche B, Mauriège P, Cantin B, Dagenais GR, Moorjani S, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. 1996;334(15):952–958. doi: 10.1056/nejm199604113341504. [DOI] [PubMed] [Google Scholar]
- 86.Triant VA. Epidemiology of coronary heart disease in HIV patients. Rev Cardiovasc Med. 2014;15(0 1):S1–S8. doi: 10.3908/ricm15S1S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Islam F, Wu J, Jansson J, Wilson D. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta–analysis. HIV medicine. 2012;13(8):453–68. [DOI] [PubMed]
- 88.Wilson P. HIV therapy and the risk for cardiovascular disease. HIV Medicine. 2012;13:453–468. doi: 10.1111/j.1468-1293.2012.00996.x. [DOI] [PubMed] [Google Scholar]
- 89.Deeks SG, Phillips AN. Clinical review: HIV infection, antiretroviral treatment, aging, and non-AIDS related morbidity. BMJ. 2009;338:288–292. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 90.Sterne J, May M, Bucher H, Ledergerber B, Furrer H, Cavassini M, et al. HAART and the heart: changes in coronary risk factors and implications for coronary risk in men starting antiretroviral therapy. J Intern Med. 2007;261(3):255–267. doi: 10.1111/j.1365-2796.2006.01761.x. [DOI] [PubMed] [Google Scholar]