Abstract
Background
Type 2 diabetes mellitus (T2DM) is a heterogenic disease with increasing incidence. The SLC30A8 gene encodes an islet zinc transporter (ZnT8), and its variants have been associated with glucose and pro-insulin levels. This study was aimed to examine the effects of a missense variant (rs13266634 C/T), and two 3’UTR variants (rs2466294 C/G and rs2466293 T/C) in SLC30A8 gene on T2DM risk in a south-east Iranian population.
Methods
In this experiment, 450 patients diagnosed with T2DM and 453 healthy subjects from the same geographic area were enrolled. Genotypes were amplified using the ARMS–PCR method. In silico analyses were performed to determine the effects of the variants on the local structure of mRNA, splicing patterns, and potential miRNA-gene interactions as well.
Results
Significant differences were noticed between cases and controls regarding the genotypic and allelic distribution of the studied variants. As regards rs2466293 and rs2466294 variants, enhanced risk of T2DM was found under allelic, dominant, recessive, and codominant models (OR > 1). Besides, different genetic models of rs13266634 were associated with decreased risk of T2DM (OR < 1). Bioinformatics analyses indicated that the rs2466293 variant might influence the binding of some miRNAs, while the G-allele of rs2466294 decreased the stability of SLC30A8-mRNA.
Conclusions
In our population, both SNPs in the 3′-untranslated region of SLC30A8 increased the risk of T2DM, while the rs13266634 variant showed a protective association against T2DM susceptibility. Investigating the effects of other variants in this gene or other ZnTs can further indicate such associations in subjects from the same ethnicity.
Keywords: Diabetes mellitus, SLC30A8, Gene Polymorphism
Introduction
Diabetes mellitus (DM) is a chronic endocrinological disorder characterized by abnormal levels of blood glucose [1]. This condition is categorized into different subtypes, affecting individuals of different ages [2], while gender, high blood pressure, and positive family history are considered as independent risk factors for DM [3]. Insulin resistance is an essential contributor to the onset and pathogenesis of type 2 diabetes mellitus (T2DM), which plays a crucial role in the occurrence of dyslipidemia and hypertension [4, 5]. The interaction between environmental factors and genetic factors remarkably affects the development and progression of T2DM [6]. However, the exact genetic architecture of common traits, including the frequency of inherited variants that contribute to the risk of this common disease, has been long debated [5].
Disturbances in zinc metabolism lead to the onset of many clinical conditions, including Alzheimer’s disease and diabetes [7]. Within the body, zinc homeostasis is maintained via two families of solute-linked carriers [Zinc Transporters (ZnT or SLC30A) and ZRT/IRT-related Proteins (ZIP or SLC39A)]. SLC30A and the other ZnTs mainly mediate the transport of zinc from the cytosolic space into cell organelles or to the extracellular space [8]. The human solute carrier family 30 member 8 (SLC30A8) gene (Gene ID: 169,026) is located in 8q24.11 position with 13 exons, codifying zinc carrier znT8 which mainly transmits zinc from the cytoplasm to insulin secretion granules in the pancreas, to improve the proper maturity, storage, and secretion of insulin [9]. SLC30A8 is highly expressed in both beta and alpha cells of the pancreatic islet and have lower expression levels in the testicles, submaxillary glands, and other organs [10].
Studies have shown that T2DM susceptibility is influenced by common single-nucleotide polymorphisms (SNPs) spanning the zinc transporter associated genes [11, 12]. This includes the SLC30A8 gene, which opens up new insight into the role of zinc in the pathophysiology DM [13]. Prior genome-wide association studies have shown the link between SNPs located in the SLC30A8 gene and the risk of T2DM in different populations [12, 14] since these variants might reflect the possible changes in glucagon release in some levels [15]. Among the SLC30A8 gene variants, rs13266634 is the most studied one. This non-synonymous SNP is a C-to-T variant (arginine to tryptophan at position 325). Many reports have shown the link between rs13266634 and enhanced risk of T2DM in different races [16].
Moreover, both rs2466294 C/G and rs2466293 A/G variants are located in 3’ untranslated region (UTR) of SLC30A8 with a minor allele frequency of 0.48 and 0.32, respectively (according to information provided by the 1000 Genome Project [17]). Previous studies have shown that SNPs in the 3’UTR of genes may create new microRNA-binding sites, or disrupt putative recognition sites, influencing the interaction between microRNAs and target mRNAs [18]. The present study is aimed to investigate the effects of a missense variant (rs13266634 C/T), and two 3’UTR variants (rs2466294 C/G and rs2466293 A/G) located in SLC30A8 gene on T2DM risk in a south-east Iranian population.
Material & methods
Study design, subjects, and assessment
The local Ethics committee of Zahedan University of Medical Sciences approved the protocol of the present work (Ethical code: IR.ZAUMS.REC.1396.31), and the study was conducted in compliance with the Declaration of Helsinki. Four hundred and fifty newly diagnosed T2DM patients (admitted to diabetes center located at Bu-Ali Hospital of Zahedan, Iran) and 453 healthy controls (referring to the same hospital for routine checkups) were enrolled between May 2018 and January 2020. T2DM diagnosis was made by at least two diabetes specialist physicians based on fasting blood sugar (FBS) ˃126 mg/dl, 2 h post-prandial (2hpp) ≥ 200 mg/dL during oral glucose tolerance test (OGTT), and glycated hemoglobin A1C (HbA1c) ˃ 6.5% [19]. The controls were in a healthy condition, with a FBS < 99 mg/dL and HbA1c < 5.7%, without any family history of diabetes. Subjects with impaired glucose tolerance were excluded from the study due to the production of misleading results in analyses of the control group. Subjects with a previous diagnosis of cancer, cardiovascular, renal, and hepatic diseases, gestational diabetes, metabolic syndrome, and type 1 diabetes, hypercholesterolemia, and hypertension were also excluded from the study in order to eliminate interference in biological variables [20]. Written consent was obtained from all the subjects.
Following 12 h of fasting, 3 mL of whole blood was collected for assessment of biochemical parameters [including fast blood sugar (FBS), triglyceride (TG), high-density lipoprotein-Cholesterol (HDL-C), low-density lipoprotein-Cholesterol (LDL-C], and HbA1C using commercially available kits (Pars Azmun Co. Tehran, Iran)[21]. Fasting serum insulin was evaluated by Monobind (Cat No: 5825 − 300; California, USA), and C-peptide levels were measured using Monobind kit (Cat No: 2725 − 300; California, USA). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the following formula: HOMA-IR= [FBS (mg/dL) × fasting serum insulin (IU)/405][22].
DNA extraction and genotyping
For DNA extraction, 2 mL of whole venous blood was collected in ethylene diamine tetra-acetic acid (EDTA)-containing tubes. Using the standard salting-out protocol, DNA was extracted from the nucleated blood cells [23]. The integrity of the extracted DNAs was evaluated using gel electrophoresis. Genotyping was performed using two inners and two outer specific primer sequences (designed by Primer1 software [24] and synthesized by Pishgam, Tehran, Iran) based on tetra-amplification refractory mutation system-polymerase chain (ARMS–PCR) method [25](Table 1).
Table 1.
The primers used for amplification of SLC30A8 gene variants.
| Variant | Primers | Sequence (5′ to 3′) | Annealing Temperature | Length of PCR product (bp) |
|---|---|---|---|---|
| rs13266634 C/T |
F (T-allele) F (C-allele) R (common) |
CTTCTTTATCAACAGCAGCCATCT CTTCTTTATCAACAGCAGCCATCC GTGGCCTGTCAAATTTGGGAAACTG |
63 °C |
200 200 200 |
| rs2466293 A/G |
F (T-allele) F (C-allele) R (common) |
CCAGATTCCAAACCAAACCGAT CCAGATTCCAAACCAAACCGAC TCGAGTTGACCCCACCAAGTTG |
60°C |
280 280 280 |
| rs2466294 C/G |
F (C-allele) F (G-allele) R (common) |
CCTCTTCCTTCATGGTGAATAG CCTCTTCCTTCATGGTGAATAC ACTCACCATTCAGATGGAATC |
56 °C |
237 237 237 |
F: Forward; R: Reverse; FO: Forward outer; RO: Reverse outer; FI: Forward inner; RI: Reverse inner
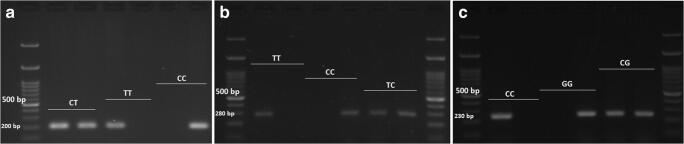
Briefly, each 20 µL reaction tube contained 1 µL of template DNA (~ 1 ng/mL), 1 µL from each primer (10 ng/mL), 5 µL of double-distilled water (Parstous Biotech Co., Iran), and 12 µL of master-mix (Ampliqon Taq 2x mastermix, Denmark). The PCR conditions were as follows: Initial denaturation at 95 °C for 6 min, 35 cycles each consisted of denaturation (30 s at 95 °C), annealing (Based on Table 1 for each SNP) and extension (40 s at 72 °C), followed by a final extension at 72 °C for 5 min. PCR products were electrophoresed on 2% agarose gel, stained in 0.5 µg/ml ethidium bromide solution and visualized by a transilluminator (Fig. 1). The amplicon size was 200 bp (for either T or C alleles of rs13266634), 280 bp (for either T or C alleles of rs2466293), and 230 bp (for either C or G alleles of rs2466294) displayed in 2% agarose gel electrophoresis (Fig. 1). Re-genotyping of one-third of the samples showed very high accuracy (> 99%).
Fig. 1.
Genotyping of a: rs13266634 C/T, b: rs2466293 T/C, and c: rs2466294 C/G variants in SLC30A8 by allele-specific amplified refractory mutation system (ARMS)-PCR resolved on a 2% agarose gel
Bioinformatics analyses
For analysis of variation effects on the mRNA structural and solidity characteristics, RNAsnp and RNAfold Web Servers were used. RNAsnp Web Server is an online tool for predicting SNP effects on local RNA secondary structures [26]. Similar to RNAsnp, RNAfold Web Server predicts the impact of variants on RNA folding as well as stability of the secondary structure of mRNA [27]. Moreover, The effect of rs13266634 C/T, rs2466293 T/C, and rs2466294 C/G variants on splicing patterns were analyzed by Human Splicing Finder v3.1 online tool [28]. The MirSNP database was employed to determine if rs2466293 T/C and rs2466294 C/G variants create or destroy the miRNA target sites [29].
Statistical analysis
SPSS v23 (SPSS Inc., Chicago, IL) software was used for data analysis. Fisher’s exact test was employed to examine the differences among the data sets when appropriate. The chi-square tests and Independent t-test were used to determine the association between the variants and T2DM. The SHEsis software platform was used to calculate the linkage disequilibrium (LD) between the SNPs [30]. A p-value of less than 0.05 was considered statistically significant.
Results
No significant difference was noticed between T2DM patients and healthy controls regarding age (P = 0.13) and sex (P = 0.47). Marked differences were observed between the studied groups concerning FBS, TG, LDL-C, BMI, HbA1C, fasting serum insulin, c-peptide, and HOMA-IR (Table 2) (P < 0.001).
Table 2.
Biochemical parameters of patients with T2DM and healthy controls
| Parameter evaluated | T2DM (n = 450)(n ± SD) | Controls (n = 453)(n ± SD) | p-value |
|---|---|---|---|
| Age (year) | 52.20 ± 11.64 | 54.43 ± 18.43 | 0.13 |
| Sex (Female/male) | 316/134 | 308/145 | 0.47 |
| FBS (mg/dL) | 198.46 ± 84.75 | 94.06 ± 8.65 | < 0.001 |
| TG (mg/dL) | 158.41 ± 83.01 | 127.41 ± 95.61 | < 0.001 |
| HDL-C (mg/dL) | 55.48 ± 20.47 | 53.89 ± 14.47 | 0.88 |
| LDL-C (mg/dL) | 97.13 ± 34.33 | 103.97 ± 28.51 | < 0.001 |
| BMI (kg/m2) | 27.64 ± 5.60 | 21.69 ± 2.42 | < 0.001 |
| HbA1C (%) | 9.01 ± 1.65 | 5.27 ± 0.55 | < 0.001 |
| Fasting serum insulin (IU/ml) | 10.4 ± 3.32 | 7.91 ± 2.86 | < 0.001 |
| c-peptide (ng/mL) | 1.97 | 2.21 | < 0.001 |
| HOMA-IR | 2.76 | 1.59 | < 0.001 |
BMI; Body Mass Index; FBS: Fast Blood Sugar; TC: Total Cholesterol; TG: Triglyceride; HDL-C; High-density lipoprotein-Cholesterol; LDL-C: Low-density lipoprotein-Cholesterol; T2DM: Type 2 diabetes; kg: kilogram; m2; square meter; mg: milligram; dL: deciliter, HOMA-IR: Homeostatic model assessment of insulin resistance. p < 0.05 was considered statistically significant
Table 3 presents the allelic genotypic distribution of SLC30A8 variants in studied groups. Regarding rs13266634, significant differences were observed among the two groups under codominant1 (CT vs. CC, OR = 0.69, 95% CI: 0.51–0.99, p-value = 0.013) and codominant2 (TT vs. CC, OR = 0.61, 95% CI: 0.42–0.86, p-value = 0.006) genetic contrast models. Significant protective role was also found between this variant and T2DM risk under dominant TT vs. CT + CC (OR = 0.66, 95% CI: 0.50–0.86, p-value = 0.002), recessive TT + CT vs. CC (OR = 0.73, 95% CI: 0.53–1.01, p-value = 0.05), and allelic T vs. C (OR = 0.61, 95% CI: 0.42–0.86, p-value = 0.006) models. As regards rs2466293 T/C, increased risk of T2DM was observed under codominant1 (TC vs. TT, OR = 1.46, 95% CI: 1.07–1.98, p-value = 0.015), codominant2 (CC vs. TT, OR = 2.10, 95% CI: 1.47–3.00, p-value < 0.001), dominant (TT vs. TC + CC, OR = 1.66, 95% CI: 1.25–2.20, p-value < 0.001), and recessive (TT + TC vs. CC, OR = 1.68, 95% CI: 1.23–2.29, p-value < 0.001) models. The presence of C allele of rs2466293 T/C enhanced T2DM susceptibility by 1.51 fold. We also found that G allele of rs2466294 C/G conferred an increased risk of T2DM (C vs. G, OR = 1.64, 95% CI: 1.36–1.97, p-value < 0.001). Regarding this SNP, enhanced risk of T2DM was also noticed under codominnat1 (CG vs. CC, OR = 1.90, 95% CI: 1.39–2.59, p-value < 0.001), codominant2 (GG vs. CC, OR = 2.65, 95% CI: 1.80–3.89, p-value < 0.001), recessive (CC + CG vs. GG, OR = 1.77, 95% CI: 1.70–2.45, p-value = 0.001), and dominant (CC vs. CG + GG, OR = 2.09, 95% CI: 1.56–2.80, p-value < 0.001) contrasted models.
Table 3.
Genotypic and allelic distribution of SLC30A8 variants in T2DM patients and control subjects
| Variant | Type | T2DM (%) | Control (%) | Model | OR(95%CI) | p-value |
|---|---|---|---|---|---|---|
| rs13266634 C/T | CC | 205 (45.6%) | 161 (35.5%) | Codominant1 | 0.69 (0.51–0.92) | 0.013 |
| CT | 164 (36.4%) | 187 (41.3%) | Codominant2 | 0.61 (0.42–0.86) | 0.006 | |
| TT | 81 (18.0%) | 105 (23.2%) | Dominant | 0.66 (0.50–0.86) | 0.002 | |
| Recessive | 0.73 (0.53–1.01) | 0.05 | ||||
| Over-dominant | 0.82 (0.62–1.07) | 0.14 | ||||
| C | 574 (63.8%) | 509 (56.02%) | 1 [reference] | |||
| T | 326 (36.2%) | 397 (43.8%) | 0.73 (0.60–0.88) | 0.001 | ||
| rs2466293 T/C | TT | 118 (26.2%) | 168 (37.1%) | Codominant1 | 1.46 (1.07–1.98) | 0.015 |
| TC | 201 (44.7%) | 196 (43.3%) | Codominant2 | 2.10 (1.47-3.00) | < 0.001 | |
| CC | 131 (29.1%) | 89 (19.6%) | Dominant | 1.66 (1.25–2.20) | < 0.001 | |
| Recessive | 1.68 (1.23–2.29) | < 0.001 | ||||
| Over-dominant | 1.06 (0.81–1.38) | 0.67 | ||||
| T | 437 (48.6%) | 532 (58.7%) | 1 [reference] | |||
| C | 463 (51.4%) | 374 (41.3%) | 1.51 (1.25–1.82) | < 0.001 | ||
| rs2466294 C/G | CC | 99 (22.0%) | 168 (37.1%) | Codominant1 | 1.90 (1.39–2.59) | < 0.001 |
| CG | 237 (52.7%) | 212 (46.8%) | Codominant2 | 2.65 (1.80–3.89) | < 0.001 | |
| GG | 114 (25.3%) | 73 (16.1%) | Dominant | 2.09 (1.56–2.80) | < 0.001 | |
| Recessive | 1.77 (1.7–2.45) | 0.001 | ||||
| Over-dominant | 1.26 (0.97–1.64) | 0.08 | ||||
| C | 435 (48.3%) | 548 (60.5%) | 1 [reference] | |||
| G | 465 (51.7%) | 358 (39.5%) | 1.64 (1.36–1.97) | < 0.001 | ||
CI: confidence interval; OR: odds ratio, T2DM: Type 2 diabetes mellitus, p < 0.05 was considered statistically significant
The results of interaction between SLC30A8 variants indicated CC/TT/CC, CT/TT/CC, CT/TT/CG, CT/TT/GG, TT/CC/CC and TT/TC/CG of rs13266634CT/rs2466293TC/rs2466294CG combinations statistically enhanced T2DM susceptibility (Table 4). As shown in Table 5(Haplotype analysis) and Fig. 2 (linkage disequilibrium patterns), the C/T/G, C/C/C, C/C/G and T/C/G of rs13266634CT/rs2466293TC/rs2466294CG haplotypes increased T2DM risk by 1.65, 1.45, 2.45, and 2.33 fold, respectively.
Table 4.
Interaction analysis of SLC30A8 variants on T2DM risk
| rs13266634 C/T | rs2466293 T/C | rs2466294 C/G | T2DM (%) | Control (%) | OR(95%CI) | p-value |
|---|---|---|---|---|---|---|
| CC | TC | CG | 69 (15.3%) | 45 (9.9%) | 1 [reference] | |
| CC | CC | CC | 9 (2.0%) | 10 (2.2%) | 0.59 (0.22–1.56) | 0.28 |
| CC | CC | CG | 15 (3.3%) | 14 (3.1%) | 0.70 (0.31–1.59) | 0.39 |
| CC | CC | GG | 21 (4.7%) | 6 (1.3%) | 2.28 (0.85–6.09) | 0.09 |
| CC | TC | CC | 16 (3.6%) | 14 (3.1%) | 0.74 (0.33–1.67) | 0.48 |
| CC | TC | GG | 11 (2.4%) | 4 (0.9%) | 1.79 (0.54–5.98) | 0.34 |
| CC | TT | CC | 9 (2.0%) | 33 (7.3%) | 0.18 (0.08–0.41) | < 0.001 |
| CC | TT | CG | 45 (10.0%) | 26 (5.7%) | 1.13 (0.61–2.08) | 0.70 |
| CC | TT | GG | 10 (2.2%) | 9 (2.0%) | 0.72 (0.27–1.92) | 0.52 |
| CT | CC | CC | 11 (2.4%) | 16 (3.5%) | 0.45 (0.19–1.05) | 0.06 |
| CT | CC | CG | 19 (4.2%) | 7 (1.5%) | 1.77 (0.69–4.55) | 0.23 |
| CT | CC | GG | 33 (7.3%) | 11 (2.4%) | 1.96 (0.90–4.26) | 0.09 |
| CT | TC | CC | 22 (4.9%) | 27 (6.0%) | 0.53 (0.27–1.04) | 0.06 |
| CT | TC | CG | 35 (7.8%) | 38 (8.4%) | 0.60 (0.33–1.09) | 0.09 |
| CT | TC | GG | 14 (3.1%) | 15 (3.3%) | 0.61 (0.27–1.38) | 0.23 |
| CT | TT | CC | 9 (2.0%) | 35 (7.7%) | 0.17 (0.07–0.38) | < 0.001 |
| CT | TT | CG | 13 (2.9%) | 22 (4.9%) | 0.38 (0.18–0.84) | 0.01 |
| CT | TT | GG | 8 (1.8%) | 16 (3.5%) | 0.33 (0.13–0.82) | 0.01 |
| TT | CC | CC | 3 (0.7%) | 13 (2.9%) | 0.15 (0.04–0.56) | 0.002 |
| TT | CC | CG | 8 (18%) | 9 (2.0%) | 0.58 (0.21–1.61) | 0.29 |
| TT | CC | GG | 12 (2.7%) | 3 (0.7%) | 2.61 (0.70–9.76) | 0.14 |
| TT | TC | CC | 10 (2.2%) | 14 (3.1%) | 0.47 (0.19–1.14) | 0.09 |
| TT | TC | CG | 19 (4.2%) | 33 (7.3%) | 0.37 (0.19–0.74) | 0.004 |
| TT | TC | GG | 5 (1.1%) | 6 (1.3%) | 0.54 (0.16–1.89) | 0.33 |
| TT | TT | CC | 10 (2.2%) | 6 (1.3%) | 1.09 (0.37–1.20) | 0.88 |
| TT | TT | CG | 14 (3.1%) | 18 (4.0%) | 0.51 (0.23–1.21) | 0.09 |
| TT | TT | GG | 0 | 3 (0.7%) | - | - |
OR: Odds ratio CI: Confidence interval; T2DM: Type 2 diabetes mellitus. p < 0.05 was considered statistically significant
Table 5.
Haplotype analysis of SLC30A8 variants in patients with T2DM and healthy subjects
| rs13266634 C/T | rs2466293 T/C | rs2466294 C/G | T2DM | Control | OR(95%CI) | p-value |
|---|---|---|---|---|---|---|
| C | T | C | 163 | 214 | 1 [reference] | |
| C | T | G | 131 | 104 | 1.65 (1.19–2.30) | 0.003 |
| C | C | C | 108 | 98 | 1.45 (1.30–2.03) | 0.033 |
| C | C | G | 172 | 92 | 2.45 (1.77–3.40) | < 0.001 |
| T | T | C | 100 | 118 | 1.11 (0.80–1.56) | 0.53 |
| T | T | G | 43 | 95 | 0.59 (0.39–0.90) | 0.013 |
| T | C | C | 64 | 117 | 0.72 (0.50–1.04) | 0.08 |
| T | C | G | 119 | 67 | 2.33 (1.62–3.35) | < 0.001 |
OR: Odds ratio CI: Confidence interval; T2DM: Type 2 diabetes mellitus. p < 0.05 was considered statistically significant
Fig. 2.
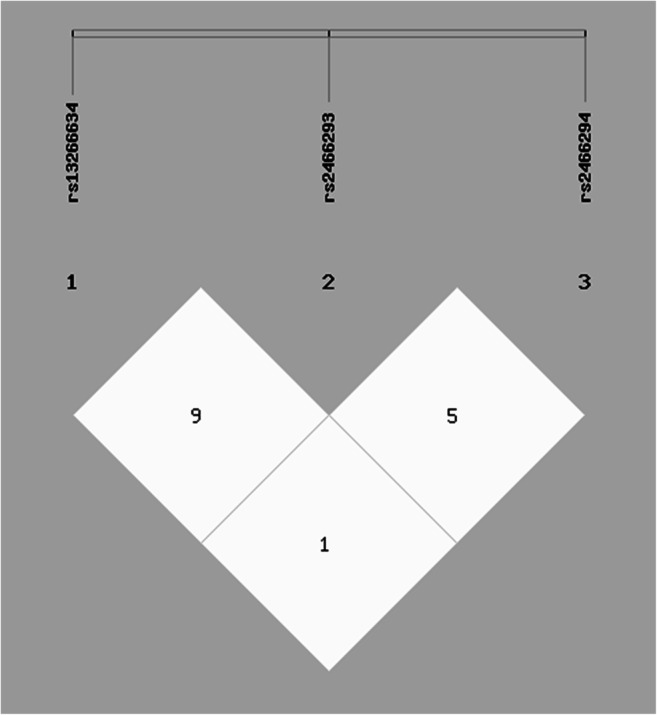
Linkage disequilibrium (LD) between multiple loci of SLC30A8 gene (rs13266634 C/T, rs2466293 T/C, and rs2466294 C/G)
The complete mRNA sequence of SLC30A8 (NM_001172811.1) was used to analyze the effect of studied variants on the whole and local mRNA structure, and the following analyses. The 100-bp flanking region of mRNA contained each SNP imported to the RNAfold server. The free energy of the thermodynamic ensemble for C and T allele of rs13266634 were − 23.15 kcal/mol and − 25.78 kcal/mol, respectively (Fig. 1, A&A’). It suggested that the local mRNA structure in the presence of the T allele is more stable. In the case of rs2466293 T/C, the free energy of the thermodynamic ensemble for C and T allele were − 19.11 kcal/mol and − 18.17 kcal/mol, respectively (Fig. 3, B&B’). The more considerable differences were observed for rs2466294 C/G. The C allele of this variant is more likely to be stable than the G allele (-22.53 kcal/mol vs. -18.47 kcal/mol)(Fig. 3, C&C’).
Fig. 3.
Analysis of rs13266634 C/T, rs2466293 T/C, and rs2466294 C/G variant effects on local mRNA structure of SLC30A8 using RNAfold server. A&A’: C and T allele of rs13266634, B&B’: C and T allele of rs2466293, C&C’: C and G allele of rs246629, D&D’: 1000-bp flanking region of SLC30A8 contained all rs13244434 C/T, rs2466293 T/C and rs2466294 C/G variants in wild type and mutant genotype, respectively. The most important structural change is related to rs2466294 C/G
Furthermore, we used a 1000 bp sequence of SLC30A8 mRNA that contained wild type alleles in the position of the studied variants and compared this sequence with the mutant model to determine the possible structural alteration by the RNASNP server. The most noticeable modification in the structure of SLC30A8 mRNA was observed in the position of rs2466294 C/G variant, which is compatible with data of the RNAfold server. In contrast, significant changes were not observed for the other two SNPs (Fig. 3, D&D’). The analysis of the mRNA sequence revealed that rs13266634 C/T might create an exonic splicing enhancer (ESE) site that probably has no impact on splicing (Data not shown). Additionally, the extracted data of HSF v3.1 showed that rs2466293 T/C and rs2466294 C/G are less likely to have a significant impact on splicing patterns even though these variations could create new and break silencer or enhancer of splicing (Data not shown). Screening of 3’ UTR region of SLC30A8 mRNA in the position of rs2466293 T/C showed that this variant might influence the binding of some miRNAs. For example, the substitution of T with C creates a recognition site for hsa-miR-1273d and hsa-miR-660-5p. In contrast, recognition sites of hsa-miR-181a-2-3p and hsa-miR-888-3p have been disrupted in the presence of the C allele (Table 6) (Fig. 4).
Table 6.
The effect of 3’ UTR variants of SLC30A8 mRNA on miRNA binding sites
| Gene | miRNA | SNP | Effect | Allele | Energy | Conservation |
|---|---|---|---|---|---|---|
| SLC30A8 | hsa-miR-1273d | rs2466293 | Create |
C T |
-18.71 | 0.002 |
| SLC30A8 | Hsa-mir181a-2-3p | rs2466293 | Break |
C T |
-14.67 | 0.001 |
| SLC30A8 | hsa-miR-660-5p | rs2466293 | Create |
C T |
-19.59 | 0.002 |
| SLC30A8 | Hsa-miR-888-3p | rs2466293 | Break |
C T |
-11.45 | 0.00 |
Fig. 4.
Base pair probability of local region analyzed by RNAsnp server. A: rs13266634 C/T, B: rs2466293 T/C, C: rs2466294 C/G
Discussion
T2DM is a polygenic metabolic disorder with a rapidly increasing number of contributing genes. The rs13266634, a non-synonymous SNP in SLC30A8, has been linked to T2DM in several reports [16, 31]. This SNP might serve as a gain-of-function mutation, thus, influence the expression of ZnT8 protein by disrupting the recognition motifs of protein kinases in this molecule [32]. Lately, genome-wide association studies (GWAS) have identified many genetic variations linked to T2DM. However, the functional roles for most of the SNPs have not been elucidated [33]. So, in this preliminary study, we aimed to investigate if the two 3’ UTR SNPs in SLC30A8 could modulate miRNA-directed regulation of gene expression and whether these SNPs in miRNA-binding sites are linked to T2DM susceptibility.
In the current work, we found significant associations between the three SLC30A8 variant and T2DM susceptibility under codominant homozygous, codominant heterozygous, dominant, and recessive genetic models. Based on our findings, rs13266634 C/T was associated with decreased risk of T2DM; therefore, it might have a protective role against the development of this endocrine disorder. In contrast, variants located in the 3’ UTR region of SLC30A8 increased T2DM incidence under different genetic models. Computational analyses indicated that rs2466293 T/C variant might influence the binding of hsa-miR-1273d, hsa-miR-660-5p, hsa-miR-181a-2-3p and hsa-miR-888-3p.
In 2017, Wang and colleagues investigated the effects of some miRNA-binding site variants and the risk of gestational diabetes mellitus (GDM) in a population from Chinese pregnant women [18]. In agreement with our results, they found that the presence of the C- allele of SLC30A8 rs2466293 T/C increased the risk of GDM by a.17 fold. Besides, this functional variant conferred an increased risk of GDM under additive CC vs. TT and recessive TC + TT vs. CC genetic models. According to their findings, the rs2466293 located on Chr. 8:117173699, could change the binding site of hsa-miR-181a-2-3p, and therefore regulate gene expression post-transcriptionally. In a similar study by Gomes et al., the SLC30A8 rs2466293 AG + GG genotype increased the risk of type 1 diabetes mellitus (T1DM) by 1.7 fold in non-European descents. Moreover, the GG genotype of the variant was correlated with a higher titer of ZnT8 autoantibody [34]. So far, the clinical significance of the other SLC30A8 3’ UTR variant, rs2466294, has not been reported, and no study has examined the effects of rs2466293 A/G variants on T2DM susceptibility.
The enthusiasm for the acknowledged effects of SLC30A8 variants in T2DM progression is driven by achieved results from large population-based genetic studies. However, the strength of this association is ill-defined [35]. Over the last decade, a growing body of evidence suggested that SLC30A8 variants are strongly associate with beta-cell dysfunction. In this regard, Kirchhoff et al. reported that the risk allele of this SNP is associated with impaired pro-insulin processing and conversion [36]. Mitchell et al. demonstrated a near-linear relationship between ZnT8 levels and glucose tolerance by selective manipulation of SLC30A8 in the mouse cells [37]. Despite the tight restriction of SLC30A8 expression to the pancreas tissue, the roles of this ZnT are mediated via interactions between multiple tissues (-cells and liver)[38]. The SLC30A8 SNPs might affect glucagon release by pancreatic cells; however, elaborated in vivo analysis involving hypoglycemic clamps are not well-studied yet [39].
A large number of studies have established a positive link between rs13266634 C/T variant and T2DM risk [40–42]. Few of these studies were on Iranian populations from different locations [16, 43]. However, these studies lacked statistical power and need to be replicated. In contrast to our findings, few studies reported that SNPs located within the SLC30A8 gene are not consistently linked to T2DM risk across different populations. In this regard, DeMenna did not find a statistically significant association between rs13266634 C/T and T2DM risk [44]. Moreover, Cheng et al. [45], as well as Cauchi et al. [46], suggested that ethnicity might contribute to the between-studies heterogeneity in such associations regarding this SNP. The effects rs13266634 C/T variants on the SLC30A8 mRNA level could be evaluated in future studies. We found that that the secondary structure of SLC30A8-mRNA is likely to be more stable in terms of the T and C alleles of rs2466293 and rs2466294, respectively. In this regard, the data extracted from the Ensembl database (available at http://www.ensembl.org) revealed that both of these alleles are more frequent in south-east Asians, compared to their counterparts. Hence, based on our in silico results, it is hypothesized that more stable alleles are more prevalent in such populations.
Evidence gathered over the last decade promises the emergence of novel strategies for diagnosis, prognosis, and treatment of metabolic disorders. Therefore, a deeper understanding of the on-going interactions between genetic variants and miRNAs could improve our knowledge on T2DM development mechanisms, and help come up with beneficial strategies that can be employed to find more accurate prognostic markers for T2DM. This exceptional field of biomedical science, called personalized medicine, is focused on the individual treatment of the patients. Bear in mind that our integrative attempt in this area is a dynamically fluid on-going project; thus, the need for future updates on the now-limited database with further investigations in larger cohorts.
Our work has limitations. We performed this study on a homogenous sample of the south-east Iranian population. Besides, we did not confirm our results by sequencing. However, re-genotyping of at least 33% of the samples showed no error in genotyping. Further studies in other races with larger sample size are encouraged to investigate such associations.
Conclusions
In our population, both SNPs in the 3′-untranslated region of SLC30A8 increased the risk of T2DM development, while rs13266634 C/T showed protective association against T2DM susceptibility. Investigating the effects of other variants in this gene or other ZnTs can further indicate such associations in subjects from the same ethnicity.
Acknowledgements
We are grateful to Dr. Maryam Piri and Dr. Hamed Taheri, the two diabetes specialist physicians, for their assistance. We also wish to thank all the individuals who voluntarily participated in this study.
Funding information
This work was financially supported by Zahedan University of Medical Sciences (Grant No. 8256).
Compliance with ethical standards
Conflict of interest
None declared.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lu G, Teng X, Zheng Z, Zhang R, Peng L, Zheng F, et al. Overexpression of a glucokinase point mutant in the treatment of diabetes mellitus. Gene Ther. 2016;23(4):323. doi: 10.1038/gt.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh A, Bhatia E, Dabadghao P, Bhatia V, Gellert S, Colman P. Role of islet autoimmunity in the aetiology of different clinical subtypes of diabetes mellitus in young north Indians. Diabet Med. 2000;17(4):275–80. doi: 10.1046/j.1464-5491.2000.00267.x. [DOI] [PubMed] [Google Scholar]
- 3.Poodineh M, Saravani R, Mirhosseini M, Sargazi S. Association of two methylenetetrahydrofolate reductase polymorphisms (rs1801133, rs1801131) with the risk of type 2 diabetes in South-East of Iran. Rep Biochem Mol Biol. 2019;8(2):178. [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Investig. 2000;106(4):473–81. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein BJ. Insulin resistance as the core defect in type 2 diabetes mellitus. Am J Cardiol. 2002;90(5):3–10. doi: 10.1016/s0002-9149(02)02553-5. [DOI] [PubMed] [Google Scholar]
- 6.Galavi H, Mollashahee-Kohkan F, Saravani R, Sargazi S, Noorzehi N, Shahraki H. HHEX gene polymorphisms and type 2 diabetes mellitus: A case‐control report from Iran. J Cell Biochem. 2019;120(10):16445–51. doi: 10.1002/jcb.28788. [DOI] [PubMed] [Google Scholar]
- 7.Devirgiliis C, Zalewski PD, Perozzi G, Murgia C. Zinc fluxes and zinc transporter genes in chronic diseases. Mutat Res. 2007;622(1–2):84–93. doi: 10.1016/j.mrfmmm.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Smidt K, Rungby J. ZnT3: a zinc transporter active in several organs. Biometals. 2012;25(1):1–8. doi: 10.1007/s10534-011-9490-x. [DOI] [PubMed] [Google Scholar]
- 9.Syring KE, Boortz KA, Oeser JK, Ustione A, Platt KA, Shadoan MK, et al. Combined deletion of Slc30a7 and Slc30a8 unmasks a critical role for ZnT8 in glucose-stimulated insulin secretion. Endocrinology. 2016;157(12):4534–41. doi: 10.1210/en.2016-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murgia C, Devirgiliis C, Mancini E, Donadel G, Zalewski P, Perozzi G. Diabetes-linked zinc transporter ZnT8 is a homodimeric protein expressed by distinct rodent endocrine cell types in the pancreas and other glands. Nutr Metab Cardiovasc Dis. 2009;19(6):431–9. doi: 10.1016/j.numecd.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Rungby J. Zinc, zinc transporters and diabetes. Diabetologia. 2010;53(8):1549–51. doi: 10.1007/s00125-010-1793-x. [DOI] [PubMed] [Google Scholar]
- 12.Xiang J, Li X-Y, Xu M, Hong J, Huang Y, Tan J-R, et al. Zinc transporter-8 gene (SLC30A8) is associated with type 2 diabetes in Chinese. J Clin Endocrinol Metab. 2008;93(10):4107–12. doi: 10.1210/jc.2008-0161. [DOI] [PubMed] [Google Scholar]
- 13.Zhao T, Huang Q, Su Y, Sun W, Huang Q, Wei W. Zinc and its regulators in pancreas. Inflammopharmacology. 2019:1–12. [DOI] [PubMed]
- 14.Weijers RN. Three-dimensional structure of β-cell-specific zinc transporter, ZnT-8, predicted from the type 2 diabetes-associated gene variant SLC30A8 R325W. Diabetol Metab Syndr. 2010;2(1):33. doi: 10.1186/1758-5996-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solomou A, Philippe E, Chabosseau P, Migrenne-Li S, Gaitan J, Lang J, et al. Over-expression of Slc30a8/ZnT8 selectively in the mouse α cell impairs glucagon release and responses to hypoglycemia. Nutr Metab. 2016;13(1):46. doi: 10.1186/s12986-016-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faghih H, Khatami S-R, Azarpira N, Foroughmand A-M. SLC30A8 gene polymorphism (rs13266634 C/T) and type 2 diabetes mellitus in south Iranian population. Mol Biol Rep. 2014;41(5):2709–15. doi: 10.1007/s11033-014-3158-x. [DOI] [PubMed] [Google Scholar]
- 17.Consortium GP. A global reference for human genetic variation. Nature. 2015;526(7571):68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Li W, Ma L, Ping F, Liu J, Wu X, et al. Investigation of miRNA-binding site variants and risk of gestational diabetes mellitus in Chinese pregnant women. Acta Diabetol. 2017;54(3):309–16. doi: 10.1007/s00592-017-0969-y. [DOI] [PubMed] [Google Scholar]
- 19.Association AD. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(Supplement 1):13–28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 20.Motavallian A, Andalib S, Vaseghi G, Mirmohammad-Sadeghi H, Amini M. Association between PRO12ALA polymorphism of the PPAR-γ2 gene and type 2 diabetes mellitus in Iranian patients. Indian J Hum Genet. 2013;19(2):239. doi: 10.4103/0971-6866.116126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosseini SRA, Hejazi K. The effects of Ramadan fasting and physical activity on blood hematological-biochemical parameters. Iran J Basic Med Sci. 2013;16(7):845. [PMC free article] [PubMed] [Google Scholar]
- 22.Asemani S, Montazeri V, Baradaran B, Tabatabiefar MA, Pirouzpanah S. The effects of Berberis vulgaris juice on insulin indices in women with benign breast disease: a randomized controlled clinical trial. Iran J Pharm Res. 2018;17(Suppl):110. [PMC free article] [PubMed] [Google Scholar]
- 23.Miller S, Dykes D, Polesky H. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins A, Ke X. Primer1: primer design web service for tetra-primer ARMS-PCR. Open Bioinforma J. 2012;6(1).
- 25.Ye S, Humphries S, Green F. Allele specific amplification by tetra-primer PCR. Nucleic Acids Res. 1992;20(5):1152. doi: 10.1093/nar/20.5.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabarinathan R, Tafer H, Seemann SE, Hofacker IL, Stadler PF, Gorodkin J. The RNAsnp web server: predicting SNP effects on local RNA secondary structure. Nucleic Acids Res. 2013;41(W1):W475–9. [DOI] [PMC free article] [PubMed]
- 27.Hashemi M, Bahari G, Sarhadi S, Eskandari E, Narouie B, Taheri M, et al. 4-bp insertion/deletion (rs3783553) polymorphism within the 3′ UTR of IL1A contributes to the risk of prostate cancer in a sample of Iranian population. J Cell Biochem. 2018;119(3):2627–35. doi: 10.1002/jcb.26427. [DOI] [PubMed] [Google Scholar]
- 28.Desmet F-O, Hamroun D, Lalande M, Collod-Béroud G, Claustres M, Béroud C. Human splicing finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37(9):e67-e. doi: 10.1093/nar/gkp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu C, Zhang F, Li T, Lu M, Wang L, Yue W, et al. MirSNP, a database of polymorphisms altering miRNA target sites, identifies miRNA-related SNPs in GWAS SNPs and eQTLs. BMC Genomics. 2012;13(1):661. doi: 10.1186/1471-2164-13-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yong Y, Lin H. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2005;15(2):97. doi: 10.1038/sj.cr.7290272. [DOI] [PubMed] [Google Scholar]
- 31.Jing Y, Sun Q, Bi Y, Shen S, Zhu D. SLC30A8 polymorphism and type 2 diabetes risk: evidence from 27 study groups. Nutr Metab Cardiovasc Dis. 2011;21(6):398–405. doi: 10.1016/j.numecd.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Kang ES, Kim MS, Kim YS, Kim CH, Han SJ, Chun SW, et al. A polymorphism in the zinc transporter gene SLC30A8 confers resistance against posttransplantation diabetes mellitus in renal allograft recipients. Diabetes. 2008;57(4):1043–7. doi: 10.2337/db07-0761. [DOI] [PubMed] [Google Scholar]
- 33.Goda N, Murase H, Kasezawa N, Goda T, Yamakawa-Kobayashi K. Polymorphism in microRNA-binding site in HNF1B influences the susceptibility of type 2 diabetes mellitus: a population based case–control study. BMC Med Genet. 2015;16(1):75. doi: 10.1186/s12881-015-0219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gomes KFB, Semzezem C, Batista R, Fukui RT, Santos AS, Correia MR, et al. Importance of zinc transporter 8 autoantibody in the diagnosis of type 1 diabetes in Latin Americans. Sci Rep. 2017;7(1):1–7. doi: 10.1038/s41598-017-00307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kulkarni H, Mamtani M, Peralta JM, Diego V, Dyer TD, Goring H, et al. Lack of association between SLC30A8 variants and type 2 diabetes in Mexican American families. J Diabetes Res. 2016;2016. [DOI] [PMC free article] [PubMed]
- 36.Kirchhoff K, Machicao F, Haupt A, Schäfer S, Tschritter O, Staiger H, et al. Polymorphisms in the TCF7L2, CDKAL1 and SLC30A8 genes are associated with impaired proinsulin conversion. Diabetologia. 2008;51(4):597–601. doi: 10.1007/s00125-008-0926-y. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell RK, Hu M, Chabosseau PL, Cane MC, Meur G, Bellomo EA, et al. Molecular genetic regulation of Slc30a8/ZnT8 reveals a positive association with glucose tolerance. Mol Endocrinol. 2016;30(1):77–91. doi: 10.1210/me.2015-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chimienti F, Devergnas S, Favier A, Seve M. Identification and cloning of a β-cell–specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes. 2004;53(9):2330–7. doi: 10.2337/diabetes.53.9.2330. [DOI] [PubMed] [Google Scholar]
- 39.Wijesekara N, Dai F, Hardy A, Giglou P, Bhattacharjee A, Koshkin V, et al. Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia. 2010;53(8):1656–68. doi: 10.1007/s00125-010-1733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabara Y, Osawa H, Kawamoto R, Onuma H, Shimizu I, Miki T, et al. Replication study of candidate genes associated with type 2 diabetes based on genome-wide screening. Diabetes. 2009;58(2):493–8. doi: 10.2337/db07-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rong R, Hanson RL, Ortiz D, Wiedrich C, Kobes S, Knowler WC, et al. Association analysis of variation in/near FTO, CDKAL1, SLC30A8, HHEX, EXT2, IGF2BP2, LOC387761, and CDKN2B with type 2 diabetes and related quantitative traits in Pima Indians. Diabetes. 2009;58(2):478–88. doi: 10.2337/db08-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cauchi S, Meyre D, Durand E, Proença C, Marre M, Hadjadj S, et al. Post genome-wide association studies of novel genes associated with type 2 diabetes show gene-gene interaction and high predictive value. PloS One. 2008;3(5):e2031. doi: 10.1371/journal.pone.0002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohaddes S, Karami F, Gharesouran J, Bahrami A. The soluble carrier 30 A8 (SLC30A8) gene polymorphism and risk of Diabetes Mellitus Type 2 in Eastern Azerbijan population of Iran. J Sci, Islamic Republic of Iran. 2012;23(1):15–20.
- 44.DeMenna J, Puppala S, Chittoor G, Schneider J, Kim JY, Shaibi GQ, et al. Association of common genetic variants with diabetes and metabolic syndrome related traits in the Arizona Insulin Resistance registry: a focus on Mexican American families in the Southwest. Hum Hered. 2014;78(1):47–58. doi: 10.1159/000363411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng L, Zhang D, Zhou L, Zhao J, Chen B. Association between SLC30A8 rs13266634 polymorphism and type 2 diabetes risk: a meta-analysis. Med Sci Monit. 2015;21:2178. doi: 10.12659/MSM.894052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cauchi S, Del Guerra S, Choquet H, D’Aleo V, Groves CJ, Lupi R, et al. Meta-analysis and functional effects of the SLC30A8 rs13266634 polymorphism on isolated human pancreatic islets. Mol Genet Metab. 2010;100(1):77–82. doi: 10.1016/j.ymgme.2010.01.001. [DOI] [PubMed] [Google Scholar]