Abstract
Aims
Inflammation is a cardinal pathogenetic mechanism in diabetic kidney injury (DKI). The detection of microalbuminuria (MA) is very important in preventing end-stage renal failure in diabetic subjects. A combination of high monocyte and low lymphocyte counts are used as a marker of inflammation. Monocyte to lymphocyte ratio (MLR) is considered as a marker in inflammatory diseases. We aimed to evaluate the MLR levels in diabetic subjects as a predictive marker in detecting MA.
Methods
A total of 212 patients with type 2 diabetes mellitus (T2DM) were included in the study. Patients with T2DM were divided into two groups as MA and normoalbuminuria (NA). MLR of the groups were compared.
Results
There were 72 patients in MA and 140 patients in NA group. MLR of the MA and NA groups were 0.247 (0.131–0.540) and 0.211 (0.052–0.390), respectively (p < 0.001). There was a statistically significant correlation between MLR and MA (r = 0.228, p = 0.001). In multivariate backward logistic regression analysis, MLR, fasting blood glucose, HbA1c and presence of comorbid clinical diseases were determined as independent predictors of DKI.
Conclusions
We suggest that MLR could serve as a predictive and effective marker for DKI in diabetic subjects due to its strong correlation with MA and inexpensive and readily available nature.
Keywords: Monocyte lymphocyte ratio; Microalbuminuria; Diabetic kidney injury; Inflammation; Type 2 diabetes mellitus, HbA1c
Introduction
Diabetic kidney injury (DKI) is a multifactorial condition and inflammation is a cardinal pathogenetic mechanism in DKI, which suggested by the research in literature [1]. Advanced glycation end-products and immune complexes have significant roles in renal infiltration by monocytes and lymphocytes in DKI [1]. The inflammation in diabetic kidney increases cellular damage and the development of fibrosis which cause the progression of kidney injury. The first evidence of DKI is development of microalbuminuria (MA) in diabetic patients. Microalbuminuria (MA) is the last reversible stage by treatment in the course of DKI. The detection of MA is very important in preventing end-stage renal failure due to diabetes.
Inflammation usually causes a decrease in lymphocyte count and increased cardiovascular risk and mortality were suggested to be related with low lymphocyte levels in circulation [2, 3]. In addition, low lymphocyte and high monocyte counts have been reported to be associated with undesirable cardiovascular outcome in patients with coronary artery disease [3, 4]. High monocyte and low lymphocyte counts are used as a marker of inflammation based on the hypothesis that MLR detects inflammation more effectively and strongly than the number of lymphocytes or monocytes separately. For instance, monocyte lymphocyte ratio (MLR) could be a more useful predictor of high vascular outcome risk in patients with atherosclerosis [5]. It has been suggested that MLR was a prognostic marker in determining the degree of coronary artery stenosis prior to coronary intervention [5]. Similarly, it has been reported as a poor prognostic marker in patients with peripheral artery occlusion [6] and lymphoma [7].
In present cross-sectional study, we aimed to evaluate whether MLR is a predictive marker in detecting DKI in subjects with type 2 diabetes mellitus.
Materials and methods
Study design
The study was designed retrospectively in descriptive nature between June 2017 and November 2018 in the Internal Medicine Department of Abant Izzet Baysal University Medical Faculty Hospital. Laboratory data of Type 2 Diabetic patients who were admitted to our clinic were recorded from institutional database. The study protocol was approved by the local Ethics Committee of the Institution (Ethical Decision Date/Number: 2018/261). Exclusion criteria were as follows: Patients with active infection, inflammatory diseases, hematological conditions, liver failure, corticosteroid use, hematuria, pregnancy and malignancy.
Clinical and laboratory assessment
Age, gender, Systolic blood pressure (SBP), diastolic blood pressure (DBP), weight, height, waist circumference (WC), morbidity and medications used by the participants, duration of type 2 diabetes mellitus were recorded from patients’ files and database. A body mass index (BMI) was calculated by the following formula: BMI = weight in kg / square of height in meters.
Biochemical analyses were held with Architect c8000 analyzer (Abbott Inc. Lake Forest, IL, USA). Total cholesterol (reference range: 0-200 mg/dL), LDL cholesterol (reference range: 80–160 mg/dL), HDL cholesterol (reference range: 35–55 mg/dL), triglyceride (reference range: ), urea (reference range: 12–42 mg/dL), creatinine (reference range: 0.57–1.11 mg/dL), HbA1c (reference range: 4-5.6%), fasting plasma glucose (FPG) (reference range: 75–100 mg/dL) were obtained and recorded form database. MA (reference range: 10–140 mg/L) level was calculated with the formula spot urine microalbumin / creatinine x100. Automatic analyzer of LH 780 model of Beckman Coulter device (Beckman Coulter In.; Bre CA) was used for complete blood count analyses in the laboratory of our institution. Monocyte (reference range: 0-0.9 k/mm3) and lymphocytes (reference range: 1.1–5.1 k/mm3) count were obtained from hemogram tests and MLR levels was calculated with dividing monocyte by lymphocytes count. The study group was divided into two groups as MA (30–300 mg / dl) and normoalbuminuria (NA) (< 30 mg / dl) according to the urinary microalbumin level. The MLR and other study parameters were compared in study groups.
Statistical analyses
Statistical analyzes were conducted with SPSS software (SPSS Inc, Chicago, IL 15.0). Kolmogorov-Smirnov test was used to analyze whether the study parameters were distributed normally in study groups. The parameters with normal distribution were compared with independent samples t test and expressed as mean ± standard deviation. The parameters without normal distribution were compared with Mann-Whitney U test and expressed as median (min-max). Chi-square test was used to compare categorical variables between groups and expressed as percentage. Correlation between MA and MLR was analyzed by Pearson’s correlation analysis. Sensitivity and specificity of MLR in detecting DKI were determined by receiver operation characteristic (ROC) analysis. Multivariate backward logistic regression analysis held for whether MLR, FBG, HbA1c and comorbid clinical diseases were independent predictors of DKI. A p < 0.05 was considered statistically significant.
Results
The study was performed with 212 patients with 72(%34) MA and 140(%66) NA. While 37 were male and 35 were female in MA group, 58 were male and 82 were female in NA group (p = 0.16). The mean age of the MA and NA groups were 60.3 ± 6.3 and 59.7 ± 9.5, respectively (p = 0.62). The duration of diabetes was 10 (1–25) years and 6 (1–21) years for T2DM patients with MA and NA, respectively (p = 0.026). There was no significant difference between the study groups in terms of SBP (p = 0.38), DBP (p = 0.83), BMI (p = 0.67) and WC (p = 0.18).
Median HbA1C was 9.5 (5.8–15.8) % and 7.7 (5.5–15.2) % in MA and NA groups, respectively. HbA1c was significantly elevated in MA group compared to NA group (p < 0.001). Similarly, FPG levels of MA group (146(85–408)) were significantly higher than NA group (115(65–296)) (p < 0.001). HDL-cholesterol was 41 (25–73) mg/dl and 47 (25–76) mg/dl in MA and NA groups, respectively, which the difference was statistically significant (p = 0.001). LDL-cholesterol (p = 0.86), total cholesterol (p = 0.85), triglyceride (p = 0.21) and serum creatinine (p = 0.15) levels were not significantly different between the MA and NA groups.
Table 1 indicates characteristics and data of study groups.
Table 1.
Characteristics and laboratory parameters of the Type 2 Diabetes Mellitus (T2DM) with microalbuminuria (MA) and normoalbuminuria (NA)
| T2DM with MA group(Mean ± StD) | T2DM with NA group(Mean ± StD) | p | ||||
| Age (year) | 60.3 ± 6.3 | 59.7 ± 9.5 | 0.62 | |||
| Total Cholesterol (mg/dl) | 194.6 ± 51.4 | 194 ± 44.5 | 0.85 | |||
| Low-Density Lipoprotein(mg/dl) | 116.4 ± 38.8 | 117.3 ± 33.7 | 0.86 | |||
| Waist Circumference (cm) | 110.4 ± 19.9 | 107.9 ± 12.6 | 0.18 | |||
| T2DM with MA group Median (Min-Max) | T2DM with NAgroup Median (Min-Max) | p | ||||
| Systolic Blood Pressure (mm/Hg) | 140(100–180) | 137.5(100–180) | 0.38 | |||
| Diastolic Blood Pressure (mm/Hg) | 80 (65–100) | 80 (50–110) | 0.83 | |||
| Duration of Diabetes (year) | 10 (1–25) | 6 (1–20) | 0.026 | |||
| Hemoglobin A 1C (%) | 9.5 (5.8–15.5) | 7.7 (5.5–15.2) | < 0.001 | |||
| Fasting Plasma Glucose (mg/dl) | 146(85–408) | 115(65–296) | < 0.001 | |||
| Triglycerides (mg/dl) | 161 (58–600) | 147 (44-1050) | 0.21 | |||
| High Density Lipoprotein (mg/dl) | 41 (25–73) | 47 (25–76) | 0.001 | |||
| Body Mass Index (kg/cm2) | 35.8(21.5–49.5) | 33.8 (20.6–49.7) | 0.67 | |||
| Creatinine (mg/dl) | 0.86 (0.6–1.2) | 0.80 (0.50–1.2) | 0.15 | |||
| Urine microalbumine/creatinine ratio x100 (mg) | 47.9 (31.7-268.2) | 14.5 (3-29.9) | < 0.001 | |||
| Monocyte to lymphocyte ratio (%) | 0.247 (0.131–0.540) | 0.211(0.052–0.442) | < 0.001 | |||
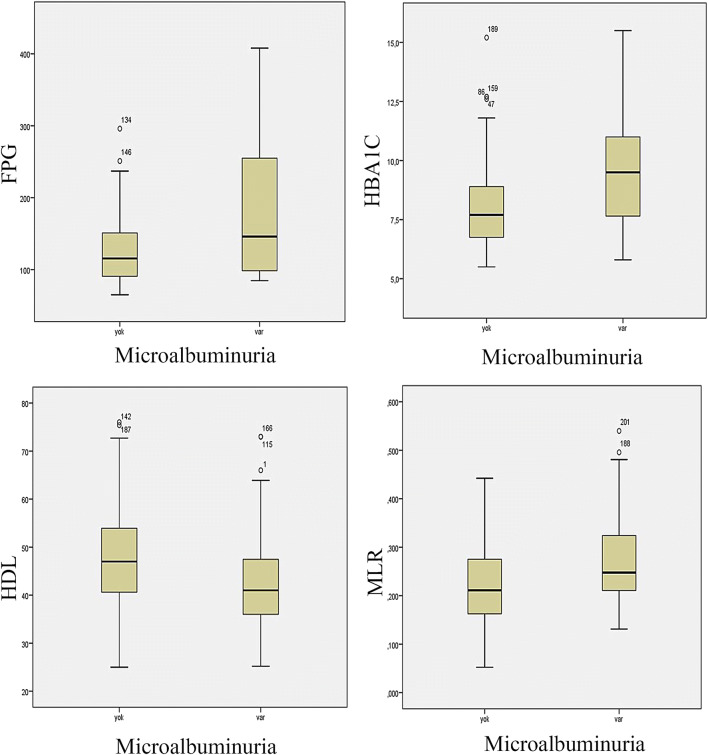
MLR was 0.247 (0.131–0.540) in MA group and 0.211 (0.052–0.390) in NA group. MLR of MA group was significantly higher than the MLR of NA group (p < 0.001). The spot urinary microalbumin/creatinine ratio was 47.9 (31.7-268.2) mg/dl and 14.5 (3-29.9) in and NA groups, respectively (p < 0.001). Association between study groups and HbA1c, FPG, MLR and HDLshown in figure 1.
Fig. 1.
Association between study groups and HbA1c, FPG, MLR and HDL
In ROC curve analysis, a MLR higher than 0.22 had a sensitivity of 69% and a specificity of 53% in prediction of MA (AUC = 0.650, p < 0.001). Figure 2 shows the ROC curve of the MLR in predicting MA.
Fig. 2.
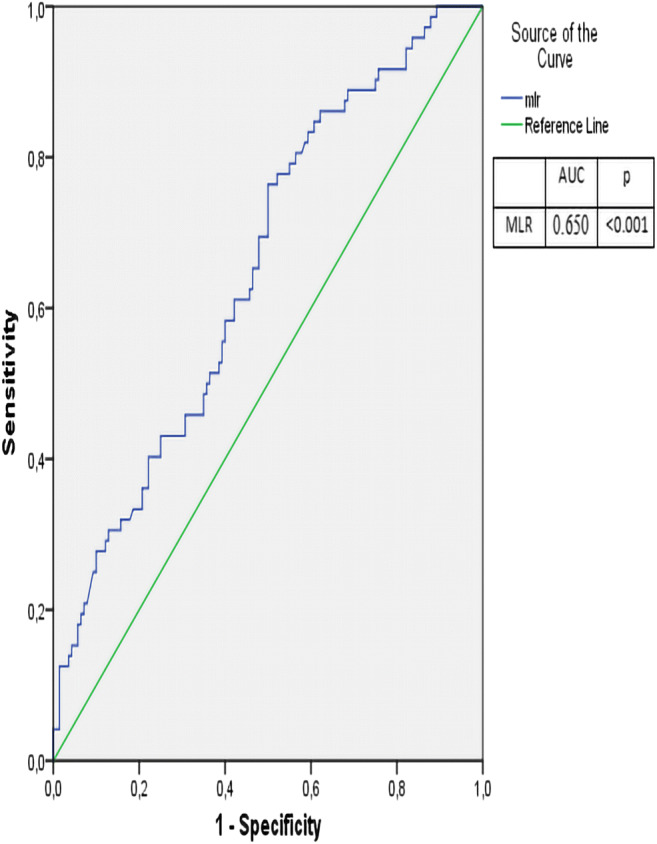
The ROC curve of MLR in predicting MA
In correlation analysis, MLR was significantly and positively correlated with MA (r = 0.228, p = 0.001). Figure 3 shows the correlation between MLR and MA (Fig. 3).
Fig. 3.
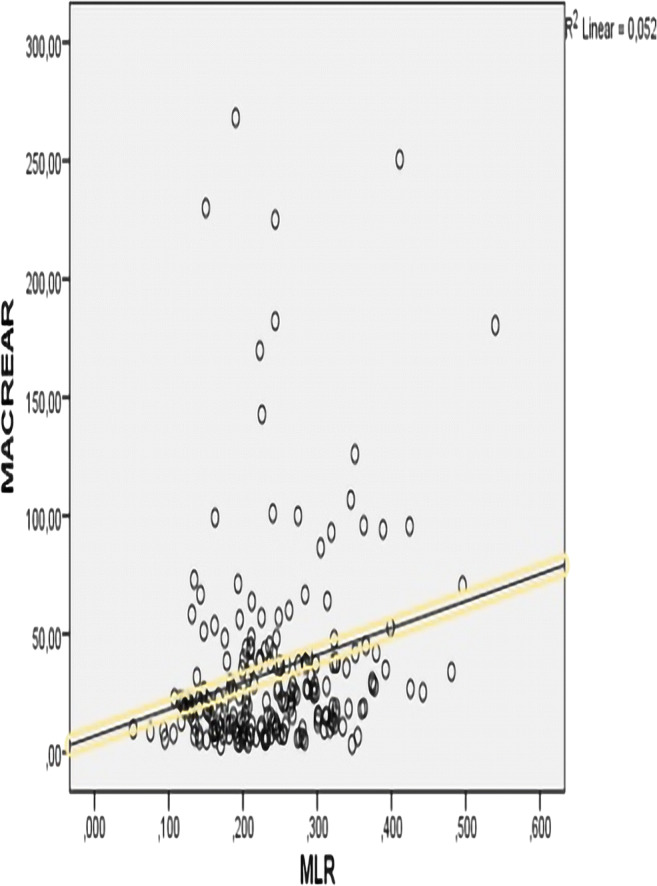
Correlation between MLR and MA
In multivariate backward logistic regression analysis, MLR (Beta: 6.276, OR:531,8, 95% CI: 8.5-33368, p = 0.003), FPG (Beta: 0.219, OR:1.245, 95%Cl:1.042–1.488, p = 0.016), HbA1c (Beta:0.219, OR:1.245, 95% Cl:1.042–1.488, p = 0.016) and presence of comorbid clinical diseases (Beta:-0.850, OR:0.427, 95% Cl:0.195–0.938, p = 0.034) were determined as independent predictors on the presence of DKI (Table 2).
Table 2.
Effects of different variables on microvascular complication in Multivariate Backward Logistic Regression Analysis (Comorbid clinical diseases: Coronary artery disease, Previous cerebro-vascular disease, peripheral artery disease, Diabetic retinopathy, Diabetic Nephropathy)
| Variable | B | OR | %95 CI | P |
|---|---|---|---|---|
| MLR | 6.276 | 531,8 | 8.5-33368 | 0.003 |
| Hemoglobin A1c (%) | 0.219 | 1.245 | 1.042–1.488 | 0.016 |
| Glucose(mg/dl) | 0.011 | 1.011 | 1.005–1.017 | 0.23 |
| Comorbid clinical diseases | -0.850 | 0.427 | 0.195–0.938 | 0.034 |
Discussion
Outstanding results of present study indicate that MLR could be a predictive marker for the detection of DKI in patients with T2DM. Other results of the study could be as following: (I) reduced HDL-cholesterol, increased FPG, elevated HbA1C and prolonged diabetes duration were associated with DKI, (II) MLR, FPG, HbA1c and comorbid clinical diseases were determined as independent predictors of DKI.
Albuminuria is considered as a clue for diabetic nephropathy and defined by MA (urinary albumin excretion < 300 mg/24 h) or macroalbuminuria (> 300 mg / 24 h) in urine samples.[8] Presence of MA indicates stage 3 diabetic nephropathy [9], which is considered as the last reversible phase of diabetic nephropathy with treatment. Therefore, determination of predictors of diabetic nephropathy in type 2 diabetic subjects is crucial for effective treatment and for reversing the deterioration in kidney functions.
The pathogenesis of DKI is complex since it has multifactorial etiology. However, it has been reported that the main pathogenetic mechanism in the development of DKI was inflammation [1]. Pro-inflammatory cytokines that could be involved in the pathogenesis of DKI were interleukin (IL) 1, IL-6 and IL-18 [10]. Serum levels of inflammatory marker vary depending on the degree of diabetic renal damage [11]. Both serum IL-6 and C-reactive protein (CRP) have been reported to be positively correlated with urinary albumin excretion in patients with type 2 diabetes mellitus, in a study from Asia [12]. These data in literature suggest the association between inflammation and DKI. As a novel inflammatory marker, MLR has been suggested to be related with inflammation and proposed as a predictive marker of DKI in type 2 diabetes mellitus, in present study.
Some other hemogram derived inflammatory markers have been studied in inflammatory conditions, too. Platelet to lymphocyte and neutrophil to lymphocyte ratios has been used in the diagnosis or estimation of prognosis in inflammatory states, such as, coronary artery disease [13], malignant tumors [14, 15], hematological neoplasms [16], inflammatory diseases (e.g. rheumatoid arthritis and ulcerative colitis) [17, 18], type 2 diabetes mellitus [19], diabetic kidney injury [20], and chronic obstructive pulmonary disease [21]. The basis of these studies was inspired by the impact of inflammation on hematopoiesis. Moreover, these markers are easy to access and inexpensive that make possible repeated measurements when necessary. MLR is also a marker that can be easily produced from hemogram parameters and has been used as an inflammation-based markers in various studies [5–7]. MLR has been shown to be an effective, inexpensive and readily available marker to demonstrate disease activity in patients with ulcerative colitis [22]. In addition, MLR has been proposed as a reliable, cost-effective and novel marker in assessing of disease severity in axial spondyloarthritis [23]. Preoperative MLR level can be used to predict recurrence in gastrointestinal stromal tumors [24].
Not only DKI, but also other microvascular complications of type 2 DM have been associated with MLR. In a study by Yue et al., MLR has been proved to be a risk factor for the development of diabetic retinopathy [25]. However, as far as we know, a study showing that MLR can be used in diagnosis in patients with developing DKI has not been done yet. In our study, it was found that MA can be determined by MLR, based on the inflammation process of the pathogenesis of DKI.
Proteinuria is correlated with several inflammatory markers. For instance positive correlation between urinary albumin excretion and inflammatory indices, such as, IL-6 and CRP has been reported in patients with T2DM [12]. Similarly, there was a statistically significant positive correlation between MA and MLR in our study.
The duration of diabetes was significantly higher in patients with MA than those in NA group in present study. Since the diabetes duration is a well-established contributing etiological factor in DKI [26], association between duration of type 2 diabetes mellitus and DKI in present study is not and unexpected outcome. Similarly, poor glycemic control and increased FPG are known risk factors in the development of DKI [26]. Therefore, increased FPG and HbA1c in MA group compared to NA were detected in our study, expectedly. In contrast, the relation between the duration of diabetes and the extent of glomerular pathology is unclear [26]. Some patients with diabetes duration of 15 years or those with shorter duration of diabetes develop renal damage, but some patients have not developed any complications despite many years of diabetes.
Along with FPG, HbA1c and presence of comorbid clinical diseases, MLR was found to be an independent predictor of DKI. Prediction of the complications of debilitating conditions is very important in primary prevention of such complications. Therefore, the results of present study are very important at this level.
Limitations of our study could be summarized as follows: (I) it is not clear how additional disease of T2DM patients affect monocyte and lymphocyte counts, (II) the effects of the anti-diabetic medications on monocyte and lymphocyte counts have not been established precisely, yet, (III) retrospective studies cannot evaluate the variations in MLR and (IV) single measurements of MLR may not show variations of these parameters. However, present study is the first in literature established association between MLR and DKI in type 2 DM.
Conclusions
We suggest that MLR could serve as a predictive and effective marker for nephropathy in diabetic subjects due to its strong correlation with MA and inexpensive and readily available nature. Despite it is not the cause of nephropathy, it may serve in estimation of the underlying inflammatory burden that promote nephropathy in diabetic subjects.
Funding information
This work has not received any funds or grants from any organizations.
Compliance with ethical standards
Conflict of interest
Authors have no conflict of interest.
Ethics approval and consent to participate
The study protocol was approved by the local Ethics Committee of the Institution (Ethical Decision Date/Number: 2018/261).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lim AK, Tesch GH. Inflammation in diabetic nephropathy. Mediat Inflamm. 2012;2012. [DOI] [PMC free article] [PubMed]
- 2.Horne BD, Anderson JL, John JM, Weaver A, Bair TL, Jensen KR, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45(10):1638–43. doi: 10.1016/j.jacc.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 3.Nunez J, Minana G, Bodi V, Nunez E, Sanchis J, Husser O, et al. Low lymphocyte count and cardiovascular diseases. Curr Med Chem. 2011;18(21):3226–33. doi: 10.2174/092986711796391633. [DOI] [PubMed] [Google Scholar]
- 4.Greene SJ, Harinstein ME, Vaduganathan M, Subačius H, Konstam MA, Zannad F, et al. Prognostic value of monocyte count in patients hospitalized for heart failure with reduced ejection fraction (from the EVEREST Trial) Am J Cardiol. 2012;110(11):1657–62. doi: 10.1016/j.amjcard.2012.07.035. [DOI] [PubMed] [Google Scholar]
- 5.Gong S, Gao X, Xu F, Shang Z, Li S, Chen W, et al. Association of lymphocyte to monocyte ratio with severity of coronary artery disease. Medicine. 2018;97(43). [DOI] [PMC free article] [PubMed]
- 6.Gary T, Pichler M, Belaj K, Eller P, Hafner F, Gerger A, et al. Lymphocyte-to-monocyte ratio: a novel marker for critical limb ischemia in PAOD patients. Int J Clin Pract. 2014;68(12):1483–7. doi: 10.1111/ijcp.12495. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Gao K, Lei W, Dong L, Xuan Q, Feng M, et al. Lymphocyte-to-monocyte ratio is associated with prognosis of diffuse large B-cell lymphoma: correlation with CD163 positive M2 type tumor-associated macrophages, not PD-1 positive tumor-infiltrating lymphocytes. Oncotarget. 2017;8(3):5414–25. doi: 10.18632/oncotarget.14289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haller H, Ji L, Stahl K, Bertram A, Menne J. Molecular mechanisms and treatment strategies in diabetic nephropathy: new avenues for calcium dobesilate-free radical scavenger and growth factor inhibition. BioMed Res Int. 2017;2017:1909258. 10.1155/2017/1909258. [DOI] [PMC free article] [PubMed]
- 9.Mogensen CE, Christensen CK, Vittinghus E. The stages in diabetic renal disease: with emphasis on the stage of incipient diabetic nephropathy. Diabetes. 1983;32(Suppl 2):64–78. doi: 10.2337/diab.32.2.s64. [DOI] [PubMed] [Google Scholar]
- 10.Navarro-Gonzalez JF, Mora-Fernandez C, Muros de Fuentes M, Garcia-Perez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7(6):327–40. doi: 10.1038/nrneph.2011.51. [DOI] [PubMed] [Google Scholar]
- 11.Cao L, Boston A, Jegede O, Newman HA, Harrison SH, Newman RH, et al. Inflammation and kidney injury in diabetic African American men. J Diabetes Res. 2019 doi: 10.1155/2019/5359635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choudhary N, Ahlawat RS. Interleukin-6 and C-reactive protein in pathogenesis of diabetic nephropathy: new evidence linking inflammation, glycemic control, and microalbuminuria. Iran J Kidney Dis. 2008;2(2):72–9. [PubMed] [Google Scholar]
- 13.Dong CH, Wang ZM, Chen SY. Neutrophil to lymphocyte ratio predict mortality and major adverse cardiac events in acute coronary syndrome: A systematic review and meta-analysis. Clin Biochem. 2018;52:131–6. doi: 10.1016/j.clinbiochem.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Zou ZY, Liu HL, Ning N, Li SY, Du XH, Li R. Clinical significance of pre-operative neutrophil lymphocyte ratio and platelet lymphocyte ratio as prognostic factors for patients with colorectal cancer. Oncol Lett. 2016;11(3):2241–8. doi: 10.3892/ol.2016.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koh CH, Bhoo-Pathy N, Ng KL, Jabir RS, Tan GH, See MH, et al. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer. 2015;113(1):150–8. doi: 10.1038/bjc.2015.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Li H, Li W, Wang L, Yan Z, Yao Y, et al. Pretreatment neutrophil/lymphocyte ratio but not platelet/lymphocyte ratio has a prognostic impact in multiple myeloma. J Clin Lab Anal. 2017;31(5). 10.1002/jcla.22107. [DOI] [PMC free article] [PubMed]
- 17.Gasparyan AY, Ayvazyan L, Mukanova U, Yessirkepov M, Kitas GD. The platelet-to-lymphocyte ratio as an inflammatory marker in rheumatic diseases. Ann Lab Med. 2019;39(4):345–57. doi: 10.3343/alm.2019.39.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fidan K, Kocak MZ. Assessment of platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in ulcerative colitis: a retrospective study. EurasianJ Med Oncol. 2017;1(4):224–7. [Google Scholar]
- 19.Mertoglu C, Gunay M. Neutrophil-Lymphocyte ratio and Platelet-Lymphocyte ratio as useful predictive markers of prediabetes and diabetes mellitus. Diabetes Metab Syndr Clin Res Rev. 2017;11:127-S31. doi: 10.1016/j.dsx.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 20.Huang W, Huang J, Liu Q, Lin F, He Z, Zeng Z, et al. Neutrophil–lymphocyte ratio is a reliable predictive marker for early-stage diabetic nephropathy. Clin Endocrinol. 2015;82(2):229–33. doi: 10.1111/cen.12576. [DOI] [PubMed] [Google Scholar]
- 21.Kocak MZ, Fidan K. Could the Neutrophil-to-Lymphocyte ratio be a marker of acute inflammation in chronic obstructive pulmonary disease? EurasianJ Med Investig. 2017;2(1):8–11. [Google Scholar]
- 22.Cherfane CE, Gessel L, Cirillo D, Zimmerman MB, Polyak S. Monocytosis and a low lymphocyte to monocyte ratio are effective biomarkers of ulcerative colitis disease activity. Inflamm Bowel Dis. 2015;21(8):1769–75. doi: 10.1097/mib.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y, Deng W, Zheng S, Feng F, Huang Z, Huang Q, et al. Relationship between monocytes to lymphocytes ratio and axial spondyloarthritis. Int Immunopharmacol. 2018;57:43–6. doi: 10.1016/j.intimp.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Cananzi FCM, Minerva EM, Sama L, Ruspi L, Sicoli F, Conti L, et al. Preoperative monocyte-to-lymphocyte ratio predicts recurrence in gastrointestinal stromal tumors. J Surg Oncol. 2019;119(1):12–20. doi: 10.1002/jso.25290. [DOI] [PubMed] [Google Scholar]
- 25.Yue S, Zhang J, Wu J, Teng W, Liu L, Chen L. Use of the monocyte-to-lymphocyte ratio to predict diabetic retinopathy. Int J Environ Res Public Health. 2015;12(8):10009–19. doi: 10.3390/ijerph120810009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caramori ML, Mauer M. Diabetes and nephropathy. Curr Opin Nephrol Hypertens. 2003;12(3):273–82. doi: 10.1097/00041552-200305000-00008. [DOI] [PubMed] [Google Scholar]