Abstract
The gut microbiota is a complex ecosystem that is involved in the development and preservation of the immune system, energy homeostasis and nutritional status of the host. The crosstalk between gut microbiota and the host cells modulates host physiology and metabolism through different mechanisms. Helicobacter pylori (H. pylori) is known to reside in the gastric mucosa, induce inflammation, and alter both gastric and intestinal microbiota resulting in a broad spectrum of diseases, in particular metabolic syndrome-related disorders. Infection with H. pylori have been shown to affect production level and physiological regulation of the gut metabolic hormones such as ghrelin and leptin which are involved in food intake, energy expenditure and body mass. In this study, we reviewed and discussed data from the literature and follow-up investigations that links H. pylori infection to alterations of the gut microbiota and metabolic hormone levels, which can exert broad influences on host metabolism, energy homeostasis, behavior, appetite, growth, reproduction and immunity. Also, we discussed the strong potential of fecal microbiota transplantation (FMT) as an innovative and promising investigational treatment option for homeostasis of metabolic hormone levels to overcome H. pylori-associated metabolic syndrome-related disorders.
Keywords: Helicobacter pylori, Gut microbiota, Gastric microbiome, Metabolic hormone, Metabolic syndrome, Endocrine disorders
Introduction
The term “gut microbiota” is referred to the microbial communities including bacteria, viruses, fungi, and protozoans inhabiting in the gut lumen [1, 2]. It has been estimated that the number of the microbiota associated genes is 150-fold higher than the host’s genes [3]; therefore, the microbiota could be considered as a distinct and functional metabolic organ [4]. During the past decade, a large amount of datasets on the gut microbial composition and diversity have been generated. As a result of the findings from the studies, the core microbiota in healthy adults consists of six dominant phyla including Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Fusobacteria and Verrucomicrobia [5].
It was indicated that imbalance of the gut microbiota composition and diversity, so-called “dysbiosis”, may happen due to unhealthy conditions, environmental factors, diet, as well as the presence of some eukaryotic microorganisms and viruses [6–9]. Many studies showed that disruption of the gut microbiota could be the origin of a broad spectrum of disorders from psychological distress [10, 11] to obesity [12, 13]. It is also believed that microbial community of the gut can communicate with one another and their hosts through metabolically active compounds to switch genes on and off [14]. In a mutualistic manner, the colonized bacteria in the gut consume undigested materials such as dietary fibers and can produce short-chain fatty acids (SCFA), which are beneficial for the physiology of their host cells while the host provides a suitable niche for the microorganisms to reside and grow [15, 16]. Moreover, it was indicated that a couple of microbial families play an important role during the food digestion, energy harvesting and uptake [17, 18]. It was well established that the gut microbiota may affect the energy harvesting through fermentation process, breaking down the dietary fibers, and producing a complex of biomolecules such as vitamins and other essential nutrients cofactors, and small molecules that the human host cannot synthesize itself [15]. Furthermore, gut bacteria can directly interact with the host’s immune cells and provoke both innate and adaptive immune responses. Therefore, interaction between bacteria and immune cells during the early years of the life leads to development of the immune system [19–21].
Helicobacter pylori (H. pylori) is a Gram-negative microaerophilic bacterium that colonizes the human stomach and affects more than half of the world’s population [22]. In 2015, it was estimated around 4.4 billion individuals were infected with H. pylori worldwide [23]. This widespread bacterial pathogen is strongly associated with various upper gastrointestinal (GI) pathologies such as chronic gastritis, peptic ulcer disease (PUD), gastric mucosa-associated lymphoid tissue (MALT) lymphoma, and/or gastric malignancies [24, 25]. Apart from gastric disorders, accumulating evidence suggests that at least in some subpopulations H. pylori infection may potentially correlate with the development of esophageal and colorectal neoplasms [26]. Furthermore, several studies indicated that H. pylori implicates in metabolic syndrome-related disorders including insulin resistance (IR), abdominal obesity, type 2 diabetes mellitus (T2DM), dyslipidemia, hypertension, cardio-cerebrovascular disease, the central nervous system (CNS) degenerative disorders, and nonalcoholic fatty liver disease (NAFLD) [27–30].
Although the human stomach has been regarded as the exclusive niche for H. pylori colonization, 16S rRNA sequencing of the fecal samples has revealed this bacterium at low relative abundance in GI tract [31]. Moreover, infection with H. pylori can hamper the secretion of gastric acid, induce chronic inflammation of the mucosal lining of the stomach, and thereby modulate the gastric microenvironment resulting in widespread alterations in the composition and diversity of gastric microbial community. These alterations in gut microbiota are associated with a wide range of GI diseases and systemic disorders.
In this review, we aim to explore and summarize accumulating evidence potentially linking H. pylori infection with alterations in the level of gut hormones and modulation of energy homeostasis specifically through gut microbiota-endocrine system axis. We also try to discuss some new hypotheses, and suggest areas to be further investigated in future studies.
Gut microbiota and endocrine system
Although during the recent years our knowledge on the beneficial genera of bacteria involved in the metabolic process has been improved, it is still vague that whether the endocrine system affects the gut microbiota composition or dysbiosis may indirectly affect the endocrine system. In this regard, it seems that gut bacteria and endocrine system can affect each other potentially through a cross-talk signaling mechanism. It was suggested that although sex-dependent, the gut microbiota play a crucial role in development of hippocampal serotonergic system [32]. Asano et al. [33] reported a critical role for the genus Lactobacillus in production of gamma aminobutyric acid (GABA) in an animal lab model. In addition, gut bacteria are able to modulate the levels of several host hormones and hormone-like biomolecules such as serotonin, norepinephrine, dopamine, pheromones, estrogen, androgen, prolactin, leptin, ghrelin, and insulin [34]. Therefore, the composition of gut microbiota and its diversity can significantly affect the psychological conditions and behavior of their hosts. In another words, composition of the gut microbiota via altering either the levels of pheromones, estrogen, androgen, and prolactin (gender-dependent) or serotonin, norepinephrine, and dopamine (gender-independent) may lead to anxiety or calming effects [34]. Recently, Schmidt et al. [35] documented that fecal microbiota transplantation (FMT) could be a promising and effective treatment to reduce the anxiety levels after a spinal surgery. FMT, previously known as bacteriotherapy, is the accepted terminology used to describe the process of transferring fecal infusion from a healthy donor into the colon of a patient particularly with debilitating GI infections, such as Clostridioides difficile infection (CDI), via colonoscopy, enema, nasogastric (NG) tube or in capsule form (popularly called “poop pills”) [36, 37]. Furthermore, in a small placebo-controlled clinical trial performed by Vrieze et al. [38], the effects of infusing intestinal microbiota from lean donors to the duodenal tube of male recipients with metabolic syndrome were studied on the composition of recipients’ microbiota and glucose metabolism. Based on their findings, insulin sensitivity of recipients was significantly elevated following 6 weeks of infusion of microbiota from lean donors along with levels of butyrate-producing intestinal microbiota. They also concluded that infusion of gut microbiota via FMT might be considered as an effective therapeutic option to increase insulin sensitivity in subjects with metabolic syndrome. However, further investigations using well-designed randomized placebo-controlled studies featuring FMT are required to deeply unravel the mechanisms through which gut microbiota interact with metabolic hormones, host metabolism, and immune system.
Together with the classical opinion on the role of exposure to microbes in development of the host immune system, recent studies highlighted the crucial indirect role of gut microbiota in educating the immune system [6, 14, 21]. Accordingly, it is believed that bidirectional communication and effects of gut microbiota and sex hormones via microbiota-gut-brain axis may play an important role in the changes in intestinal physiological function and immune responses [32]. Recently, Flak et al. [39] presented a novel concept termed as “microgenderome” or sexual dimorphism, which defines the bidirectional interaction and relationship between sex hormones, gut microbiota, immune system and disease susceptibility. It was also shown that certain bacterial species such as Clostridium scindens can utilize glococorticoids and convert them into androgens by side-chain cleavage [40]. On the other hand, it was demonstrated that the gut microbiota composition and abundance are sex-dependent and modulated by the sex hormones. Regarding the key role of sex hormones in immune responses, it seems that gut microbiota can modulate the immune system via alteration in the levels of sex hormones [41].
Gut microbiota and energy homeostasis
Apart from the direct impact of the gut microbiota composition on the digestion of foods, the microbial communities are able to control metabolism of energy via altering the levels of hormones through either secretion of specific hormones [42] or via gut-brain axis [43].
From the classical point of view, the gut microbiota can break down polysaccharides to monosaccharides and fermenting them to SCFAs [44]. An in vivo study conducted by Samuel et al. [45] represented that the caecal concentration of acetate in mice was significantly correlated with colonization of both B. thetaiotaomicron and M. smithii than either one of them or in germ-free mice. On the other hand, it was suggested that B. thetaiotaomicron not only increased the capacity of polysaccharides to glucose conversion [46], but also increased the expression of sodium/glucose co-transporter-1 (Slc5a1) which plays a crucial role in the glucose uptake in the ileum by the enterocytes [47].
Experimental evidence indicated that gut microbiota might be involved in the dehydroxylation and deconjugation of bile acids. Therefore, the secondary structure of the bile acids due to gut microbiota composition can lead to alteration during the lipid digestion and absorption [48–50]. Notably, the microbial community of the gut can affect the appetite and metabolism via controlling the levels of digestive hormones. It was established that gut microbiota affects the levels of leptin and ghrelin, two important appetite-related hormones. The experimental studies revealed that although the level of leptin was positively correlated with quantity of Bifidobacterium and Lactobacillus, the level of ghrelin was reported to be negatively related with these microorganisms [51].
H. pylori infection and alterations in gut microbiota
Although the stomach was traditionally considered as a sterile environment due to high acidity and a low number of cultured bacteria, the recent studies, particularly those which employed metagenomics approaches, indicated a large diverse group of colonizing bacteria in the gastric tissues [52]. Nevertheless, the density of bacteria in this organ is estimated to be significantly lower than that in the colon from 101 to 102 vs. 1010 to 1012 (CFU/g) [53]. In addition, despite of differences in the richness of the bacteria between different parts of the stomach, it seems that the diversity of microbial composition through the all parts of the stomach is almost similar [52, 54].
Recent studies strongly suggested a modulator role for H. pylori on endocrine system and the gut microbiota composition [52]. It seems that there are microbe-microbe and host-microbe cross-talks between H. pylori and the other bacterial taxa residing in the human stomach [55]. The exact mechanisms in which the altered gut microbiota cooperates with H. pylori to trigger gastric carcinogenesis is not completely clarified. In this regard, several studies demonstrated that the combination between H. pylori and other bacteria may synergistically increase the risk of gastric cancer development. For instance, Lactobacillus, Staphylococcus, and Escherichia coli are among the microorganisms which can transform nitrogen compounds into carcinogenic N-nitroso compounds [54, 56, 57]. In contrast, certain commensal bacteria including Prevotella, Neisseria and Streptococcus are reported to associate with a lower risk of development of gastric carcinoma [58, 59].
Apart from the effects of H. pylori on the microbial composition of the stomach, a couple of studies reported an important role for this bacterium in the composition of microbial population through the intestine [60, 61]. Additionally, it was shown that H. pylori not only alter the gut microbiota, but also this bacterium may change the fungi composition throughout the intestine [62]. Besides, it was demonstrated that the presence of H. pylori disrupts the Firmicutes/Bacteroidetes (F/B) ratio, which may affect the physiological conditions of H. pylori-infected subjects, particularly in asymptomatic individuals [54, 63, 64]. Gut microbiota analysis of rodents revealed an increase in Staphylococcus aureus and Enterococcus spp., and decrease of Lactobacillus in gerbils, as well as an increase of E. coli, Bacteroides, and Prevotella in mice [63, 65]. However, it is strongly suggested that H. pylori alters the composition of the gut microbiota via three probable ways: 1) changing the acidity level of the stomach [66–68], 2) providing substrates favor for colonizing some other taxa of bacteria [69, 70], and 3) changes in the life style and dietary patterns of the H. pylori-positive subjects [71, 72].
H. pylori infection and its impact on gut hormones
Apart from the classical influence of H. pylori infection on the gastric microbiota composition, this bacterium may alter the composition of the microbiome of the gut via provoking or decreasing the secretion of gastrointestinal hormones. It was revealed that the release of gastrin, a peptide hormone released from G cell which stimulates acid secretion, was increased in H. pylori-positive subjects [73, 74]. It was well-studied that the presence of H. pylori can affect the metabolism in human subjects through modulating the hormones. Therefore, H. pylori can directly or indirectly modulate the energy harvesting, food metabolism, and the body weight in infected human patients.
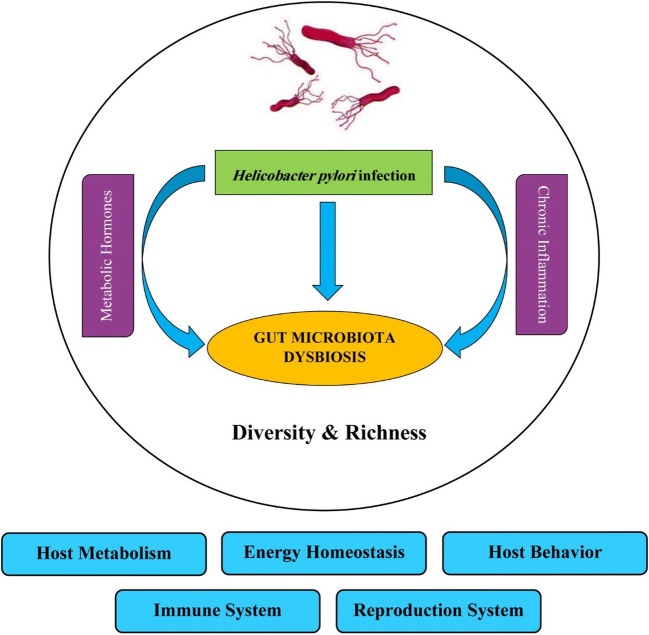
Acbay et al. [75] showed that the colonization of H. pylori in the stomach could increase the serum level of food-stimulated insulin release probably in a gastrin-dependent manner. However, recent studies using experimental animals [76] as well as humans demonstrated that infection by H. pylori induces insulin resistance in nondiabetic subjects [77, 78]. Leptin and ghrelin are two hormones that play a central role in energy homeostasis. Ghrelin is mainly produced by the stomach and is known as hunger hormone. Ghrelin increases appetite and energy harvesting, and leads to weight gain. In contrast to ghrelin, leptin is primary produced by adipose cell and enterocytes through the small intestine. This hormone decreases appetite and fat storage, and increases weight loss [79, 80]. A couple of studies demonstrated that H. pylori may regulate the energy homeostasis via not only indirectly through alteration in the gut microbiota composition, but also by directly manipulating the secretion of leptin and ghrelin, as well [81, 82]. Khosravi et al. [83] investigated the plasma level of ghrelin in three groups of mice, germ-free, specific-pathogen germ-free (SPF), and H. pylori-infected, and showed that H. pylori-infected mice represented increased level of ghrelin in comparison to other two groups. These results were in contrast with previous studies by Tatsuguchi et al. [84], and Isomoto et al. [85] who reported an inverse correlation between the plasma level of ghrelin and presence of H. pylori. Furthermore, in a study conducted by Francoise et al., [82] the meal-associated level of ghrelin and leptin increased after successful eradication of H. pylori. In a study by Yap et al. [86], the levels of pre-prandial active amylin, total peptide YY (PYY) and pancreatic polypeptide (PP), and the levels of post-prandial gut metabolic hormones including glucagon-like peptide-1 (GLP-1), total PYY, active amylin, and PP were significantly higher 12 months post-eradication as compared to baseline levels. However, the values of body mass index (BMI) and anthropometric did not significantly alter following the H. pylori eradication therapy. The potential impact of H. pylori infection on gut microbiota dysbiosis via changes in metabolic hormone levels and induction of host inflammatory responses is shown in Fig. 1. Moreover, Pierantozzi et al. [87] demonstrated that H. pylori infection may increase the risk for Parkinson’s disease (PD) by affecting L-DOPA levels. They also suggested that H. pylori eradication may ameliorate the clinical status of infected patients with PD and motor fluctuations by modifying L-dopa pharmacokinetics. Taken together, these results demonstrate that the presence of H. pylori infection may affect the energy homeostasis in infected human subjects, but require further investigations.
Fig. 1.
Impact of H. pylori infection on gut microbiota dysbiosis through changes in metabolic hormone levels (ghrelin, leptin, amylin, GLP-1, PYY, PP) and induction of host inflammatory responses. H. pylori infection may lead to alterations of the gut microbiota and metabolic hormone levels, which could exert broad impacts on host metabolism, energy homeostasis, behavior, immune and reproduction systems
Conclusions
In conclusion, the number of studies implicating the role and impact of H. pylori infection on both gastric and intestinal microbiota is rapidly growing. This study provides further evidence that gastric H. pylori infection is involved in the alterations of gut microbiota composition and diversity, which can lead to changes in production level and physiologic regulation of the gut metabolic hormones released from the host endocrine system. Furthermore, this work supports the rationale that links H. pylori infection to hormone production and the gut microbiota composition, which can exert broad effects on host metabolism, energy homeostasis, behavior, appetite, growth, reproduction and immune system development. In our vision, we see how restoration of the gut microbiome through FMT could be a promising and effective option for homeostasis of metabolic hormone levels to overcome H. pylori-associated metabolic syndrome-related disorders. However, in order to validate such novel approaches, the accurate mechanisms of H. pylori pathogenesis, and its precise bacterial and endocrine-microbiome crosstalk must all be thoroughly deciphered in future studies.
Acknowledgements
The authors would like to thank all laboratory members from Foodborne and Waterborne Diseases Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Authors’ contributions
S.O.M. reviewed the literature and collected the relevant data. A.Y. worked on concept and design of the study. A.Y. and M.K. supervised the findings of this work. A.Y. and H.M. contributed to manuscript writing. A.Y. and F.K. critically revised the manuscript. All authors provided critical feedback, helped shape the research, and contributed to the final version of the manuscript.
Funding information
This study was supported by a PhD grant from the Department of Microbiology, Jahrom Branch, Islamic Azad University, Jahrom, Iran.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Scarpellini E, Ianiro G, Attili F, Bassanelli C, de Santis A, Gasbarrini A. The human gut microbiota and virome: potential therapeutic implications. Dig Liver Dis. 2015;47(12):1007–12. doi: 10.1016/j.dld.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cani PD. Human gut microbiome: hopes, threats and promises. Gut. 2018;67(9):1716–1725. doi: 10.1136/gutjnl-2018-316723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu B, Wang X, Li L. Human gut microbiome: the second genome of human body. Protein Cell. 2010;1(8):718–725. doi: 10.1007/s13238-010-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baquero F, Nombela C. The microbiome as a human organ. Clin Microbiol Infect. 2012;18(Suppl 4):2–4. doi: 10.1111/j.1469-0691.2012.03916.x. [DOI] [PubMed] [Google Scholar]
- 5.Arumugam M, et al. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17(4):219–232. doi: 10.1038/nri.2017.7. [DOI] [PubMed] [Google Scholar]
- 7.Weiss GA, Hennet T. Mechanisms and consequences of intestinal dysbiosis. Cell Mol Life Sci. 2017;74(16):2959–2977. doi: 10.1007/s00018-017-2509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan SZ, Liu C, Huang CK, Luo FY, Zhu X. Ursolic acid improves intestinal damage and bacterial Dysbiosis in liver fibrosis mice. Front Pharmacol. 2019;10:1321. doi: 10.3389/fphar.2019.01321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tito RY, Chaffron S, Caenepeel C, Lima-Mendez G, Wang J, Vieira-Silva S, Falony G, Hildebrand F, Darzi Y, Rymenans L, Verspecht C, Bork P, Vermeire S, Joossens M, Raes J. Population-level analysis of Blastocystis subtype prevalence and variation in the human gut microbiota. Gut. 2019;68(7):1180–1189. doi: 10.1136/gutjnl-2018-316106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naude PJW, et al. Association of maternal prenatal psychological stressors and distress with maternal and early infant faecal bacterial profile. Acta Neuropsychiatr. 2019:1–31. [DOI] [PMC free article] [PubMed]
- 11.Browne PD, Aparicio M, Alba C, Hechler C, Beijers R, Rodríguez JM, Fernández L, de Weerth C. Human Milk microbiome and maternal postnatal psychosocial distress. Front Microbiol. 2019;10:2333. doi: 10.3389/fmicb.2019.02333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moran-Ramos S, et al. Environmental and intrinsic factors shaping gut microbiota composition and diversity and its relation to metabolic health in children and early adolescents: A population-based study. Gut Microbes. 2020:1–18. [DOI] [PMC free article] [PubMed]
- 13.Chen X, Sun H, Jiang F, Shen Y, Li X, Hu X, Shen X, Wei P. Alteration of the gut microbiota associated with childhood obesity by 16S rRNA gene sequencing. PeerJ. 2020;8:e8317. doi: 10.7717/peerj.8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, Li T, Yin Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol. 2018;8:13. doi: 10.3389/fcimb.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pascale A, Marchesi N, Govoni S, Coppola A, Gazzaruso C. The role of gut microbiota in obesity, diabetes mellitus, and effect of metformin: new insights into old diseases. Curr Opin Pharmacol. 2019;49:1–5. doi: 10.1016/j.coph.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano G, Gasbarrini A, Mele M. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1):14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy EF, Cotter PD, Healy S, Marques TM, O'Sullivan O, Fouhy F, Clarke SF, O'Toole PW, Quigley EM, Stanton C, Ross PR, O'Doherty RM, Shanahan F. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut. 2010;59(12):1635–1642. doi: 10.1136/gut.2010.215665. [DOI] [PubMed] [Google Scholar]
- 18.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Q, Elson CO. Adaptive immune education by gut microbiota antigens. Immunology. 2018;154(1):28–37. doi: 10.1111/imm.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pickard JM, Zeng MY, Caruso R, Núñez G. Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. 2017;279(1):70–89. doi: 10.1111/imr.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geuking MB, Köller Y, Rupp S, McCoy KD. The interplay between the gut microbiota and the immune system. Gut Microbes. 2014;5(3):411–418. doi: 10.4161/gmic.29330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farzi N, Yadegar A, Sadeghi A, Asadzadeh Aghdaei H, Marian Smith S, Raymond J, Suzuki H, Zali MR. High prevalence of antibiotic resistance in Iranian helicobacter pylori isolates: importance of functional and mutational analysis of resistance genes and virulence genotyping. J Clin Med. 2019;8(11):2004. doi: 10.3390/jcm8112004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global prevalence of helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153(2):420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 24.Yadegar A, Mohabati Mobarez A, Zali MR. Genetic diversity and amino acid sequence polymorphism in helicobacter pylori CagL hypervariable motif and its association with virulence markers and gastroduodenal diseases. Cancer Med. 2019;8(4):1619–1632. doi: 10.1002/cam4.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farzi N, Yadegar A, Aghdaei HA, Yamaoka Y, Zali MR. Genetic diversity and functional analysis of oipA gene in association with other virulence factors among helicobacter pylori isolates from Iranian patients with different gastric diseases. Infect Genet Evol. 2018;60:26–34. doi: 10.1016/j.meegid.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Kountouras J, Polyzos SA, Doulberis M, Zeglinas C, Artemaki F, Vardaka E, Deretzi G, Giartza-Taxidou E, Tzivras D, Vlachaki E, Kazakos E, Katsinelos P, Mantzoros CS. Potential impact of helicobacter pylori-related metabolic syndrome on upper and lower gastrointestinal tract oncogenesis. Metabolism. 2018;87:18–24. doi: 10.1016/j.metabol.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Mokhtare M, Mirfakhraee H, Arshad M, Samadani Fard SH, Bahardoust M, Movahed A, Masoodi M. The effects of helicobacter pylori eradication on modification of metabolic syndrome parameters in patients with functional dyspepsia. Diabetes Metab Syndr. 2017;11(Suppl 2):S1031–s1035. doi: 10.1016/j.dsx.2017.07.035. [DOI] [PubMed] [Google Scholar]
- 28.Yu Y, et al. Association of Helicobacter pylori infection with metabolic syndrome in aged Chinese females. Exp Ther Med. 2019;17(6):4403–4408. doi: 10.3892/etm.2019.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu X, Li W, Qin L, Yang W, Yu G, Wei Q. Relationship between helicobacter pylori infection and obesity in Chinese adults: a systematic review with meta-analysis. PLoS One. 2019;14(9):e0221076. doi: 10.1371/journal.pone.0221076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu MY, et al. Nonalcoholic fatty liver disease is associated with helicobacter pylori infection in north urban Chinese: a retrospective study. Gastroenterol Res Pract. 2020;2020:9797841. doi: 10.1155/2020/9797841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yap TW-C, Gan HM, Lee YP, Leow AHR, Azmi AN, Francois F, Perez-Perez GI, Loke MF, Goh KL, Vadivelu J. Helicobacter pylori eradication causes perturbation of the human gut microbiome in young adults. PLoS One. 2016;11(3):e0151893. doi: 10.1371/journal.pone.0151893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vemuri R, Sylvia KE, Klein SL, Forster SC, Plebanski M, Eri R, Flanagan KL. The microgenderome revealed: sex differences in bidirectional interactions between the microbiota, hormones, immunity and disease susceptibility. Semin Immunopathol. 2019;41(2):265–275. doi: 10.1007/s00281-018-0716-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, Koga Y, Sudo N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. 2012;303(11):G1288–G1295. doi: 10.1152/ajpgi.00341.2012. [DOI] [PubMed] [Google Scholar]
- 34.Neuman H, Debelius JW, Knight R, Koren O. Microbial endocrinology: the interplay between the microbiota and the endocrine system. FEMS Microbiol Rev. 2015;39(4):509–521. doi: 10.1093/femsre/fuu010. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt EKA, Torres-Espin A, Raposo PJF, Madsen KL, Kigerl KA, Popovich PG, Fenrich KK, Fouad K. Fecal transplant prevents gut dysbiosis and anxiety-like behaviour after spinal cord injury in rats. PLoS One. 2020;15(1):e0226128. doi: 10.1371/journal.pone.0226128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mullish BH, Quraishi MN, Segal JP, McCune VL, Baxter M, Marsden GL, Moore DJ, Colville A, Bhala N, Iqbal TH, Settle C, Kontkowski G, Hart AL, Hawkey PM, Goldenberg SD, Williams HRT. The use of faecal microbiota transplant as treatment for recurrent or refractory <em>Clostridium difficile</em> infection and other potential indications: joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. Gut. 2018;67(11):1920. doi: 10.1136/gutjnl-2018-316818. [DOI] [PubMed] [Google Scholar]
- 37.Azimirad M, Yadegar A, Asadzadeh Aghdaei H, Kelly CR. Enterotoxigenic Clostridium perfringens infection as an adverse event after Faecal microbiota transplantation in two patients with ulcerative colitis and recurrent Clostridium difficile infection: a neglected agent in donor screening. J Crohns Colitis. 2019;13(7):960–961. doi: 10.1093/ecco-jcc/jjz006. [DOI] [PubMed] [Google Scholar]
- 38.Vrieze A, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–916.e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 39.Flak MB, Neves JF, Blumberg RS. Welcome to the Microgenderome. Science. 2013;339(6123):1044–1045. doi: 10.1126/science.1236226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ridlon JM, Ikegawa S, Alves JMP, Zhou B, Kobayashi A, Iida T, Mitamura K, Tanabe G, Serrano M, de Guzman A, Cooper P, Buck GA, Hylemon PB. Clostridium scindens: a human gut microbe with a high potential to convert glucocorticoids into androgens. J Lipid Res. 2013;54(9):2437–2449. doi: 10.1194/jlr.M038869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rizzetto L, Fava F, Tuohy KM, Selmi C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: the role of sex. J Autoimmun. 2018;92:12–34. doi: 10.1016/j.jaut.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Zhuang M, Shang W, Ma Q, Strappe P, Zhou Z. Abundance of probiotics and butyrate-production microbiome manages constipation via short-chain fatty acids production and hormones secretion. Mol Nutr Food Res. 2019;63(23):1801187. doi: 10.1002/mnfr.201801187. [DOI] [PubMed] [Google Scholar]
- 43.Grasset E, Burcelin R. The gut microbiota to the brain axis in the metabolic control. Rev Endocr Metab Disord. 2019;20(4):427–438. doi: 10.1007/s11154-019-09511-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012;3(4):289–306. doi: 10.4161/gmic.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samuel BS, Gordon JI. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc Natl Acad Sci U S A. 2006;103(26):10011–10016. doi: 10.1073/pnas.0602187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez-Castano GP, et al. Bacteroides thetaiotaomicron starch utilization promotes Quercetin degradation and butyrate production by Eubacterium ramulus. Front Microbiol. 2019;10:1145. doi: 10.3389/fmicb.2019.01145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291(5505):881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 48.Staley C, Weingarden AR, Khoruts A, Sadowsky MJ. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl Microbiol Biotechnol. 2017;101(1):47–64. doi: 10.1007/s00253-016-8006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joyce SA, Gahan CG. Disease-associated changes in bile acid profiles and links to altered gut microbiota. Dig Dis. 2017;35(3):169–177. doi: 10.1159/000450907. [DOI] [PubMed] [Google Scholar]
- 50.Woting A, Blaut M. The intestinal microbiota in metabolic disease. Nutrients. 2016;8(4):202. doi: 10.3390/nu8040202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Queipo-Ortuno MI, et al. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS One. 2013;8(5):e65465. doi: 10.1371/journal.pone.0065465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wroblewski LE, Peek RM. Helicobacter pylori, Cancer, and the gastric microbiota. Adv Exp Med Biol. 2016;908:393–408. doi: 10.1007/978-3-319-41388-4_19. [DOI] [PubMed] [Google Scholar]
- 53.Sheh A, Fox JG. The role of the gastrointestinal microbiome in helicobacter pylori pathogenesis. Gut Microbes. 2013;4(6):505–531. doi: 10.4161/gmic.26205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pero R, et al. A Novel View of Human Helicobacter pylori Infections: Interplay between Microbiota and Beta-Defensins. Biomolecules. 2019;9(6). [DOI] [PMC free article] [PubMed]
- 55.Engstrand L, Lindberg M. Helicobacter pylori and the gastric microbiota. Best Pract Res Clin Gastroenterol. 2013;27(1):39–45. doi: 10.1016/j.bpg.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 56.Mowat C, Williams C, Gillen D, Hossack M, Gilmour D, Carswell A, Wirz A, Preston T, McColl KEL. Omeprazole, helicobacter pylori status, and alterations in the intragastric milieu facilitating bacterial N-nitrosation. Gastroenterology. 2000;119(2):339–347. doi: 10.1053/gast.2000.9367. [DOI] [PubMed] [Google Scholar]
- 57.Wang L, Zhou J, Xin Y, Geng C, Tian Z, Yu X, Dong Q. Bacterial overgrowth and diversification of microbiota in gastric cancer. Eur J Gastroenterol Hepatol. 2016;28(3):261–266. doi: 10.1097/MEG.0000000000000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferreira RM, Pereira-Marques J, Pinto-Ribeiro I, Costa JL, Carneiro F, Machado JC, Figueiredo C. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut. 2018;67(2):226–236. doi: 10.1136/gutjnl-2017-314205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang I, Woltemate S, Piazuelo MB, Bravo LE, Yepez MC, Romero-Gallo J, Delgado AG, Wilson KT, Peek RM, Correa P, Josenhans C, Fox JG, Suerbaum S. Different gastric microbiota compositions in two human populations with high and low gastric cancer risk in Colombia. Sci Rep. 2016;6:18594. doi: 10.1038/srep18594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park CH, Lee AR, Lee YR, Eun CS, Lee SK, Han DS. Evaluation of gastric microbiome and metagenomic function in patients with intestinal metaplasia using 16S rRNA gene sequencing. Helicobacter. 2019;24(1):e12547. doi: 10.1111/hel.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao JJ, Zhang Y, Gerhard M, Mejias-Luque R, Zhang L, Vieth M, Ma JL, Bajbouj M, Suchanek S, Liu WD, Ulm K, Quante M, Li ZX, Zhou T, Schmid R, Classen M, Li WQ, You WC, Pan KF. Association between gut microbiota and helicobacter pylori-related gastric lesions in a high-risk population of gastric Cancer. Front Cell Infect Microbiol. 2018;8:202. doi: 10.3389/fcimb.2018.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dash NR, Khoder G, Nada AM, al Bataineh MT. Exploring the impact of helicobacter pylori on gut microbiome composition. PLoS One. 2019;14(6):e0218274. doi: 10.1371/journal.pone.0218274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khosravi Y, et al. Proteomics analysis revealed that crosstalk between helicobacter pylori and Streptococcus mitis may enhance bacterial survival and reduces carcinogenesis. Front Microbiol. 2016;7:1462. doi: 10.3389/fmicb.2016.01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kienesberger S, Cox LM, Livanos A, Zhang XS, Chung J, Perez-Perez GI, Gorkiewicz G, Zechner EL, Blaser MJ. Gastric helicobacter pylori infection affects local and distant microbial populations and host responses. Cell Rep. 2016;14(6):1395–1407. doi: 10.1016/j.celrep.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yin YN, Wang CL, Liu XW, Cui Y, Xie N, Yu QF, Li FJ, Lu FG. Gastric and duodenum microflora analysis after long-term helicobacter pylori infection in Mongolian gerbils. Helicobacter. 2011;16(5):389–397. doi: 10.1111/j.1523-5378.2011.00862.x. [DOI] [PubMed] [Google Scholar]
- 66.Navabi N, Johansson MEV, Raghavan S, Lindén SK. Helicobacter pylori infection impairs the mucin production rate and turnover in the murine gastric mucosa. Infect Immun. 2013;81(3):829–837. doi: 10.1128/IAI.01000-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Espinoza JL, Matsumoto A, Tanaka H, Matsumura I. Gastric microbiota: an emerging player in helicobacter pylori-induced gastric malignancies. Cancer Lett. 2018;414:147–152. doi: 10.1016/j.canlet.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 68.Goddard AF, Spiller RC. The effect of omeprazole on gastric juice viscosity, pH and bacterial counts. Aliment Pharmacol Ther. 1996;10(1):105–109. doi: 10.1111/j.1365-2036.1996.tb00183.x. [DOI] [PubMed] [Google Scholar]
- 69.Bauerfeind P, Garner R, Dunn BE, Mobley HL. Synthesis and activity of helicobacter pylori urease and catalase at low pH. Gut. 1997;40(1):25–30. doi: 10.1136/gut.40.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ziebarth D, Spiegelhalder B, Bartsch H. N-nitrosation of medicinal drugs catalysed by bacteria from human saliva and gastro-intestinal tract, including helicobacter pylori. Carcinogenesis. 1997;18(2):383–389. doi: 10.1093/carcin/18.2.383. [DOI] [PubMed] [Google Scholar]
- 71.Dong Q, Xin Y, Wang L, Meng X, Yu X, Lu L, Xuan S. Characterization of gastric microbiota in twins. Curr Microbiol. 2017;74(2):224–229. doi: 10.1007/s00284-016-1176-8. [DOI] [PubMed] [Google Scholar]
- 72.Noto JM, Peek RM., Jr The gastric microbiome, its interaction with helicobacter pylori, and its potential role in the progression to stomach cancer. PLoS Pathog. 2017;13(10):e1006573. doi: 10.1371/journal.ppat.1006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pucci, A. and R.L. Batterham, Endocrinology of the Gut and the Regulation of Body Weight and Metabolism, in Endotext, K.R. Feingold, et al., Editors. 2000, MDText.com, Inc.: South Dartmouth (MA).
- 74.Waldum HL, Rehfeld JF. Gastric cancer and gastrin: on the interaction of helicobacter pylori gastritis and acid inhibitory induced hypergastrinemia. Scand J Gastroenterol. 2019;54(9):1118–1123. doi: 10.1080/00365521.2019.1663446. [DOI] [PubMed] [Google Scholar]
- 75.Acbay O, Celik AF, Gundogdu S. Does helicobacter pylori-induced gastritis enhance food-stimulated insulin release? Dig Dis Sci. 1996;41(7):1327–1331. doi: 10.1007/BF02088555. [DOI] [PubMed] [Google Scholar]
- 76.He C, Yang Z, Cheng D, Xie C, Zhu Y, Ge Z, Luo Z, Lu N. Helicobacter pylori infection aggravates diet-induced insulin resistance in association with gut microbiota of mice. EBioMedicine. 2016;12:247–254. doi: 10.1016/j.ebiom.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Allam AS, Bawady S, Abdel-Moaty AS, el-Nakeep S. Effect of helicobacter pylori on insulin resistance in nonobese, nondiabetic, and normolipidemic Egyptian patients. Egypt Liver J. 2018;8(1):23–28. [Google Scholar]
- 78.Vafaeimanesh J, et al. Helicobacter pylori infection and insulin resistance in diabetic and nondiabetic population. SciWorldJ. 2014;2014:391250. doi: 10.1155/2014/391250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yeung, A.Y. and P. Tadi, Physiology, Obesity Neurohormonal Appetite And Satiety Control, in StatPearls. 2020, StatPearls Publishing StatPearls Publishing LLC.: Treasure Island (FL). [PubMed]
- 80.de Candia P, Matarese G. Leptin and ghrelin: Sewing metabolism onto neurodegeneration. Neuropharmacology. 2018;136(Pt B):307–316. doi: 10.1016/j.neuropharm.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 81.Khosravi Y, Bunte RM, Chiow KH, Tan TL, Wong WY, Poh QH, Doli Sentosa IM, Seow SW, Amoyo AA, Pettersson S, Loke MF, Vadivelu J. Helicobacter pylori and gut microbiota modulate energy homeostasis prior to inducing histopathological changes in mice. Gut Microbes. 2016;7(1):48–53. doi: 10.1080/19490976.2015.1119990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Francois F, et al. The effect of H. pylori eradication on meal-associated changes in plasma ghrelin and leptin. BMC Gastroenterol. 2011;11:37. doi: 10.1186/1471-230X-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khosravi Y, Seow SW, Amoyo AA, Chiow KH, Tan TL, Wong WY, Poh QH, Sentosa IMD, Bunte RM, Pettersson S, Loke MF, Vadivelu J. Helicobacter pylori infection can affect energy modulating hormones and body weight in germ free mice. Sci Rep. 2015;5:8731. doi: 10.1038/srep08731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tatsuguchi A, Miyake K, Gudis K, Futagami S, Tsukui T, Wada K, Kishida T, Fukuda Y, Sugisaki Y, Sakamoto C. Effect of helicobacter pylori infection on ghrelin expression in human gastric mucosa. Am J Gastroenterol. 2004;99(11):2121–2127. doi: 10.1111/j.1572-0241.2004.30291.x. [DOI] [PubMed] [Google Scholar]
- 85.Isomoto H, Nakazato M, Ueno H, Date Y, Nishi Y, Mukae H, Mizuta Y, Ohtsuru A, Yamashita S, Kohno S. Low plasma ghrelin levels in patients with helicobacter pylori-associated gastritis. Am J Med. 2004;117(6):429–432. doi: 10.1016/j.amjmed.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 86.Yap TW, et al. Changes in metabolic hormones in Malaysian young adults following helicobacter pylori eradication. PLoS One. 2015;10(8):e0135771. doi: 10.1371/journal.pone.0135771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pierantozzi M, Pietroiusti A, Brusa L, Galati S, Stefani A, Lunardi G, Fedele E, Sancesario G, Bernardi G, Bergamaschi A, Magrini A, Stanzione P, Galante A. Helicobacter pylori eradication and l-dopa absorption in patients with PD and motor fluctuations. Neurology. 2006;66(12):1824–1829. doi: 10.1212/01.wnl.0000221672.01272.ba. [DOI] [PubMed] [Google Scholar]