Abstract
Objective
In this study, zebrafish was used as a biological model to induce type 2 diabetes mellitus through glucose. Then, the effect of metformin and silibinin combination was examined on elevated blood glucose, intestinal tissues, liver enzymes, and TNF-α, IFN-γ, INL1β genes as inflammation marker genes.
Methods
The liver enzymes (AST, ALT, and ALP) derived from fish viscera homogenate supernatants were assayed in an auto-analyzer. The expression of target genes was quantified on RNA extracted from the tails by an in-house RT-PCR method, with fine intestine tissue staining performed by hematoxylin and eosin protocol (H&E).
Result
In the glucose-free treatments, metformin and silymarin decreased the levels of AST, ALT, and ALP enzymes in the blood. The combination of these two drugs had also a significant role in reducing glucose levels. The body weight increased significantly in the control group which was affected by glucose concentration, with the lowest body weight gain observed in the metformin group. The expression of INL-1β gene was significantly enhanced in the control group and the highest IFN-γ expression was observed in both control groups with glucose (G + CTRL) and without glucose (G-CTRL) (p < 0.05). The lowest level of TNF-α gene expression was observed in the control + glucose group (G + CTRL) (p < 0.05). Diabetic state causes weak absorption whereby the fish body demands to increase absorption level by enhancing the amount of acidic goblet cells thereby acidifying the environment in the gastric tracts.
Conclusion
Collectively, this study indicated that treatment with metformin and Silibinin could improve metabolic-mediated performances by reducing the expression of inflammatory genes and blood glucose, modulating liver enzymes, and ameliorating the intestinal inflammation in type 2 diabetic zebrafish model.
Keywords: Zebrafish, Type 2 diabetes, Metformin, Silibinin
Introduction
Type 2 diabetes, obesity, and cardiovascular problems, linked to unhealthy life styles and metabolic-driven disorders, have grown dramatically for decades globally [1, 2]. Nowadays, the disease associated with obesity and high blood glucose are becoming a leading cause of death. Metformin is one of the popular medicines for patients with type 2 diabetes. Metformin is not insulin dependent and functions by lowering glucose in blood [3, 4]. Metformin also affects the immune system through infiltration of T-cells while reducing inflammation [5]. Silymarin is a flavonoid; flavonoids are plant chemicals of diverse phytonutrients, which can be found in many fruits and vegetables. Silymarin has a structure called silibinin which can protect the liver [6]. Silymarin has been reported as an effective compound in patients with type 2diabetes [7]., such as lack of useful medication and side effects in most of cases [8, 9]. Zebra fish has been established as a suitable model among vertebrate for biomedical research with more than 85% genetic similarity to humans [10]. Zebrafish also established a powerful platform as a model in metabolic diseases and hyperglycemia research, a cutting edge in diabetes investigation [11]. Zebrafish as a model for T2DM has already proved a promising platform to mechanistic, therapeutic, and heath management research for diet-induced, blood glucose level, and insulin resistance [12]. Zebrafish has been observed as a promising underline model as hyperglycemic, with significant response to high glucose treatment and insulin response which can be used as anti-diabetic drugs research [11, 13]. Regarding metformin and diabetes research, Wyett et al. reported a new protocol which can be applied for enhancement of beta cell based on beta-cell ablation in zebrafish using metformin. This study indicated that beta-cell number could be significantly increased using metformin [14].Inflammatory biomarker genes are found in diabetic and obese individuals, and these biomarkers can predict the progression of type 2 diabetes. In the present study, we investigate the possibility of tracking the inflammation in diabetes type 2 induced model of Zebrafish in the intestine and expression of TNF-α [15], IFN-γ [16] and IL-1β genes [17] by chemical (metformin) and herbal (Silymarin) alone and as combined drugs. Indeed, we intend to introduce a novel herbal and chemical combination compound to reduce the effects the possible inflammation caused by hyperglycemia due to high concentration of glucose uptake in zebrafish. This novel combination therapy could lead to diminished tissue damage by inflammation and improve the blood glucose level for diabetes type 2 management.
Materials and methods
Ethical statement
All zebrafish research experiments were performed according to the Endocrinology and Metabolism Research Center (EMRI), Tehran university of Medical Sciences, Tehran, Iran ethical guideline (IR.IAU.Babol.REC.1398.046).
Zebrafish (Danio rerio) maintenance
AB (wide type) Adult zebrafish strain was used in our study provided by core facility of Zebrafish research center, University of Tampere, Finland. Fish were acclimatized for 10 days to the temperature and photoperiod of our Zebrafish laboratory.
Experimental design and fish group definition
Seven-month-old adult fish were randomly chosen and divided into two main groups and four subgroups. Eight tanks were used in which there were 15 fish per 2-l water. All 8 fish tanks were kept under constant dissolved oxygen and pH around 7 and temperature of 28 ± 1 °C. The average weight per fish was 3 g. Each group was repeated as 3 duplicates. Two main experimental groups were evaluated as Induced glucose = G+) and (without glucose = G-) with each main group containing 4 subgroups as (Control = CTRL), (Silymarin = Si), (Metformin = Me), (Silymarin and Metformin = SiMe), making a total of 8 incubators in this study. Fish were fed on commercial Gemma micro (500) diet daily with 0.2 g per 10 fish twice a day. Water was replaced every other day (Fig. 1).
Fig. 1.
Trail group preparation. Fish were divided in to 2 main groups as (glucose induced) and (without glucose). Each of them contained four subgroups as CTRL, Si, Me, SiMe. Each tank contained 15 fish in 2 L of water. Tanks were kept under constant conditions and aerated with oxygen plus filtration at a constant temperature of 28 ± 1 °C. Water was replaced every other day
Preparing induced hyperglycemia zebrafish
In our present study, we induced zebrafish as a hyperglycemia model with increasing the glucose concentration in their tank water. After 5 days of acclimation (induction of glucose), the group of fish were treated through adding glucose monohydrate gradually into the tank. We started the initial induction with a concentration of 50 mM glucose (Merck, Germany). We kept the fish at this glucose concentration for 4 days, and monitored the health and mortality, followed by changing the water each day. On day 5, we elevated the glucose concentration up to 100 mM, and in order to prevent the fatality, we kept the fish for another 3 days while constantly monitoring the situation. The fish were adapted to the new glucose concentration and then we again heightened the glucose level (glucose-induced) to further treat the fish with 200 mM glucose.
Metformin and Silibinin treatments
Silymarin used in our present study was obtained from Zarband Pharmaceuticals, Iran. Silymarin liquid stock was prepared and stored at 4 °C. 10 drops (concentration 10 mM) were added to 2 L water tank containing 15 Fish for the groups: (G+ Si), (G+ SiMe), (G-Si), (G-SiMe).
Me 500 mg tablet was purchased from Chemidarou, Iran. To prepare the Me stock solution, we crashed 1 tablet of 500 mg Me to form a fine powder. The powder was gently collected and dissolved in 10 mL of RO water using falcon tube and vortex to reach fine mixers. 1 tablet equivalent to a concentration of 20 μL in 2 L of water (250 ppm) Also, 2 ml of the above stock solution was added to 2 L tank of 15 fish for group (G+ Me), (G+ SiMe), (G-Me) and (G-SiMe).
Zebrafish biometric analysis
In order measure the biometric parameters, we randomly collected three fish from each tank. To avoid the stress effect, the fish were then allowed to rest for 1 min after which they were euthanized with 3 drops of Eugenol in 50 mL of RO water. The fish length and weight were measured and recorded followed by fin clipping for further gene analysis and then immediately prepared for blood glucose measurement [18]. The fish length was measured from the anterior-most point of the mouth, to the posterior-most region of the caudal peduncle, using digital calipers. Next, the weight was measured using a microbalance (Mettler MT5, Mettler Toledo, Spain).
Blood glucose measurement and enzyme determinations
We conducted the sample collection in duplicate. Prior to blood collection experiment, three fish were randomly collected from each tank and kept fast in normal RO water for 12 h. The temperature and dissolved oxygen remained the same as other experimental tanks. The fish were euthanized in the same procedures as mentioned above. The posterior tail was cut and a drop of blood was directly applied to strip on glucometer and then blood glucose was recorded. The fish were then immediately dissected for the intestine sample collection [19]. The fish organs were separated and homogenized using a homogenizer and then placed into 0.15 M of NACL in ice -filled boxes. The samples were then centrifuged in a microtube at 2500 rpm (4 °C) for 15 min. After centrifugation, the soluble supernatant was used to measure the enzymes: aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkannin phosphatase (ALP) [20].
Histopathological studies
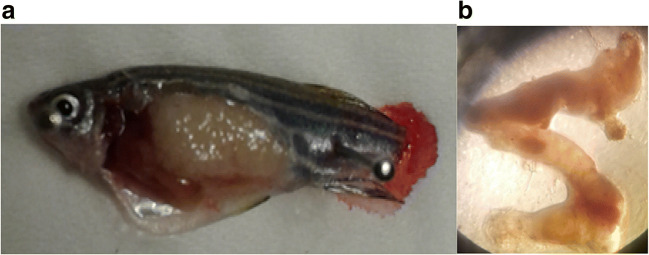
After recording biometric data and blood glucose level, the same fish were used for collecting intestine sample from all groups. Intact intestine was extracted and immediately stored in 10% formalin followed by storing in +4C° until further histological analysis Fig. 2a. The intact intestine was removed and kept in formalin. The middle part of intestine was then cut and stained for histological analysis Fig. 2b. Intestine staining experiments were performed by hematoxylin and eosin methods (H&E) [21]. Tissue extraction was performed from the middle part of intestine and fixed on 4% paraformaldehyde followed by washing with PBS after 10 min and incubated in to 0.5% periodic acid for 4–5 min. The tissue was stained with Schiff’s reagent for 15 min, while counterstaining using hematoxylin solution for 2 min. The entire staining procedure was performed at room temperature, with the tissue samples rinsed with water after each staining step. For imaging the histological sections, Olympus CX41 system microscope (Olympus USA) was used, and then DS-5 M-L1 digital sight camera (Nikon) captured the images.
Fig. 2.
Fish dissection. a Fish dissected immediately after biometric record and blood glucose measurement. b The intact intestine has been removed and immediately stored in 10% formalin and kept at +4C° for histology
Gene expression analysis and real time PCR
Total RNA extraction was performed from zebrafish tail using (RNA XPLUS Sina Colon) according to manufacture protocol. RNA extract concentrations were determined using Nano Drop_ 1000 Spectrophotometer (Thermo Scientific). Specifically, 3 μL of RNA was used for cDNA synthesis through reverse transcription Kit (Pars Toos, Iran). Prior to real-time PCR, 1 μL of cDNA was used as template in reaction to diluted with containing 100 μL PCR primers (Pars Toos. Iran). Real-time polymerase chain reaction (RTPCRs) were carried out with Fluo Cycle 2TM SYBR® method containing master mix containing SYBR green fluorescent. Real-Time PCR was performed using (StepOneTM, Applied Biosystems). Triplicate RTPCRs reaction was applied in each analysis. All reactions were set using a 96-well plate. The mixture contained 2.5 mL cDNA, 7 mL distilled water, and 12.5 mL of concentrated SYBR Fluo Cycle 2TM SYBR®. Also, 1.5 mL of forward and 1.5 mL of reverse primer were added in the reaction mixtures.
For all the reactions, we followed the thermal profile consisting of 5 min 95 °C with 40 cycles of 15 s at 95 °C, 20 s at 55 °C, and 30 s at 72 °C. Fluorescence emission occurred in each cycle followed by curve analysis. β-actin was considered as a housekeeping gene applied in our set of experiments as an internal standard gene to standardize the results to estimate the quantity and quality variations in mRNA. The relative value of each mRNA expression was then calculated from the threshold of β-actin cycles’ values with the values of each cycle presented by standard curves [22].The obtained date were analyzed using iQ5 optical software version 2.0. GeneEx Macro iQ5 Conversion and files were included (BioRad system). The primer sequences used are listed in Table 1.
Table 1.
List of primers used for real-time PCR experiment; tumor necrosis factor (TNF-α), Interferon gamma (IFN-γ), Interleukin-1β (IL-1β), Beta actin (bACT)
| Gene name | forward primer (5–3) Seq (5–3) | Gene bank accession number |
|---|---|---|
| TNF-α - F | GCTGGATCTTCAAAGTCGGGTGTA | AY427649 |
| TNF-α _R | TGTGAGTCTCAGCACACTTCCATC | AY427649 |
| IFN-γ _F | GAATGGCTTGGCCGATACAGGATA | AB126869 |
| IFN-γ _R | TCCTCCACCTTTGACTTGTCCATC | AB126869 |
| IL-1β _F | CATTTGCAGGCCGTCACA | CU695198 |
| IL-1β _R | GGACATGCTGAAGCGCACTT | CU695198 |
| bACT_F | ACAGGGAAAAGATGACACAGATCA | AF057040 |
| bACT_R | CAGCCTGGATGGCAACGTA | AF057040 |
Statistical analysis
Data analysis was performed by ANOVA. p < 0.05 was considered as statistically significant.
Results
Body weight
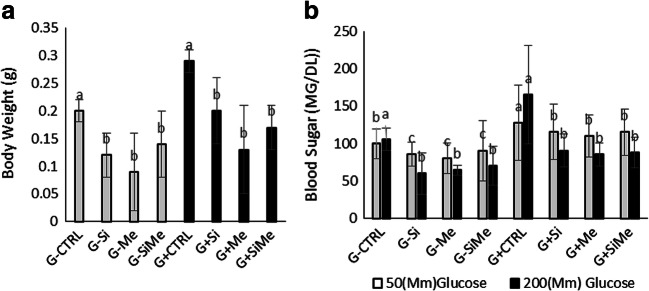
The use of metformin in obese non-diabetic adults leads to a decrease in food intake and weight loss along with a reduction in fasting blood glucose and insulin levels. The highest body weight (BW) was observed in glucose containing control group, while the lowest BW was seen in the metformin group. The BW was significantly higher in the control group receiving glucose-containing treatment than in other groups (p < 0.05) (Fig. 3a).
Fig. 3.
a Body weight. a The highest body weight index (BWI) observed in glucose-containing control group, while the lowest BWI was seen in the metformin group. The BWI was significantly higher in the control group receiving glucose-containing treatment than other groups (p < 0.05). b Blood glucose measurement. The highest level of blood glucose at the beginning and the end of experiment found in the glucose-containing control group, which was significantly higher compared to other groups. At the end of the experiment (after 20 days), the lowest level of blood glucose was seen in the glucose induced group treated with metformin, silymarin, and metformin plus silymarin groups (p < 0.05)
Blood glucose measurement
Increased blood glucose leads to elevated body weight, blood lipid levels, insulin resistance, and impaired insulin secretion. Also, the release of some peptide hormones such as cytokines from adipose tissue and other body tissues are involved in the elevated blood glucose in patients with diabetes. Thus, lower blood glucose with less side effects would be beneficial for patients with diabetes type 2 [23]. Silymarin therapy reduces both blood glucose and insulin secretion, and this has a beneficial effect on the treatment of hyperglycemia together with hyperinsulinemia in type 2 diabetes [24]. Silymarin, similar to other herbal extracts, has many compounds, whose biological impact cannot be linked to a single compound. Flavonoids are a group of compounds present in silymarin that, in addition to their very strong antioxidant properties, stabilize the cell membrane and increase cellular glutathione, which has been shown to lower the blood glucose and improve liver metabolism [24]. Me had a significant effect on glycemic control with lower plasma insulin levels without weight gain. Me and insulin combination together help obese patients with type 2 diabetes and could improve glycemic control reducing the need for exogenous insulin by approximately 30% [25]. In our study, we observed the highest level of blood glucose at the beginning and the end of experiment in the glucose-containing control group which was significantly higher in compared to other groups. At the end of the trail period (day 20), the lowest level of blood glucose was observed in the glucose-containing group treated with metformin, Silymarin, and metformin-Silymarin groups (p < 0.05) (Fig. 3b).
Enzyme analysis
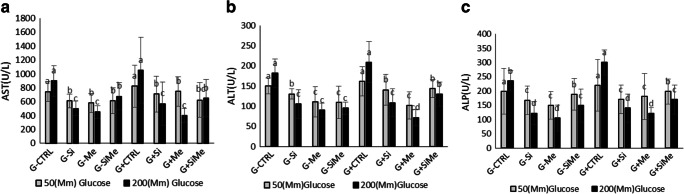
Drug- induced changes in liver enzymes which occur rapidly in a dose-dependent manner also play a major role in inflammation of the liver in many disorders [26]. Blood parameters are used as indicators of the physiological stress in fish as well, in response to endogenous or exogenous changes. As physiological characteristics of fish are very important in their rearing and considering that blood is one of the most vital substances in the body, thus, knowing the blood condition of fish plays an important role in advancing the goals of preserving, reproducing, and breeding these fish. Different types of diabetes, especially type 2, are usually associated with lipoprotein and lipid plasma abnormalities. These lipid abnormalities are linked with different enzymes such as aspartate amino transferase (AST) and alanine amino transferase (ALT) which are found in various tissues such as the liver, heart, skeletal muscles kidney, pancreas, spleen, red blood cells, and fish gills. These enzymes are also important in examining the health status of fish. Thus, decreased activity of this enzyme is a reason for proper liver function and suitable nutritional conditions [20]. In our present study, the result indicated that the highest level of liver enzyme AST was observed in the glucose-containing control group (p < 0.05). The highest level of liver enzyme ALT at the beginning and the end of experiment was seen in the both control groups with and without glucose (p < 0.05). At the end of the experiment, the lowest level of (ALT) enzyme observed in the glucose-containing metformin treated group (p < 0.05). The highest level of liver enzyme Alkaline Phosphatase (ALP) at the beginning and the end of experiment was also found in the control group containing glucose (p < 0.05). At the end of the experiment, the lowest level of this enzyme was seen in the glucose-containing metformin treated group and metformin group (p < 0.05) (Fig. 4).
Fig. 4.
Evaluation of enzyme analysis. Aspartate Aminotransferase (AST), Alanine aminotransferase (ALT), Alkanine Phosphatase (ALP), treatments with metformin and silymarin in glucose-free and glucose-containing treated fish. a The highest level of liver enzyme aspartate aminotransferase (AST) was observed in the glucose-containing control group at the beginning and the end of the experimental period (p < 0.05). b The highest level of liver enzyme alanine amino transferase (ALT). At the beginning and the end of experiment was seen in in the glucose containing control group and the control group without glucose (p < 0.05). At the end of the experiment, the lowest level of this enzyme was observed in the glucose-containing metformin treated group (p < 0.05). c The highest level of liver enzyme Alkaline Phosphatase (ALP) at the beginning and the end of experiment was observed in the glucose containing control group (p > 0.05). At the end of the experiment, the lowest level of this enzyme was seen in the glucose-containing metformin treated group and metformin group (p < 0.05)
Gene factors
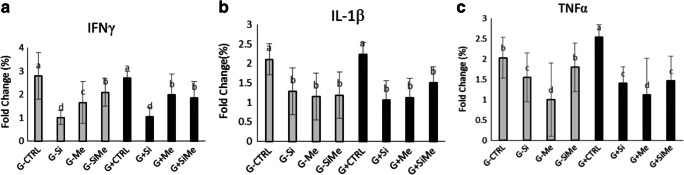
The structure of genes involved in the metabolism may be affected by different factors, causing changes in the way calories are stored and consumed in the body.Osteoblasts secrete cytokines such as IL-1β, where its inflammatory properties reduce cellular function and induce inflammatory responses in body tissues [17]. As a key cytokine, interferon-gamma plays an essential role in innate immunity and adaptive immunity against viruses and intracellular bacteria. Interferon-gamma stimulates phagocytosis by macrophages, regulates pro-inflammatory cytokines, and induces proteins that bind to iron and restrict iron availability to pathogens [27]. In fish, TNF-α is a central and a cytokine that influences inflammatory and antimicrobial responses. Various studies have shown that genetic and environmental factors are related to insulin resistance. The role of various genes in insulin resistance has been investigated among which TNF-α is prominent. Recent reports suggest that TNF-α disrupts insulin signaling pathway through the reduced kinase activity of insulin receptor [28] . The highest expression rate of IL-1β gene was observed in the control and glucose-containing control groups, and there was a significant difference with the other groups (p < 0.05). The highest expression rate of IFN-γ gene was observed in the control and glucose-containing control groups (p < 0.05). The lowest expression of this gene was seen in the silymarin group and the glucose-containing silymarin group (p < 0.05). We observed the highest expression rate of TNF-α in the control and glucose-containing control groups (p < 0.05). The lowest expression rate of TNF-α gene was observed in the silymarin group and the glucose-containing silymarin treated group (p < 0.05) (Fig. 5).
Fig. 5.
Expression rate of inflammation genes in fish samples. Interferon gamma gene (IFN-γ), interleukin-1 beta, and (TNF-α). a The highest expression rate of interferon gamma gene (IFN-γ) was observed in the control and glucose-containing control groups (p < 0.05). The lowest expression of the same gene was seen in the silymarin group and the glucose-containing silymarin group (p < 0.05). b The highest expression rate of interleukin-1 beta gene was found in the control and glucose-containing control groups and there was a significant difference with the other groups (p < 0.05). c Highest expression rate of (TNF-α) was observed in the control and glucose-containing control groups (p < 0.05). The lowest expression rate of this gene was seen in the silymarin group and the glucose-containing silymarin treated group (p < 0.05)
Intestinal tissue analysis
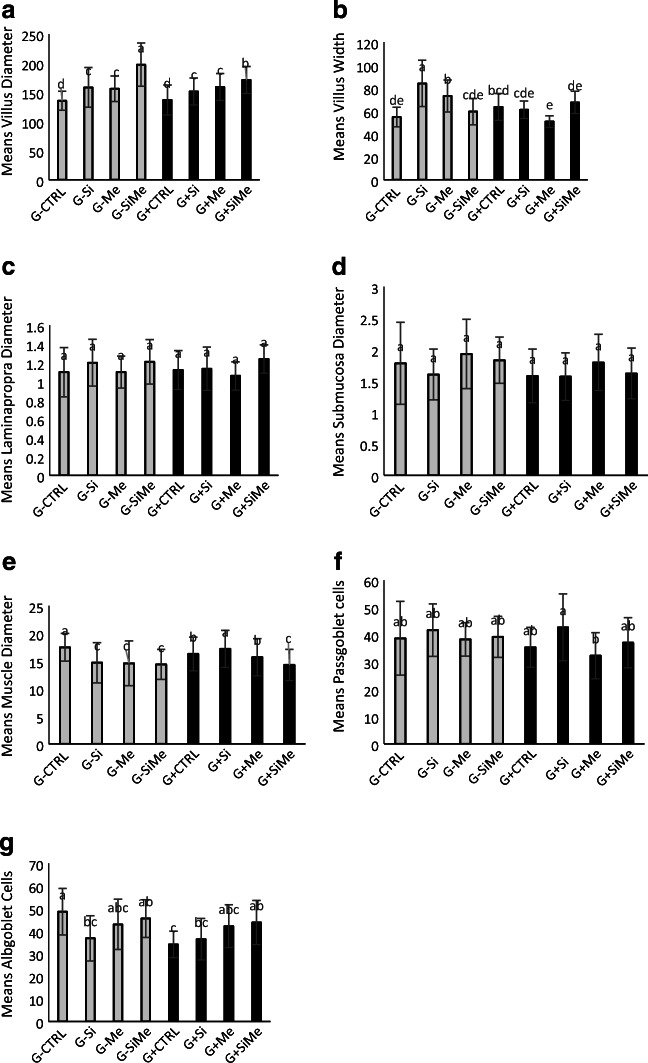
During development, the height of the villi in the intestine increases after birth. Studies have shown that there is a direct relationship between villus height and intestinal absorption capacity in diabetes. The highest villus diameter was observed in the silymarin-metformin combination group which was significantly higher than in other groups (p < 0.05). One can conclude that silymarin and metformin have been implicated in the improvement of blood supply through the mechanism described in intestinal mucosal healing, and have been able to bring mucosal adhesion to the former state. The highest villus width was observed in the silymarin group, while the lowest width was seen in the glucose-containing metformin treated group. There was a significant difference in the group treated with silymarin which was significantly higher than in other groups (p < 0.05). There were goblet cells within the tissue cells with the mucosal layer containing the cylindrical cells. Crypts of Lieberkühn, responsible for mucus secretion and epithelial cell production, are in the intestinal laminapropria (Fig. 6).
Fig. 6.
Intestinal tissue analysis, means of villus diameter, means of villus width, means of laminapropria diameter, means of submocusa diameter, means of muscle diameter, means of passgoblet cells, means of albgoblet cells, in experimental treatments with metformin and silymarin in glucose-free and glucose-containing treated fish. a The highest villus diameter was observed in the silymarin and metformin combination group which was significantly higher than in the other groups (p < 0.05). b The highest villus width was observed in the silymarin group, while the lowest amount was seen in the glucose-containing metformin treated group. There was a significant difference in the group treated with silymarin which was significantly higher than in other groups (p < 0.05). c Evaluation of Laminapropria diameter did not have a significant difference between the various study groups(p > 0.05).d Evacuation of Submocusa diameter did not reveal a significant difference between the study group (p > 0.05). e The highest muscle diameter was observed in the glucose-containing silymarin treated group which was significantly higher than in other groups. (p < 0.05). f The highest amount of Passgoblet Cells was observed in glucose containing treatment with silymarin which revealed a significant difference compared to other groups (p < 0.05).g The highest level of Albgoblet cells was observed in the control group, while the lowest was in the glucose-containing control group. The control group had the highest level of Albgoblet Cells with a significant difference (p < 0.05)
Evaluation of Laminapropria diameter did not show any significant difference across the various study groups (p > 0.05). The assessment of submucosa diameter did not reveal any significant difference either between the study group (p > 0.05). The type of mucus secreted differs according to the species of fish and the different pathways of the gastrointestinal tract, and the quality of the intestinal mucosa is fully correlated with environmental conditions as well as the type of feeding channel functioning. The highest amount of muscle diameter was observed in the glucose-containing silymarin treated group with significant difference with than other groups and was higher (p < 0.05). The highest amount of Passgoblet cells was observed in glucose containing treatment with silymarin which revealed a significant difference compared to other groups as well (p < 0.05). The highest level of Albgoblet cells was seen in the control group, while the lowest was in the glucose-containing control group. The control group had the highest level of Albgoblet cells with a significant difference (p < 0.05) (Fig. 7).
Fig. 7.
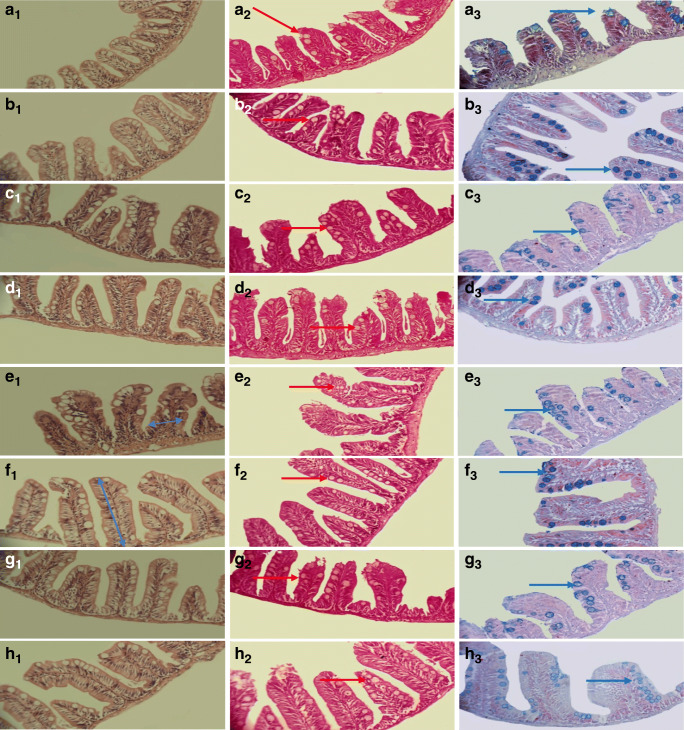
H&E staining in fish treatment. Midgut intestinal tissue. (A1–3): control, (B1–3): control + glucose,(C1–3): metformin, (D1–3): metformin + glucose, (E1–3): silibinin, (F1–3): silibinin + glucose, (G1–3): silibinin + metformin,(H1–3): silibinin + metformin + glucose .red arrow: Neutral goblet cells. Blue arrow: Acidic goblet cells. (G1–3) The highest villus diameter was observed in the silymarin and metformin combination group (p < 0.05).(E1–3) The highest villus width observed in the silymarin group.(D1–3), while the lowest was seen in the glucose-containing metformin treated group. (F1–3) The highest muscle diameter was observed in the glucose-containing silymarin treated group (p < 0.05). (F1–3) Also, the highest amount of Passgoblet Cells was observed in glucose containing treatment with silymarin which revealed a significant difference (p < 0.05). (A1–3) The highest level of Albgoblet cells was observed in the control group, (B1–3), while the lowest was in the glucose-containing control group. (A1–3) The control group had the highest level of Albgoblet cells with a significant difference (p < 0.05). (F1-F3) The highest villi height was observed in the glucose containing-treatment with silibinin. The height of the villi increased the level of absorption and have the lowest levels in the control group since becoming a diabetic. (G1–3) The greatest villus width was in the silibinin group and metformin, respectively, (E1–3) and the difference was statistically significant in the silibinin group (p < 0.05). (F1–3) Highest levels of goblet cells were observed in glucose-containing treatment with silibinin group and (E1–3) the silibinin group. (D1–3) The lowest level of neutral goblet cells observed in the glucose--containing treatment with metformin. (A1–3). The highest amount of Alcine blocking goblet cells was observed in the control group
Discussion
Blood glucose analysis
According to the Fig. 3, increased glucose uptake led to elevated glucose levels in (G + CTRL) treatment, but in the silymarin (G-Si) and metformin (G + Me) treatments, blood glucose levels dropped (p < 0.05). The combination of the two compounds had also a significant role in reducing glucose levels as much as silymarin lowered blood sugar levels. Metformin belongs to a class of medicines called Biguanide, which work in a similar way. In this class, metformin in this group reduces the amount of glucose produced by the liver, thereby lowering the body’s absorption of glucose, and increasing the effect of insulin on the body. With this in mind, a proper blood glucose control alone is not sufficient to delay the diabetic degenerative process. As shown by our findings in the present study, metformin seems to be a suitable drug both in terms of pharmaceutical composition as well as its hypoglycemic, anti-lipidemic, and antioxidant effects. Thus, the administration of metformin in obese diabetic patients is very useful.
BWI
As displayed in Fig. 3, the highest body weight index was observed in the glucose control group (G + CTRL) (P < 0.05). The lowest body weight gain was seen in the metformin (G-Me) group. There have been several studies regarding the effect of metformin on insulin function and insulin sensitivity in diabetes patients [29]. Meanwhile, the effect of metformin has been more pronounced in type 2 diabetes patients. Types of diabetes, especially type 2, are commonly associated with lipoprotein abnormalities and lipid plasma. Numerous studies have shown that metformin is an anti-hyperglycemic agent that causes weight loss, and is widely used in diabetic patients [30]. Studies suggest that metformin treatment of obese adults with type 2 diabetes could lead to weight loss and improved glucose tolerance as well as fat reserves. Metformin mainly exerts its anti-hyperglycemic effect by reducing the excretion of hepatic glucose through inhibiting gluconeogenesis causing reduced weight and blood glucose in type 2 diabetic patients [31]. Metformin appears to be effective in blood glucose control, lipid status, and weight control and can be a good therapy in obese type 2 diabetic patients. The mechanism of efficacy of silymarin administration in the protection of pancreatic cells and prevention of high glucose-induced metabolic disorders have been reported in several studies before. The Silibinin available in silymarin extract prevents the insulin release through glucose stimulation [32].
Enzyme
According to the Fig. 4, in the glucose-containing treatment, metformin alone had the highest effect after which silymarin alone was able to decrease these enzymes (P < 0.05). The combination of the two therapies also helped reduce the level of enzymes. Metformin is one of the drugs used to control blood glucose. Metformin reduces hepatic glucose production through inhibition of glucogenesis and increases glucose uptake by stimulating insulin in muscle and adipose tissue. Silymarin-stimulated DNA polymerase increases ribosomal RNA synthesis, resulting in liver cell regeneration. Increased cellular glutathione concentrations also stabilize superoxide dismutase and glutathione peroxidase. It has been confirmed that silymarin can induce liver cell repair and liver cell remodeling by affecting the outer membrane of liver cells and preventing the penetration of substances as well as enhancing ribosomal protein synthesis. This, in turn, improves liver function, and effectively blocks fat absorption [33]. In general, it can be concluded that silymarin reduces the liver enzymes AST, ALT, ALP in fish receiving glucose-containing treatment. In another study, addition of silymarin to the feed had no significant effect on ALP levels (p > 0.05). Many experiments have induced physiological stress responses in fish. Further, the effects of nutrients and dietary supplements have mechanisms that change serum cortisol concentration and subsequently blood glucose so it does not have a clear and regular pattern.
Gene factors
Myeloperoxidase, a marker for measuring the activity of phagocytic cells and inflammatory cytokine IL-1β, plays a key role in mediating an acute inflammatory response to infection-causing agents [34]. According to Fig. 5, the highest expression of interleukin-1 beta gene was observed in the control group (G-CTRL) and the control group (G + CTRL) (p < 0.05). Different expressions of cytokines in the body determine the type of Th1 or Th2 immune responses. Despite the many chemical drugs used to treat inflammation, inflammation is still incompletely treated, especially its chronic type, and the widespread side effects of synthetic drugs further complicate it. Flavonoids decrease intracellular calcium by inhibiting N-methyl-D aspartate receptor activity. The expression rate of cytokines and their secretion from immune cells depends on various factors, including treatment of infectious agents, hormonal status, genetic polymorphisms in cytokine genes, etc. [35]. The protective effect of Salvia officinalis extract not only conjugates with harmful free radicals but also suppresses pro-inflammatory responses induced by increased levels of TGF B1 and TNF-a [36]. The function of interferon-gamma in the cell can be independent of other cytokines such as interleukin-12 (IL-12) and may produce and increase TH1 levels. Th1 (interferon-gamma) also suppresses specific antigen response by blocking the inflammatory response induced by cytokines such as inter-leukine-10 (IL-10) [37]. It seems that by suppressing IFN-γ production, the suppression of this cytokine on TH17 cells also diminishes, resulting in an increase in IL17 production [16].As shown by Fig. 5, the highest expression of interferon gamma gene was observed in the control and glucose-containing control groups (G-CTRL, G + CTRL) (p < 0.05). The lowest was observed in silymarin-containing (G-Si) treatment.It has also been reported that the anti-inflammatory effects of flavonoids are due to the inhibition of inflammatory cytokines such as tumor necrosis factor, which are secreted by macrophages that are active in inflammation and increase prostaglandins. Through their inhibition of catechol O-methyl transferase and maintaining catecholamines, some flavonoids exert their antioxidant and anti-inflammatory properties [38]. Silibinin stimulates RNA production in hepatocytes. It acts as a radical scavenger and inhibits proliferation of HSC cells and inhibits collagen production. In vivo studies have shown that silymarin reduces collagen accumulation in animal models of secondary biliary fibrosis [39]. Cytokines are divided into several classes, including interferons, interleukins, chemokines, and tumor necrosis factor-alpha [40]. TNF- α is capable of inducing fever, inflammation, and tumor inhibition, viral proliferation and response to infection through the production of IL1 and IL6 cells. Disruption of the regulation of TNF production has been implicated in a variety of human diseases, including Alzheimer’s disease; however, the association between depression as well as inflammatory bowel disease and TNF levels has remained under dispute [41]. According to Fig. 5, the highest expression of TNF- α gene was observed in the G + CTRL group (p < 0.05), which was significant. The expression of adipose tissue-derived TNF-α gene in obese insulin-resistant individuals reported an unusually increasing pattern [42]. Decreased expression of TNF- α gene may eliminate insulin signaling pathway. Taken together, our findings suggest TNF- α as a suitable therapeutic candidate for the treatment of patients with insulin resistance, type 2 diabetes, obesity, blood lipid disorders, and metabolic syndrome [43]. The most important physiological action of TNF-α is related to the immune system; in the presence of microbial agents such as lipopolysaccharides, TNF- α is produced and causes vascular endothelial cells to express adherent molecules such as selectins at the site of infection. Thus, leukocytes such as neutrophils and monocytes can attach to the surface of these vessels and enter the site of infection [44]. It also enhances phagocytic activity of neutrophils and macrophages and stimulates endothelial cells and macrophages to secrete chemokines, which increase the binding affinity of leukocyte integrins for their ligand and leukocyte chemotaxis. They also affect single phagocytes and stimulate the secretion of interleukin-1 (IL-1), which acts similar to TNF- α. With this in mind, it can be concluded that the primary role of TNF-α is to induce local inflammatory responses to microbes [45]. TNF-α is involved in the onset of acute response as it affects the liver cells to produce specific serum proteins such as C-reactive protein, fibrinogen, and amyloid A, which are responsible for the acute phase response to germs [45]. When TNF-α levels rise in the blood, the contraction of the heart muscle and vascular smooth muscle is inhibited leading to a lower blood pressure which ends up to sepsis [46].
Intestinal tissue analysis
By appropriately lowering blood glucose levels, silymarin and metformin prevent the increase of glucose within the cell which leads to advanced glycation products thereby preventing ascites and thus improving blood supply and repair of damaged tissues. Meanwhile, it prevents the accumulation of glucose and thus sorbitol in the nerve cells that damage these cells thereby preventing neuropathy. As a result, the function of the nerve cells injected into the villi improved and their atrophy slightly increased. The rise of the villi can be justified by both of these mechanisms. The mucosal surface of the gastrointestinal tract is protected by a continuous epithelium that prevents the germs from entering. The destruction of this epithelium activates inflammatory factors [47]. This prevents the entry of pathogenic agents that activate the immune system, thereby reducing the activity of inflammatory agents and their complications, such as exudate, destruction, bleeding, and necrosis. According to Fig. 6, the results of tissue work revealed that the highest villi height was observed in the glucose containing-treatment with silibinin (G + Si). Fish treated with glucose, silibinin, and metformin were observed to have increased height of villi and to somehow elevated level of absorption, and had the lowest levels in the control group since becoming a diabetic. The greatest villus width was found in the silibinin group (G-Si) and metformin (G-Si), respectively, where the difference was statistically significant in the silibinin group (p < 0.05). There was no significant difference between the groups regarding laminapropriaparin, mucus, and muscle. In the case of the intestinal muscles, a trivial fluctuation was observed. It appears that in response to the loss of function and fluctuation in this group, the muscles do not have a proper contraction state and take a more relaxed state causing a minor difference due to the immobility triggered in the gut. Regarding goblet cells, the highest levels of goblet cells were observed in the glucose-containing treatment with silibinin group containing glucose (G + Si) and the silibinin group without glucose (G-Si), but there was no significant difference between the groups. The lowest level of neutral goblet cells was observed in the glucose--containing treatment with metformin (G + Me(. As mentioned earlier, goblet cells augment food solubility, and subsequently increase food absorption levels with no significant difference between the groups. Goblet cells, which have a higher acidic environment, boosted nutrient uptake, but the difference was not significant. The highest amount of Alcine blocking goblet cells was observed in the control group (G-Si.(.The lowest amount of Alcine blocking goblet cells was observed in the glucose-containing control group (G + Si). It seems that the diabetic state causes a weak absorption, and that the fish’s body has been able to heighten the absorption level by enhancing acidic goblet cells and acidifying the environment.
Conclusion
In this study, fasting blood glucose (FBG) and insulin resistance increased among fish. Zebrafish proved to be a promising model to investigate the inflammation, toxicity, and gene expression under the effect of type 2 diabetes. Our research result during 20 days of treatment with metformin and silymarin indicated a significant improvement in blood glucose, inflammation caused by high concentration of glucose. Our zebrafish model provides rapid model of T2DM compared to other models among mammals. The new combination therapy for T2DM we developed here was effective and fast, and can be used for the early phase of T2DM research. Our findings provide a new and useful tool for further research on drug discovery and toxicity research against diabetes.
Acknowledgments
We kindly acknowledge Dr. Mataleena Parikka director of zebrafish core facility, Tamere University, Finland for providing us with the AB wild type strain of zebrafish.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
The original online version of this article was revised due to a retrospective Open Access cancellation.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
12/7/2020
A Correction to this paper has been published: 10.1007/s40200-020-00698-8
Contributor Information
Hassan Mohammadi, Email: Babolmohammadi@gmail.com.
Hamed Manouchehri, Email: hdmanuchehri@gmail.com.
Reza Changizi, Email: rech76ir@gmail.com.
Fatemeh Bootorabi, Email: fatemeh.Bootorabi@tuni.fi.
Mohammad Reza Khorramizadeh, Email: khoramza@Tums.ac.ir.
References
- 1.Falcinelli S, et al. Dietary lipid content reorganizes gut microbiota and probiotic L. rhamnosus attenuates obesity and enhances catabolic hormonal milieu in zebrafish. Sci Rep. 2017;7(1):5512. doi: 10.1038/s41598-017-05147-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 3.Dunn CJ, Peters DH. Metformin. A review of its pharmacological properties and therapeutic use in non-insulin-dependent diabetes mellitus. Drugs. 1995;49(5):721–749. doi: 10.2165/00003495-199549050-00007. [DOI] [PubMed] [Google Scholar]
- 4.Hundal RS, Inzucchi SE. Metformin: new understandings, new uses. Drugs. 2003;63(18):1879–1894. doi: 10.2165/00003495-200363180-00001. [DOI] [PubMed] [Google Scholar]
- 5.de Oliveira S, Houseright RA, Graves AL, Golenberg N, Korte BG, Miskolci V, Huttenlocher A. Metformin modulates innate immune-mediated inflammation and early progression of NAFLD-associated hepatocellular carcinoma in zebrafish. J Hepatol. 2019;70(4):710–721. doi: 10.1016/j.jhep.2018.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vargas-Mendoza N, Madrigal-Santillán E, Morales-González A, Esquivel-Soto J, Esquivel-Chirino C, García-Luna Y González-Rubio M, Gayosso-de-Lucio JA, Morales-González JA. Hepatoprotective effect of silymarin. World J Hepatol. 2014;6(3):144–149. doi: 10.4254/wjh.v6.i3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piazzini V, et al. Nanostructured lipid carriers for oral delivery of silymarin: improving its absorption and in vivo efficacy in type 2 diabetes and metabolic syndrome model. Int J Pharm. 2019;572:118838. doi: 10.1016/j.ijpharm.2019.118838. [DOI] [PubMed] [Google Scholar]
- 8.Mang B, Wolters M, Schmitt B, Kelb K, Lichtinghagen R, Stichtenoth DO, Hahn A. Effects of a cinnamon extract on plasma glucose, HbA, and serum lipids in diabetes mellitus type 2. Eur J Clin Investig. 2006;36(5):340–344. doi: 10.1111/j.1365-2362.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- 9.Khalili L, Alipour B, Asghari Jafarabadi M, Hassanalilou T, Mesgari Abbasi M, Faraji I. Probiotic assisted weight management as a main factor for glycemic control in patients with type 2 diabetes: a randomized controlled trial. Diabetol Metab Syndr. 2019;11:5. doi: 10.1186/s13098-019-0400-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assunção JA, Zhou Y, Gu Y, Yen J, Vogel JH, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Elliott D, Threadgold G, Harden G, Ware D, Begum S, Mortimore B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P, Barker N, Lloyd C, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K, Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, Whitehead S, Kay M, Brown J, Murnane C, Gray E, Humphries M, Sycamore N, Barker D, Saunders D, Wallis J, Babbage A, Hammond S, Mashreghi-Mohammadi M, Barr L, Martin S, Wray P, Ellington A, Matthews N, Ellwood M, Woodmansey R, Clark G, Cooper JD, Tromans A, Grafham D, Skuce C, Pandian R, Andrews R, Harrison E, Kimberley A, Garnett J, Fosker N, Hall R, Garner P, Kelly D, Bird C, Palmer S, Gehring I, Berger A, Dooley CM, Ersan-Ürün Z, Eser C, Geiger H, Geisler M, Karotki L, Kirn A, Konantz J, Konantz M, Oberländer M, Rudolph-Geiger S, Teucke M, Lanz C, Raddatz G, Osoegawa K, Zhu B, Rapp A, Widaa S, Langford C, Yang F, Schuster SC, Carter NP, Harrow J, Ning Z, Herrero J, Searle SMJ, Enright A, Geisler R, Plasterk RHA, Lee C, Westerfield M, de Jong PJ, Zon LI, Postlethwait JH, Nüsslein-Volhard C, Hubbard TJP, Crollius HR, Rogers J, Stemple DL. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496(7446):498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capiotti KM, Antonioli JR, Kist LW, Bogo MR, Bonan CD, Da Silva RS. Persistent impaired glucose metabolism in a zebrafish hyperglycemia model. Comp Biochem Physiol B: Biochem Mol Biol. 2014;171:58–65. doi: 10.1016/j.cbpb.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Zang L, Shimada Y, Nishimura N. Development of a novel zebrafish model for type 2 diabetes mellitus. Sci Rep. 2017;7(1):1–11. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Driessen M, Kienhuis AS, Pennings JL, Pronk TE, van de Brandhof E, Roodbergen M, Spaink HP, van de Water B, van der Ven L. Exploring the zebrafish embryo as an alternative model for the evaluation of liver toxicity by histopathology and expression profiling. Arch Toxicol. 2013;87(5):807–823. doi: 10.1007/s00204-013-1039-z. [DOI] [PubMed] [Google Scholar]
- 14.Wyett G, Gibert Y, Ellis M, Castillo HA, Jan Kaslin, Aston-Mourney K. Metformin, beta-cell development, and novel processes following beta-cell ablation in zebrafish. Endocrine. 2018;59(2):419–425. doi: 10.1007/s12020-017-1502-3. [DOI] [PubMed] [Google Scholar]
- 15.Grayfer L, Walsh JG, Belosevic M. Characterization and functional analysis of goldfish (Carassius auratus L.) tumor necrosis factor-alpha. Dev Comp Immunol. 2008;32(5):532–543. doi: 10.1016/j.dci.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Harjula SE, et al. Interleukin 10 mutant zebrafish have an enhanced interferon gamma response and improved survival against a Mycobacterium marinum infection. Sci Rep. 2018;8(1):10360. doi: 10.1038/s41598-018-28511-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kralisch S, Weise S, Sommer G, Lipfert J, Lossner U, Bluher M, Stumvoll M, Fasshauer M. Interleukin-1beta induces the novel adipokine chemerin in adipocytes in vitro. Regul Pept. 2009;154(1–3):102–106. doi: 10.1016/j.regpep.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Eames SC, Philipson LH, Prince VE, Kinkel MD. Blood sugar measurement in zebrafish reveals dynamics of glucose homeostasis. Zebrafish. 2010;7(2):205–213. doi: 10.1089/zeb.2009.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gleeson M, Connaughton V, Arneson LS. Induction of hyperglycaemia in zebrafish (Danio rerio) leads to morphological changes in the retina. Acta Diabetol. 2007;44(3):157–163. doi: 10.1007/s00592-007-0257-3. [DOI] [PubMed] [Google Scholar]
- 20.Li S, Jiang Y, Sun Q, Coffin S, Chen L, Qiao K, Gui W, Zhu G. Tebuconazole induced oxidative stress related hepatotoxicity in adult and larval zebrafish (Danio rerio) Chemosphere. 2020;241:125129. doi: 10.1016/j.chemosphere.2019.125129. [DOI] [PubMed] [Google Scholar]
- 21.Chan JK. The wonderful colors of the hematoxylin-eosin stain in diagnostic surgical pathology. Int J Surg Pathol. 2014;22(1):12–32. doi: 10.1177/1066896913517939. [DOI] [PubMed] [Google Scholar]
- 22.Gioacchini G, Giorgini E, Olivotto I, Maradonna F, Merrifield DL, Carnevali O. The influence of probiotics on zebrafish Danio rerio innate immunity and hepatic stress. Zebrafish. 2014;11(2):98–106. doi: 10.1089/zeb.2013.0932. [DOI] [PubMed] [Google Scholar]
- 23.Vancea D, Vancea JN, Pires MI, Reis MA, Moura RB, Dib SA. Effect of frequency of physical exercise on glycemic control and body composition in type 2 diabetic patients. Arq Bras Cardiol. 2009;92(1):23–30. doi: 10.1590/S0066-782X2009000100005. [DOI] [PubMed] [Google Scholar]
- 24.Fraschini F, Demartini G, Esposti D. Pharmacology of silymarin. Clinical drug investigation. 2002;22(1):51–65. doi: 10.2165/00044011-200222010-00007. [DOI] [Google Scholar]
- 25.Fritsche A, Schmülling RM, Häring HU, Stumvoll M. Intensive insulin therapy combined with metformin in obese type 2 diabetec patients. Acta Diabetol. 2000;37(1):13–18. doi: 10.1007/s005920070030. [DOI] [PubMed] [Google Scholar]
- 26.Wong J, et al. A comparative study of blood endotoxin detection in haemodialysis patients. J Inflamm. 2016;13(1):24. doi: 10.1186/s12950-016-0132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cope A, et al. The Th1 life cycle: molecular control of IFN-gamma to IL-10 switching. Trends Immunol. 2011;32(6):278–286. doi: 10.1016/j.it.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Tafalla C, Granja AG. Novel insights on the regulation of B cell functionality by members of the tumor necrosis factor superfamily in jawed fish. Front Immunol. 2018;9:1285. doi: 10.3389/fimmu.2018.01285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu Z, Gilbert ER, Liu D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr Diabetes Rev. 2013;9(1):25–53. doi: 10.2174/157339913804143225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freemark M, Bursey D. The effects of metformin on body mass index and glucose tolerance in obese adolescents with fasting hyperinsulinemia and a family history of type 2 diabetes. Pediatrics. 2001;107(4):e55–5. [DOI] [PubMed]
- 31.Solymar M, et al. Metformin induces significant reduction of body weight, total cholesterol and LDL levels in the elderly - a meta-analysis. PLoS One. 2018;13(11):e0207947. doi: 10.1371/journal.pone.0207947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kazazis CE, Evangelopoulos AA, Kollas A, Vallianou NG. The therapeutic potential of milk thistle in diabetes. Rev Diabet Stud. 2014;11(2):167–174. doi: 10.1900/RDS.2014.11.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venkataramanan R, et al. Milk thistle, a herbal supplement, decreases the activity of CYP3A4 and Uridine Diphosphoglucuronosyl Transferase in human hepatocyte cultures. Drug Metab Dispos. 2000;28(11):1270. [PubMed] [Google Scholar]
- 34.Balkwill FR, Burke F. The cytokine network. Immunol Today. 1989;10:299–301. doi: 10.1016/0167-5699(89)90085-6. [DOI] [PubMed] [Google Scholar]
- 35.Gianoukakis AG, Smith TJ. The role of cytokines in the pathogenesis of endocrine disease. Can J Diabet. 2004;28(1):30–42. [Google Scholar]
- 36.Pradeep K, Mohan CVR, Gobianand K, Karthikeyan S. Silymarin modulates the oxidant–antioxidant imbalance during diethylnitrosamine induced oxidative stress in rats. Eur J Pharmacol. 2007;560(2–3):110–116. doi: 10.1016/j.ejphar.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 37.Cope A, le Friec G, Cardone J, Kemper C. The Th1 life cycle: molecular control of IFN-γ to IL-10 switching. Trends Immunol. 2011;32(6):278–286. doi: 10.1016/j.it.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Toker G, Küpeli E, Memisoğlu M, Yesilada E. Flavonoids with antinociceptive and anti-inflammatory activities from the leaves of Tilia argentea (silver linden) J Ethnopharmacol. 2004;95(2–3):393–397. doi: 10.1016/j.jep.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Jia J-D, Bauer M, Cho JJ, Ruehl M, Milani S, Boigk G, Riecken EO, Schuppan D. Antifibrotic effect of silymarin in rat secondary biliary fibrosis is mediated by downregulation of procollagen α1 (I) and TIMP-1. J Hepatol. 2001;35(3):392–398. doi: 10.1016/S0168-8278(01)00148-9. [DOI] [PubMed] [Google Scholar]
- 40.Akbari M, et al. The key role of tumor necrosis factor alpha (TNF-α) in vaccinated rainbow trout via irradiated Ichthyophthirius multifiliis trophont. Veterinarski arhiv. 2017;87(2):229–237. [Google Scholar]
- 41.Swardfager W, Lanctôt K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer's disease. Biol Psychiatry. 2010;68(10):930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 42.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95(5):2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pashaei S, Bakhtiyari S, Haghani K. Investigating the effects of TNF-α knockdown gene on insulin resistance in C2C12 muscle cells in the presence and absence of Palmitate. Iranian Journal of Endocrinology and Metabolism. 2013;15(4):387–394. [Google Scholar]
- 44.Kelly SA, Goldschmidt-Clermont PJ, Milliken EE, Arai T, Smith EH, Bulkley GB. Protein tyrosine phosphorylation mediates TNF-induced endothelial-neutrophil adhesion in vitro. Am J Phys Heart Circ Phys. 1998;274(2):H513–H519. doi: 10.1152/ajpheart.1998.274.2.H513. [DOI] [PubMed] [Google Scholar]
- 45.Chu W-M. Tumor necrosis factor. Cancer Lett. 2013;328(2):222–225. doi: 10.1016/j.canlet.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfeffer K. Biological functions of tumor necrosis factor cytokines and their receptors. Cytokine Growth Factor Rev. 2003;14(3–4):185–191. doi: 10.1016/S1359-6101(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 47.Abbas AK, Lichtman AH, Pillai S, Cellular and molecular immunology E-Book. 2011: Elsevier Health Sciences.