Abstract
Objective
The present review aims to provide an overview of traditional medicinal plants known to be of anti-diabetic potential.
Methods
A literature search was conducted using the scientific databases including PubMed, EMBASE and google scholar and a total of fifty herbs have been described and their possible mechanism of anti-diabetic action has been mentioned. Among them, in-depth discussion on five most potent anti-diabetic herbs has been provided with respect to their mechanism of action, in-vivo studies and clinical efficacies.
Results
The present review has highlighted the usefulness of the herbal source for the treatment and management of diabetes mellitus. With the help of previous literature published on In-vivo animal studies and human clinical studies; the effectiveness of Gymnema sylvestre, Momordica charantia, Trigonella foenum graecum, Tinospora cordifolia and Curcuma longa in the treatment and management of Diabetes has been proved.
Conclusion
Based on this review it can be concluded that herbs can serve as more efficient, safer, and cost-effective adjuvant therapy in the management and treatment of diabetes. Further investigations mainly focusing on the isolation of phytocompounds from these herbs can lead to the discovery of newer antidiabetic agents.
Keywords: Anti-diabetic, Diabetes mellitus, Herbs, Hyperglycaemia, Medicinal plants, Phytocompounds
Introduction
Diabetes mellitus is one of the most prevalent diseases found in all parts of the world and is becoming a serious threat to mankind’s health [1]. It is a complex heterogeneous group of metabolic disorders including hyperglycemia and is associated with the imbalance in carbohydrate, protein, and lipid metabolism [2]. According to WHO, “Diabetes mellitus is a chronic disease caused by inherited and/or acquired deficiency in production of insulin by the pancreas, or by the ineffectiveness of the insulin produced. Such a deficiency results in increased concentrations of glucose in the blood, which in turn damage many of the body’s systems, in particular the blood vessels and nerves” [3]. According to the recent data by International Diabetes Federation (IDF) Atlas claims that around 463 million adults are currently living with diabetes and estimates that there will be 578 million adults with diabetes by 2030, and 700 million by 2045 [4].
The management of diabetes mellitus is considered a global problem. In current allopathic therapy the oral hypoglycaemic agents and insulin, are subsequently used to control the diabetic conditions, however, complications associated with them, limited tolerability, cost, and other side effects reduce its wide acceptance. This could be the main reason for the shift of common people to Ayurveda form allopathic system nowadays [5].
Since ancient times traditional herbal drugs with multiple phytoconstituents and properties have been used as medicines for the treatment of a wide range of diseases [6]. Herbal medicines have been considered to be intrinsically safe, due to their natural occurrence, efficacy, and fewer side effects [7]. India has a long history of use of medicinal plants for the management of diabetes. World ethnobotanical information has reported the usage of about 800 plants for the control of diabetes mellitus, amongst them only 410 are experimentally proven for having anti-diabetic properties but the complete mechanism of action is available only for about 109 plants [8]. The treatment of diabetes using herbs has more advantageous effects and does not cause much side effects. These herbal drugs act by different mechanisms and consequently protect the β-cells during the diabetic condition and reduce the amount of glucose level in the blood [9].
This review aims to provide an overview of the use of medicinal plants in the management of diabetes, focusing on their mechanism of action. Furthermore, an emphasis on the five most commonly available and potent anti-diabetic herbs has been given. These include Gymnema sylvestre, Momordica charantia, Trigonella foenum graceum, Tinospora cardifolia, and Curcuma longa.
Methods
A literature search was conducted using the scientific databases including PubMed, EMBASE and google scholar. The aim was to identify published data on traditionally used medicinal plant for the treatment and management of Diabetes mellitus. The search terms used were “diabetes and plants”, “traditional plants”, “medicinal plants and diabetes”, “anti-hyperglycemic plants”, and “mechanism of anti-diabetic action”. Based on the above criteria extensive literature search was carried out and a total of fifty herbs have been described with their possible mechanism of anti-diabetic action. Amongst the fifty herbs in-depth discussion on five most potent and easily available anti-diabetic herbs has been provided with respect to their mechanism of action, in-vivo studies and clinical efficacies.
Traditional anti-diabetic plants
Since the time of Charaka and Sushruta, traditional medicines have been used for the management of diabetes mellitus [10]. Medicinal plants have always been a valuable source of drugs and many of the currently available drugs such as aspirin, quinine, vincristine, vinblastine, and digitalis have been derived directly or indirectly from them [11]. Most of the anti-diabetic drugs derived from plants are from the phytochemical class of polyphenols, terpenoids, tannins, and steroids. These affect various metabolic cascades, which further affect the level of glucose in the human body [12].
A list of medicinal plants used traditionally for diabetes with proven anti-diabetic and related beneficial effects are compiled along with their family, active principles responsible for diabetes, mechanism of action and use (Table 1).
Table 1.
List of Traditional plants used in management and treatment of Diabetes
| Plant name | Family | Parts used | Active Principles | Mechanism of action | Uses | Reference |
|---|---|---|---|---|---|---|
| Acacia arabica | Leguminoceae | Bark |
Gallic acid, pyrocatechol, (+)- catechin, (-) epigallocatechin-7-gallate, (-) epicatechin, quercetin, (+) catechin-5-gallate. |
Act as secretagouge to release insulin | Hypoglycemic activity | [13, 14] |
| Achyranthes aspera | Amaranthaceae | Leaves, seeds. | Betaine, achyranthine, β ecdysone | Carbohydrate digestion and absorption | Hypoglycemic effect | [15, 16] |
| Adhatoda vasica | Acanthaceae | leaves | Vasicine Vasicinol | α-Glucosidase-inhibiting activity | Antidiabetic | [17, 18] |
| Aegle marmelose | Rutaceae | leaves | Aegelin, marmesin and marmelosin | Regeneration of pancreatic β cells and insulin secretion | Hypoglycaemic effect | [16, 19] |
| Ageratum conyzoides | Asteraceae | leaves | Mono- and sesquiterpenes | Increase peripheral utilization of glucose | Hypoglycaemic effect | [20, 21] |
| Allium cepa | Amaryllidaceae | bulb | S-methyl cysteine sulfoxide, S-allyl cysteine sulfoxide | Stimulates pancreatic β-cells | Hypoglycaemic effect | [22, 23] |
| Allium sativum | Amaryllidaceae | bulb | Allicin, apigenin, alliin | Stimulates pancreatic β-cells | Antidiabetic and anti-oxidant | [16, 23, 24] |
| Aloe barbadensis | Asphodelaceae | leaves | Aloin, barbaloin, isobarbaloine, aloetic acid. | Insulin secretion and synthesis | Hypoglycemic effect. | [16, 25] |
| Andrographis paniculata | Acanthaceae | Whole plant | Andrographolide, | Regeneration of pancreatic β cells, insulin secretion | Antidiabetic & hepatoprotective. | [26, 27] |
| Annona squamosa | Annonaceae | leaves | Acetogenin | Enhances insulin level from pancreatic islets, increased utilization of glucose in muscle. | Hypoglycemic and antihyperglycemic activities | [28, 29] |
| Areca catechu | Palmitaceae | Leaves, flowers, seeds | Nitrosamines, arecoline, arecaidine | Carbohydrate digestion and absorption | Hypoglycemic | [16, 30] |
| Azadirachta indica | Meliaceae | leaves |
Azadirachtin, nimbolinin, nimbin, nimbidin, quercetin. |
Improves the insulin signaling molecules and glucose utilization in the skeletal muscle. | Antidiabetic, Antibacterial, antioxidant | [31, 32] |
| Bacopa manneri | Serophulariacea | Aerial part | Bacosine, brahmine, bacopaside I, II, III, IV and V. | Increase in peripheral glucose consumption | Antihyperglycemic agent | [33, 34] |
| Bauhinia forficuta | Fabaceae | leaves | Kaempferitrin | Glycolysis, insulinonematic activity. | Hypoglycemic effect, antioxidant. | [35, 36] |
| Berberis aristata | Berberidaceae | Stem bark, roots, leaves | Barberin, | Glucose transport, carbohydrate digestion and absorption, DPP-IV inhibition | Hypoglycemic effect | [37, 38] |
| Boerhavia diffusa | Nyctaginaceae | leaves | Punarnavine, Boeravinone A-F | Increase in hexokinase activity, increase plasma insulin level, antioxidant | Antidiabetic | [39, 40] |
| Camellia sinensis | Thecaceae | leaves | Epigallocatechin-gallate, gallocatechin, epicatechin, (+) catechin, (−) epicatechin | Free radical scavenging activity, insulinonematic activity | Antihyperglycemic activity, antioxidant | [41, 42] |
| Caseria esculenta | Salicaceae | roots | Leucopelargonidin, Dulcitol, Beta sitosterole. | Insulin secretion | Antihyperglycemic activity | [43] |
| Cassia auriculata | Fabaceae | roots | Bis (2-ethyl hexyl) phthalate | α-Glucosidase-inhibiting activity | Antihyperglycemic effect | [44, 45] |
| Centella asiatica | Apiaceae | Whole plant | asiaticoside | Antihyperglycemic activity | [46, 47] | |
| Coccinia indica | Cucurbitaceae | Aerial parts | - β- Amyrin Acetate, Lupeol, Cucurbitacin B, Taraxerone, Taraxerol, β-carotene, Lycopene, | Initiate insulin secretion, carbohydrate digestion and absorption. | Hypoglycemic effect. | [48, 49] |
| Commelina communis | Commelinaceae | Leaves, stem | 1-deoxynojirimycin, (2R,3R,4R,5R)2,5-bis(hydroxymethyl)-3,4-dihydroxypyrrolidine | Inhibition of α-glucosidase | Antihyperglycemic agent. | [50, 51] |
| Curcuma longa | Zingiberaceae | rhizomes | Curcumin, termerone, germacrone, zingiberene | Inhibition of α-glucosidase, inhibition of GSK-3β | Antidiabetic, Antihyperlipidemic, antioxidant | [52, 53] |
| Cyprus rotandus | Cyperaceae | Whole plant | α cyperone, cyperene, cyperol. | Inhibites intestinal glucose absorption and promoting glucose consumption. | Hypoglycemic agent | [54, 55] |
| Emblica officinalis | Euphorbiaceae | fruits | Gallic acid, ellagic acid, vitamin c. | Hypoglycemic, Decreases lipid peroxidation, antioxidant. | Hypoglycemic and antioxidant. | [56, 57] |
| Enicostema littorale | Gentianaceae | Whole plant | Swertiamarin, apigenin, isovitexin, swertisin, saponarin, 5-o glucosylswertisin | Glucose-induced insulin release through K(+)-ATP channel. | Hypoglymcemic effect. | [58, 59] |
| Ficus benghalensis | Moraceae | Bark, leaves | Leucocyanidin, pelarogonidin | Insulin secretion, glycogen synthesis | Antidiabetic | [60, 61] |
| Ficus racemosa | Moraceae | Bark, leaves | β-sitosterol, racemosic acid, Bergenin. | Glycogenolysis and gluconeogenesis | Hypoglycemic activity | [62, 63] |
| Glycyrrhiza glabra | Leguminoceae | roots | Glycyrrhizin, glycyrrhizic acid liquirtin, isoliquirtin. | Potent PPAR-γ ligand binding activity thus, reduces the blood glucose level | Hypoglycemic agent. | [64, 65] |
| Gymnema sylvestre | Asclepidaceae | leaves | Gymnenic acid, Stigmasterol, Gurmarin, betaine, gymnemosides. | Regeneration of pancreatic β cells, α-glucosidase inhibitor, insulin secretion | Antidiabetic agent. | [66–68] |
| Ginkgo biloba | Ginkgoceae | leaves | Kaempferol, isorhamnetin | Inhibition of α-amylase and α-glucosidase activity | Hypoglycemic agent. | [69] |
| Mangifera indica | Anacardiaceae | leaves | Mangiferin | α-Glucosidase-inhibiting activity | Hypoglycemic agent | [70] |
| Momordica charantia | Cucurbitaceae | fruits | Momordin, momordicine, charantin | Insulin secretion, glycogen synthesis | Hypoglycemic agent | [71, 72] |
| Morus indica | Moraceae | leaves | Chrysin, isoquercitrin | Insulin secretion | Hypoglycemic agent | [73] |
| Ocimum sanctum | Lamiaceae | leaves | Eugenol, trans-β ocimene, Carvacrol, linalool. | Insulin secretion, carbohydrate digestion and absorption | Hypoglycemic agent. | [74, 75] |
| Panax ginseng | Araliaceae | roots | Ginsenosides Rg2, panaxan A, B, C, D, E | Regeneration of pancreatic β cells, free radical scavenging | Antihyperglycemic activity | [76, 77] |
| Phyllanthus amarus | Phyllanthaceae | leaves | Brevifolin carboxylic acid, ethyl brevifolin carboxylate | α-Amylase inhibitory activity | Hypoglycemic, Anti-oxidant activity. | [78] |
| Pterocarpus marsupium | Leguminoceae | Stem wood | Marsupsin, pterosupin, pterostilbene | Insulinomematic activity | Antidiabetic | [79] |
| Swertia chirata | Gentinaceae | Whole plant | Amarogentin, swerchirin, chirantin | Stimulates insulin release from islets | Antihyperglycemic agent | [80] |
| Syzygium aromaticum | Myrtaceae | Flower buds | Eugenol, Caryophylline | Insulin secretion, carbohydrate digestion and absorption | Hypoglycemic agent | [81] |
| Syzygium cumini | Myrtaceae | Bark, seeds | Jambosine, jambolin, anthocyanins. | α-Glucosidase-inhibiting activity | Anti-hyperglycemic | [82] |
| Terminalia arjuna | Comberetaceae | Stem bark | Arjunic acid, arjunolic acid, gallic acid. | Stimulates insulin release from islets | Hypoglycemic ativity | [83] |
| Terminalia chebula | Comberetaceae | fruits | Gallic acid, chebulic acid, chebulanin, ellagic acid, chebulegic acid, chebulinic acid | Secretion of insulin from the β-cells. | Hypoglycemic ativity | [84] |
| Terminalia belerica | Comberetaceae | fruits | ß- sitosterol, gallic acid, ellagic acid, ethyl gallate, chebulaginic acid. | Insulin secretion, carbohydrate digestion and absorption | Hypoglycemic ativity | [85] |
| Tinospora cardifolia | Menispermaceae | Leaves and stem | Tinosporine, cordifolide, tinosporide, Barberin. | α-Glucosidase-inhibiting activity, glycolysis | Antidiabetic agent. | [86, 87] |
| Trigonella foenum graceum | Fabaceae | seeds | Trigonellin, Fenugreekine. | Regeneration of pancreatic β cells, insulin secretion | Antidiabetic activity. | [88, 89] |
| Vinca rosea | Apocynaceae | Whole plant | Catharanthine, vindoline, vindolinene vinblastine, vincristine | Regeneration of pancreatic β cells, insulin release | Hypoglycemic activity | [90] |
| Vitis vinifera | Vitaceae | Leaves, stem | E-resveratrol, E-ε-viniferin, anthocyanins. | Insulinonematic activity | Anti-hyperglycemic activity | [91] |
| Withania somnifera | Solanaceae | Leaves, roots | Withaferin A, withanolides | Insulin release from pancreatic β cells | Hypoglycemic activity | [92] |
| Zingiber officinalis | Zingiberaceae | rhizomes | Gingirol, shoagol, zingerone. |
Increase insulin level & decrease fasting glucose level |
Hypoglycemic activity | [93] |
Amongst the fifty herbs described in Table 1 we have identified five most potent and easily available anti-diabetic herbs namely, Gymnema sylvestre, Momordica charantia, Trigonella foenum graecum, Tinospora cordifolia and Curcuma longa. In-depth discussion on these herbs has been provided with respect to their mechanism of action, in vivo studies and clinical efficacies. The rationale behind selection of these five herbs owes to their traditional usage, easy availability, effectiveness and most importantly to their proven clinical significance. The selected plants are a choice of herbal medicine in the treatment of diabetes. They are also utilized by multiple pharmaceutical herbal industries and most of the herbal anti-diabetic preparation consist of these herbs. Although there are other plants which are utilized in the diabetes management as folklore medicine but their commercial utilization is less as compare to the chosen five plants. Hence a need has been felt for understanding their various mechanism of actions and efficacy in management of diabetes.
Gymnema sylvestre
Gymnema sylvestre is an indigenous herb, belonging to the family Asclepiadaceae. It is popularly known as “gurmar” for its distinct property as sugar destroyer, it is a reputed herb in the Ayurvedic system of medicine. The plant is indigenous to western and central India, Australia, and tropical Africa [94].
Phytochemistry of G. sylvestre
G. sylvestre is a good source of a large number of bioactive substances. The leaves contain Triterpene saponins like gymnemic acids, gymnemasaponins, and gymnemasides. Apart from this, other phytoconstituents include flavones, anthraquinones, pentatriacontane, hentri-acontane, α and β-chlorophylls, phytin, stigmasterol, dquercitol, resins, etc. The major secondary metabolites present in Gymnema includes Gymnemic acid. The Gymnemic acids consist of numerous members termed as gymnemic acids I–VII, gymnemasaponins, and gymnemosides A–F. Gurmarin is another essential phytoconstituent isolated from G. sylvestre [95].
Mechanism of Action
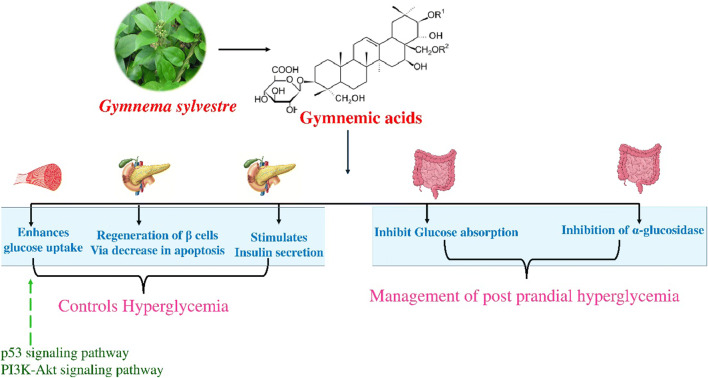
Antidiabetic activity of Gymnemic acids appears to be due to a combination of mechanisms. It acts through stimulation in insulin secretion from the pancreas. It also shows a similar effect by delaying the glucose absorption in the blood. In the intestine it attaches to the receptor present in the external layer of the intestine, thereby preventing the absorption of sugar molecules by the intestine, resulting in low blood glucose levels [95]. In a study extract of the plant has showed its effectiveness in regeneration of pancreatic β cells [66]. Gymnemic acid the major phytocompounds present in the plant is reported to interact with glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a key enzyme in glycolysis pathway [96]. Moreover, G. sylvestre has been reported to exhibit significant inhibitory activity against α-glucosidase; Fig. 1.
Fig. 1.
Schematic representation of Probable molecular mechanism for anti-diabetic effect of G. sylvestre
Antidiabetic effects of Gymnema sylvestre
Various in vivo animal studies and clinical expeiments have repeatedly shown hypoglycemic effects of leaves of G. sylvestre. In a study, the methanol extract of G. sylvestre leaf and callus showed pronounced anti-diabetic activity through the regeneration of β-cells [97]. Sugihara Y., et al. reported the antihyperglycemic effect of gymnemic acid IV, isolated from the leaves of G. sylvestre. The study reported that gymnemic acid IV decreased blood glucose levels by 13.5 − 60.0% within 6 hours of administration in comparison with glibenclamide, also increased plasma insulin levels was observed in STZ-diabetic mice at a concentration of 13.4 mg/kg due to gymnemic acid IV [66].
Momordica charantia
Momordica charantia also known as karela, the bitter guard is a flowering wine, belonging to family Cucurbitaceae. The herb is commonly used by Ayurvedic and other traditional systems of medicine as an anti-diabetic agent. The plant is widely cultivated in Asia, India, East Africa, and South America [72].
Phytochemistry of M. charantia
Bitter melon is rich in constituents such as glycosides, saponins, alkaloids, reducing sugars, resins, phenolic constituents, fixed oil, and free acids [98]. The main phytoconstituents present in M. charantia are charantine, charine, momordicin, momordin, cucurbitins, cucurbitacins momorcharins, etc. [72]. Most of the anti-diabetic potential of M. charantia is ascribed to Charantin. The hypoglycemic activity of the compound is similar to that of insulin [99].
Mechanism of action
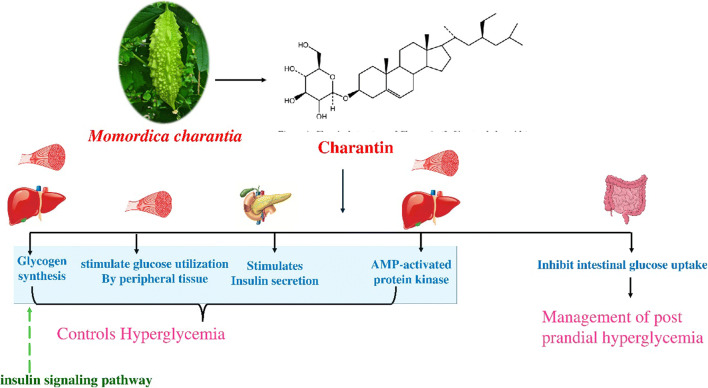
M. charantia exerts its hypoglycemic effects via multiple mechanisms. The possible mechanism of the hypoglycemic action of M. charantia is mainly due to insulin secretion and glycogen synthesis [71, 72]. Some studies indicate that bitter melon may stimulate glucose utilization by peripheral and skeletal muscle, inhibit intestinal glucose uptake, and increase hepatic glycogen synthesis [72]. Hsin-Yi Lo et al., reported that seed extract of M. charantia regulates glucose metabolism mainly via the insulin signaling pathway [100]. In an experimental study using cell-based screening assay Hsueh-ling Cheng identified and reported that triterpenoids are the potential hypoglycaemic agents responsible for anti-diabetic action of the plant, also the underlying mechanism to bring about the action was attributed to AMP-activated protein kinase [101]; Fig. 2.
Fig. 2.
Schematic representation of Probable molecular mechanism for anti-diabetic effect of M. charantia
Antidiabetic effects of M. charantia
Experimental studies have confirmed the hypoglycaemic effect of M. charantia on various animal models. A study demonstrated the dose-dependent hypoglycaemic activity exhibited by methanolic fruit extract of M. charantia in alloxan-induced diabetic rats [102]. Mahmoud MF et al., studied the antidiabetic activity of M. charantia fruit juice in streptozotocin-induced diabetic rats [103]. In an investigational study Joo-Hui Han et al., isolated four new cucurbitane-type triterpenoids (C1-C4) from the ethanol extract of M. charantia and investigated whether the compounds affect insulin sensitivity both in vitro and in vivo models. The results reported significant decreases in blood glucose level and enhanced glycogen storage by compound C2 in STZ‐injected mice [104].
Trigonella foenum graceum
Trigonella foenum graceum also known as fenugreek is used primarily as an alternative therapy for diabetes. A member of the Fabaceae family, the plant cultivated in India and North African countries. The herb has a long history of usage as a potent anti-diabetic agent in Ayurvedic and folklore medicine [94, 105].
Phytochemistry of T. foenum graceum
The phytochemical studies have largely been focused on seeds. The main chemical constituents of seeds are alkaloids approximately 36%, steroidal saponins, mucilage, fibers [105]. Among the alkaloid content of fenugreek seed, Trigonelline is major phytoconstituents which is responsible for most of the activity of the herb. The mucilage (25–30%) is mostly a galactomannan. Steroidal saponins such as diosgenin and yamogenin constitute about 0.1–2.2%. Fenugreekine a sapogenin peptide ester is also present in the seeds. The free amino acids in the seeds are present as 4-hydroxyisoleucine, which is reported to have directly stimulated insulin [94, 106].
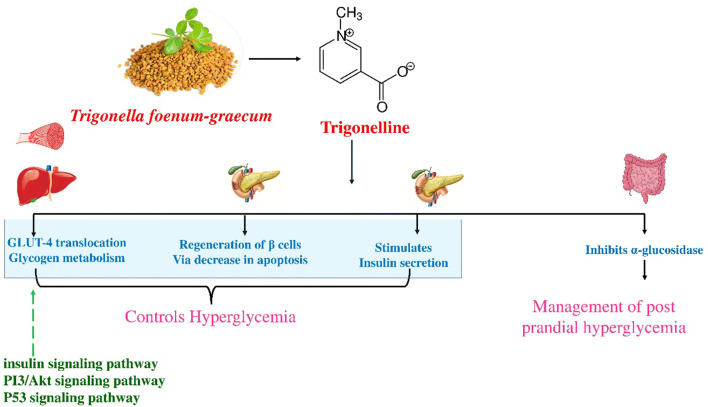
Mechanism of action
The mode of action of the herb is through Regeneration of pancreatic β cells and insulin secretion [88, 89]. A study reported the inhibitory role of Trigonelline on the activity of glycogen synthase kinase isoforms in the regulation of glycogen metabolism, to bring about the hypoglycaemic action [106]. Trigonelline has also been found to enhance glucose and lipid hemostasis via the improvement of the insulin signaling pathway [107]. Another possible mode of action of T. foenum graecum, are the Inhibition of glucose uptake, GLUT-4 translocation, and improved insulin resistance [108, 109]; Fig. 3.
Fig. 3.
Schematic representation of Probable molecular mechanism for anti-diabetic effect of T. foenum-graecum
Antidiabetic effects of T. foenum graecum
The pharmacological studies have proven the anti-diabetic potential of various extracts of T. foenum graecum. In a study, the ethanolic extract of fenugreek seeds was investigated for anti-diabetic action on streptozotocin-induced diabetic rats. The results demonstrated a significant decrease in serum glucose, total cholesterol, triacylglycerol, while an increase in serum insulin in diabetic rats was observed [110]. Shah et al., studied the hypoglycaemic effect of Trigonelline in alloxan-induced diabetic mice. They reported a reduction in blood glucose level and identified the presence of islet cells in the pancreatic duct suggesting its beneficial effect on β cells [111]. Multiple studies on seed extracts, raw powder, and active constituents by investigators have demonstrated the hypoglycaemic action of the herb, confirming its use as a potent herbal remedy for the management of diabetes.
Tinospora cordifolia
Tinospora cordifolia (Willd.) Miers, belonging to family Menispermaceae, is a potent herb used to combat diabetes. The herb reported to possess anti-diabetic activity in Ayurvedic literature and is present in many Ayurvedic formulations. The herb is commonly known as Guduchi and is indigenous to the tropical areas of India, Myanmar, and Sri Lanka [94].
Phytochemistry of T. cordifolia
T. cordifolia consists of a variety of phytoconstituents belonging to different classes such as alkaloids, glycosides, steroids diterpenoid, sesquiterpenoid, phenolics, proteins, etc. The major active constituents responsible for the anti-diabetic effect belongs to a class of alkaloids; these consist of Berberine, Palmatine, Tembetarine, Magnoflorine, Tinosporin. The other constituents present are Tinocordiside, Tinocordifolioside, Cordioside, Cordifolioside, Tinosporon, Tinosporides, etc. [94, 112].
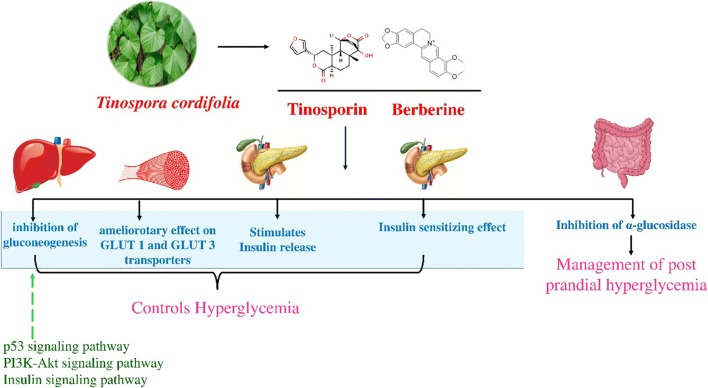
Mechanism of Action
T. cordifolia is reported to act by a different mechanism of action. The possible mechanism to bring about hypoglycemic action is due to inhibition of α-glucosidase activity and glycolysis. In a study Chougale et al. reported the inhibitory effect of T. cordifolia on the α-glucosidase enzyme [86]. Joladarashi et al., proved the Glucose uptake-stimulatory effect of stem extracts of T. cordifolia by conducting experiments with Ehrlich ascites tumor cell model. The study reported the ameliorotary effect of T. cordifolia on GLUT 1 and GLUT 3 transporters involved in basal glucose uptake; suggesting it as a possible mechanism to exert the hypoglycemic effect [113]. Another study reported the insulin-releasing, insulin-sensitizing, and inhibition of gluconeogenesis as the possible mechanism exhibited by an alkaloidal fraction of T. cordifolia [114]; Fig. 4.
Fig. 4.
Schematic representation of Probable molecular mechanism for anti-diabetic effect of T. cordifolia
Antidiabetic effects of T. cordifolia
The stem has been the maximum investigated part of the plant for its anti-diabetic activity. It has been reported that methanol extract of T. cordifolia significantly reduces the fasting blood glucose levels in streptozotocin-induced diabetic rats. Improvement in the insulin and C-peptide levels were also reported which indicated the regeneration of β cells in the pancreas [115]. Manikkam et al. isolated a polysaccharide from methanolic extract of T. cordifolia stem and demonstrated the β-cell regenerative property of the isolated polysaccharide in streptozotocin-induced diabetic rats suggesting its usage as a potent hypoglycemic agent [116].
Curcuma longa
Curcuma longa Linn, belonging to family Zingiberaceae, is reported as a potent herb in Ayurveda system of medicine to combat diabetes. It is commonly known as Turmeric, Haldi, Haridra, etc. The herb is native to India and is widely cultivated particularly in West Bengal, Tamil Nadu, and Maharashtra [94].
Phytochemistry of C. longa
The rhizomes of C. longa consist of a large number of phenolic compounds. Curcuminoids are the major active constituents present in the rhizomes. Curcuminoids are the mixture of three related compounds namely Curcumin, Demethoxycurcumin, and Bisdemethoxycurcumin among this curcumin constitute about 60% of total curcuminoids. Curcumin is a major active principle responsible for most of the biological activity of C. longa [94].
Mechanism of action
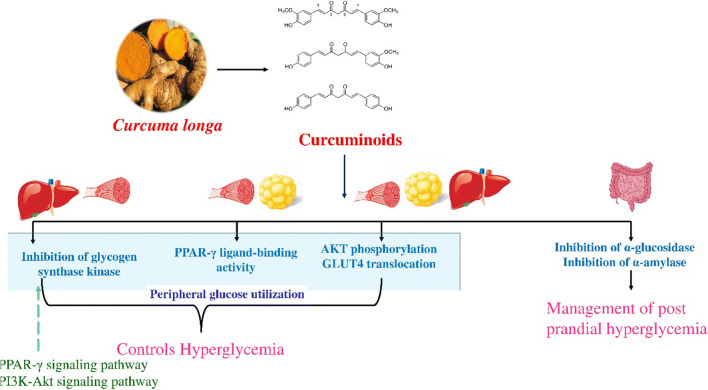
C. longa is known to exert the hypoglycemic action via different mechanisms, of which the most common being the inhibition of α-glucosidase and α-amylase enzyme [52, 117, 118]. Gutierres et al.., reported that increased levels of AKT phosphorylation and GLUT4 translocation in skeletal muscles could be the possible mechanism responsible for the antidiabetic activity of curcumin [53]. Kuroda et al., reported that the hypoglycemic effect exerted by curcumin, demethoxycurcumin, bisdemethoxycurcumin, and ar-turmerone is mainly attributed to PPAR-γ ligand-binding activity of the compound [119]. In a molecular docking study Yasser et al., reported that the hypoglycemic effects of curcumin may be due to the Inhibition of glycogen synthase kinase 3β [120]; Fig. 5.
Fig. 5.
Schematic representation of Probable molecular mechanism for anti-diabetic effect of C. longa
Antidiabetic effect of C. longa
Various studies have shown the hypoglycemic activity of rhizomes of C. longa. Seo et al. investigated the glucose-lowering potential of curcumin in diabetic db/db mice. A significant decrease in blood glucose and HbA1c levels were observed in animals treated with curcumin. A further study reported the improvement in glucose homeostasis, glucose tolerance, and elevated plasm insulin levels by the administration of curcumin [121]. A study reported the suppression of increased blood glucose levels in Genetically Diabetic KK-Ay Mice by ethanolic extract of C. longa [119].
Clinical studies
Clinical trials plays an important role in accessing the safety and efficacy of a particular medication in humans. Based on the literature obtained on the clinical efficacy of the selected five herbs, only recent publications from the year 2010 onwards have been identified from the database search. The details of clinical experiments for the investigation of anti-diabetic effects of the five herbs has been summarized in Table 2.
Table 2.
Clinical studies on Anti-diabetic efficacy of selected five plants
| Author (year) | Plant name | Study | Duration | Dose | Number (Cases/ Control) |
Outcome | References |
|---|---|---|---|---|---|---|---|
| Gaytan Martinez L. et al., (2020) | G. sylvestre |
RCT Double-blind |
12 weeks |
600 mg/day (GS capsule) |
15/15 | ↓ 2-h OGTT (p = 0.003) and A1C (p = 0.025), ↑ insulin sensitivity | [122] |
| Al-Romaiyan A. et al., (2010) | G. sylvestre | Cohort study | 2 months |
1 gm/day (Novel GS extract) |
11 |
↓ blood glucose ↑plasma insulin and C-peptide levels |
[123] |
| Nanda Kumar S. et al., (2010) | G. sylvestre | quasi-experimental design | 3 months |
500 mg/day (GS capsule) |
39/19 | ↓ blood glucose (fasting and post-prandial), and glycated hemoglobin | [124] |
| Trakoon-osot W. et al., (2013) | M. charantia |
RCT Double-blind |
16 weeks | 6 g/day of MC dried-fruit pulp | 19/19 | ↓ A1C from baseline (p = 0.042, ↓ of total advanced glycation end products (AGEs) in serum (p = 0.028) | [125] |
| Fuangchan A. et al., (2011) | M. charantia | Multicentric double-blind RCT | 4 weeks |
G1-500 mg/day, G2-1000 mg/day, G3-2000 mg/day, dry fruit pulp (G4-1000 mg/day metformin) |
G1 = 33 G2 = 32 G3 = 31 G4 = 33 |
↓ fructosamine levels in G3 and G4. | [126] |
| Lim ST. et al., (2010) | M. charantia |
RCT double-blind |
G1- 60 mg/kg/day G2-80 mg/kg/day G3-100 mg/kg/day G4 = Placebo |
G1 = 10 G2 = 10 G3 = 10 G4 = 10 |
G3 showed a more rapid (15 minutes) stimulation of insulin secretion than placebo | [127] | |
| Najdi RA. et al. (2019) | T. foenum graecum | RCT | 12 weeks | 2 gm/day TGF | 6/6 | ↑ fasting insulin level (P = 0.04). | [128] |
| Verma N. et al., (2016) | T. foenum graecum | Multicentric double-blind RCT | 3 month |
1000 mg/day Fenfuro (TGF seed extract) capsule |
154 |
↓ fasting plasma, post-prandial blood sugar levels and HbA1c levels. ↑ fasting and post-prandial C-peptide levels. |
[129] |
| Kumar V. et al., (2015) | T. cordifolia | RCT | 15 days | 50 mg/ kg body weight/ day TC stem powder | 90 | ↓ fasting blood sugar | [130] |
| Roy K. (2015) | T. cordifolia | RCT | 2 Months | 500 mg/day encapsulated stem of TC | 29/30 | ↓ HbA1c levels | [131] |
| Rahimi HR. et al., (2016) | C. longa |
RCT Double-blind |
3 months | 80 mg/day Nano-curcumin (as nano-micelle) | 39/41 |
↓ fasting blood sugar (p = 0.004) ↓ HbA1c levels (p = 0.02) |
[132] |
| Chuengsamarn S. et al. (2012) | C. longa |
RCT Double-blind |
9 months | 1500 mg/day curcuminoids capsule | 119/116 | ↓ fasting plasma glucose and HbA1c levels (p = < 0.01), better β cell functions. ↑HOMA-β (p = < 0.01) | [133] |
| Na LX. et al. (2012) | C. longa |
RCT Double-blind |
3 months | 300 mg/day curcuminoids | 50/50 |
↓ fasting blood glucose (p < 0.01), HbA1c (p = 0.031), and insulin resistance index (HOMA-IR) (p < 0.01) |
[134] |
Conclusion
Diabetes mellitus is the most common endocrine disorder marked by persistent hyperglycaemia resulting from impaired insulin production or insulin resistance. Regardless of all the developments in therapeutics, diabetes still remains a major cause of morbidity and mortality in the world. Allopathic therapies available currently for the treatment of diabetes have a number of serious side effects; consequently, there is a need for investigation of more effective and safer hypoglycaemic agents. Traditional medicine and ethno-botany have an ever-emerging role to play in the treatment and management of diabetes mellitus.
The present review has highlighted the usefulness of the herbal source for the treatment and management of diabetes mellitus. Around 50 herbs known for their usefulness in diabetes have been reviewed and a possible mechanism of the action exerted by them to bring about the anti-diabetic action has been highlighted. Among them, light has been shed upon 5 most potent anti-diabetic herbs with respect to their phytochemistry, underlying mechanism of action, and anti-diabetic effect exerted by them.
From the evidences gathered from literature it is noticeable that the herbs acts by various mechanism to bring about the anti-diabetic effect. As evident from the literature a single herbs displays multiple mechanism of action for example Regeneration of pancreatic β cells, inhibition α-glucosidase enzyme, insulin secretion, PPAR-γ ligand binding activity, etc. this may be due to the presence of a variety of phytoconstituents in a herb. This can in turn bring about the synergistic effects leading to reduction in hyperglycaemic action. As described in the review, most of the reported mechanism of actions exerted by the herbs have been described. In addition to that, more information on G. sylvestre, M. charantia, T. foenum graecum, T. cordifolia and C. longa has been provided owing to their extensive utilization by herbal industries for development of anti-diabetic products. By looking at the diverse phytoconstituents, their mechanism of action, and clinical evidences it is clear that these herbs possess anti-diabetic effect.
Based on this comprehensive overview we can conclude that herbal medicines can play a pivotal role in the management and treatment of diabetes with fewer side effects. More investigations mainly focusing on the isolation of phytocompounds from these herbs can lead to the discovery of newer anti-diabetic agents.
Acknowledgements
The author are thankful to principal KLE College of Pharmacy, Belagavi for his encouragement to write this review.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Onal S, Timur S, Okutucu B, Zihnioglu F. Inhibition of α-Glucosidase by Aqueous Extracts of Some Potent Antidiabetic Medicinal Herbs. Prep Biochem Biotech. 2005;35(1):29–36. doi: 10.1081/PB-200041438. [DOI] [PubMed] [Google Scholar]
- 2.Lin Y, Sun Z. Current views on type 2 diabetes. J Endocrinol. 2009;204(1):1–11. doi: 10.1677/JOE-09-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO | Diabetes mellitus. https://www.who.int/mediacentre/factsheets/fs138/en/. Accessed 7 Aug 2020.
- 4.Worldwide toll of diabetes. Diabetesatlas.org. 2020. https://www.diabetesatlas.org/en/sections/worldwide-toll-of-diabetes.html. Accessed 7 Aug 2020.
- 5.Parasuraman S, Thing G, Dhanaraj S. Polyherbal formulation: concept of Ayurveda. Pharmacogn Rev. 2014;8(16):73. doi: 10.4103/0973-7847.134229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calixto JB. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents) Braz J Med Biol Res. 2000;33(2):179–89. doi: 10.1590/s0100-879x2000000200004. [DOI] [PubMed] [Google Scholar]
- 7.Prabhakar P, Kumar A, Doble M. Combination therapy: A new strategy to manage diabetes and its complications. Phytomedicine. 2014;21(2):123–30. doi: 10.1016/j.phymed.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Prabhakar P, Doble MA. Target based therapeutic approach towards diabetes mellitus using medicinal plants. Curr Diabetes Rev. 2008;4(4):291–308. doi: 10.2174/157339908786241124. [DOI] [PubMed] [Google Scholar]
- 9.Jeeva S, Sheebha A. A review of antidiabetic potential of ethnomedicinal plants. Med Aromat Plants. 2014;3(4):1–8.
- 10.Kashikar VS, Kotkar T. Indegenous remedies for Diabetes mellitus. Int J Pharm Pharm Sci. 2011;3(3):22–9. [Google Scholar]
- 11.Jasmin R, Ganesh kumar R, Rajaram R. Probing the mechanism of the anti-diabetic potential of terpenoids from Elephentopus scaber L., an Indian ethanomedical plant in STZ diabetic rats In-vivo and In-silico analysis. Indian J Biochem Biophys. 2018;55:384–88. [Google Scholar]
- 12.Zhang L, Reddy N. Bioactive molecules from medicinal herbs for life threating diseases. J Mol Sci. 2018;2(4):1–11. [Google Scholar]
- 13.Rajvaidhya S, Nagori BP, Singh GK, Dubey BK, Desai P, Alok S, Jain SA. Review on Acacia arabica - An Indian medicinal plant. Int J Pharm Sci Res. 2012;3(7):1995–2005. [Google Scholar]
- 14.Hegazy GA, Alnoury AM, Gad HG. The role of Acacia Arabica extract as an antidiabetic, antihyperlipidemic, and antioxidant in streptozotocin-induced diabetic rats. Saudi Med J. 2013;34(7):727–723. [PubMed] [Google Scholar]
- 15.Vijayaraj R, Sri Kumaran N. Evaluation of in vivo antidiabetic and antioxidant activity of Achyranthes aspera Linn. seeds by streptozotocin induced diabetic rats. Int J Green Pharm. 2018;12(1):29–37. [Google Scholar]
- 16.Bharti S, Krishnan S, Kumar A, Kumar A. Antidiabetic phytoconstituents and their mode of action on metabolic pathways. Ther Adv Endocrinol Metab. 2018;9(3):81–100. doi: 10.1177/2042018818755019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao H, Huang Y, Gao B, Li P, Inagaki C, Kawabata J. Inhibitory effect on α-glucosidase by Adhatoda vasica Nees. Food Chem. 2008;108(3):965–72. doi: 10.1016/j.foodchem.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Kumar V, Prakash O, Kumar S, Narwal S. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn Rev. 2011;5(9):19. doi: 10.4103/0973-7847.79096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arawwawala M, Jayaratne M. Aegle marmelos (L.) Correa as a potential candidate for treatment of diabetes mellitus: A review. J Herbmed Pharmacol. 2017;6(4):141–7. [Google Scholar]
- 20.Rafe MR. A review of five traditionally used anti-diabetic plants of Bangladesh and their pharmacological activities. Asian Pac J Trop Med. 2017;10(10):933–9. doi: 10.1016/j.apjtm.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Nyunai N, Njikam N, Abdennebi EH, Mbafor JT, Lamnaouer D. Hypoglycaemic and antihyperglycaemic activity of Ageratum conyzoides L. in rats. Afr J Tradit Complement Altern Med. 2009;6(2):123–30. [PMC free article] [PubMed] [Google Scholar]
- 22.Vu NK, Kim CS, Ha MT, Mai Ngo TQ, Park SE, Kwon H, et al. Antioxidant and antidiabetic activities of flavonoid derivatives from the outer skins of Allium cepa L. J Agric Food Chem. 2020 doi: 10.1021/acs.jafc.0c02122. [DOI] [PubMed] [Google Scholar]
- 23.Sabiu S, Madende M, Ajao AA, Aladodo RA, Nurain IO, Ahmad JB. The Genus Allium (Amaryllidaceae: Alloideae): Features, phytoconstituents, and mechanisms of antidiabetic potential of Allium cepa and Allium sativum. In: Watson RR, Preedy VR, eds. Bioactive food as dietary interventions for diabetes. Cambridge: Academic; 2019. pp. 137–154.
- 24.Mamun MA, Hasan N, Shirin F, Belal MH, Khan MAJ, Tasnin MN, et al. Antihyperglycemic and antihyperlipidemic activity of ethanol extract of garlic (Allium sativum) in streptozotocin-induced diabetic miceInternational. J Med Health Res. 2017;3(2):63–6. [Google Scholar]
- 25.Yagi A, Hegazy S, Kabbash A, Wahab EA. Possible hypoglycemic effect of Aloe vera L. high molecular weight fractions on type 2 diabetic patients. Saudi Pharm J. 2009;17:209–15. doi: 10.1016/j.jsps.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komalasari T, Harimurti S. A Review of The Anti-diabetic Activity of Andrographis paniculata (Burm. f.) Nees based in-vivo Study. Int J Med Sci Public Health. 2015;4(4):265–263. [Google Scholar]
- 27.Akhtar M, Bin Mohd Sarib M, Ismail I, Abas F, Ismail A, Lajis N. Anti-diabetic activity and metabolic changes induced by Andrographis paniculata plant extract in obese diabetic rats. Molecules. 2016;21(8):1026. doi: 10.3390/molecules21081026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomar RS, Sisodia SS. Antidiabetic activity of Annona squamosa Linn. In alloxan – induced diabetic rats. Int J Green Pharm. 2014;8(4):237–41. [Google Scholar]
- 29.Davis JA, Sharma S, Mittra S, Sujatha S, Kanaujia A, Shukla G, et al. Antihyperglycemic effect of Annona squamosa hexane extract in type 2 diabetes animal model: PTP1B inhibition, a possible mechanism of action. Indian J Pharmacol. 2012;44(3):326–32. doi: 10.4103/0253-7613.96304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mannan N, Boucher BJ, Evans SJW. Increased waist size and weight in relation to consumption of Areca catechu (betel-nut); a risk factor for increased glycaemia in Asians in East London. Br J Nutr. 2000;83:267–75. doi: 10.1017/s0007114500000349. [DOI] [PubMed] [Google Scholar]
- 31.Satyanarayana K, Sravanthi K, Shaker IA, Ponnulakshmi R. Molecular approach to identify antidiabetic potential of Azadirachta indica. J Ayurveda Integr Med. 2015;6(3):165–74. doi: 10.4103/0975-9476.157950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Omnia M. Molecular and biochemical effect of neem extract on experimental Diabetes. IOSR J Appl Chem. 2014;7(7):24–9. [Google Scholar]
- 33.Ghosh T, Maity T, Singh J. Antihyperglycemic activity of bacosine, a triterpene from Bacopa monnieri, in alloxan-induced diabetic rats. Planta Med. 2011;77:804–8. doi: 10.1055/s-0030-1250600. [DOI] [PubMed] [Google Scholar]
- 34.Kishore L, Kaur N, Singh R. Renoprotective effect of Bacopa monnieri via inhibition of advanced glycation end products and oxidative stress in STZ-nicotinamide-induced diabetic nephropathy. Ren Fail. 2016 doi: 10.1080/0886022X.2016.1227920. [DOI] [PubMed] [Google Scholar]
- 35.Franco RR, Alves VH, Zabisky LF, Justino AB, Martins MM, Saraiva AL, et al. Antidiabetic potential of Bauhinia forficata Link leaves: a non-cytotoxic source of lipase and glycoside hydrolases inhibitors and molecules with antioxidant and antiglycation properties. Biomed Pharmacother. 2020;123:1–8. [DOI] [PubMed]
- 36.Jorge AP, Horst H, de Sousa E, Pizzolatti MG, Silva FR. Insulinomimetic effects of kaempferitrin on glycaemia and on 14C-glucose uptake in rat soleus muscle. Chem Biol Interact. 2004;149(2–3):89–96. doi: 10.1016/j.cbi.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Al masri IM, Mohammad MK, Tahaa MO. Inhibition of dipeptidyl peptidase IV (DPP-IV) is one of the mechanisms explaining the hypoglycemic effect of berberine. J Enzyme Inhib Med Chem. 2009;24:1061–6. doi: 10.1080/14756360802610761. [DOI] [PubMed] [Google Scholar]
- 38.Cicero AFG, Tartagni E. Antidiabetic Properties of Berberine: From Cellular Pharmacology to Clinical Effects. Hosp Pract. 2012;40(2):57–63. doi: 10.3810/hp.2012.04.970. [DOI] [PubMed] [Google Scholar]
- 39.Alam P, Shahzad N, Gupta AK, Mahfoz AM, Bamagous GA, Al-Ghamdi SS, et al. Anti-diabetic Effect of Boerhavia diffusa L. root extract via free radical scavenging and antioxidant mechanism. Toxicol Environ Health Sci. 2018;10:220–7. [Google Scholar]
- 40.Brahima M, Eugene A, Justin B, Madjid A, Machioud S, Rodrigue A, et al. The Effect of Methanolic Leaf Extract of Boerhavia diffusa Linn. (Nictaginaceae) on the Activities of Antidiabetic, Anti-inflammatory and Antioxidant Enzymes in Experimental Diabetes. J Pharm Res Int. 2018;24(5):1–25. [Google Scholar]
- 41.Wang H, Shi S, Bao B, Li X, Wang S. Structure characterization of an arabinogalactan from green tea andits anti-diabetic effect. Carbohydr Polym. 2015;124:98–108. doi: 10.1016/j.carbpol.2015.01.070. [DOI] [PubMed] [Google Scholar]
- 42.Hininger-Favier I, Benaraba R, Coves S, Anderson RA, Roussel AM. Green Tea Extract Decreases Oxidative Stress and Improves Insulin Sensitivity in an Animal Model of Insulin Resistance, the Fructose-Fed Rat. J Am Coll Nutr. 2009 doi: 10.1080/07315724.2009.10718097. [DOI] [PubMed] [Google Scholar]
- 43.Prakasam A, Sethupathy S, Pugalendia KV. Antiperoxidative and antioxidant effects of Casearia esculenta root extract in streptozotocin induced diabetic rats. Yale J Biol Med. 2005;78:15–23. [PMC free article] [PubMed] [Google Scholar]
- 44.Govindasamy C, Al-Numair KS, Alsaif MA, Viswanathan KP. Influence of 3-hydroxymethyl xylitol, a novel antidiabetic compound isolated from Casearia esculenta (Roxb.) root, on glycoprotein components in streptozotocin-diabetic rats. J Asian Nat Prod Res. 2011;13(8):700–6. [DOI] [PubMed]
- 45.Benalla W, Bellahcen S, Bnouham M. Antidiabetic medicinal plants as a source of alpha glucosidase inhibitors. Curr Diabetes Rev. 2010;6(4):247–54. doi: 10.2174/157339910791658826. [DOI] [PubMed] [Google Scholar]
- 46.Kabir AU, Samad MB, D’Costa NM, Akhter F, Ahmed A, Hannan JM. Anti-hyperglycemic activity of Centella asiatica is partly mediated by carbohydrase inhibition and glucose-fiber binding. BMC Complement Altern Med. 2014;18:14–31. doi: 10.1186/1472-6882-14-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nugroho AE, Lindawati NY, Herlyanti K, Widyastuti L, Pramono S. Anti-diabetic effect of a combination of andrographolide-enriched extract of Andrographis paniculata (Burm f.) Nees and asiaticoside-enriched extract of Centella asiatica L. in high fructose-fat fed rats. Indian J Exp Biol. 2013;51(12):1101–8. [PubMed] [Google Scholar]
- 48.Jamwal A, Kumar S. Antidiabetic activity of isolated compound from Coccinia indica. Indian J Pharm Educ. 2019;53(1):151–9.
- 49.Kohli S, Kumar PN. Combined effect of Coccinia indica leaf extract with acarbose in type II diabetes induced neuropathy in rats. J Innov Pharm Biol Sci. 2014;1(2):77–87. [Google Scholar]
- 50.Youn JY, Park HY, Cho KH. Anti-hyperglycemic activity of Commelina communis L.: inhibition of alpha-glucosidase. Diabetes Res Clin Pract. 2004;66:149-55. doi: 10.1016/j.diabres.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 51.Shibano M, Kakutani K, Taniguchi M, Yasuda M, Baba K. Antioxidant constituents in the dayflower (Commelina communis L.) and their alpha-glucosidase-inhibitory activity. J Nat Med. 2008;62(3):349–53. doi: 10.1007/s11418-008-0244-1. [DOI] [PubMed] [Google Scholar]
- 52.Riyaphan J, Jhong C, Lin S, Chang C, Tsai M, Lee D, et al. Hypoglycemic efficacy of docking selected natural compounds against α-Glucosidase and α-Amylase. Molecules. 2018;23:2260. doi: 10.3390/molecules23092260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gutierres V, Campos M, Arcaro C, Assis R, Baldan-Cimatti H, Peccinini R, et al. Curcumin Pharmacokinetic and Pharmacodynamic Evidences in Streptozotocin-Diabetic Rats Support the Antidiabetic Activity to Be via Metabolite(s). Evid Based Complement Alternat Med. 2015;1–13. [DOI] [PMC free article] [PubMed]
- 54.Singh P, Khosa RL, Mishra G, Jha KK. Antidiabetic activity of ethanolic extract of Cyperus rotundus rhizomes in streptozotocin-induced diabetic mice. J Pharm Bioallied Sci. 2015;7(4):289–92. doi: 10.4103/0975-7406.168028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tran H, Nguyen MC, Le HT, Nguyen TL, Pham TB, Chau VM, et al. Inhibitors of a-glucosidase and a-amylase from Cyperus rotundus. Pharm Biol. 2013 doi: 10.3109/13880209.2013.814692. [DOI] [PubMed] [Google Scholar]
- 56.Fatima N, Hafizur RM, Hameed A, Ahmed S, Nisar M, Kabir N. Ellagic acid in Emblica officinalis exerts anti-diabetic activity through the action on β-cell of pancreas. Eur J Nutr. 2015. 10.1007/s00394-015-1103-y. [DOI] [PubMed]
- 57.Nain P, Saini V, Sharma S, Nain J. Antidiabetic and antioxidant potential of Emblica officinalis Gaertn. leaves extract in streptozotocin-induced type-2 diabetes mellitus (T2DM) rats. J Ethnopharmacol. 2012;142(1):65–71. doi: 10.1016/j.jep.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 58.Murali B, Upadhyaya UM, Goyal RK. Effect of chronic treatment with Enicostemma littorale in non-insulin-dependent diabetic (NIDDM) rats. J Ethnopharmacol. 2002;81(2):199–204. doi: 10.1016/s0378-8741(02)00077-6. [DOI] [PubMed] [Google Scholar]
- 59.Maroo J, Vasu VT, Aalinkeel R, Gupta S. Glucose lowering effect of aqueous extract of Enicostemma littorale Blume in diabetes: a possible mechanism of action. J Ethnopharmacol. 2002;81(3):317–20. doi: 10.1016/s0378-8741(02)00095-8. [DOI] [PubMed] [Google Scholar]
- 60.Chikaraddy A, Maniyar Y. Evaluation and comparison of hypoglycemic activity of bark and leaf of Ficus bengalensis linn. in alloxan induced diabetes in albino rats. Indian J Pharm Pharmacol. 2017;4(3):138–42. [Google Scholar]
- 61.Khanal P, Patil BM. Gene set enrichment analysis of alpha-glucosidase inhibitors from Ficus benghalensis. Asian Pac J Trop Biomed. 2019;9(6):263–70. [Google Scholar]
- 62.Veerapur VP, Prabhakar KR, Thippeswamy BS, Bansal P, Srinivasan KK, Unnikrishnan MK. Antidiabetic effect of Ficus racemosa Linn. stem bark in high-fat diet and low-dose streptozotocin-induced type 2 diabetic rats: a mechanistic study. Food Chem. 2012;132(1):186–93. doi: 10.1016/j.foodchem.2011.10.052. [DOI] [PubMed] [Google Scholar]
- 63.Amin MM, Bhakta S, Das SK. Anti-diabetic potential of Ficus racemosa: current state and prospect especially in the developing countries. J Biosci Agric Res. 2015;05(02):65–72. [Google Scholar]
- 64.Karthikeson PS, Laxmi T. Anti-Diabetic Activity of Glycyrrhiza glabra - An In vitro Study. Int J Pharm Sci Rev Res. 2017;44(1):80–1. [Google Scholar]
- 65.Harwansh RK, Parea KC, Pareta SK, Singh J. Pharmacological studies of Glycyrrhiza glabra- a review. Pharmacologyonline. 2011;2:1032–8. [Google Scholar]
- 66.Sugihara Y, Nojima H, Matsuda H, Murakami T, Yoshikawa M, Kimura I. Antihyperglycemic Effects of Gymnemic Acid IV, a Compound Derived from Gymnema sylvestre Leaves in Streptozotocin-Diabetic Mice. J Asian Nat Prod Res. 2000;2:321–7. doi: 10.1080/10286020008041372. [DOI] [PubMed] [Google Scholar]
- 67.Chen G, Guo M. Rapid Screening for α-Glucosidase Inhibitors from Gymnema sylvestre by Affinity Ultrafiltration-HPLC-MS. Front Pharmacol. 2017;8:1-8. [DOI] [PMC free article] [PubMed]
- 68.Dholi SK, Raparla R. Kannappan. In vivo anti diabetic evaluation of gymnemic acid in streptozotocin induced rats. Pharma Innov J. 2014;3(7):82–6. [Google Scholar]
- 69.Tanaka S, Han LK, Zheng YN, Okuda H. Effects of the flavonoid fraction from Ginkgo biloba extract on the postprandial blood glucose elevation in rats. Yakugaku Zasshi. 2004;124(9):605–11. doi: 10.1248/yakushi.124.605. [DOI] [PubMed] [Google Scholar]
- 70.Ngo DH, Ngo DN, Vo TT, Vo TS. Mechanism of Action of Mangifera indica Leaves for Anti-Diabetic Activity. Sci Pharm. 2019;87(13):1–12. [Google Scholar]
- 71.Miura T, Itoh C, Iwamoto N, Kato M, Kawai M, Park SR, Suzuki I. Hypoglycemic activity of the fruit of the Momordica charantia in type 2 diabetic mice. J Nutr Sci Vitaminol. 2001;47(5):340–4. doi: 10.3177/jnsv.47.340. [DOI] [PubMed] [Google Scholar]
- 72.Joseph B, Jini D. Antidiabetic effects of Momordica charantia (bitter melon) and its medicinal potency. Asian Pac J Trop Dis. 2013;3(2):93–102. [Google Scholar]
- 73.Pradeep Kumar R, Sujatha D, Mohamed Saleem TS, Madhusudhana Chetty C, Ranganayakulu D. Potential antidiabetic and antioxidant activities of Morus indica and Asystasia gangetica in alloxan-induced diabetes mellitus. J Exp Pharmacol. 2010;2:29–36. doi: 10.2147/jep.s8947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raja TAR, Reddy RV, Priyadharshini, Buchineni M. An evaluation of anti-hyperglycemic activity of Ocimum sanctum Linn (leaves) in Wister rats. Pharma Innov. 2016;5(1):1–3. [Google Scholar]
- 75.Patil R, Patil R, Ahirwar B, Ahirwar D. Isolation and characterization of anti-diabetic component (bioactivity-guided fractionation) from Ocimum sanctum L. (Lamiaceae) aerial part. Asian Pac J Trop Med. 2011;4(4):278–82. doi: 10.1016/S1995-7645(11)60086-2. [DOI] [PubMed] [Google Scholar]
- 76.Qiu S, Yang WH, Yao CL, Shi XJ, Li JY, Lou Y, et al. Malonylginsenosides with Potential Antidiabetic Activities from the Flower Buds of Panax ginseng. J Nat Prod. 2017;80:899–908. doi: 10.1021/acs.jnatprod.6b00789. [DOI] [PubMed] [Google Scholar]
- 77.Kang OH, Shon MY, Kong R, Seo YS, Zhou T, Kim DY. Anti-diabetic effect of black ginseng extract by augmentation of AMPK protein activity and upregulation of GLUT2 and GLUT4 expression in db/db mice. BMC Complem Alter M. 2017;17(341):1–11. doi: 10.1186/s12906-017-1839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adedapo AA, Ofuegbe SO. The evaluation of the hypoglycemic effect of soft drink leaf extract of Phyllanthus amarus (Euphorbiaceae) in rats. J Basic Clin Physiol Pharmacol. 2014;25(1):47–57. doi: 10.1515/jbcpp-2013-0033. [DOI] [PubMed] [Google Scholar]
- 79.Pant DR, Pant ND, Saru DB, Yadav UN, Khanal DP. Phytochemical screening and study of antioxidant, antimicrobial, antidiabetic, anti-inflammatory and analgesic activities of extracts from stem wood of Pterocarpus marsupium Roxburgh. J Intercult Ethnopharmacol. 2017;6(2):170–6. doi: 10.5455/jice.20170403094055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kavitha KN, Dattatri AN. Experimental Evaluation of antidiabetic activity of Swertia Chirata – Aqueous Extract. J Pub Health Med Res. 2013;1(2):71–5. [Google Scholar]
- 81.Kuroda M, Mimaki Y, Ohtomo T, Yamada J, Nishiyama T, Mae T, et al. Hypoglycemic effects of clove (Syzygium aromaticum flower buds) on genetically diabetic KK-Ay mice and identification of the active ingredients. J Nat Med. 2012;66(2):394–9. doi: 10.1007/s11418-011-0593-z. [DOI] [PubMed] [Google Scholar]
- 82.Kumar A, Ilavarasan R, Jayachandran T, Deecaraman M, Aravindan P, Padmanabhan N, et al. Anti-diabetic activity of Syzygium cumini and its isolated compound against streptozotocin-induced diabetic rats. J Med Plant Res. 2008;2(9):246–9. [Google Scholar]
- 83.Barman S, Das S. Hypoglycemic effect of ethanolic extract of bark of Terminalia arjuna Linn. In normal and alloxan–induced noninsulin-dependent diabetes mellitus albino rats. Int J Green Pharm. 2012;6(4):279–284.
- 84.Akram N, Srivastava M, Mishra MK. Antidiabetic Activity of root of Terminalia chebula on alloxan induced diabetic rat. World J Pharm Med Res. 2019;5(3):108–12. [Google Scholar]
- 85.Sabu MC, Kuttan R. Antidiabetic and antioxidant activity of Terminalia belerica Roxb. Indian J Exp Biol. 2009;47(4):270–5. [PubMed] [Google Scholar]
- 86.Chougale AD, Ghadyale VA, Panaskar SN, Arvindekar AU. Alpha glucosidase inhibition by stem extract of Tinospora cordifolia. J Enzyme Inhib Med Chem. 2009;24(4):998–1001. doi: 10.1080/14756360802565346. [DOI] [PubMed] [Google Scholar]
- 87.Sharma R, Amin H, Galib, Prajapati PK. Antidiabetic claims of Tinospora cordifolia (Willd.) Miers: critical appraisal and role in therapy. Asian Pac J Trop Biomed. 2015;5(1):68–78. [Google Scholar]
- 88.Yoshinari O, Igarashi K. Anti-Diabetic Effect of Trigonelline and Nicotinic Acid, on KK-Ay Mice. Curr Med Chem. 2010;17:2196–202. doi: 10.2174/092986710791299902. [DOI] [PubMed] [Google Scholar]
- 89.Zhou J, Zhou S, Zeng S. Experimental diabetes treated with trigonelline: effect on β cell and pancreatic oxidative parameters. Fundam Clin Pharmacol. 2011;27(3):279–87. doi: 10.1111/j.1472-8206.2011.01022.x. [DOI] [PubMed] [Google Scholar]
- 90.Ahmed MF, Kazim SM, Ghori S, Mehjabeen SS, Ahmed SR, Ali SM, Ibrahim M. Antidiabetic activity of Vinca rosea extracts in alloxan-induced diabetic rats. Int J Endocrinol. 2010;2010:1–6. doi: 10.1155/2010/841090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Orhan N, Aslan M, Orhan DD, Ergun F, Yeşilada E. In-vivo assessment of antidiabetic and antioxidant activities of grapevine leaves (Vitis vinifera) in diabetic rats. J Ethnopharmacol. 2006;108(2):280–6. doi: 10.1016/j.jep.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 92.Kumar V, Dey A, Chatterjee SS. Phytopharmacology of Ashwagandha as an Anti-Diabetic Herb, In: Kaul S., Wadhwa R, editors. Science of Ashwagandha: Preventive and Therapeutic Potentials. Berlin: Springer. 2017.
- 93.Otunola GA, Afolayan AJ. Antidiabetic effect of combined spices of Allium sativum, Zingiber officinale and Capsicum frutescens in alloxan-induced diabetic rats. Front Life Sci. 2015 doi: 10.1080/21553769.2015.1053628. [DOI] [Google Scholar]
- 94.Khare CP. Indian Medicinal Plants An Illustrated Dictionary. Berlin: Springer. 2008.
- 95.Kanetkar P, Singhal R, Kamat M. Gymnema sylvestre: A Memoir. J Clin Biochem Nutr. 2007;41:77–81. doi: 10.3164/jcbn.2007010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ishijima S, Takashima T, Ikemura T, Izutani Y. Gymnemic acid interacts with mammalian glycerol-3-phosphate dehydrogenase. Mol Cell Biochem. 2008;310:203–8. doi: 10.1007/s11010-007-9681-5. [DOI] [PubMed] [Google Scholar]
- 97.Ahmed A, Rao AS, Rao M. In vitro callus and in vivo leaf extract of Gymnema sylvestre stimulate β-cells regeneration and anti-diabetic activity in Wistar rats. Phytomedicine. 2010;17(13):1033–9. doi: 10.1016/j.phymed.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 98.Saeed MK, Shahzadi I, Ahmad I, Ahmad R, Shahzad K, Ashraf M, et al. Nutritional analysis and antioxidant activity of bitter gourd (Momordica charantia) from Pakistan. Pharmacologyonline. 2010;1:252–60. [Google Scholar]
- 99.Budrat P, Shotipruk A. Extraction of phenolic compounds from fruits of bitter melon (Momordica charantia) with subcritical water extraction and antioxidant activities of these extracts. Chiang Mai J Sci. 2008;35(1):123–30. [Google Scholar]
- 100.Lo H, Ho T, Lin C, Li C, Hsiang C. Momordica charantia and Its Novel Polypeptide Regulate Glucose Homeostasis in Mice via Binding to Insulin Receptor. J Agric Food Chem. 2013;61:2461–8. doi: 10.1021/jf3042402. [DOI] [PubMed] [Google Scholar]
- 101.Hsueh-Ling C, Hsin-Kai H, Chi -IC, Chung-Pao T, Chang-Hung C. A Cell-Based Screening Identifies Compounds from the Stem of Momordica charantia that Overcome Insulin Resistance and Activate AMP-Activated Protein Kinase. J Agric Food Chem. 2008;56:6835–43. doi: 10.1021/jf800801k. [DOI] [PubMed] [Google Scholar]
- 102.Nkambo W, Anyama NG, Onegi B. In vivo hypoglycemic effect of methanolic fruit extract of Momordica charantia L. Afr Health Sci. 2013;13(4):933–9. doi: 10.4314/ahs.v13i4.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mahmoud MF, El Ashry FE, El Maraghy NN, Fahmy A. Studies on the antidiabetic activities of Momordica charantia fruit juice in streptozotocin-induced diabetic rats. Pharm Biol. 2017;55(1):758–65. doi: 10.1080/13880209.2016.1275026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Han JH, Tuan NQ, Park MH, Quan K, Oh J, Heo KS, et al. Cucurbitane Triterpenoids from the Fruits of Momordica Charantia Improve Insulin Sensitivity and Glucose Homeostasis in Streptozotocin-Induced Diabetic Mice. Mol Nutr Food Res. 2018;62(7):1700–769. doi: 10.1002/mnfr.201700769. [DOI] [PubMed] [Google Scholar]
- 105.Bahmani M, Shirzad H, Mirhosseini M, Mesripour A, Rafieian-Kopaei M. A Review on Ethnobotanical and Therapeutic Uses of Fenugreek (Trigonella foenum-graceum L) Evid Based Complement Alternat Med. 2016;21(1):53–62. doi: 10.1177/2156587215583405. [DOI] [PubMed] [Google Scholar]
- 106.Roshana Devi V, Sharmila C, Subramanian S. Molecular Docking Studies Involving the Inhibitory Effect of Gymnemic Acid, Trigonelline and Ferulic Acid, the Phytochemicals with Antidiabetic Properties, on Glycogen Synthase Kinase 3 (α and β) J Appl Pharm Sci. 2018;8(04):150–60. [Google Scholar]
- 107.Amat-Alrazaq A, Aldakinah, Muhammad Y, Al-Shorbagy, Dalaal M, Abdallah, et al. Trigonelline and vildagliptin antidiabetic effect: improvement of insulin signalling pathway. J Pharm Pharmacol. 2017:1–9. [DOI] [PubMed]
- 108.Jaiswal N, Maurya CK, Venkateswarlu K, Sukanya P, Srivastava AK, Narender T, Tamrakar AK. 4-Hydroxyisoleucine stimulates glucose uptake by increasing surface GLUT4 level in skeletal muscle cells via phosphatidylinositol-3-kinase-dependent pathway. Eur J Nutr. 2012;51(7):893–8. doi: 10.1007/s00394-012-0374-9. [DOI] [PubMed] [Google Scholar]
- 109.Maurya CK, Singh R, Jaiswal N, Venkateswarlu K, Narender T, Tamrakar AK. 4-Hydroxyisoleucine ameliorates fatty acid-induced insulin resistance and inflammatory response in skeletal muscle cells. Mol Cell Endocrinol. 2014;395(1–2):51–60. doi: 10.1016/j.mce.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 110.Eidia A, Eidib M, Sokhteha M. Effect of fenugreek (Trigonella foenum-graecum L) seeds on serum parameters in normal and streptozotocin-induced diabetic rats. Nutr Res. 2007;27:728–33. [Google Scholar]
- 111.Shah SN, Bodhankar SL, Badole SL, Kamble HV, Mohan V. Effect of trigonelline: An active compound from Trigonella foenum graecum Linn. In alloxan induced diabetes in mice. Cell Tissue Res. 2006;6(1):585–90. [Google Scholar]
- 112.Singh SS, Pandey SC, Srivastava S, Gupta VS, Patro B, Ghosh AC. Chemistry and medicinal properties of Tinospora cordifolia (Guduchi) Indian J Pharmacol. 2003;35:83–91. [Google Scholar]
- 113.Joladarashi D, Chilkunda ND, Salimath PV. Glucose uptake-stimulatory activity of Tinospora cordifolia stem extracts in Ehrlich ascites tumor cell model system. J Food Sci Technol. 2014;51(1):178–82. doi: 10.1007/s13197-011-0480-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Patel MB, Mishra S. Hypoglycemic activity of alkaloidal fraction of Tinospora cordifolia. Phytomed. 2011;18:1045–52. doi: 10.1016/j.phymed.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 115.Rajalakshmi M, Eliza J, Cecilia E, Nirmala A, Daisy P. Anti-diabetic properties of Tinospora cordifolia stem extracts on streptozotocin- induced diabetic rats. Afr J Pharm Pharmacol. 2009;3(5):171–80. [Google Scholar]
- 116.Rajalakshmi R, Roy A. b-cell regenerative efficacy of a polysaccharide isolated from methanolic extract of Tinospora cordifolia stem on streptozotocin-induced diabetic Wistar rats. Chem-Biol Interact. 2016;243:45–53. doi: 10.1016/j.cbi.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 117.Ghani U. Re-exploring promising a-glucosidase inhibitors for potential development into oral anti-diabetic drugs: Finding needle in the haystack. Eur J Med Chem. 2015;103:133–62. doi: 10.1016/j.ejmech.2015.08.043. [DOI] [PubMed] [Google Scholar]
- 118.Jhong C, Riyaphan J, Lin J, Chia Y, Weng C. Screening alpha-glucosidase and alpha-amylase inhibitors from natural compounds by molecular docking in silico. BioFactors. 2015;41(4):242–51. doi: 10.1002/biof.1219. [DOI] [PubMed] [Google Scholar]
- 119.Kuroda M, Mimaki Y, Nishiyama T, Mae T, Kishida H, Tsukagawa M, et al. Hypoglycemic Effects of Turmeric (Curcuma longa L. Rhizomes) on Genetically Diabetic KK-Ay Mice. Biol Pharm Bull. 2005;28(5):937–9. doi: 10.1248/bpb.28.937. [DOI] [PubMed] [Google Scholar]
- 120.Bustanji Y, Taha MO, Almasri IM, Al-Ghussein M, Mohammad MK, Alkhatib HS. Inhibition of glycogen synthase kinase by curcumin: Investigation by simulated molecular docking and subsequent in vitro/invivo evaluation. J Enzyme Inhib Med Chem. 2009;24(3):771–8. doi: 10.1080/14756360802364377. [DOI] [PubMed] [Google Scholar]
- 121.Seo K, Choi M, Jung U, Kim H, Yeo J, Jeon S, et al. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol Nutr Food Res. 2008;52:995–1004. doi: 10.1002/mnfr.200700184. [DOI] [PubMed] [Google Scholar]
- 122.Gaytan Martinez L, Sanchez-Ruiz L, Zuniga L, Gonzalez-Ortiz M, Martinez-Abundis E. Effect of Gymnema sylvestre Administration on Glycemic Control, Insulin Secretion, and Insulin Sensitivity in Patients with Impaired Glucose Tolerance. J Med Food. 2020 doi: 10.1089/jmf.2020.0024. [DOI] [PubMed] [Google Scholar]
- 123.Al-Romaiyan A, Liu B, Asare-Anane H, Maity C, Chatterjee S, Koley N, et al. A novel Gymnema sylvestre extract stimulates insulin secretion from human islets in vivo and in vitro. Phytother Res. 2010;24(9):1370–6. doi: 10.1002/ptr.3125. [DOI] [PubMed] [Google Scholar]
- 124.Nanda Kumar S, Mani UV, Mani I. An Open Label Study on the Supplementation of Gymnema sylvestre in Type 2 Diabetics. J Diet Suppl. 2010;7(3):273–82. [DOI] [PubMed]
- 125.Trakoon-osot W, Sotanaphun U, Phanachet P, Porasuphatana S, Udomsubpayakul U, Komindr S. Pilot study: Hypoglycemic and antiglycation activities of bitter melon (Momordica charantia L.) in type 2 diabetic patients. J Pharm Res. 2013;6:859–64. [Google Scholar]
- 126.Fuangchana A, Sonthisombata P, Seubnukarnb T, Chanouanc R, Chotchaisuwatd P, Sirigulsatien V, et al. Hypoglycemic effect of bitter melon compared with metformin in newly diagnosed type 2 diabetes patients. J Ethnopharmacol. 2011;134:422–8. doi: 10.1016/j.jep.2010.12.045. [DOI] [PubMed] [Google Scholar]
- 127.Lim ST, Jimeno CA, Razon-Gonzales EB, Velasquez ME. The MOCHA DM study: The Effect of MOmordica CHArantia tablets on glucose and insulin levels during the postprandial state among patients with Type 2 Diabetes Mellitus. Philipp J Intern Med. 2010;48(2):19–25. [Google Scholar]
- 128.Najdi RA, Hagras MM, Kamel FO, Magadmi RM. A randomized controlled clinical trial evaluating the effect of Trigonella foenum-graecum (fenugreek) versus glibenclamide in patients with diabetes. Afr Health Sci. 2019;19(1):1594–601. doi: 10.4314/ahs.v19i1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Verma N, Usman K, Patel N, Jain A, Dhakre S, Swaroop A, et al. A multicenter clinical study to determine the efficacy of a novel fenugreek seed (Trigonella foenum-graecum) extract (Fenfuro™) in patients with type 2 diabetes. Food Nutr Res. 2016;60(1):32382. [DOI] [PMC free article] [PubMed]
- 130.Kumar V, Mahdi F, Singh R, Mahdi A, Singh RK. A clinical trial to assess the antidiabetic, antidyslipidemic and antioxidant activities of Tinospora cordifolia in management of type – 2 Diabetes Mellitus. Int J Pharm Sci Res. 2016;7(2):757–64.
- 131.Roy K, Shah R, Iyer U. Tinospora cordifolia stem supplementation in diabetic dyslipidemia: an open labelled randomized controlled trial. Funct Food Health Dis. 2015;5(7):265–74. [Google Scholar]
- 132.Rahimi HR, Mohammadpour AH, Dastani M, Jaafari MR, Abnous K, Mobarhan MG, et al. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: a randomized clinical trial. Avicenna J Phytomed. 2016;6(5):567–77. [PMC free article] [PubMed] [Google Scholar]
- 133.Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C, Jirawatnotai S. Curcumin Extract for Prevention of Type 2 Diabetes. Diabetes Care. 2012;35(11):2121–7. doi: 10.2337/dc12-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Na LX, Li Y, Pan HZ, Zhou XL, Sun DJ, Meng M, et al. Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: a double-blind, placebo-controlled trial. Mol Nutr Food Res. 2012;56:1–9. doi: 10.1002/mnfr.201200131. [DOI] [PubMed] [Google Scholar]