Summary
In vivo interrogation of the functional role of genes implicated in colorectal cancer (CRC) is limited by the need for physiological models that mimic the disease. Here, we describe a protocol that provides the steps required for the orthotopic co-implantation of tumoral and stromal cells into the cecum and rectum to investigate the crosstalk between the tumor and its microenvironment. This protocol recapitulates metastases to the lymph nodes, liver, and lungs observed in human CRC.
For complete details on the use and execution of this protocol, please refer to Kasashima et al. (2020).
Subject areas: Cancer, Model organisms, Organoids
Graphical Abstract
Highlights
-
•
Orthotopic implantation in the cecum or rectum is a useful tool to study CRC
-
•
These methods recapitulate metastases of CRC to the lymph nodes, liver, and lungs
-
•
Co-implantation of tumor and stromal cells results in faster tumor progression
In vivo interrogation of the functional role of genes implicated in colorectal cancer (CRC) is limited by the need for physiological models that mimic the disease. Here, we describe a protocol that provides the steps required for the orthotopic co-implantation of tumoral and stromal cells into the cecum and rectum to investigate the crosstalk between the tumor and its microenvironment. This protocol recapitulates metastases to the lymph nodes, liver, and lungs observed in human CRC.
Before you begin
Note: All animal work was performed in accordance with and approved by the IACUC committee at Weill Cornell Medicine (WCM).
All mouse experiments must be approved by an Animal Care Committee in your research institution. Personal protective equipment, autoclaved sterile surgical instruments and frequent disinfection of equipment with 70% ethanol are essential for handling mice.
8–12 weeks old mice of either sex can be used in this protocol.
Note: Although the injection in rectum does not require laparotomy, and it is an easier and less invasive technique in principle, the end point is earlier than in cecum injections and the probability of metastases development is reduced. The reason is that an injection in rectum can cause intestinal obstruction earlier than an injection in cecum, causing death or forcing euthanasia before the metastasis appears.
Preparation of mouse surgery
Timing: 30 min
-
1.
Prepare 1 mL aliquots of Cultrex Basement Membrane Matrix, Type2 (BME) and store at −20°C. Thaw them on ice 2 h before use.
-
2.
Prepare a set of autoclaved surgical instruments per mouse as follows: (cecum injection) Sharp and blunt scissors; 1 straight fine forceps; 1 straight blunt forceps; (rectum injection) 1 straight fine forceps; 1 Hartman hemostats.
-
3.
Weigh and prepare the mice for the following procedure.
-
4.
For anesthesia of mice, an isoflurane chamber with nose cone attachment is needed.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| PBS (no calcium, no magnesium) | Thermo Fisher Scientific | Cat# 10010-023 |
| DMEM [+] 4.5 g/L glucose, sodium pyruvate [−] L-glutamine | VWR | Cat# 45000-316 |
| Gentamicin | GIBCO | Cat# 15750045 |
| Insulin, human recombinant, zinc solution | Thermo Fisher Scientific | Cat# 12585014 |
| Apo-transferrin | Sigma | Cat# T2252-500MG |
| HBSS (no calcium, no magnesium) | Thermo Fisher Scientific | Cat# 14175095 |
| Advanced DMEM/F12 | Thermo Fisher Scientific | Cat# 12634010 |
| GlutaMAX supplement | Thermo Fisher Scientific | Cat# 35050061 |
| Fetal bovine serum | Omega Scientific | Cat# FB-01 |
| HEPES | Thermo Fisher Scientific | Cat# 12587001 |
| Trypsin-EDTA (0.25%), phenol red | GIBCO | Cat# 25200056 |
| Cultrex basement membrane matrix, type2 (BME) | Trevigen | Cat# 3532-001-02 |
| B27 supplement minus vitamin A | Thermo Fisher Scientific | Cat# 12587001 |
| Murine EGF | Thermo Fisher Scientific | Cat# PMG8045 |
| Cell recovery solution | Corning | Cat# 354253 |
| Trypan blue solution | Gibco | Cat# 15250061 |
| Experimental models: cell lines | ||
| Mouse tumor organoids (MTOs)a | (Tauriello et al., 2018) | N/A |
| Mouse colonic fibroblasts (CFs) | (Kasashima et al., 2020) | N/A |
| Experimental models: organisms/strains | ||
| Mouse: WT (C57BL/6)b | Moscat lab | N/A |
| Other | ||
| Insulin syringe, 0.3 mL with 31-gauge needle | Becton Dickinson | Cat# 328438 |
| 15 mL polypropylene centrifuge tubes | Genesee Scientific | Cat# 28-103 |
| Bright-field microscope | EVOS M5000 | Cat# AMF5000 |
| Caliper | Mitutoyo | Cat# 500-171-30 |
| Scale (capable of measuring mouse weight) | Ohaus | Cat# SP202 |
| Sterile gauze | N/A | N/A |
| Electric small animal heated pad | K&H PET PRODUCTS | N/A |
| Isoflurane | VETONE | Vet-Rx-MW 502017 |
| Curved fine forceps | FST | Cat# 11159-10 |
| Straight brunt forceps | FST | Cat# 11002-12 |
| Straight scissors | FST | Cat# 14040-10 |
| Hartman hemostats | FST | Cat# 13002-10 |
| Autoclip applier 9-mm autoclips | Kent Scientific | Cat# INS750345; Cat# INS75045 |
| PERMA-HAND silk 5-0 | VWR | Cat# 95057-064 |
| Germinator 500 | Braintree Scientific | Cat# GER 5287-120V |
| Surgical microscope | Leica | N/A |
| Wound clip remover | FST | Cat#: 12033-00 |
This protocol can be used with other colorectal cell lines, such as MC-38 cells.
This protocol can be used in immunocompromised mice such as NSG mice if tumor producing rate is low.
Materials and equipment
MTO maintenance media
| Reagent | Final concentration | Stock concentration | Volume (mL) |
|---|---|---|---|
| Advanced DMEM/F12 | – | – | 95.95 |
| GlutaMAX supplement | 1% | 100% | 1 |
| HEPES | 1% | 100% | 1 |
| B27 supplement minus vitamin A (add before using) | 2% | 100% | 2 |
| Murine EGF (add before using) | 50 ng/mL | 100 μg/mL | 0.05 |
CFs maintenance media
| Reagent | Final concentration | Stock concentration | Volume (mL) |
|---|---|---|---|
| DMEM | - | - | 88.778 |
| Gentamicin | 10 μg/mL | 10 mg/mL | 0.1 |
| FBS | 10% | 100% | 10 |
| GlutaMAX supplement | 1% | 100% | 1 |
| Insulin | 10 μg/mL | 10 mg/mL | 0.1 |
| Apo-transferrin | 10 μg/mL | 50 mg/mL | 0.02 |
| Murine EGF | 2 ng/mL | 100 ng/mL | 0.002 |
Step-by-step method details
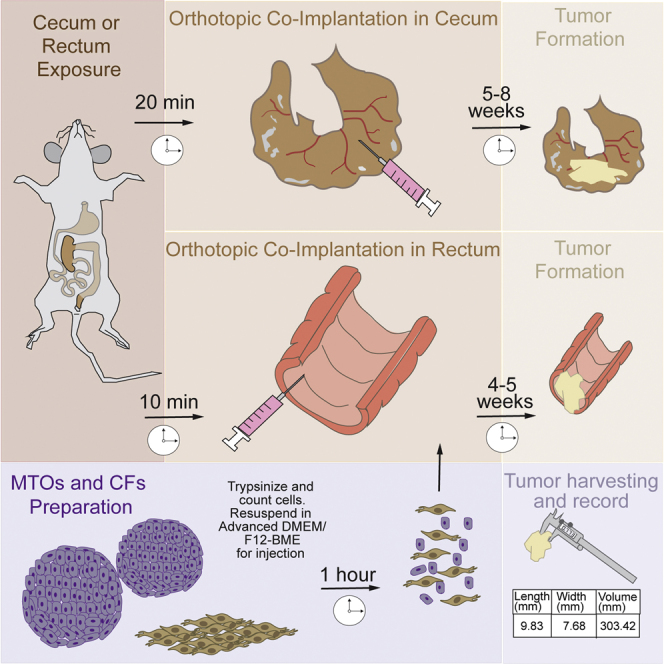
This protocol is aimed at co-injecting CRC cells and colonic fibroblasts to study tumor initiation and progression. As a result of the co-injection, tumor progression is more efficient, and metastases in lymph nodes, lungs, and liver will appear. We described two different approaches for mouse models of CRC. The first one is the co-implantation of mouse tumor organoids (MTOs) and colonic fibroblasts (CFs) in the cecum. The tumor develops in about 5–8 weeks allowing the metastases. The second one is the co-implantation in the rectum. In this method, the tumors develop in 4–5 weeks which allows you to study tumor progression in a shorter time than other models. Before the injection, MTOs and CFs are trypsinized, mixed and resuspended in Advanced DMEM/F12 and BME, and placed in ice. Following the anesthesia of the mouse, the peritoneal cavity is opened to expose the cecum. After the orthotopic injection in the sub-serosal layer, the peritoneum and the skin are closed. In the case of the rectum, the rectal mucosa is exposed after anesthesia of the mouse by grabbing the ventral wall of the rectum. The injection is performed in the submucosa. In both models, mice will be monitored for tumor progression, especially in the rectum model due to the possibility of intestinal obstruction.
Preparation of mouse tumor organoids (MTOs) and colonic fibroblasts (CFs) for injection
Timing: 1–1.5 h
Here, MTOs and CFs are prepared for injection. MTOs and CFs are mixed 1:1 in HBSS, centrifuged and resuspended in Advanced DMEM/F12 with BME. The proportion of BME is different depending on the injection site: for cecum, BME will be 30% of the solution, and for rectum will be 50%.
-
1.
Prewarm 0.25% trypsin-EDTA at 25°C before use.
-
2.
Remove the medium from the dish of MTOs at around 80% confluence (3 days after passage).
CRITICAL: Avoid cell passaging for at least 2 days before implantation.
-
3.
Rinse the dish with PBS (2–3 mL per 1 well of 6-well plate) and aspirate the PBS.
-
4.
Repeat step 3.
-
5.
Add cold Cell Recovery Solution (1.5 mL per 1 well of 6-well plate) followed by mechanical disaggregation of the organoid fragments by pipetting using a 1 mL micropipette.
-
6.
Transfer the organoid suspension to a 15 mL polypropylene tube and keep on ice for 40 min.
-
7.
Centrifuge the tube at 400 × g for 5 min at 4°C.
-
8.
Aspirate supernatant and wash the cell pellet with 2 mL of HBSS.
-
9.
Centrifuge the tube at 400 × g for 5 min at 4°C.
-
10.
Aspirate supernatant and add 500 μL of 0.25% trypsin-EDTA. Place the tube in the 37°C incubator for 20–30 min.
-
11.
Add 9.5 mL of HBSS to dilute the trypsin.
-
12.
Centrifuge the tube at 400 × g for 5 min at 4°C.
-
13.
Resuspend with 1 mL of HBSS and transfer to a 1.5 mL tube
-
14.
Keep the cell suspension on ice. Transfer 10 μL of cell suspension to 1.5 mL tube and mix with 10 μL of trypan blue for counting the number of live cells.
-
15.
Remove the medium from the dish of CFs at around 80% confluence (3 days after passage).
CRITICAL: Avoid cell passaging for at least 2 days before implantation.
-
16.
Rinse the dish with PBS (6 mL per a 10 cm dish) and aspirate the PBS.
-
17.
Repeat step 16.
-
18.
Add 2 mL of 0.25% trypsin-EDTA. Place the dish in the 37°C incubator for 1–3 min.
-
19.
Pipet the cells with a 1 mL micropipette to detach the cells and add 8 mL of maintenance medium to dilute the trypsin.
-
20.
Centrifuge the tube at 300 × g for 5 min at 4°C.
-
21.
Resuspend with 1 mL of HBSS and transfer to a 1.5 mL tube
-
22.
Keep the cell suspension on ice. Transfer 10 μL of cell suspension to 1.5 mL tube and mix with 10 μL of trypan blue for counting the number of live cells.
-
23.
Mix 5 x 105 cells from MTOs and 5 x 105 CFs per mouse in a 1.5 mL tube.
-
24.
Centrifuge the cells mix for 7–8 s at 12,000 × g in a table-top centrifuge with fixed rotor.
CRITICAL: Do not centrifuge more than 7–8 s.
-
25.
For cecum injection, resuspend the cell mix (5 x 105 cells from MTOs + 5 x 105 CFs = 1 × 106 cells per mouse) in 10 μL of 30% BME diluted in Advanced DMEM/F12 supplemented with GlutaMAX and HEPES per mouse. For rectum injection, resuspend the cell mix (5 x 105 cells from MTOs + 5 x 105 CFs = 1 × 106 cells per mouse) in 10 μL of 50% BME diluted in Advanced DMEM/F12 supplemented with GlutaMAX Supplement and HEPES per mouse.
CRITICAL: Prepare 10% more volume than the exact volume in the case of the loss during resuspending the cells or with leakage during the injection. Keep the cell suspension on wet ice.
Orthotopic co-implantation of syngeneic MTOs and CFs in cecum (sub-serosal injection)
Timing: ∼10–15 min per mouse
Here, MTOs and CFs are injected into cecum. After anesthetizing the mouse, the cecum is carefully exposed and flattened. The cell suspension is injected slowly and as shallow as possible. A swelling of the cecum surface can be seen after a successful injection. After the injection, the cecum is returned to the peritoneal cavity and the peritoneum is sutured. Skin is closed using the clips.
-
26.
Place the required materials in the biological safety cabinet to prepare for orthotopic implantation (Figure 1).
-
27.
Anesthetize the mouse by using 2%–3% isoflurane inhalation with 1–1.5 L/min air flow. Monitor respiratory and heart rate.
Figure 1.
Surgical area
Prepare the surgical instruments in a clean safety cabinet as follows: (A) tubes with cell suspension on ice and 0.3-mL 31-gauge needle insulin syringes, (B) gauzes with povidone-iodine solution, (C) gauzes with 70% ethanol, (D) sterilized surgical instruments, (E) autoclip applicator plus 9 mm clips, (F) suture, (G) bead sterilizer, (H) ophthalmic ointment, (I) analgesia.
See Methods Video S1.
-
28.
Apply the ophthalmic ointment and administer the analgesia of choice. Check level of anesthesia by pedal reflex (firm toe pinch).
-
29.
Use sterile gauzes with a 10% povidone-iodine solution to disinfect and clean the shaved abdominal region. Wait until the skin is dry from povidone-iodine and rinse with 70% ethanol. Repeat this step three times.
-
30.
Apply pre-operative analgesia of choice subcutaneously. Either meloxicam SR or buprenorphine can be used, depending on your IACUC protocol.
This video shows how to anesthetize the mouse using isoflurane inhalation.
See Methods Video S2.
-
31.
Gently grab the skin with a pair of straight blunt forceps and perform a midline abdominal incision of the skin (starting ∼2 cm under the xiphoid process).
-
32.
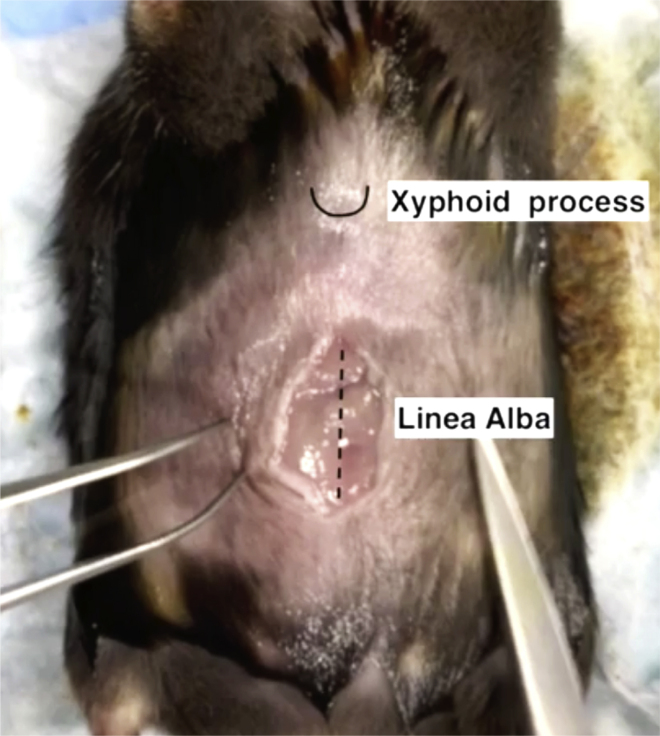
Identify the linea alba in the midline of the peritoneum and make a 1.0–1.5 cm incision along the linea alba (Figure 2).
-
33.
Identify the cecum and expose by using a pair of straight blunt forceps.
CRITICAL: Cecum is usually located at the caudal side of stomach.
-
34.
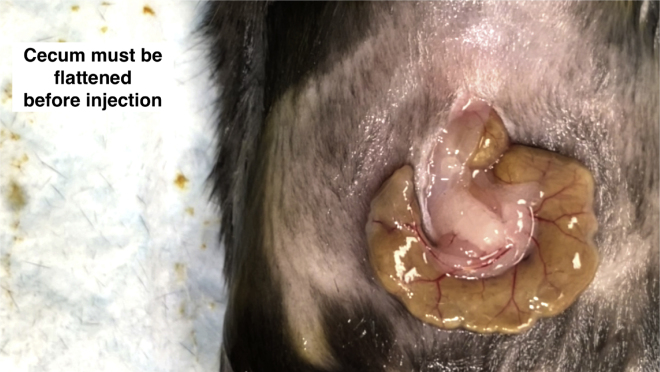
Gently flatten the cecal wall by using a pair of moistened cotton tips. This procedure weakens intestinal peristalsis and makes a good angle of injection (Figure 3).
Figure 2.
Schematic of linea alba and xiphoid process in mouse for the cecum injection
For cecum injection, incision in the peritoneum should be performed 2 cm below the xiphoid process and along the linea alba.
Figure 3.
Cecum preparation for injection
In order to diminish the peristaltic movement, the cecum must be flattened using a pair of cotton tips.
This video shows how to apply ophthalmic ointment and the analgesia, and how to disinfect the abdominal area.
See Methods Video S3.
-
35.
Withdraw 10 μL of the cell suspension with a 0.3-mL insulin syringe attached to a 31-gauge needle.
CRITICAL: Keep the BME-containing solution on ice to prevent gel solidification.
-
36.
Adjust the focus of the surgical microscope on the injection site. Fix the injection site and perform a counter-traction by using a pair of straight fine forceps.
CRITICAL: Do not injure branched vessels in submucosa of cecum or mesentery. The blood branches are visible after exposing the cecum. There must be 2–3 mm between the injection site and the blood vessels.
-
37.
Gently insert the 31-gauge needle of the 0.3-mL insulin syringe, approximately 1–2 mm, into the sub-serosal layer.
CRITICAL: Face a needle tip to upward and insert as shallow as possible.
-
38.
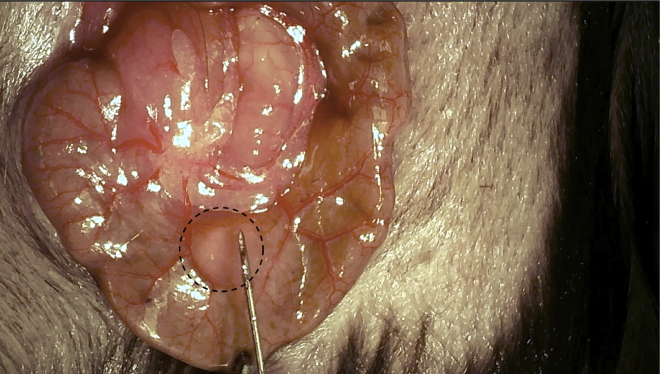
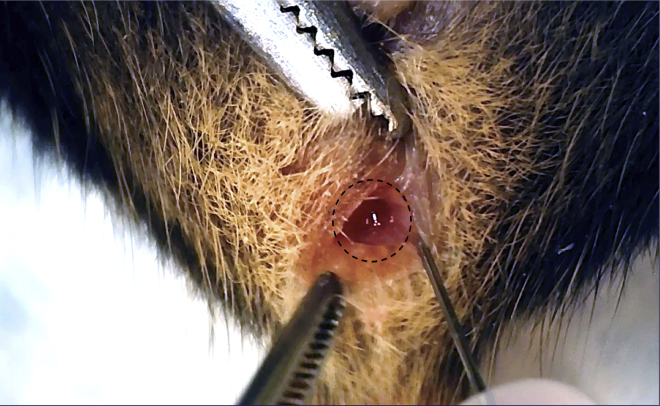
Slowly inject 10 μL of the cell suspension into the sub-serosal layer. Sub-serosal swelling with distinct border will be observed if injection is performed successfully (Figure 4).
CRITICAL: This procedure requires surgical skill and practice to obtain reproducible results. In order to get more reproducible results, it is critical to inject viable cells and this is achieved by a shorter time of the procedure.
Figure 4.
Injection in the cecum
Sub-serosal swelling is observed after a successful injection in the cecum.
This video shows how to do the initial incision to expose the cecum. Cecum must be flattened before injection.
See Methods Video S4.
-
39.
Check the injection site for leakage and bleeding.
-
40.
Close the peritoneum of the abdominal wall by an interrupted suture using PERMA- HAND SILK 5-0. Close the skin layer of the abdominal wall by Reflex 9 mm Wound Clips using the autoclip applier.
The injection in the cecum needs to be as shallow and slow as possible. Swelling in the subserosa must be confirmed.
See Methods Video S5.
Note: Alternatively, you can use the continuous suture technique to close the peritoneum.
You can use the continuous suture to close the peritoneum of the abdominal wall, followed by the closure of the skin using clips.
See Methods Video S6.
-
41.
Leave the mouse on the heating pad and allow the mouse to recover from anesthesia.
An alternative technique to close the peritoneum is the continuous suture technique.
See Methods Video S7.
Isotonic liquid is administered to alleviate the body fluids loss during surgery. Mouse are left to recover on a clean cage under a heat lamp.
Orthotopic co-implantation of syngeneic MTOs and CFs in rectum (sub-mucosal injection)
Timing: 5–10 min per mouse
Here, MTOs and CFs are injected into rectum. After anesthetizing the mouse, the rectum is open carefully to expose the mucosa. As parallel as possible, the cell suspension is injected in the sub-mucosal layer very slowly to avoid leakage. A swelling of the rectum wall can be seen after a successful injection. After the injection, the mouse is left on a clean cage to recover from anesthesia.
-
42.
Place the required materials in the biological safety cabinet to prepare for orthotopic implantation. Anesthetize the mice by using isoflurane inhalation.
-
43.
Place the mouse in a supine position on the heating pad to avoid hypothermia under a surgical microscope.
-
44.
Apply pre-operative analgesia subcutaneously. Either meloxicam SR or buprenorphine can be used, depending on your IACUC protocol.
-
45.
Apply the ophthalmic ointment.
-
46.
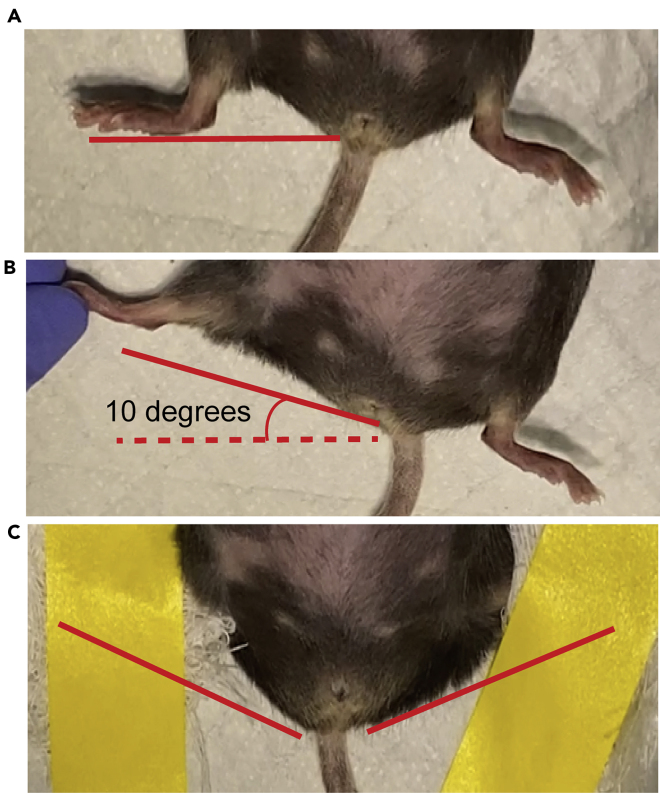
Secure the lower extremities raised to the cranial side with a gauze-covered tape (Figure 5).
CRITICAL: This procedure makes anus faces toward a surgical microscope.
-
47.
Insert a blunt-ended Hartman hemostat (∼1 cm) into the anus.
-
48.
Open the hemostat slightly and grab the ventral part of the rectum.
-
49.
Flip the hemostat to the cranial side to exteriorize the rectal mucosa.
-
50.
Withdraw 10 μL of the cell suspension with a 0.3-mL insulin syringe attached to a 31-gauge needle.
CRITICAL: Keep the BME-containing solution on ice to prevent gel solidification.
-
51.
Adjust the focus of the surgical microscope on the injection site.
-
52.
Gently grab the dorsal part of the rectum with a pair of straight fine forceps to fix the injection site and perform a counter-traction.
-
53.
Gently insert (approximately 1–2 mm) a 31-gauge needle of 0.3-mL insulin syringe with the cell solution into a sub-mucosal layer.
CRITICAL: Face a needle tip to a lumen side and insert as shallow as possible.
-
54.
Slowly inject 10 μL of the cell solution (containing the equivalent to 1 × 106 cells) in the sub-mucosal layer. Sub-mucosal swelling will be observed if the injection is performed successfully (Figure 6).
Figure 5.
Preparation of the mouse for Injection in the rectum
(A) Normal position of lower extremities.
(B) Lower extremities are raised to the cranial side in a 10° angle to expose the rectum before the injection.
(C) The extremities are secured using gauze and tape.
Figure 6.
Injection in the rectum
Sub-mucosal swelling after successful injection in the rectum.
See Methods Video S8.
-
55.
Check the injection site for leakage and bleeding.
-
56.
Leave the mouse on the heating pad and allow the mouse to recover from anesthesia.
The rectal mucosa needs to be exposed using a hemostat and forceps. The successful injection of the cell suspension will cause the swelling in the submucosa.
Monitoring mouse condition
Timing: 5–8 weeks (cecum injection), 4–5 weeks (rectum injection)
-
57.
(Cecum injection) Wound clips must be removed in 10–14 days once the incision has healed.
-
58.
Closely monitor the mice for tumor progression and continue to assess their health until the experiment will be finished.
CRITICAL: If severe weight loss occurs (>20% loss of starting body weight), the mouse should be sacrificed according to your institution’s animal protocol.
-
59.
See the Quantification and statistical analysis section for more detail into the analysis of the results.
Expected outcomes
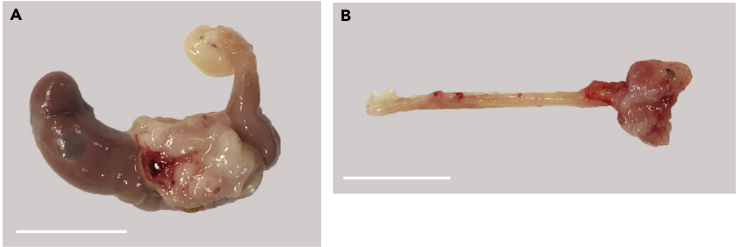
Tumors in cecum (Figure 7A) take 5 to 8 weeks to engraft and grow enough for analysis, meanwhile in rectum, tumors take 4 to 5 weeks to be analyzed (Figure 7B). By 8 weeks (cecum injection) or 5 weeks (rectum injection), if there are no tumors, it is unlikely that a tumor will form. Tumor growth in the rectum can be assessed by checking the anus periodically, while tumors in cecum cannot be assessed until sacrificing the mice. Evaluation of lymph node, liver, and lung metastases can be used to assess the metastatic abilities.
Figure 7.
Expected outcomes from cecum and rectum co-implantation
(A) Tumor in cecum. Scale bar, 1 cm.
(B) Tumor in rectum. Scale bar, 1 cm.
Quantification and statistical analysis
-
1.
Create an excel file to record data pre-injection and after tumor harvesting. Data to be recorded should include: sex, date of birth, age, and body weight before surgery, body weight at the endpoint.
-
2.
At the endpoint, diameters (short and long) of tumor, the number of lymph node, liver, and lung metastases should be recorded. Tumor volume is calculated using the following formula: 1/6 x 3.14 x (short diameter)2 x (long diameter).
Limitations
The effort for building surgical expertise is needed due to the difficulty of these procedures. Potential issues for the orthotopic tumor implantation in cecum are leakage and “accidental” peritoneal metastasis. To avoid these problems, we recommend (1) small-volumes for the cell suspension (10 μL per injection) and (2) 0.3-mL insulin syringes with 31-gauge needles. Another potential issue after injection in the rectum is the early development of intestinal obstruction which will not allow the study of metastases.
Troubleshooting
Problem 1
Difficulty to find the cecum.
Potential solution
Mouse cecum is usually located at the caudal side of stomach. If not, search cecum by its color (darker brown than small intestine) and shape (bigger and connected to colon).
Problem 2
Leakage from the injection site.
Potential solution
Inject cell suspension as slow as possible and hold for at least 30 s. Reduce the injection volume. Increase the ratio of BME in the cell solution.
Problem 3
Sub-serosal swelling in cecum cannot be seen.
Potential solution
Cecum injection is too deep. Face a needle tip to upward and insert as shallow as possible.
Problem 4
Sub-mucosal swelling in rectum cannot be seen.
Potential solution
Rectum injection is too deep. Face a needle tip to a lumen side and insert as shallow as possible.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jorge Moscat (jom4010@med.cornell.edu).
Materials availability
MTOs will be available upon request with a completed Materials Transfer Agreement.
CFs will be available upon request with a completed Materials Transfer Agreement.
Data and code availability
This study did not generate datasets.
Acknowledgments
Research was supported by grants by NCI and NIDDK of the National Institutes of Health under award numbers R01DK108743, R01CA211794, R01CA207177 to J.M. and R01CA218254 to M.T.D.-M. J.M. and M.T.D.-M. are the Homer T. Hirst III Professors of Oncology in Pathology.
Author contributions
Conceptualization, H. Kasashima, M.T.D.-M., and J.M.; Investigation, H. Kasashima, A.D., T.C.-D., Y.M., and H. Kinoshita; Resources, E.B; Writing – Original Draft, H. Kasashima and A.D.; Writing – Review & Editing, A.D., T.C.-D., M.T.D.-M., and J.M.; Funding Acquisition, M.T.D.-M. and J.M.; Supervision, M.T.D.-M. and J.M.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2021.100297.
Contributor Information
Hiroaki Kasashima, Email: m1165063@med.osaka-cu.ac.jp.
Maria T. Diaz-Meco, Email: mtd4001@med.cornell.edu.
Jorge Moscat, Email: jom4010@med.cornell.edu.
References
- Kasashima H., Duran A., Martinez-Ordonez A., Nakanishi Y., Kinoshita H., Linares J.F., Reina-Campos M., Kudo Y., L'Hermitte A., Yashiro M. Stromal SOX2 upregulation promotes tumorigenesis through the generation of a SFRP1/2-expressing cancer-associated fibroblast population. Dev. Cell. 2020 doi: 10.1016/j.devcel.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauriello D.V.F., Palomo-Ponce S., Stork D., Berenguer-Llergo A., Badia-Ramentol J., Iglesias M., Sevillano M., Ibiza S., Canellas A., Hernando-Momblona X. TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538–543. doi: 10.1038/nature25492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This video shows how to anesthetize the mouse using isoflurane inhalation.
This video shows how to apply ophthalmic ointment and the analgesia, and how to disinfect the abdominal area.
This video shows how to do the initial incision to expose the cecum. Cecum must be flattened before injection.
The injection in the cecum needs to be as shallow and slow as possible. Swelling in the subserosa must be confirmed.
You can use the continuous suture to close the peritoneum of the abdominal wall, followed by the closure of the skin using clips.
An alternative technique to close the peritoneum is the continuous suture technique.
Isotonic liquid is administered to alleviate the body fluids loss during surgery. Mouse are left to recover on a clean cage under a heat lamp.
The rectal mucosa needs to be exposed using a hemostat and forceps. The successful injection of the cell suspension will cause the swelling in the submucosa.
Data Availability Statement
This study did not generate datasets.