Abstract
Sulforaphane (SFN) extracted from broccoli sprout has previously been investigated for its potential properties in cancers, however, the underlying mechanisms of the anticancer activity of SFN remain not fully understood. In the present study, we investigate the effects of SFN on cell proliferation, cell cycle, cell apoptosis, and also the expression of several cell cycle and apoptosis-related genes by MTT assay, flow cytometry and western blot analysis in gastric cancer (GC) cells. The results showed that SFN could impair the colony-forming ability in BGC-823 and MGC-803 cell lines compared with the control. In addition, SFN significantly suppressed cell proliferation by arresting the cell cycle at the S phase and enhancing cell apoptosis in GC cells in a dose-dependent manner. Western blot results showed that SFN treatment significantly increased the expression levels of p53, p21 and decreased CDK2 expression, which directly regulated the S phase transition. The Bax and cleaved-caspase-3 genes involved in apoptosis executive functions were significantly increased in a dose-dependent manner in BGC-823 and MGC-803 cells. These results suggested that SFN-induced S phase cell cycle arrest and apoptosis through p53-dependent manner in GC cells, which suggested that SFN has a potential therapeutic application in the treatment and prevention of GC.
Subject terms: Cancer, Gastroenterology, Health care, Oncology, Apoptosis
Introduction
Sulforaphane is an isothiocyanate compound mainly derived from cruciferous vegetables such as broccoli, Brussels sprouts and cabbage1. Previous research has demonstrated that SFN has a variety of important biological activities, including anti-oxidation2, anti-inflammation3, anti-aging4 and antibacterial effects5, and so on. More importantly, SFN has been found to exert anticancer effects by inhibiting cell proliferation6, promoting apoptosis7, inhibiting metastasis8 and anti-angiogenesis properties9 in cancer cells.
GC is one of the most common fatal malignancies worldwide and poses a serious threat to human health10. The global GC incidence rate accounted for 5.7% of all cancer cases (ranked fifth), and the mortality rate accounted for 8.2% of all cancer deaths (ranked third) in 201811. In China, there are 679,000 new cases and 498,000 deaths of GC in 2015, both of which ranked second in malignant tumors12. In spite of the rapid advances in surgery, radiation and chemotherapy during recent decades, the prognosis of GC patients is still remains unsatisfactory13, therefore, it is urgent to find new and effective treatment methods for GC patients. Among chemotherapy agents, phytochemicals have attracted widespread attention in recent years because of their high curative effect, low side effect and high safety, and numerous studies have proved that resveratrol14, curcumin15, genipin16, chrysin17 and eugenol18 have anticancer effects in GC cells.
SFN is one of phytochemicals and has become a promising anticancer chemotherapeutic agent because of its low toxicity19. Studies have shown that SFN plays an anti-tumor role in breast20, colon21, prostate22 and bladder cancer23 as well as GC. Several studies have demonstrated that SFN inhibits the proliferation and promotes apoptosis of GC cells through various mechanism and targets24–27. However, the anti-cancer mechanism of SFN has not been fully elucidated in GC. In this study, we choose GC as our aim because SFN can quickly and directly acts on gastric cancer cells and which could achieve higher therapeutic effects, and investigated the potential novel mechanisms involved in SFN-induced apoptosis and cell cycle arrest in GC, our studies will assist us in developing new anticancer drugs for GC patients.
Results
Effect of SFN on cell viability of GC cells
SFN has a relative molecular weight of 177.3 with a molecular formula of C6H11NOS2 (Fig. 1A). In order to investigate the potential toxic effects on GC cell lines and gastric mucosal immortalized cells GES-1, we first determined the viabilities of GC cells followed by treatment in a series of gradient SFN at concentrations of 0–22.5 μM (with an increasing increment between every 1.5 μM) for 48 h. As shown in Fig. 1B–D, MTT assays indicated that SFN obviously reduced the cell viabilities of BGC-823, MGC-803 and GES-1 cells in dose-dependent manners. The IC50 values of SFN on BGC-823, MGC-803 and GES-1 cells were 14.4 μM, 18.7 and 20.1 μM, respectively (Fig. 1B–D), the results also indicated that GES-1 cells have higher SFN tolerance than BGC-823 and MGC-803 cells. To reduce the toxicity of SFN on normal cells, the concentration of SFN we choose in the subsequent functional experiments was much less than the IC50 of GC cells.
Figure 1.
SFN decreased the cell viability in GC cells. (A) The chemical structure of SFN. (B–D) The cell viability of GES-1 (B), BGC-823 (C) and MGC-803 (D) cells were measured by MTT assay after treated with different concentrations of SFN for 48 h, and the IC50 values of BGC-823, MGC-803 and GES-1 cells were 14.4, 18.7 and 20.1 μM, respectively.
SFN inhibits colony formation of GC cells
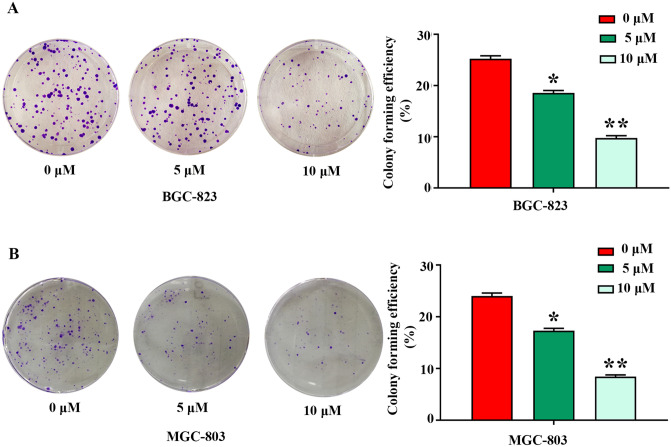
To investigate the influence of SFN on the capacity of colony formation of GC cells, the colony-forming efficiency of BGC-823 and MGC-803 cells with or without SFN was assessed. As demonstrated in Fig. 2A,B, colony formation assay showed that SFN induced a dose-dependent decline in colony forming efficiency in BGC-823 and MGC-803 cell lines. Compared with control cells, treatment with 5 and 10 µM of SFN caused decreased cell colony numbers by 26%, 61% and 28%, 65% in BGC-823 and MGC-803 cells (Fig. 2A,B), respectively. These findings indicated that SFN serves a key role in the inhibition of colony formation and as a potential drug for clinical applications in GC.
Figure 2.
SFN inhibits the cell colony formation abilities of GC cells. BGC-823 (A) and MGC-803 (B) cells were treated with SFN at the indicated concentrations (0, 5 and 10 µM) for 48 h. The data represent the mean ± SD from three independent experiments, *P < 0.05; **P < 0.01 vs control.
SFN suppresses GC cells proliferation by arresting the cell cycle at the S phase
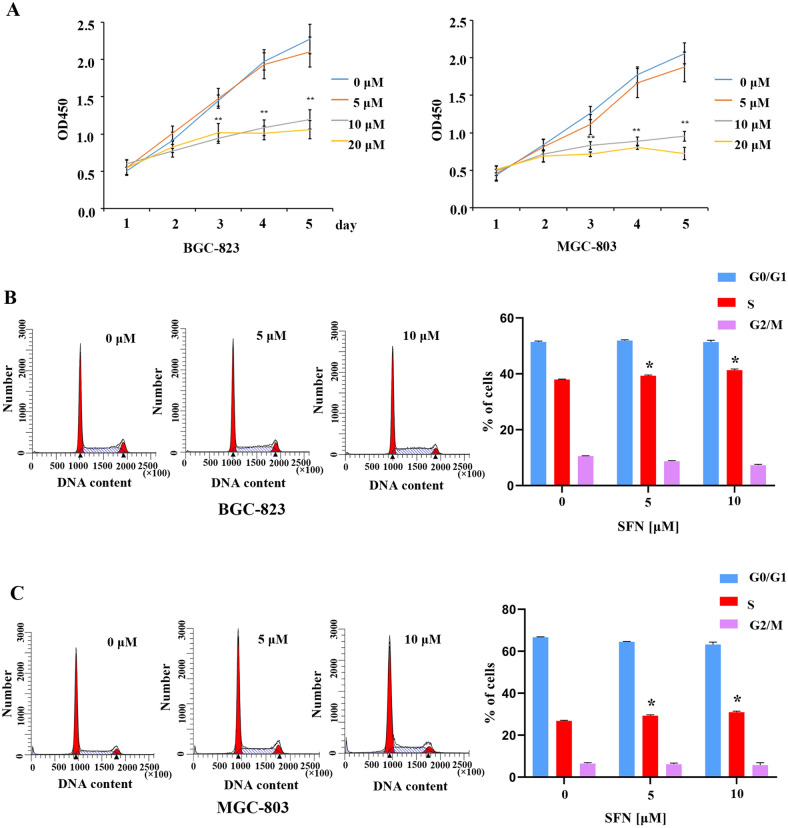
Abnormal cell proliferation is a typical characteristic of cells that have undergone malignant transformation. Studies have shown that SFN was involved in the regulation of cell proliferation in human cancer cells23,28–30. To evaluate the effect of SFN on cell proliferation ability of GC cells, BGC-823 and MGC-803 cells were treated with 0, 5, 10 and 20 μM of SFN for 48 h, MTT assay revealed that SFN significantly suppressed GC cells proliferation of GC cells at a concentration of 10 μM compared with control cells (Fig. 3A). Uncontrolled proliferation is a major feature of cancer cells which often triggered by the malfunction of cell cycle, to further explore the effects of SFN on the cell cycle, the GC cells were treated with 0, 5 and 10 μΜ of SFN for 48 h, cell cycle distribution analysis by flow cytometry indicated that with the increase of the concentration of SFN, the number of cells in S-phase obviously increased after SFN treatment in BGC-823 (Fig. 3B) and MGC-803 (Fig. 3C) cells. These results indicate that SFN suppresses GC cells proliferation by arresting the cell cycle at the S phase in GC cell lines.
Figure 3.
Effects of SFN on GC cell proliferation and cell cycle progression. (A) The cell proliferation of BGC-823 and MGC-803 cells was measured by MTT assay after cells were treated with indicated concentration of SFN for 48 h. (B, C) The distribution of cell cycle phases in BGC-823 (B) and MGC-803 (C) were examined using flow cytometry assay. The percentages of cells in the G1, S, and G2/M phases are shown in the bar chart, and the values indicate the mean ± SD for three independent experiments, statistical analyses were performed by Student’s t-test compared with the control group (*P < 0.05, **P < 0.01).
SFN induces apoptosis in GC cells
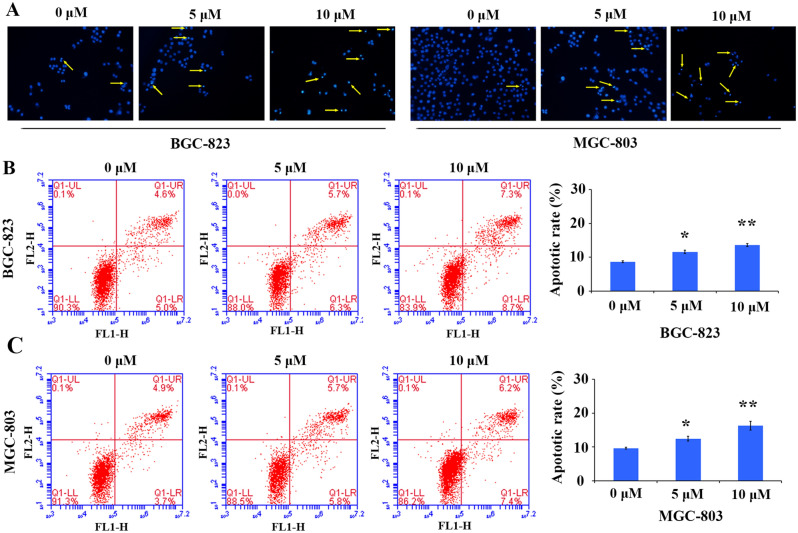
SFN has previously been investigated for their potential apoptosis-inducing activity in nasopharyngeal cancer cells and macrophages31,32. However, few studies concerned about the apoptosis of GC cells induced by SFN, so we performed Hoechst staining and Annexin V-FITC/PI double staining assay to confirm and quantify the apoptosis-inducing activity of SFN exhibited in GC cells. Hoechst staining (Fig. 4A) showed that SFN obviously induced GC cells apoptosis, after treatment with 5 and 10 μM of SFN for 48 h, some typical apoptosis-related morphological changes such as nuclear shrinkage and nuclear condensation were observed in BGC-823 and MGC-803 cells, whereas the control group cells were stained evenly with regular shape (Fig. 4A). Those results suggest that SFN could induce apoptosis of GC cells.
Figure 4.
SFN induce cell apoptosis in GC cells. (A) Increased apoptosis induction by SFN in BGC-823 and MGC-803 cells by Hoechst staining (× 200), the arrow indicates the apoptotic cells. The apoptosis rate in BGC-823 (B) and MGC-803 (C) cells treated with different concentration of SFN for 48 h was detected by flow cytometry after Annexin V/PI double staining. The apoptosis rate was calculated and depicted in a bar chart, and the values indicate the mean ± SD for three separate experiments, the statistical values were determined by Student’s t-test, *P < 0.05, **P < 0.01.
To further verify SFN-induced apoptotic activity in GC cells, we detected the cell apoptosis rate using flow cytometry after Annexin V-FITC/PI double fluorescence staining. As shown in Fig. 4B,C, when GC cell lines were treated with 5 and 10 µM of SFN for 48 h, we observed a marked increase in the level of apoptosis following SFN treatment in GC cells, and ~ 12% and 15% of the cells were Annexin V positive, respectively, whereas only ~ 9.5% in the control group. These data indicate that SFN could significantly induce apoptosis of GC cells in a dose-dependent manner.
Effect of SFN on the expression of cell cycle and apoptosis-related proteins in GC cells
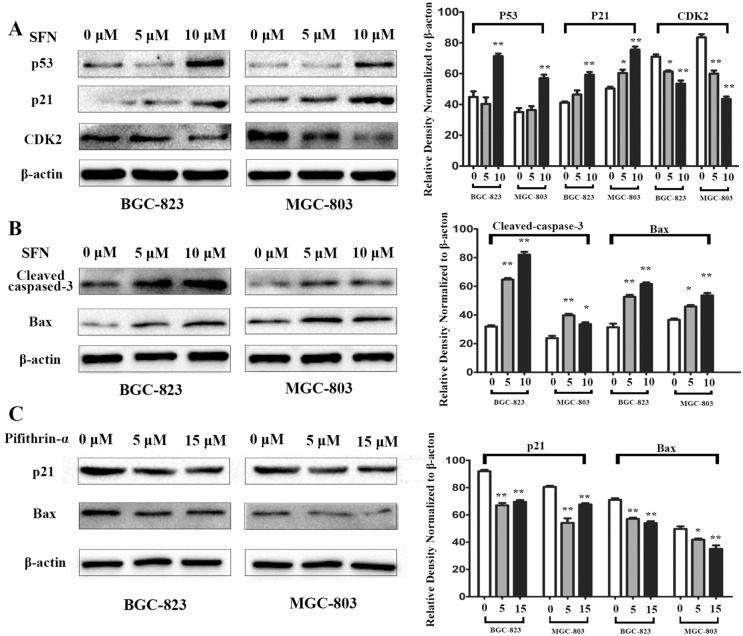
Inhibition of cell cycle progression and induction of apoptosis in GC cells mediated by SFN which have revealed by our experiments (Figs. 3 and 4), to further characterize the mechanisms of SFN on cell S phase arrest, we detected the expression levels of CDK2, p21 and p53 by western blotting, which directly regulated the S-phase transition33–36. As shown in Fig. 5A by western blot analysis, compared to that in control cells, the expression levels of cell cycle regulatory proteins such as p53 and p21 were increased in a dose-dependent manner when the GC cells were treated with 5 and 10 μM of SFN for 48 h, while the expression level of S phase related proteins CDK2 was significantly decreased upon SFN induction in BGC-823 and MGC-803 cells (Fig. 5A, Supplementary Information). The results above indicated that the SFN effectively inhibited cell proliferation via inducing cell cycle arrest at S phase partly by regulating the expression of cell cycle-related genes in GC cells.
Figure 5.
The effect of SFN on the expression of cell cycle and apoptosis related proteins in a dose-dependent manner in GC cells. (A) The S phase arrest associated proteins p53, p21 and CDK2 associated proteins were examined by western blot analysis after treated with the increased concentration of SFN for 48 h in BGC-823 and MGC-803 cells. (B) Western blotting was used to analyze the effect of SFN on the expression of apoptosis-related proteins caspase-3 and Bax in BGC-823 and MGC-803 cells. (C) Inhibition of p53 activity caused by pifithrin-α reduces the expression of p21 and Bax in GC cells. (*P < 0.05, **P < 0.01).
Additionally, the effects of SFN on the expression of apoptosis-related proteins caspase-3 and Bax, which are involved in apoptosis executive functions were also assessed in GC cells37–39. As shown in Fig. 5B, the expressions of Bax and cleaved-caspase-3 were significantly increased in a dose-dependent manner in the experimental group at 48 h compared with the control in BGC-823 cell, and the similar results were also obtained in MGC-803 cell (Fig. 5B, Supplementary Information). Because p53 acts upstream of p21 and Bax, it is expected that p53 inactivation may decrease their expression. Indeed, it is proved that the inhibition of p53 activity caused by pifithrin-α could significantly attenuated the expression of p21 and Bax in GC cells (Fig. 5C, Supplementary Information). Taken together, our results indicate that SFN induces cells apoptosis and inhibits cell proliferation in GC cells via p53-dependent manner.
Discussion
Cyclic abnormalities and anti-apoptosis are commonly observed in cancer cells, and the ability to induce cell cycle arrest and promote apoptosis is a criterion for selecting potential anti-tumor chemotherapeutics40. Studies have shown that phytochemicals can affect the cell cycle and apoptosis in cancer cells41,42. Therefore, the focus of this study was to evaluate the effects of SFN on cell cycle and apoptosis in GC cells.
Previous studies have reported that SFN induces sub-G143,44, G0/G145,46 and G2/M27,47 cell cycle arrest in human non-small cell lung carcinoma, leukemia and melanoma cells, etc. Our study showed that SFN induced S phase arrest in BGC-823 and MGC-803 GC cells, the results were consistent with previous reports by Juengel et al., that SFN induces S phase arrest in kidney carcinoma Caki-1 cell48. Interestingly, studies have shown that SFN can induce G0/G1, S and G2/M phase arrest in AGS and MGC-803 GC cells26,27, which may be related to tumor heterogeneity and SFN concentration49. To analyze the molecular mechanisms behind SFN-induced cell cycle arrest, the relative protein levels of S phase arrest-related gene CDK2 which involved in the cell cycle progression50 and the transition from G1 to S phase51–53 was measured by western blot assay. Our data indicate that SFN causes S-phase arrest in BGC-823 and MGC-803 cells by inhibiting the expression of CDK2 protein in dose-dependent manner. In addition, studies have demonstrated that the reduction in CDK2 production was related to the up-regulation level of p21, a cyclin-dependent kinase inhibitor54. Furthermore, when the tumor suppressor gene p5355 was activated, it activates the downstream gene p21 and blocks normal cell cycle progression56,57. Therefore, we examined whether the expression of p53 and p21 were changed after treated with SFN for 48 h. The similar results were obtained that SFN can significantly induce the expression of p53 and p21 (Fig. 5A). Taken together, our results indicated that SFN causes S phase arrest via the p53-mediated p21-CDK2 axis in BGC-823 and MGC-803 cells (Fig. 6).
Figure 6.
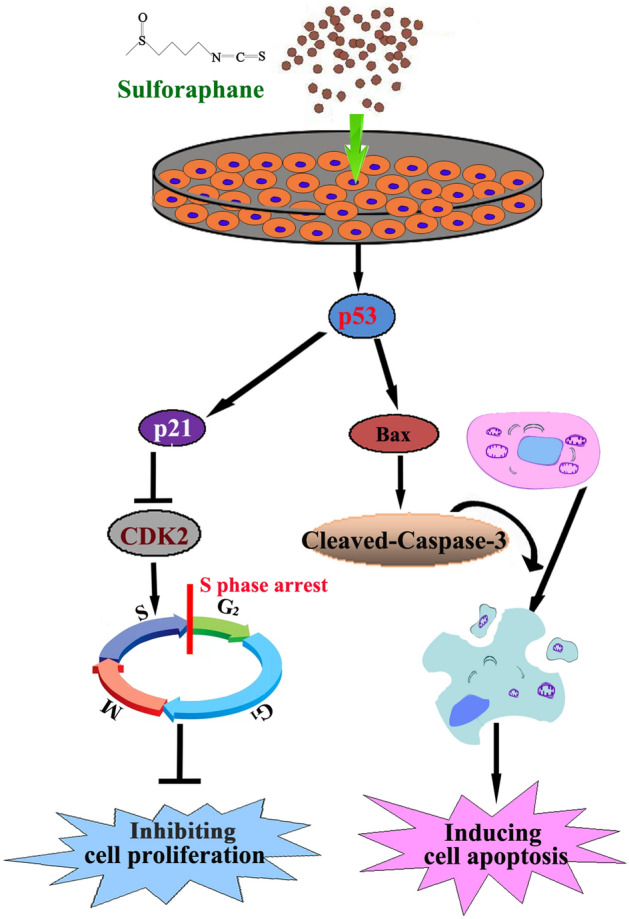
SFN induces S-phase arrest and apoptosis via p53-dependent manner in gastric cancer cells in GC cells.
Apart from cell cycle arrest, we also observed apoptosis induced by SFN in GC cells. Apoptosis is a programmed cell death controlled by genes to maintain homeostasis58. The mitochondrial pathway is one of the main pathways of apoptosis59. Mitochondrial-mediated apoptosis is promoted by members of the Bcl-2 protein family, which includes apoptotic proteins (such as bax, Bak and Bik) and anti-apoptotic proteins (such as bcl-2, Bcl-w and bcl-xl)60. Previous studies have found that SFN can induce apoptosis of cancer cells through endoplasmic reticulum stress61, targeting STAT3 signaling pathway31 and the type 1 IP3 receptor62. Therefore, we first explored the role of apoptotic protein Bax in SFN treated BGC-823 and MGC-803 cells. Our data showed that SFN induced apoptosis by enhancing expression of Bax after treatment for 48 h. The imbalance between Bcl-2 family members can activate caspases family to induce apoptosis63, and caspase-3 is one of the key effectors64. In our study, the cleaved-caspase-3 (activation form of caspase-3) levels were increased after 48 h of treatment with SFN (Fig. 5B). The results showed that SFN induced apoptosis of GC cells through mitochondrial dependent pathway. In addition, studies have shown that activated p53 not only induces cell cycle arrest, but also induces apoptosis65,66. When DNA damage is serious and irreparable, p53 can induce the expression of Bax and activate caspase-3 to induce apoptosis of cancer cells66, and our results also showed that inhibition of p53 activity caused by pifithrin-α could significantly attenuated the expression of Bax (Fig. 5C). Taken together, our results indicated that SFN can induce apoptosis through the p53-dependent mitochondrial pathway in human GC cells although the detailed molecular mechanism needs further exploration (“Supplementary Information”).
In conclusion, our study found that SFN induces S phase arrest via the p53-dependent antiproliferation and apoptosis induction in BGC-823 and MGC-803 cells (Fig. 6), these studies not only clarify the molecular mechanisms of SFN involved in GC cell cycle and apoptosis, but provide a potential novel agent for the treatment of GC.
Materials and methods
Cell lines and reagents
Human gastric cancer cell lines (MGC-803 and BGC-823) were purchased from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). All cells were cultured in a humidified incubator maintained DMEM medium containing 10% fetal bovine serum (Wisent, St. Bruno, QC, Canada), 100 mg/ml streptomycin and 100 U/ml penicillin (Invitrogen, Carlsbad, CA, USA) at 37 °C with 5% CO2. SFN and Pifithrin-α (PFT-α) was obtained from Sigma-Aldrich (St Louis, MO, USA) and Beyotime Biotechlonogy (Shanghai, China).
MTT assays
The cells (5 × 103 cells per well) were seeded in 96-well plate for 24 h, then treated with different concentrations of SFN for 0, 24, 48, 72 and 96 h. Thereafter, cell proliferation was measured using the MTT assay according to the kit instructions (Beyotime Biotechnology, Shanghai, China).
Colony-forming assay
Cells were inoculated into 6-well plates (1000 cells per well) for 24 h, then the medium was removed and the fresh medium containing different concentrations of SFN was added, and the cells were cultured for 10–14 days until colonies were visible. The cells were fixed with methanol for 20 min, then stained with 2% crystal violet for 20 min and finally photographed the number of colonies.
Hoechst 33258 staining assay
Hoechst 33258 (Beyotime Biotechnology, Shanghai, China) staining is used to distinguish condensed nuclei in apoptotic cells. Cells were treated with different concentrations of SFN for 48 h, fixed by fixative for 10 min, and then the cells were washed twice with PBS. Next, the fixed cells were stained with Hoechst 33258 for 5 min, washed by PBS five times, and observed under a fluorescence microscope (Olympus, Tokyo, Japan).
Flow cytometric analysis of apoptosis
Cells were inoculated into 6-well plates for 24 h, then the old medium was removed and the medium containing different concentrations of SFN was added. After 48 h of culture, the cells were collected, washed three times with cold PBS, and resuspended in 200 µL binding buffer. Then, 10 µL Annexin-V-FITC and 10 µL PI were added according to the kit instructions, and incubated at room temperature for 30 min. Finally, the cells were analyzed by flow cytometry (Becton Dickinson, Franklin Lakes, NJ, USA).
Cell cycle analysis
Cells were inoculated into 6-well plates for 24 h, then the old medium was removed and the medium containing different concentrations of SFN was added. After 48 h of culture, the cells were collected and washed with PBS. Then the cells were fixed in cold 70% ethanol and stored at 4 °C overnight. The next day, the cells were centrifuged and washed twice with PBS, then stained with PI, and incubated at 37 °C for 30 min, and at last the cells were analyzed by flow cytometry (Becton Dickinson, Franklin Lakes, NJ, USA).
Western blotting assay
The cells were lysed with RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China) to extract total protein. Then the total protein solution was quantified by the BCA kit (Beyotime Biotechnology, Shanghai, China). The samples were heated for 5 min at 100 °C before the protein samples were separated by 10% SDS-PAGE gel electrophoresis, the target proteins are transferred from the gel to a PVDF membrane (the PVDF membrane was cut to 2 cm wide to transfer the target proteins instead of using a full-length PVDF membrane to the gels), then the PVDF membranes were washed with Tris-buffered saline containing 0.1% Tween-20 (TBST) and blocked with 5% skimmed milk at room temperature for 2 h. After three times of TBST washing, the membrane was incubated with a special primary antibody at 4 °C overnight. Subsequently, the membranes were washed with TBST and then incubated with the secondary antibody for 1 h at room temperature. The immunoreactive proteins were detected using an enhanced chemiluminescence western blotting detection kit (Beyotime Biotechnology, Shanghai, China) and ChemDoc XRS and quantified with Quantity One software (Bio-Rad, Hercules, CA, USA). All antibodies are from Abbkine (Abbkine Scientific Co., Ltd, Wuhan, China).
Statistical analysis
Data were analyzed using GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, USA) and SPSS 23.0 software (IBM Corporation, Armonk, NY, USA). Results were presented as mean ± SD. For all experiments, one-way ANOVA or Student’s t-test were used to analyze the differences between groups. All P-values were derived from two-sided tests and P < 0.05 was considered as statistically significant.
Supplementary Information
Author contributions
Y.L.Z. and H.Z.W. designed the experiments and commented the manuscript. Y.W. and H.Z.W. performed the experiments and wrote the manuscript. N.N.D., X.S., M.X.D., Y.Q.W., J.W., G.F.L. and Q.J.P. participate in experiments and data analysis.
Funding
This work was supported by National Natural Science Foundation of China (21707002); the Natural Science Foundation of Anhui Province (1908085MH257); Foundation for Young Talents and Natural Science in Higher Education of Anhui Province (gxyq2018035, KJ2019A0361, KJ2017A228); Special Fund for Translational Medicine of Bengbu Medical College (BYTM2019008).
Data availability
All data during this study are included in the article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41598-024-82511-7"
Change history
12/10/2024
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41598-024-82511-7
Contributor Information
Huazhang Wu, Email: whzhang1025@163.com.
Yunli Zhao, Email: yunli201@126.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-81815-2.
References
- 1.Lubecka-Pietruszewska, K. et al. Sulforaphane alone and in combination with clofarabine epigenetically regulates the expression of DNA methylation-silenced tumour suppressor genes in human breast cancer cells. J. Nutrigenet. Nutrigenom.8, 91–101 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Angulo, J. et al. Short-term pharmacological activation of Nrf2 ameliorates vascular dysfunction in aged rats and in pathological human vasculature. A potential target for therapeutic intervention. Redox Biol.26, 101271 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoo, I. H. et al. The anti-inflammatory effect of sulforaphane in mice with experimental autoimmune encephalomyelitis. J. Korean Med. Sci.34, e197 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomez-Linton, D. R. et al. Some naturally occurring compounds that increase longevity and stress resistance in model organisms of aging. Biogerontology20, 583–603 (2019). [DOI] [PubMed]
- 5.Nowicki, D., Rodzik, O., Herman-Antosiewicz, A. & Szalewska-Palasz, A. Isothiocyanates as effective agents against enterohemorrhagic Escherichia coli: Insight to the mode of action. Sci. Rep.6, 22263 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milczarek, M. et al. Autophagic cell death and premature senescence: New mechanism of 5-fluorouracil and sulforaphane synergistic anticancer effect in MDA-MB-231 triple negative breast cancer cell line. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc.111, 1–8 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Zhou, Y. et al. Sulforaphane metabolites cause apoptosis via microtubule disruption in cancer. Endocr. Relat. Cancer25, 255–268 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Zheng, Z. et al. Sulforaphane metabolites inhibit migration and invasion via microtubule-mediated Claudins dysfunction or inhibition of autolysosome formation in human non-small cell lung cancer cells. Cell Death Disease10, 259 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu, P. et al. Sulforaphane exerts anti-angiogenesis effects against hepatocellular carcinoma through inhibition of STAT3/HIF-1alpha/VEGF signalling. Sci. Rep.7, 12651 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang, H., Yang, T., Lu, P. & Ma, Y. Gene expression profiling of gastric cancer. Eur. Rev. Med. Pharmacol. Sci.18, 2109–2115 (2014). [PubMed] [Google Scholar]
- 11.Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.68, 394–424 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Chen, W. et al. Cancer statistics in China, 2015. CA Cancer J. Clin.66, 115–132 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Shen, L. et al. Management of gastric cancer in Asia: Resource-stratified guidelines. Lancet Oncol.14, e535–e547 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Rauf, A. et al. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr.58, 1428–1447 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Barati, N., Momtazi-Borojeni, A. A., Majeed, M. & Sahebkar, A. Potential therapeutic effects of curcumin in gastric cancer. J. Cell. Physiol.234, 2317–2328 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Kim, J. M. et al. Chemopreventive properties of genipin on AGS cell line via induction of JNK/Nrf2/ARE signaling pathway. J. Biochem. Mol. Toxicol.30, 45–54 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Xia, Y. et al. Chrysin inhibits cell invasion by inhibition of Recepteur d’origine Nantais via suppressing early growth response-1 and NF-kappaB transcription factor activities in gastric cancer cells. Int. J. Oncol.46, 1835–1843 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Jaganathan, S. K. & Supriyanto, E. Antiproliferative and molecular mechanism of eugenol-induced apoptosis in cancer cells. Molecules17, 6290–6304 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herman-Antosiewicz, A., Xiao, H., Lew, K. L. & Singh, S. V. Induction of p21 protein protects against sulforaphane-induced mitotic arrest in LNCaP human prostate cancer cell line. Mol. Cancer Ther.6, 1673–1681 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Mielczarek, L. et al. In the triple-negative breast cancer MDA-MB-231 cell line, sulforaphane enhances the intracellular accumulation and anticancer action of doxorubicin encapsulated in liposomes. Int. J. Pharm.558, 311–318 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Milczarek, M. et al. In vitro evaluation of sulforaphane and a natural analog as potent inducers of 5-fluorouracil anticancer activity. Molecules 23, 3040 (2018). [DOI] [PMC free article] [PubMed]
- 22.Hac, A. et al. Mechanism of selective anticancer activity of isothiocyanates relies on differences in DNA damage repair between cancer and healthy cells. Eur. J. Nutr. 5, 1421–1432 (2020). [DOI] [PMC free article] [PubMed]
- 23.Xia, Y., Kang, T. W., Jung, Y. D., Zhang, C. & Lian, S. Sulforaphane inhibits nonmuscle invasive bladder cancer cells proliferation through suppression of HIF-1alpha-Mediated glycolysis in hypoxia. J. Agric. Food Chem.67, 7844–7854 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Ge, M. et al. Sulforaphane inhibits gastric cancer stem cells via suppressing sonic hedgehog pathway. Int. J. Food Sci. Nutr.70, 570–578 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Kiani, S. et al. Purified sulforaphane from broccoli (Brassica oleracea var. italica) leads to alterations of CDX1 and CDX2 expression and changes in miR-9 and miR-326 levels in human gastric cancer cells. Gene678, 115–123 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Dong, Q. Q. et al. SMYD3-associated pathway is involved in the anti-tumor effects of sulforaphane on gastric carcinoma cells. Food Sci. Biotechnol.27, 1165–1173 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi, Y. H. ROS-mediated activation of AMPK plays a critical role in sulforaphane-induced apoptosis and mitotic arrest in AGS human gastric cancer cells. Gen. Physiol. Biophys.37, 129–140 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Kerr, C., Adhikary, G., Grun, D., George, N. & Eckert, R. L. Combination cisplatin and sulforaphane treatment reduces proliferation, invasion, and tumor formation in epidermal squamous cell carcinoma. Mol. Carcinog.57, 3–11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pappa, G., Strathmann, J., Lowinger, M., Bartsch, H. & Gerhauser, C. Quantitative combination effects between sulforaphane and 3,3′-diindolylmethane on proliferation of human colon cancer cells in vitro. Carcinogenesis28, 1471–1477 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Han, Z., Xu, Q., Li, C. & Zhao, H. Effects of sulforaphane on neural stem cell proliferation and differentiation. Genesis 55, e2322 (2017). [DOI] [PubMed]
- 31.Li, X. et al. Sulforaphane promotes apoptosis, and inhibits proliferation and self-renewal of nasopharyngeal cancer cells by targeting STAT signal through miRNA-124-3p. Biomed. Pharmacother. Biomed. Pharmacother.103, 473–481 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Bonay, M. et al. Caspase-independent apoptosis in infected macrophages triggered by sulforaphane via Nrf2/p38 signaling pathways. Cell Death Discov.1, 15022 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka, T. & Iino, M. Knockdown of Sec8 promotes cell-cycle arrest at G1/S phase by inducing p21 via control of FOXO proteins. FEBS J.281, 1068–1084 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Doan, P. et al. Alkylaminophenol induces G1/S phase cell cycle arrest in glioblastoma cells through p53 and cyclin-dependent kinase signaling pathway. Front. Pharmacol.10, 330 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mai, W., Liu, H., Chen, H., Zhou, Y. & Chen, Y. RGNNV-induced cell cycle arrest at G1/S phase enhanced viral replication via p53-dependent pathway in GS cells. Virus Res.256, 142–152 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu, J. W., Sun, P., Zhang, D. X., Xiong, W. J. & Mi, J. Hexokinase 2 regulates G1/S checkpoint through CDK2 in cancer-associated fibroblasts. Cell. Signal.26, 2210–2216 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Zhang, D. X. et al. Potent inhibition of human gastric cancer by HER2-directed induction of apoptosis with anti-HER2 antibody and caspase-3 fusion protein. Gut59, 292–299 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Rukoyatkina, N. et al. Protein kinase A activation by the anti-cancer drugs ABT-737 and thymoquinone is caspase-3-dependent and correlates with platelet inhibition and apoptosis. Cell Death Disease8, e2898 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hou, D. et al. Suppression of AURKA alleviates p27 inhibition on Bax cleavage and induces more intensive apoptosis in gastric cancer. Cell Death Disease9, 781 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morsi, H. M. et al. Apoptosis, bcl-2 expression, and proliferation in benign and malignant endometrial epithelium: An approach using multiparameter flow cytometry. Gynecol. Oncol.77, 11–17 (2000). [DOI] [PubMed] [Google Scholar]
- 41.Abbasi, B. A. et al. Potential phytochemicals in the prevention and treatment of esophagus cancer: A green therapeutic approach. Pharmacol. Rep.71, 644–652 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Qamar, H., Rehman, S. & Chauhan, D. K. Current status and future perspective for research on medicinal plants with anticancerous activity and minimum cytotoxic value. Curr. Drug Targets20, 1227–1243 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Chatterjee, S., Rhee, Y. H. & Ahn, J. C. Sulforaphene-carboplatin combination synergistically enhances apoptosis by disruption of mitochondrial membrane potential and cell cycle arrest in human non-small cell lung carcinoma. J. Med. Food19, 860–869 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Shang, H. S. et al. Sulforaphane-induced apoptosis in human leukemia HL-60 cells through extrinsic and intrinsic signal pathways and altering associated genes expression assayed by cDNA microarray. Environ. Toxicol.32, 311–328 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Royston, K. J., Paul, B., Nozell, S., Rajbhandari, R. & Tollefsbol, T. O. Withaferin A and sulforaphane regulate breast cancer cell cycle progression through epigenetic mechanisms. Exp. Cell Res.368, 67–74 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kntayya, S. B. et al. Induction of apoptosis and cytotoxicity by isothiocyanate sulforaphene in human hepatocarcinoma HepG2 cells. Nutrients10, 717 (2018). [DOI] [PMC free article] [PubMed]
- 47.Arcidiacono, P. et al. Antitumor activity and expression profiles of genes induced by sulforaphane in human melanoma cells. Eur. J. Nutr.57, 2547–2569 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juengel, E. et al. Sulforaphane as an adjunctive to everolimus counteracts everolimus resistance in renal cancer cell lines. Phytomed. Int. J. Phytother. Phytopharmacol.27, 1–7 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Kang, M. et al. Theracurmin efficiently inhibits the growth of human prostate and bladder cancer cells via induction of apoptotic cell death and cell cycle arrest. Oncol. Rep.35, 1463–1472 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Ma, D. & Gk, S. Development of cell-cycle inhibitors for cancer therapy. Curr. Oncol.16, 36–43 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han, S. H., Chung, J. H., Kim, J., Kim, K. S. & Han, Y. S. New role of human ribosomal protein S3: Regulation of cell cycle via phosphorylation by cyclin-dependent kinase 2. Oncol. Lett.13, 3681–3687 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hydbring, P., Castell, A. & Larsson, L. G. MYC modulation around the CDK2/p27/SKP2 axis. Genes8, 174 (2017). [DOI] [PMC free article] [PubMed]
- 53.Chen, K. C. et al. Pemetrexed induces S-phase arrest and apoptosis via a deregulated activation of Akt signaling pathway. PLoS ONE9, e97888 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Oakes, V. et al. Cyclin A/Cdk2 regulates Cdh1 and claspin during late S/G2 phase of the cell cycle. Cell Cycle13, 3302–3311 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Godar, S. et al. Growth-inhibitory and tumor-suppressive functions of p53 depend on its repression of CD44 expression. Cell134, 62–73 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El-Deiry, W. S. et al. WAF1, a potential mediator of p53 tumor suppression. Cell75, 817–825 (1993). [DOI] [PubMed] [Google Scholar]
- 57.Overton, K. W., Spencer, S. L., Noderer, W. L., Meyer, T. & Wang, C. L. Basal p21 controls population heterogeneity in cycling and quiescent cell cycle states. Proc. Natl. Acad. Sci. USA111, E4386–E4393 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol.2007, 495–516 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Igney, F. H. & Krammer, P. H. Death and anti-death: Tumour resistance to apoptosis. Nat. Rev. Cancer2, 277–288 (2002). [DOI] [PubMed] [Google Scholar]
- 60.Tsujimoto, Y. Bcl-2 family of proteins: Life-or-death switch in mitochondria. Biosci. Rep.2002, 47–58 (2002). [DOI] [PubMed] [Google Scholar]
- 61.Zou, X., Qu, Z., Fang, Y., Shi, X. & Ji, Y. Endoplasmic reticulum stress mediates sulforaphane-induced apoptosis of HepG2 human hepatocellular carcinoma cells. Mol. Med. Rep.15, 331–338 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Hudecova, S. et al. Sulforaphane-induced apoptosis involves the type 1 IP3 receptor. Oncotarget7, 61403 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nusse, R. et al. Wnt signaling and stem cell control. Cold Spring Harb. Symp. Quant. Biol.73, 59–66 (2008). [DOI] [PubMed] [Google Scholar]
- 64.Rudel, T. Caspase inhibitors in prevention of apoptosis. Herz1999, 3 (1999). [DOI] [PubMed] [Google Scholar]
- 65.Speidel, D. The role of DNA damage responses in p53 biology. Arch. Toxicol.89, 501–517 (2015). [DOI] [PubMed] [Google Scholar]
- 66.Chen, J. The cell-cycle arrest and apoptotic functions of p53 in tumor initiation and progression. Cold Spring Harbor Perspect. Med.6, a026104 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data during this study are included in the article.