Abstract
This study aimed to clarify the effects of the interruption of cardiac rehabilitation (CR) and refraining from going outside due to the COVID-19 pandemic on hemodynamic response and rating of perceived exertion (RPE) during exercise including differences by age in phase 2 CR outpatients. Among 76 outpatients participating in consecutive phase 2 CR in both periods from March to April and June to July 2020, which were before and after CR interruption, respectively, at Sanda City Hospital were enrolled. The inclusion criterion was outpatients whose CR was interrupted due to COVID-19. We compared the data of hemodynamic response and RPE during exercise on the last day before interruption and the first day after interruption when aerobic exercise was performed at the same exercise intensity in the < 75 years group and ≥ 75 years group. Fifty-three patients were enrolled in the final analysis. Post-CR interruption, peak heart rate increased significantly (p = 0.009) in the < 75 years group, whereas in the ≥ 75 years group, weight and body mass index decreased significantly (p = 0.009, 0.011, respectively) and Borg scale scores for both dyspnea and lower extremities fatigue worsened significantly (both, p < 0.001). CR interruption and refraining from going outside due to the COVID-19 pandemic affected the hemodynamic response, RPE during exercise and body weight in phase 2 CR outpatients. In particular, patients aged ≥ 75 years appeared to be placed at an increased risk of frailty.
Keywords: COVID-19, Phase 2 cardiac rehabilitation patient, Hemodynamic response, Rating of perceived exertion
Introduction
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may increase mortality in affected patients with cardiovascular disease (CVD) [1]. In addition, because it spreads from person to person via close contact, the delivery of cardiac rehabilitation (CR) is currently being hampered in many countries due to the COVID-19 pandemic [2, 3].
The Japanese Association of Cardiac Rehabilitation announced guidelines to consider discontinuing phase 2 outpatient CR, particularly group exercise therapy, in endemic areas of COVID-19 in Japan. Outpatient phase 2 CR was actually interrupted in many areas, especially during the April–May state-of-emergency declaration. Furthermore, the government requested citizens to refrain from going outside. It is thus expected that physical activity performed during phase 2 CR has decreased drastically. The problem of decreased physical activity due to COVID-19 is a global concern [4–7]. In fact, the introduction of a quarantine due to COVID-19 had a detrimental effect on the level of habitual physical activity performed by heart failure patients [8]. Reduced physical activity significantly lowers peak oxygen uptake (VO2) [9], which is the gold standard indicator of cardiorespiratory fitness, and its decrease indicates a poor prognosis in patients with CVD [10, 11]. As well, the effects of COVID-19, such as weight gain, also increase the risk of heart disease [12]. As a result of staying at home, physical activity and exercise levels drastically decline while dietary habits remain unchanged or fail to offset this inactivity, thus producing a positive energy balance [13]. In fact, it was observed in northern Italy that obese outpatients gained significant weight 1 month after the beginning of the lockdown period there [14]. However, as it has also been reported that prolonged sedentary bouts were associated with worse frailty in CVD patients, it is possible that CVD patients may lose weight [15].
Thus, there is concern that interruption of CR or refraining from going outside may adversely affect the physical and cardiopulmonary function of outpatients participating in phase 2 CR. However, what effect it would have on hemodynamic response during exercise is unknown. It is important to clarify the effects of the interruption of phase 2 CR and refraining from going outside on patients and to predict their condition at the resumption of CR and use this knowledge for exercise therapy and patient education. Above all, it is also useful as a measure against a recurrence of the pandemic.
Therefore, in this study, we hypothesized that the physical function of phase 2 CR patients would decline due to the pandemic of COVID-19. However, the change in these functions might differ depending on age and should be considered separately in patients < 75 years old and those ≥ 75 years old [16]. Hence, the aim of this study was to clarify the effects of the COVID-19 pandemic on hemodynamic response, rating of perceived exertion (RPE) during exercise and body weight including differences by age in outpatients in whom phase 2 CR was interrupted due to the pandemic.
Methods
Study design and patients
This was a retrospective, single-center, observational study. Seventy-six outpatients who participated in consecutive phase 2 CR in the two periods from March to April and June to July 2020, which were before and after interruption, respectively, of CR at Sanda City Hospital were enrolled. The inclusion criterion was outpatients whose CR was interrupted due to COVID-19, and the exclusion criteria were patients with a change in exercise intensity and medications or who experienced cardiovascular events during the period of interruption. We compared the data obtained on the last day before interruption of CR with that obtained on the first day after resumption of CR when aerobic exercise was performed at the same exercise intensity. Patients’ characteristics and clinical parameters including age, sex, body mass index, left ventricular ejection fraction, medical history, medications, CR interruption period, exercise intensity and parameters of usual aerobic exercise in CR were obtained from the electronic medical records by two physical therapists. A nurse questioned each of the patients to determine if they had decreased their level of exercise during the interruption of CR. The present study complied with the Declaration of Helsinki with respect to investigation in humans and was approved by the Ethics Committee of Sanda City Hospital (approval no. 2020006). Written informed consent was obtained from each patient.
CR exercise program
Aerobic exercise was performed based on the Guidelines for Rehabilitation in Patients with Cardiovascular Disease (JCS 2012) [17]. Exercise intensity is prescribed according to exercise at anaerobic threshold level, 40–60% of the peak V̇O2, 40–60% of maximum heart rate (HR) or a Borg scale score of 12–13 or resting HR + 30 bpm (resting HR + 20 bpm for patients receiving β-blockers). Some patients underwent high-intensity interval training at 85% peak HR. Aerobic exercise was performed on a cycle ergometer or uphill treadmill, and the exercise time was 20 min. All patients exercised while wearing a surgical or cloth mask.
Hemodynamic assessment during CR
The variables chosen for monitoring were HR and systolic blood pressure (SBP) as indicators of hemodynamic response. We measured the resting (Rest) and peak (Peak) HRs. For SBP, we measured the resting (Rest) SBP and the immediate post-exercise (Post) SBP. HR (Rest) was measured from lead II of a 3-lead electrocardiogram recorded using the limb lead method during exercise. All SBPs were measured with the patient in the sitting position. These measurements were obtained by one physical therapist and two nurses.
Rating of perceived exertion (RPE) assessment during CR
The RPEs for dyspnea and lower extremity fatigue were assessed using the Borg scale score [18] 10 min after the start of exercise and just before cooldown. The Borg scale score has been suggested to be a valid tool for monitoring and prescribing exercise intensity independent of sex, age, exercise modality, physical activity level and coronary artery disease status [19]. The same assessment panel was used for all patients to ensure minimal interindividual variability.
Statistical analysis
Continuous variables are expressed as medians and interquartile ranges, categorical variables are expressed as percentages (numbers). Continuous variables were compared using the Mann–Whitney U test and categorical data were compared using the χ2 test or the Fisher exact test, as appropriate. The Wilcoxon rank sum test was used to compare pre- and post-CR interruption. A p value of < 0.05 was considered to indicate statistical significance. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics.
Results
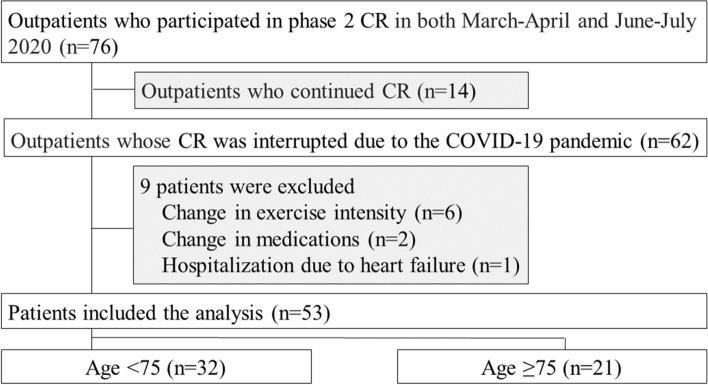
Seventy-six outpatients participated in phase 2 CR in the two periods from March to April and June to July 2020. Of them, 62 patients with interruption of CR were enrolled, with the exception of 14 patients who continued CR in individual 1:1 CR sessions. Patients with a change in exercise intensity (n = 6) or medications (n = 2) and patients admitted to hospital due to heart failure (n = 1) were excluded. Thus, 53 patients were included in the final analysis (Fig. 1). Table 1 presents their clinical characteristics. There were no significant differences between the patients in the < 75 years group and the ≥ 75 years group in sex, left ventricular ejection fraction, medical history, medications, CR interruption period, decreased exercise during CR interruption and exercise intensity at CR. Body weight, hemodynamics and RPE pre- and post-CR interruption can be compared in Table 2. Among the hemodynamic variables measured post-CR interruption, HR (Peak) showed significant increases (p = 0.001) in all patients. The peak Borg scale scores for both dyspnea and lower extremity fatigue also showed significant increases (p = 0.001, 0.003, respectively). Following post-CR interruption, the < 75 years group experienced a significant increase in HR (Peak) (p = 0.009), whereas the ≥ 75 years group experienced significant decreases in weight (p = 0.009) and BMI (p = 0.011) and worsening of the Borg scale scores for both dyspnea and lower extremities fatigue (both, p < 0.001).
Fig. 1.
Patient flow through the study. CR: cardiac rehabilitation; COVID-19: Coronavirus disease 2019
Table 1.
Clinical characteristics of the patients
| All patients, n = 53 | Age < 75 years, n = 32 | Age ≥ 75 years, n = 21 | χ2 value | p value | |
|---|---|---|---|---|---|
| Age (years) | 73 (67–76) | 68 (61–72) | 76 (76–80) | < 0.001 | |
| Male, n (%) | 41 (77.4) | 23 (71.9) | 18 (85.7) | 0.323 | |
| LVEF (%) | 58.6 (46.9–65.6) | 58.9 (48.6–65.6) | 56.1 (45.2–65.5) | 0.547 | |
| Medical history | |||||
| Previous MI, n (%) | 24 (45.3) | 16 (50.0) | 8 (38.1) | 0.324 | 0.596 |
| Angina pectoris, n (%) | 13 (24.5) | 6 (18.8) | 7 (33.3) | 0.775 | 0.379 |
| CHF, n (%) | 23 (43.4) | 15 (46.9) | 8 (38.1) | 0.121 | 0.728 |
| Hypertension, n (%) | 35 (66.0) | 23 (71.9) | 12 (57.1) | 0.658 | 0.417 |
| Diabetes mellitus, n (%) | 27 (50.9) | 18 (56.2) | 9 (42.9) | 0.453 | 0.501 |
| Medications | |||||
| Beta blocker, n (%) | 42 (79.3) | 28 (87.5) | 14 (66.7) | 0.090 | |
| ACE-I/ARB, n (%) | 36 (67.3) | 25 (78.1) | 11 (52.4) | 2.766 | 0.101 |
| CCB, n (%) | 20 (37.7) | 13 (40.6) | 7 (33.3) | 0.060 | 0.806 |
| Diuretic, n (%) | 22 (41.5) | 14 (43.8) | 8 (38.1) | 0.015 | 0.902 |
| Period of CR interruption (days) | 77 (63–92) | 70 (59.6–98) | 84 (70.0–91) | 0.334 | |
| Decreased exercise during CR interruption, n (%) | 46 (86.8) | 27 (84.4) | 19 (90.5) | 0.690 | |
| Exercise intensity (watts)a | 50 (40–60) | 50 (40–60) | 50 (40–60) | 0.869 |
Values shown are n (%), medians (interquartile ranges)
ACE-I angiotensin-converting enzyme inhibitor, ARB angiotensin II receptor blocker, CCB calcium channel blocker, CHF congestive heart failure, CR cardiac rehabilitation, LVEF left ventricular ejection fraction, MI myocardial infarction
aOnly cycle ergometer (n = 49)
Table 2.
Patients’ body weight, hemodynamics and RPE pre- and post-CR interruption
| All patients, n = 53 | Age < 75 years, n = 32 | Age ≥ 75 years, n = 21 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-interruption | Post-interruption | p value | Pre-interruption | Post-interruption | p value | Pre-interruption | Post-interruption | p value | |
| Body weight (kg) | 65.1 (58.6–71.4) | 65.3 (57.9–71.5) | 0.359 | 67.2 (59.8–74.0) | 66.6 (59.2–73.3) | 0.254 | 61.7 (57.4–69.4) | 60.4 (56.0–67.9) | 0.009 |
| BMI (kg/m2) | 23.4 (22.1–25.5) | 23.4 (21.6–25.4) | 0.239 | 23.7 (21.6–26.4) | 23.8 (22.5–26.4) | 0.367 | 23.3 (22.1–24.9) | 22.9 (22.2–24.4) | 0.011 |
| Rest HR (bpm) | 64 (61–71) | 67 (60–76) | 0.114 | 64 (58.8–70.3) | 65.5 (58.8–75.3) | 0.143 | 64 (62.0–72.0) | 71.0 (60.0–77.0) | 0.324 |
| Peak HR (bpm) | 101 (91–109) | 104 (92–112) | 0.001 | 101.5 (90.8–109.3) | 104.5 (90–112) | 0.009 | 100.0 (92.0–108.0) | 103.0 (95–110) | 0.052 |
| Rest SBP (mmHg) | 130 (116–140) | 126 (113–143) | 0.690 | 131 (121.5–140) | 124.5 (112.5–143) | 0.281 | 128 (115.0–137) | 131.0 (115.0–147) | 0.538 |
| Post-exercise SBP (mmHg) | 127 (110–139) | 125 (114–142) | 0.495 | 121.5 (105.8–145.0) | 124.5 (112.8–142) | 0.104 | 128.0 (112.0–145.0) | 126.0 (116.0–137) | 0.554 |
| Peak Borg scale: dyspnea | 12 (12–13) | 12.5 (12–13) | 0.001 | 12 (12.0–13) | 12 (12.0–13) | 0.260 | 12 (11.5–12) | 13 (12.5–13) | < 0.001 |
| Peak Borg scale: lower extremities | 12 (12–13) | 12.5 (12–13) | 0.003 | 12 (12–13) | 12.5 (12.0–13) | 0.440 | 12 (12–12) | 13.0 (12.5–13) | < 0.001 |
CR cardiac rehabilitation; BMI body mass index; HR heart rate; RPE rating of perceived exertion; SBP systolic blood pressure. Values shown are medians (interquartile ranges)
Discussion
Interruption of CR and refraining from going outside due to the COVID-19 pandemic affected the hemodynamic response, rating of perceived exertion during exercise and body weight in outpatients who were participating in phase 2 CR. Further, the impact of these findings was different between the group aged < 75 and that ≥ 75 years of age. The results in all patients showed that post-CR interruption, HR increased and Borg scale score worsened when assessed at the same intensity of exercise as that performed pre-CR interruption. However, age-specific examinations revealed that the findings in the outpatients under 75 years old and those over 75 years old were completely different.
Only HR (Peak) changed in the group under 75 years of age, whereas weight loss and worsening of RPE were observed in the group over 75 years old. The most likely cause of these changes are the decreases in exercise and physical activity. There have been reports of decreased physical activity due to the COVID-19 pandemic [5–7, 20]; and in the present study as well, 86.8% of patients responded that the amount of exercise they performed was reduced. A decrease in physical activity has various adverse effects. Cessation of regular exercise rapidly abolished all training adaptations for cardiorespiratory fitness and cardiometabolic health within one month after cessation [21]. Even if exercise is not completely interrupted, reductions of more than one- to two-thirds in training frequency and/or duration reduce exercise capacity. Furthermore, despite maintaining training frequency and the duration of exercise, a decrease of one- to two-thirds in exercise training intensity reduces both V̇O2max and submaximal endurance time [22]. There are several reports of an increase in HR occurring at the same exercise intensity after exercise interruption in subjects < 75 years of age. Increases in HR that occur with tapering, reduced training and complete cessation of training may reflexively arise to counteract the reduction in ventricular filling and maximal stroke volume that occurs when the volume of blood decreases [22, 23]. However, there was no significant increase in HR (Peak) in our ≥ 75 years group. In this regard, the decline in β-receptor density with age may be due to a slowing heart rate response at moderate exercise intensity with low catecholamine levels [24].
In contrast, the ≥ 75 years group experienced weight loss and deterioration of the Borg scale score at the same exercise intensity, suggesting concern that these are indications of an increased risk of frailty. Weight loss is a typical characteristic of frailty [25], and one of the causes of weight loss is a decrease in skeletal muscle mass [26]. Physical inactivity and a sedentary life lead to specific alterations in the skeletal muscle that contribute to insulin resistance, impairment of oxidative function and rapid hypotrophy [27]. These changes in the muscle pathophysiology usually take place after a couple of weeks, but they can also occur more quickly in older people [27]. The changes in the Borg scale score in the ≥ 75 years group was greater than the value reported as the minimally clinically important difference in both dyspnea and lower extremity fatigue [28]. The worsening of the Borg Scale score for lower extremity fatigue may reflect this decrease in skeletal muscle mass and may also indicate a decrease in skeletal muscle metabolism [29]. Furthermore, as the Borg scale score is useful as a marker of impending decline in mobility-intact older adults [30], and it decrease is a serious issue with regard to the prognosis of patients with cardiac disease. The ≥ 75 years group had performed independent activities of daily living and was able to exercise at 50 W, the same level as that in the < 75 years group. The percentage of the outpatients who reported that their physical activity decreased was also not significantly different from that of the < 75 years group. Nonetheless, the factors leading to this result were the functional reserve capacity of the heart and skeletal muscle decline with age [31]. Several studies have proved that the losses in strength and function due to detraining are higher in the elderly than in younger people [27, 32].
Currently, a system to provide phase 2 CR during the COVID-19 pandemic is under consideration [2, 3]. The present study suggested that more careful intervention is needed with a focus on the decline of physical function, especially in patients aged 75 years and older.
Study limitations
Several limitations of this study need to be acknowledged. First, this was a single-center, retrospective study comprising a very small number of patients. Second, detailed physical activity and exercise during CR interruption were not investigated. As we were not able to actually measure skeletal muscle mass and VO2, quantitative evaluation will be required in a future study. Third, Table 1 shows that the 77.4% of patients were men and the authors found no difference between men and women in the present study. However, it is well known that women were less likely to perform physical activity and that sarcopenia strongly affected postmenopausal women [33]. Thus, gender-related differences, it needs to do more research work in the future trial. Finally, the parameters obtained from usual exercise therapy (HR and SBP) may reduce the overall quality of the present assessment. However, in the current situation where exercise tests in hospitals are difficult to perform due to the COVID-19 pandemic, these parameters measured during usual exercise may be useful as the easiest and safest indicators that can be obtained.
Conclusion
Interruption of CR and refraining from going outside due to the COVID-19 pandemic affected the hemodynamic response, rating of perceived exertion during exercise and body weight in outpatients participating in phase 2 CR. The effect was particularly large on patients aged 75 and over and appeared to place these patients at an increased risk of frailty.
Acknowledgements
The authors would like to thank all of the participating patients in Sanda City Hospital. We thank the staff members of Kobe University who collaborated in this study. This study was also benefitted by the support and encouragement of Sae Ono of the Faculty of Health Sciences, Kobe University, and Hiroto Ogi, Kodai Ishihara, Masahiro Kitamura, Yuji Kanejima, Masato Ogawa, and Shinichi Shimada, all of the Graduate School of Health Sciences, Kobe University. We also thank Dr. Minato Nakazawa, Department of Public Health, Graduate School of Health Sciences, Kobe University, for statistical support of the present study.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferrari R, G. Pasquale GD, Rapezzi C, Commentary: what is the relationship between Covid-19 and cardiovascular disease? Int J Cardiol. 2020;310:167–168. doi: 10.1016/j.ijcard.2020.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Besnier F, Gayda M, Nigam A, Juneau M, Bherer L. Cardiac rehabilitation during quarantine in COVID-19 pandemic: challenges for center-based program. Arch Phys Med Rehabil. 2020;101(10):1835–1838. doi: 10.1016/j.apmr.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kemps HMC, Brouwers RWM, Cramer MJ, Jorstad HT, Kluiver EP, Kraaijenhagen RA, Kuijpers PMJC, Linde MR, Melker E, Rodrigo SF, Spee RF, Sunamura M, Vromen T, Wittekoek ME. Recommendations on how to provide cardiac rehabilitation services during the COVID-19 pandemic. Neth Heart J. 2020;28:387–390. doi: 10.1007/s12471-020-01474-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peçanha T, Gressler KF, Roschel H, Gualano B. Social isolation during the COVID-19 pandemic can increase physical inactivity and the global burden of cardiovascular disease. Am J Physiol Heart Circ Physiol. 2020;318:H1441–H1446. doi: 10.1152/ajpheart.00268.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall G, Laddu DR, Phillips SA, Lavie CJ, Arena R. A tale of two pandemics: how will COVID-19 and global trends in physical inactivity and sedentary behavior affect one another? Prog Cardiovasc Dis. 2020 doi: 10.1016/j.pcad.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tison GH, Avram R, Kuhar P, Abreau S, Marcus GM, Pletcher MJ, Olgin JE. Worldwide effect of COVID-19 on physical activity: a descriptive study. Ann Intern Med. 2020 doi: 10.7326/M20-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lippi G, Henry BM, Sanchis-Gomar F. Physical inactivity and cardiovascular disease at the time of coronavirus disease 2019 (COVID-19) Eur J Prev Cardiol. 2020;27:906–908. doi: 10.1177/2047487320916823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vetrovsky T, Frybova T, Gant I, Semerad M, Cimler R, Bunc V, Siranec M, Miklikova M, Vesely J, Griva M, Precek J, Pelouch R, Parenica J, Belohlavek J. The detrimental effect of COVID-19 nationwide quarantine on accelerometer-assessed physical activity of heart failure patients. ESC Heart Fail. 2020;7(5):2093–2097. doi: 10.1002/ehf2.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowden Davies KA, Sprung VS, Norman JA, Thompson A, Mitchell KL, Halford JCG, Harrold JA, Wilding JPH, Kemp GJ, Cuthbertson DJ. Short-term decreased physical activity with increased sedentary behavior causes metabolic derangements and altered body composition: effects in individuals with and without a first-degree relative with type 2 diabetes. Diabetologia. 2018;61:1282–1294. doi: 10.1007/s00125-018-4603-5. [DOI] [PubMed] [Google Scholar]
- 10.Arena R, Myers J, Abella J, Pinkstaff S, Brubaker P, Kitzman PDW, Peberdy MA, Bensimhon D, Chase P, Guazzi M. Cardiopulmonary exercise testing is equally prognostic in young, middle-aged and older individuals diagnosed with heart failure. Int J Cardiol. 2011;151:278–283. doi: 10.1016/j.ijcard.2010.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tashiro H, Tanaka A, Ishii H, Motomura N, Arai K, Adachi T, Okajima T, Iwakawa N, Kojima H, Mitsuda T, Hirayama K, Hitota Y, Hayashi M, Furusawa K, Yoshida R, Imai H, Ogawa Y, Kawaguchi K, Murohara T. Reduced exercise capacity and clinical outcomes following acute myocardial infarction. Heart Vessels. 2020;35:1044–1050. doi: 10.1007/s00380-020-01576-2. [DOI] [PubMed] [Google Scholar]
- 12.Mattioli AV, Puviani MB, Nasi M, Farinetti A. COVID-19 pandemic; the effects of quarantine on cardiovascular risk. Eur J Clin Nutr. 2020;74:852–855. doi: 10.1038/s41430-020-0646-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Ferran M, Guía-Galipienso F, Sanchis-Gomar F, Pareja-Galeano H. Metabolic impacts of confinement during the COVID-19 pandemic due to modified diet and physical activity habits. Nutrients. 2020;12:1549. doi: 10.3390/nu12061549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pellegrini M, Ponzo V, Rosato R, Scumaci E, Goitre I, Benso A, Belcastro S, Crespi C, Michieli FD, Ghigo E, Broglio F, Bo S. Changes in weight and nutritional habits in adults with obesity during the “Lockdown” period caused by the COVID19 virus emergency. Nutrients. 2020;12(7):2016. doi: 10.3390/nu12072016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kehler DS, Clara I, Hiebert B, Stammers AN, Hay J, Schultz A, Arora RC, Tangri N, Duhamel TA. The association between patterns of physical activity and sedentary time with frailty in relation to cardiovascular disease. Aging Med (Milton) 2019;2:18–26. doi: 10.1002/agm2.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouchi Y, Rakugi H, Arai H, Akishiro M, Ito H, Toba K, Kai I. Redefining the elderly as aged 75 years and older: proposal from the joint committee of Japan gerontological society and the Japan geriatrics society. Geriatr Gerontol Int. 2017;17:1045–1047. doi: 10.1111/ggi.13118. [DOI] [PubMed] [Google Scholar]
- 17.JCS Joint Working Group Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012) Circ J. 2014;78:2022–2093. doi: 10.1253/circj.CJ-66-0094. [DOI] [PubMed] [Google Scholar]
- 18.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 19.Scherr J, Wolfarth B, Christle JW, Pressler A, Wagenpfeil S, Halle M. Associations between Borg’s rating of perceived exertion and physiological measures of exercise intensity. Eur J Appl Physiol. 2013;113:147–155. doi: 10.1007/s00421-012-2421-x. [DOI] [PubMed] [Google Scholar]
- 20.Hamer M, Kivimäki M, Gale CR, Batty GD. Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: a community-based cohort study of 387,109 adults in UK. Brain Behav Immun. 2020;87:184–187. doi: 10.1016/j.bbi.2020.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nolan PB, Keeling SM, Robitaille CA, Buchanan CA, Dalleck LC. The effect of detraining after a period of training on cardiometabolic health in previously sedentary individuals. Int J Environ Res Public Health. 2018;15:2303. doi: 10.3390/ijerph15102303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neufer PD. The effect of detraining and reduced training on the physiological adaptations to aerobic exercise training. Sports Med. 1989;8:302–320. doi: 10.2165/00007256-198908050-00004. [DOI] [PubMed] [Google Scholar]
- 23.Zavorsky GS. Evidence and possible mechanisms of altered maximum heart rate with endurance training and tapering. Sports Med. 2000;29:13–26. doi: 10.2165/00007256-200029010-00002. [DOI] [PubMed] [Google Scholar]
- 24.Birnbaumer P, Traninger H, Borenich A, Falgenhauer M, Modre-Osprian R, Harpf H. Heart rate performance curve is dependent on age, sex, and performance. Front Public Health. 2020;8:98. doi: 10.3389/fpubh.2020.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bortz WM., 2nd A conceptual framework of frailty: a review. J Gerontol A Biol Sci Med Sci. 2002;57:M283–M288. doi: 10.1093/gerona/57.5.M283. [DOI] [PubMed] [Google Scholar]
- 26.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 27.Stefano VD, Battaglia G, Giustino V, Gagliardo A, D’Aleo M, Giannini O, Palma A, Brighina F. Significant reduction of physical activity in patients with neuromuscular disease during COVID-19 pandemic: the long-term consequences of quarantine. J Neurol. 2020 doi: 10.7326/M20-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khair RM, Nwaneri C, Damico RL, Kolb T, Hassoun PM, Mathai SC. The minimal important difference in Borg dyspnea score in pulmonary arterial hypertension. Ann Am Thorac Soc. 2016;13:842–849. doi: 10.1513/AnnalsATS.201512-824OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stendardi L, Grazzini M, Gigliotti F, Lotti P, Scano G. Dyspnea and leg effort during exercise. Respir Med. 2005;99:933–942. doi: 10.1016/j.rmed.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Simonsick EM, Glynn NW, Jerome GJ, Shardell M, Schrack JA, Ferrucci L. Fatigued, but not frail: perceived fatigability as a marker of impending decline in mobility-intact older adults. J Am Geriatr Soc. 2016;64:1287–1292. doi: 10.1111/jgs.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldspink DF. Ageing and activity: their effects on the functional reserve capacities of the heart and vascular smooth and skeletal muscles. Ergonomics. 2005;48:1334–1351. doi: 10.1080/00140130500101247. [DOI] [PubMed] [Google Scholar]
- 32.Coswig VS, Barbalho M, Raiol R, Vecchio FBD, Ramirez-Campillo R, Gentil P. Effects of high vs moderate-intensity intermittent training on functionality, resting heart rate and blood pressure of elderly women. J Transl Med. 2020;18:88. doi: 10.1186/s12967-020-02261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sciomer S, MoscucciMaffei Gallina Mattioli FSSAV. Prevention of cardiovascular risk factors in women: the lifestyle paradox and stereotypes we need to defeat. Eur J Prev Cardiol. 2019;26:609–610. doi: 10.1177/2047487318810560. [DOI] [PubMed] [Google Scholar]