Highlights
-
•
SV2A variants are linked to epilepsy in animal studies and rare human reports.
-
•
Animal studies of SV2A loss of function support decreased efficacy of levetiracetam (LEV).
-
•
We describe a child with epilepsy and LEV-induced seizure worsening due to a de novo SV2A variant.
-
•
In patients with rare SV2A variants, LEV should not be used as an antiseizure medication.
-
•
More studies are needed to establish the spectrum of SV2A-related epilepsy and treatment regimen.
Keywords: SV2A, Levetiracetam, Epilepsy, Status epilepticus, Genetic
Abstract
SV2A encodes a neuronal synaptic vesicle glycoprotein essential for neurotransmitter release. Altered SV2A function leads to epilepsy in animal models, yet only two reports of human variants have linked SV2A to syndromic drug-resistant epileptic encephalopathies and epilepsy. SV2A is also the binding site for the commonly used antiseizure medication levetiracetam (LEV). However, information about how rare SV2A variants influence LEV response is lacking. Here, we report a two-year-old child with new-onset epilepsy found to have a de novo heterozygous rare variant in SV2A (NM_014849.5:c.1978G>A;p.Gly660Arg) who developed refractory status epilepticus after escalation of LEV treatment for initial baseline seizure control. This report provides additional evidence that monoallelic pathogenic SV2A variants cause epilepsy and that genetic variation in SV2A could lead to paradoxical seizure worsening when treated with LEV.
1. Introduction
The synaptic vesicle glycoprotein SV2A is essential for proper regulation of calcium-dependent synaptic vesicle release and is therefore necessary in regulating neurotransmission [1], [2], [3]. Furthermore, SV2A is the target of the widely used antiseizure medication (ASM) levetiracetam (LEV) [4], [5] and the only known synaptic vesicle target of an ASM. Mice and chickens with homozygous SV2A loss-of-function (LOF) have severe epilepsy and decreased lifespan [6], [7], and heterozygous SV2A LOF mice are ten times more likely to develop epilepsy compared to wildtype litter mates [6]. Additionally, both homozygous and heterozygous SV2A LOF mice show decreased neuronal response when treated with LEV [8]. However, the exact mechanism of this decreased response is unknown. In humans, only two families with epilepsy linked to SV2A variants have been reported. A homozygous SV2A variant (NM_014849.5:c1148G>A:p.Arg383Gln) was found in a patient with drug-resistant syndromic epileptic encephalopathy with microcephaly, developmental delay/intellectual disability, movement disorder, and growth retardation [9] (Table 1). More recently, a heterozygous SV2A variant (NM_014849:c1708C > T:p.Arg570Cys) was found in a patient and mother with epilepsy and poor treatment response to LEV [10]. Here, we report a two-year-old child with new-onset epilepsy and a de novo rare heterozygous variant in SV2A (NM_014849.5:c.1978G > A:p.Gly660Arg) who developed refractory status epilepticus (SE) after treatment with LEV.
Table 1.
Comparison of SV2A variant and clinical information.
CADD-Combined Annotation Dependent Depletion, GTC- generalized tonic-clonic seizure, hmz-homozygous, htz-heterozygous, DD-developmental delay, ID-intellectual disability.
2. Case report
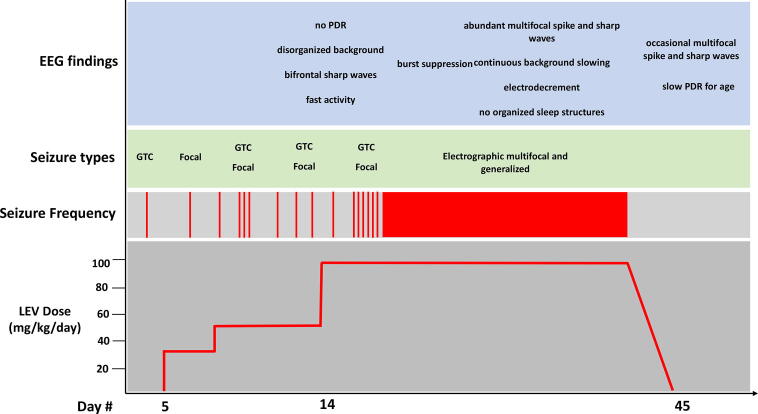
A two-year-old ambidextrous and developmentally appropriate female with two prior episodes of simple febrile seizures at one year of age was transferred to the pediatric intensive care unit (PICU) from an outside facility for difficult to control seizures. Two weeks prior to arrival the patient developed a first unprovoked seizure characterized as generalized tonic-clonic (GTC) movements without focality lasting one to two minutes (Fig. 1). Five days later she had a second unprovoked seizure described as irregular respirations, orolingual movements, and right arm flexion lasting one to two minutes. She was diagnosed with epilepsy and started on low dose LEV (27 mg/kg/day). The following week the patient had another GTC seizure and developed frequent seizure clusters following a sedated outpatient MRI brain. She was admitted to an outside hospital for treatment of SE, received midazolam (MDZ), started on valproic acid (VPA), and LEV was increased to 50 mg/kg/day. MDZ was weaned and the patient remained seizure free for 24 h. She subsequently required reinitiation of MDZ due to seizure recurrence, at which point she was transferred to our facility for higher level of care. MDZ was weaned, VPA and LEV continued, and clobazam (CBZ) initiated. Continuous EEG (cEEG) monitoring revealed a high amplitude disorganized background, bifrontal sharp waves, occipital spikes, and lack of posterior dominant rhythm but no seizures (Fig. 2A). The following day, LEV was maximized to 100 mg/kg/day with the goal of LEV monotherapy, and MDZ was discontinued. On day three of admission, the patient had a two-minute focal seizure. Lorazepam was administered and CBZ was increased given good response to MDZ. She was transferred to the inpatient service and remained seizure free for 24 h prior to developing frequent seizure clusters which evolved into drug-resistant SE requiring transfer back to the PICU. cEEG revealed electrographic SE (Fig. 2B) and MDZ infusion was restarted. Due to lack of response to MDZ, she required escalation to pentobarbital infusion. Burst suppression was achieved (Fig. 2C) and she remained suppressed for 48 h. MDZ and pentobarbital were weaned slowly over the next several days, but seizures recurred and EEG showed lateralized periodic epileptiform discharges and abundant multifocal epileptiform activity. The decision was made to continue MDZ infusion in preparation for in-hospital initiation of the ketogenic diet.
Fig. 1.
Timeline of the patient’s clinical course in response to levetiracetam treatment. LEV-levetiracetam, PDR-posterior dominant rhythm, GTC-generalized tonic clonic.
Fig. 2.
Sample EEG traces throughout the patient’s hospitalization. (A) EEG trace on day 17 showing occipital spikes. (B) EEG trace at the onset of refractory status epilepticus. (C) EEG trace of burst-suppression. (D) Awake EEG trace after levetiracetam treatment was discontinued.
Up until this point, the patient’s workup, including basic metabolic and CSF studies were unrevealing. Magnetic resonance imaging (MRI) of the brain showed increased T2/FLAIR hyperintensities in the bilateral hippocampi attributed to ongoing seizure activity (Fig. 3), and magnetic resonance spectroscopy was normal. Autoimmune antibody workup from CSF and serum were negative (Mayo Clinic Laboratories, Rochester, MN). Critical trio ES was performed to evaluate for a genetic etiology of explosive onset drug-resistant epilepsy. ES was negative for any variants in known epilepsy genes but resulted with a de novo heterozygous rare variant in SV2A (NM_014849.5:c.1978G>A:p.Gly660Arg, Fig. 4A). The affected amino acid residue is fully conserved across species, suggestive of evolutionary importance (Fig. 4B). Furthermore, the variants is rare with a gnomAD allele frequency of 3.979e-6, is predicted damaging by multiple in silico algorithms (MutationTaster: 0.81, disease-causing; SIFT: 0.01, damaging; PolyPhen: 0.93, damaging), and has a Combined Annotation Dependent Depletion (CADD) score of 32. With this information and a prior report of a patient with a rare heterozygous SV2A variant with worsening seizures while treated with LEV [10], LEV was discontinued. At the same time the ketogenic diet was started. Seizures stopped very quicky over the course of 48 h after LEV discontinuation (Fig. 2D). She was subsequently discharged on the ketogenic diet and VPA monotherapy due to sedative effects with CBZ. Her pre-hospital discharge EEG improved and showed occasional multiform epileptiform activity and slow posterior dominant rhythm for age. At her last neurology office visit at age three (one year after hospital discharge), she is doing well but had two breakthrough seizures in the setting of weaning the ketogenic diet at which time CBZ was restarted due to prior efficacy in seizure control.
Fig. 3.
Patient brain magnetic resonance imaging (A) Axial T2 FLAIR. (B) Coronal T2 FLAIR. Red arrows-bilateral hippocampal hyperintensity. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 4.
Patient pedigree and SV2A variant information. (A) Family pedigree showing variant information. Note parents are homozygous wildtype and patient has heterozygous de novo SV2A variant. (B) Conservation of the affected amino acid residue across species, (C) schematic of SV2A protein indicating genetic variants. Black variants- previously reported, red variant- this report, blue squares- transmembrane domains. Note that the variant reported here affects transmembrane domain 10.
3. Discussion
SV2A is a widely expressed synaptic vesicle glycoprotein important in neurotransmission. However, the precise role of SV2A in human disease remains to be elucidated, and thus far only two families linking SV2A to epilepsy have been reported [9], [10]. Here, we report a third child with new-onset epilepsy and paradoxical worsening of seizures resulting in drug-resistant SE after treatment with LEV who was found to have a rare de novo heterozygous variant in SV2A (NM_014849.5:c.1978G>A:p.Gly660Arg). This report provides further evidence linking SV2A to epilepsy in humans.
SV2A is expressed throughout the brain and functions in protein trafficking and neurotransmitter release at the synapse [2], [3], [11], [12]. More specifically, SV2A regulates the readily available synaptic vesicle pool and synaptic vesicle size [11], [13]. Physiologically, SV2A is involved in calcium-dependent neurotransmitter exocytosis and interacts with the synaptotagmin family of genes [3], [9], [11], [12], [13], [14], [15]. Moreover, SV2A is the target for the widely used ASM LEV and the only synaptic vesicle target of an ASM [4], [5], [16], [17], [18]. Studies in animal models using both mice and chickens have shown that SV2A LOF causes severe epilepsy and premature mortality [6], [7]. Homozygous SV2A LOF mice, although viable at birth, quickly develop severe seizures, growth retardation, and die within the first 3 weeks of life [6]. Heterozygous SV2A LOF mice are ten times more likely to develop seizures than wildtype litter mates and have a physiologically lower seizure threshold [6]. Therefore, SV2A is essential is normal brain function and both homozygous and heterozygous LOF results in epilepsy in model organisms. Furthermore, both complete and partial SV2A LOF animals show decreased responsiveness when treated with LEV [8], suggesting that LEV binding to its target site is impaired and that LEV is unable to exert its downstream effects. Downstream effects include inhibiting depolarization-induced neurotransmitter release and decreasing neuronal calcium levels by affecting high-voltage activated calcium channels [16], [17], [18].
Despite compelling studies of SV2A in animal models and in vitro studies, only two prior reports have linked SV2A to human disease. A homozygous SV2A variant (NM_014849.5:c.1148G>A:p.Arg383Gln) was reported in a patient with consanguineous parents. The patient’s phenotype comprised syndromic drug-resistant infantile-onset epileptic encephalopathy, microcephaly, developmental delay/intellectual disability, movement disorder, and optic atrophy [9] (Table 1). The patient’s epilepsy was unresponsive to treatment with LEV. In vitro functional studies in mouse hippocampal neurons expressing SV2A:p.Arg383Gln showed altered synaptotagmin expression and impaired trafficking as a potential mechanism of seizure activity [19]. Furthermore, a heterozygous SV2A variant (NM_014849.5:c.1708C>T:p.Arg570Cys) was identified in a child with infantile-onset myoclonic epilepsy and her less severely affected mother with epilepsy [10]. The patient’s seizures were unresponsive to treatment with LEV, and she developed an additional seizure type characterized by spasm clusters after treatment with LEV that completely resolved following LEV discontinuation.
The patient presented in this report is only the third case connecting a rare SV2A variant to human disease and the second heterozygous patient. The patient has a de novo heterozygous variant (NM_014849.5:c.1978G>A:p.Gly660Arg) in transmembrane domain 10 (Fig. 4C) that is predicted damaging based on multiple lines of evidence, including amino acid residue conservation, allele frequency, in silico prediction algorithms, and CADD score. Additionally, glycine to arginine substitutions in transmembrane domains are among the most common disease-causing mutations with implication on protein folding [20]. The patient’s phenotype varies from the other two reported patients in that she has a history of febrile seizures but did not develop epilepsy until age two and achieved early milestones appropriately. Her seizure types included GTCs and focal seizures compared to the other two patients who developed spasms and myoclonic seizures [9], [10] (Table 1). Furthermore, the response to LEV differed in that treatment with LEV paradoxically induced increased seizures in a dose-dependent manner that resulted in refractory SE requiring midazolam and pentobarbital infusions to induce burst suppression. Her seizures resolved and her EEG markedly improved with discontinuation of LEV and initiation of the ketogenic diet (Fig. 3D). By comparison, the first child with a heterozygous SV2A variant developed a new seizure type with initiation of LEV that completely resolved after LEV discontinuation [10]. The precise reason why the patient responded to initiation of the ketogenic diet, on which she was also discharged from the hospital, is unknown and likely a combination of factors not directly involved with SV2A. Nevertheless, she additionally responded to benzodiazepines, which should also be considered in patients with SV2A-related epilepsy.
The precise reason for this paradoxical seizure worsening in our patient is not understood. However, there are several possibilities. Given that complete SV2A LOF in animal models and humans cause a severe phenotype with intractable epilepsy [6], [9], LEV may inactivate the second functional allele in patients with a heterozygous SV2A LOF variant, temporarily mimicking a bilallelic LOF and thus resulting in severe paradoxical increase in seizure frequency and new seizure types. This paradoxical response appears fully reversible after LEV discontinuation. Another possibility is that LEV does not simply act as an antagonist of SV2A but that LEV binding to SV2A instead inhibits abnormal bursting in epileptogenic circuits, a function that when lost in LOF animals leads to severe epilepsy [5]. The severe seizure phenotype in SV2A LOF mice additionally supports the hypothesis that SV2A influences seizure generation and propagation. Alternatively, a third possibility is that LEV binding to dysfunctional mutant SV2A causes abnormal neuronal firing, leading to seizure generation. Further functional studies are needed to elucidate a potential mechanism.
Since the implementation of LEV as a widely used antiseizure medication effective for many seizure types in most epilepsy patients, it has been noted that a few select patients lack a response. To investigate this, Lynch et al. 2009 [21] studied the role of common SV2A single nucleotide polymorphisms with an allele frequency of 0.08 to 0.19 in the response to LEV treatment. While a statistically significant association between common SV2A variants and LEV response was not found, no conclusions about rare SV2A variants can be drawn from the design of this study. Additionally, no association between common SV2A variants and predisposition to epilepsy was found [21], [22], [23]. Importantly, the variant identified in our patient as well as the bi-allelic and heterozygous variants previously described are exceedingly rare with gnomAD allele frequencies of 3.979e-6, 2.39e-5, and 3.979e-6, respectively (Table 1). Taken together, these observations provide evidence that rare variants in SV2A variants are linked to certain genetically-based epilepsy syndromes.
4. Conclusion
We provide additional evidence for a role of SV2A in human disease by describing a rare heterozygous variant in association with epilepsy and paradoxical worsening of seizures when treated with LEV. Further cohort studies of patients with SV2A variants are needed to elucidate the spectrum of SV2A-related epilepsy. Additionally studies are needed to elucidate the impact of pathogenic SV2A variants on neuronal function and LEV response in order to determine optimal treatment regimens for patients with SV2A-related epilepsy.
SV2A ethical statement
Informed consent was obtained from the family of the patient described in this manuscript.
Conflict of interest
The authors have nothing to disclose.
References
- 1.Yao J., Nowack A., Kensel-Hammes P., Gardner R.G., Bajjalieh S.M. Cotrafficking of SV2 and synaptotagmin at the synapse. J Neurosci. 2010;30:5569–5578. doi: 10.1523/JNEUROSCI.4781-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feany M.B., Lee S., Edwards R.H., Buckley K.M. The synaptic vesicle protein SV2 is a novel type of transmembrane transporter. Cell. 1992;70:861–867. doi: 10.1016/0092-8674(92)90319-8. [DOI] [PubMed] [Google Scholar]
- 3.Nowack A., Yao J., Custer K.L., Bajjalieh S.M. SV2 regulates neurotransmitter release via multiple mechanisms. Am J Physiol Cell Physiol. 2010;299:C960–C967. doi: 10.1152/ajpcell.00259.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukuyama K., Tanahashi S., Nakagawa M. Levetiracetam inhibits neurotransmitter release associated with CICR. Neurosci Lett. 2012;518:69–74. doi: 10.1016/j.neulet.2012.03.056. [DOI] [PubMed] [Google Scholar]
- 5.Lynch B.A., Lambeng N., Nocka K. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. PNAS. 2004;101:9861–9866. doi: 10.1073/pnas.0308208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crowder K.M., Gunther J.M., Jones T.A. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A) PNAS. 1999;96:15268–15273. doi: 10.1073/pnas.96.26.15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douaud M., Feve K., Pituello F., Gourichon D., Boitard S., Leguern E. Epilepsy caused by an abnormal alternative splicing with dosage effect of the SV2A gene in a chicken model. PLoS ONE. 2011;6:e26932. doi: 10.1371/journal.pone.0026932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaminski R.M., Gillard M., Leclercq K. Proepileptic phenotype of SV2A-deficient mice is associated with reduced anticonvulsant efficacy of levetiracetam. Epilepsia. 2009;50:1729–1740. doi: 10.1111/j.1528-1167.2009.02089.x. [DOI] [PubMed] [Google Scholar]
- 9.Serajee F.J., Huq A.M. Homozygous mutation in synaptic vesicle glycoprotein 2A gene results in intractable epilepsy, involuntary movements, microcephaly, and developmental and growth retardation. Pediatr Neurol. 2015;52(6):642–646.e1. doi: 10.1016/j.pediatrneurol.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Wang D.i., Zhou Q., Ren L., Lin Y., Gao L., Du J. Levetiracetam induced a new seizure type in a girl with a novel SV2A gene mutation. Clin Neurol and Neurosurg. 2019;181:64–66. doi: 10.1016/j.clineuro.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Chang WP, Sudhof TC. SV2 renders primed synaptic vesicles competent for Ca2+-induced exocytosis. 2009. 29:883-897. [DOI] [PMC free article] [PubMed]
- 12.Bajjalieh S.M., Peterson K., Linial M., Scheller R.H. Brain contains two forms of synaptic vesicle protein 2. PNAS. 1993;90:2150–2154. doi: 10.1073/pnas.90.6.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Budzinski K.L., Allen R.W., Fujimoto B.S., Kensel-Hammes P., Belnap D.M., Bajjalieh S.M. Large structural change in isolated synaptic vesicles upon loading with neurotransmitter. Biophys J. 2009;97:2577–2584. doi: 10.1016/j.bpj.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogl C., Mochida S., Wolff C., Whalley B.J., Stephens G.J. The synaptic vesicle glycoprotein 2A ligand levetiracetam inhibits presynaptic Ca2+ channels through an intracellular pathway. Mol Pharmacol. 2012;82:199–208. doi: 10.1124/mol.111.076687. [DOI] [PubMed] [Google Scholar]
- 15.Xu T., Bajjalieh S.M. SV2 modulates the size of the readily releasable pool of secretory vesicles. Nat Cell Biol. 2001;3:691–698. doi: 10.1038/35087000. [DOI] [PubMed] [Google Scholar]
- 16.Meehan A.L., Yang X., Yan L.L. Levetiracetam has an activity-dependent effect on inhibitory transmission. Epilepsia. 2012;53:469–476. doi: 10.1111/j.1528-1167.2011.03392.x. [DOI] [PubMed] [Google Scholar]
- 17.Nagarkatti N., Deshpande L.S., DeLorenzo R.J. Levetiracetam inhibits both ryanodine and IP3 receptor activated calcium induced calcium release in hippocampal neurons in culture. Neurosci Lett. 2008;436:289–293. doi: 10.1016/j.neulet.2008.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nowack A., Malarkey E.B., Yao J., Bleckert A., Hill J., Bajjalieh S.M. Levetiracetam reverses synaptic deficits produced by overexpression of SV2A. PLoS ONE. 2011;6:e29560. doi: 10.1371/journal.pone.0029560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molnár J., Szakács G., Tusnády G.E., Zhao M. Characterization of disease-associated mutations in human transmembrane proteins. PLoS ONE. 2016;11(3):e0151760. doi: 10.1371/journal.pone.0151760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harper C.B., Small C., Davenport E.C., Low D.W., Smillie K.J., Martínez-Mármol R. An epilepsy-associated SV2A mutation disrupts synaptotagmin-1 expression and activity-dependent trafficking. J Neurosci. 2020;40(23):4586–4595. doi: 10.1523/JNEUROSCI.0210-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynch J.M., Tate S.K., Kinirons P. No major role of common SV2A variation for predisposition or levetiracetam response in epilepsy. Epilepsy Res. 2009;83(1):44–51. doi: 10.1016/j.eplepsyres.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Cavalleri G.L., Weale M.E., Shianna K.V., Singh R., Lynch J.M., Grinton B. Multicentre search for genetic susceptibility loci in sporadic epilepsy syndrome and seizure types: a case—control study. Lancet Neurol. 2007;6:970–980. doi: 10.1016/S1474-4422(07)70247-8. [DOI] [PubMed] [Google Scholar]
- 23.Dibbens L.M., Hodgson B.L., Helbig K.L. Rare protein sequence variation in SV2A gene does not affect response to levetiracetam. Epilepsy Res. 2012;101(3):277–279. doi: 10.1016/j.eplepsyres.2012.04.007. [DOI] [PubMed] [Google Scholar]