Abstract
Background: High dietary fiber intake has been associated with reduced risk of Helicobacter pylori infection and co-morbidities such as gastric cancer but also with reduced risk of cardiovascular disease. It has been suggested that fermented rye could affect Helicobacter pylori bacterial load and that high- fiber rye may be superior to wheat for improvement of several cardiometabolic risk factors, but few long-term interventions with high fiber rye foods have been conducted.
Objective: To examine the effect of high-fiber wholegrain rye foods with added fermented rye bran vs. refined wheat on Helicobacter pylori infection and cardiometabolic risk markers in a Chinese population with a low habitual consumption of high fiber cereal foods.
Design: A parallel dietary intervention was set up and 182 normal- or overweight men and women were randomized to consume wholegrain rye products containing fermented rye bran (FRB) or refined wheat (RW) for 12 weeks. Anthropometric measurements, fasting blood sample collection and 13C-urea breath test (13C-UBT) were performed at baseline and after 6 and 12 weeks of intervention as well as 12 weeks after the end of the intervention.
Results: No difference between diets on Helicobacter pylori bacterial load measured by 13C-UBT breath test or in virulence factors of Helicobacter pylori in blood samples were found. Low density lipoprotein cholesterol (LDL-C) and high sensitivity C-reactive protein (hs-CRP) were significantly lower in the FRB group, compared to the RW group after 12 weeks of intervention. The intervention diets did not affect markers of glucose metabolism or insulin sensitivity.
Conclusions: While the results of the present study did not support any effect of FRB on Helicobacter pylori bacterial load, beneficial effects on LDL-C and hs-CRP were clearly shown. This suggest that consumption of high fiber rye foods instead of refined wheat could be one strategy for primary prevention of cardiovascular disease.
Clinical Trial Registration: The trial was registered at www.clinicaltrials.gov, Identifier: NCT03103386.
Keywords: wholegrain, rye, cardiovascular disease, Helicobacter pylori, inflammation, LDL cholesterol, C-reactive protein, cereal fiber
Introduction
Helicobacter pylori (H. pylori) is a common pathogen infecting approximately half the world's population (1). In China, the prevalence has been estimated to 56% but may differ substantially between regions (1). Epidemiological and clinical studies show that H. pylori infections lead to increased risk for several diseases including superficial gastritis, gastric and duodenal ulcers and gastric cancer (2–5). A growing number of experiments including in vitro and animal studies, as well as clinical trials have revealed the definite role of H. pylori in gastric carcinogenesis (6). H. pylori has been identified as a Group I carcinogen by the International Agency for Research on Cancer and is currently recognized as a major cause of gastric adenocarcinoma (7, 8). It has been estimated that about 78% of all gastric cancers can be attributable to H. pylori infection (9). Studies proposed that dietary patterns may play a crucial role in defining the final outcome of a H. pylori infection and diet may play an interactive role with H. pylori for development of gastric cancer (6, 10, 11). Probiotics, in the form of yogurts and supplements has shown a modest potential for H. pylori eradication (12). However, further clinical trials are required for improved understanding of the role of specific dietary components in H. pylori pathogenesis and development (13). Direct methods for investigating the development and progression of diseases in the upper GI-tract are typically invasive and are therefore difficult to use in a wide context (14, 15). Urea breath test (UBT) is a commonly used, non-invasive diagnostic tool to measure presence or absence of H. pylori infection. It measures the urease activity of H. pylori in the stomach after intake of labeled urea and detects 13C labeled CO2. It is a widely used diagnostic tool due to its high accuracy and low impact on patients, as compared to invasive methods such as endoscopic examinations of the gastrointestinal tract (14, 15).
Cardiovascular disease (CVD) accounts for 30% of global mortality and is the main cause of death overall with 17.3 million people dying from the disease every year (16). In China, CVD accounts for 45 and 43% of all deaths in rural and urban areas, respectively (17). Moreover, the prevalence of type 2 diabetes (T2D) is increasing rapidly all over the world. In China, almost 10% of all adults have T2D (18). Results from large cohort studies have clearly shown that healthy eating and an active lifestyle are important aspects for prevention and control of cardiometabolic diseases such as CVD and T2D (19–22). Based on observational data, high wholegrains and cereal fiber intakes have consistently been associated with lower incidence and mortality from non-communicable diseases, such as CVD and T2D (23–26). In addition to other well-established mechanisms (27), wholegrains and/or cereal fiber may have a favorable effect on local and systemic inflammatory processes (28–31). Reduced inflammation has been associated with lowered incidence and less adverse outcome in CVD and T2D, and biomarkers such as high sensitivity C-reactive protein (hs-CRP) and interleukin 6 have been forwarded as independent risk markers of CVD (32–36). Due to its higher fiber content, rye has been suggested to be superior to other cereals in terms of improving metabolic risk markers (37) with some supportive evidence from a few conducted studies (38–40).
Based on positive findings from in vitro studies showing reduced adherence and colonization of H. pylori in response to supernatants from fermented rye bran and feasibility of consuming fermented rye bran from a small pilot study in humans (www.epo.org, patent no.: EP1450626), the aim of the present study was to examine the effects of high-fiber wholegrain rye foods with added fermented rye bran vs. refined wheat as a strategy to reduce the H. pylori bacterial load among H. pylori positive men and women from a Chinese population, in a 12 week randomized controlled trial. We further investigated the effect of the intervention on cardio-metabolic risk factors, as secondary outcomes. We hypothesized that consumption of wholegrain rye with fermented rye bran, compared with refined wheat, would reduce H. pylori bacterial load and have a favorable effect on cardio-metabolic risk factors.
Methods
A 12-week randomized parallel intervention study was conducted to compare the effects of wholegrain rye products with added fermented rye bran (FRB) vs. refined wheat (RW) products (control) on normal weight and overweight participants with H. pylori infection. Participants who completed the study were invited to participate in a follow up visit 12 weeks after completing the intervention. The study was conducted at Zhongye Hospital, Shanghai, in April-October 2015. Participants gave written consent before each visit to the clinic, as well as oral consent before each procedure. The study was approved by the Institutional Review Board at Fudan University and the study was conducted in accordance with the Declaration of Helsinki.
Intervention Diets
The intervention diets consisted of four pieces of crisp bread and two portion packs of extruded cereal puffs per day, providing ~515 kcal/d (Table 1). Participants were free to consume the products at any time of the day to facilitate compliance. Products were packed in neutral packaging materials and participants were not informed which diet they were allocated to, but as wheat and rye products differ visually some participants might have guessed their allocation. Hospital staff conducting the outcome assessment were not aware of the participants' treatment allocation.
Table 1.
Daily nutritional composition of fermented rye bran and refined wheat diets.
| Product | Amount (g) | Energy (kcal) | Protein (g) | Fat (g) | Carbohydratea (g) | Total fiberb (g) | Soluble fiber (g) | Water (g) | Ash (g) |
|---|---|---|---|---|---|---|---|---|---|
| Fermented rye bran (FRB) | |||||||||
| Crisp bread | 44.8 | 145.5 | 4.3 | 1.2 | 23.9 | 11.1 | 3.9 | 3.2 | 1.2 |
| Puffs | 110.0 | 372.6 | 11.4 | 3.3 | 61.2 | 26.3 | 3.5 | 5.0 | 2.8 |
| Sum | 154.8 | 518.1 | 15.7 | 4.5 | 85.1 | 37.4 | 7.4 | 8.2 | 4.0 |
| Refined wheat (RW) | |||||||||
| Crisp Bread | 58.8 | 210.5 | 6.8 | 1.0 | 41.2 | 3.6 | 0.9 | 4.5 | 1.1 |
| Puffs | 84.0 | 302.7 | 9.7 | 1.6 | 58.1 | 8.6 | 4.0 | 4.9 | 1.4 |
| Sum | 142.8 | 513.2 | 16.4 | 2.6 | 99.3 | 12.2 | 4.9 | 9.4 | 2.3 |
Calculated by difference.
Fiber content as analyzed by the Uppsala method with inclusion of fructans. The bold rows are the summary rows.
The FRB products were produced from dried fermented rye bran, incorporated into wholegrain rye crisp bread product and extruded wholegrain rye puffs (25% on weight basis). The RW products were refined wheat crisp bread and extruded refined wheat puffs. The fermented rye bran (provided by Lantmännen, Stockholm, Sweden) was prepared by mixing rye bran with autoclaved tap water in a proportion of 1:5 w/w. Lactobacillus plantarum (DSMZ 13890) was cultured at 37°C for 24 h on MRS-agar, and thereafter it was suspended in autoclaved tap water and added in the rye bran-water mixture to final concentration of 105 bacteria/mL. The mixture was incubated at 37° under gentle agitation for 24 h in 4 different batches. The final concentration of L. plantarum in the fermented rye bran was estimated by count of colony forming units to about 109/mL. The fermented rye bran was dried and used in the production of products.
The crisp bread with fermented rye bran contained wholegrain rye flour (7.5 kg), fermented rye bran (2.5 kg), water (13.5 kg) and salt (100 g). The dough was mixed, baked in the oven for 10 min (gradient temperature) and dried for 30 min at 80°C, resulted in a final water content of 7%. The RW crisp bread contained wheat flour (10 kg), dextrose (400 g) water (3.6 kg), salt (133 g) and yeast (1.92 kg, 8% solution). The dough was mixed, pre-warmed for 35 min, baked in the oven for 7.5 min (gradient temperature) and dried for 30 min at 80°C, resulted in a final water content of 7%.
Both FRB and RW puffs were extruded on a co-rotating twin screw extruder APV MPF 19/25 (Baker Perkins Group Ltd., Peterborough, UK), with a screw speed of 450 rpm at 103°C. The feed rate of flour mixture was 50 g/min and water was added at a rate of 2.5–6 ml/min. After extrusion the puffs were oven dried for 45 min at 100°C. The flour mixture RW puffs consisted of refined wheat flour (98.2% w/w), salt and ascorbic acid, whereas the flour mixture for FRB puffs contained whole grain rye flour (74.2% w/w), fermented rye bran (25% w/w) and salt.
Composition of Intervention Products
Samples of crisp bread and puffs were freeze dried and milled with a cyclone sample mill (Retsch, Haan, Germany). Dietary fiber content was analyzed according to the Uppsala method (41). Fructan, and starch content were analyzed using a K-FRUC kit (42), and a K-TSTA kit (43), respectively (Megazyme, Bray, Ireland). Crude fat was determined according to the method described in the Official Journal of the European Communities (1984) and protein according to the Kjeldahl method (N × 6.25). Dry matter was determined by drying the samples at 105°C for 16 h according to the AACC method 44-15A.
Participants, Screening, and Randomization
Males and females, aged 20–70 years old, were invited to attend a screening visit at Zhongye Hospital. Participants were screened for H. pylori infection with 13C-urea breath test (13C-UBT), and as per manufacturer's recommendation a delta-over-baseline (DOB) value >4%0 were considered positive for H. pylori infection. H. pylori negative participants, as well as participants who reported smoking, use of medications (except for mild hypertension medication), chronic disease such as diabetes, CVDs or cancer, active peptic ulcers, pregnancy or planning pregnancy within the duration of the study, allergies or food intolerances or travel plans within coming 4 months were excluded.
The aim was that 50% of participants recruited should have BMI ≤ 24.00 (normal weight) and 50% should have BMI > 24.00 (overweight), as H. pylori infection has been shown to be associated with BMI (44, 45). BMI of 24 kg/m2 was chosen as cut-off as it has been shown to be the optimal distinction between overweight and normal weight in an adult Chinese population (46).
Eligible participants were randomly assigned to either the FRB or RW diet (1:1) by the study investigators in Shanghai. The randomization sequence was generated in Microsoft Excel and stratified for weight status (overweight or normal weight) to ensure equal distribution of normal weight and overweight subjects in each group. Participants in the two weight groups were ranked according to their 13C-UBT value obtained at the screening visit and every other person were then allocated to FRB or RW, respectively. Two participants chose to withdraw from the study shortly after randomization, before baseline, and two additional participants were chosen from the pool of screened volunteers to increase the sample size. Those two participants belonged to the same weight group and had 13C-UBT values in the same range as the withdrawn participants and were therefore allocated based on the withdrawn participants place in the ranked lists (both were allocated to FRB). One of these participants were however excluded at a later stage due having consumed antibiotics during screening and therefore not fulfilling the inclusion criteria. Furthermore, two participants, one in each intervention group, were mistakenly provided wrong foods at baseline and were re-allocated to the diet group matching the provided food.
Study Design, Procedures, and Sample Size
Participants attended an examination visit at the clinic at baseline, and after 6 and 12 weeks of intervention. All participants who completed the 12-week intervention were invited to attend a follow-up visit 12 weeks after finalizing the intervention (24 weeks after baseline). Participants were instructed to avoid food and beverages, with the exception of water, from 18:00 the day before each examination.
At the examination, participants underwent anthropometric measurements and bacterial load was estimated using 13C-UBT. Blood samples were drawn into a 7 ml EDTA tube and 7 ml serum tube, following a 5 min rest in a supine position. EDTA tubes were centrifuged immediately after sampling, whereas serum tubes were kept at room temperature for 30 min before centrifugation. Samples were centrifuged 10 min at 4°C under 2,000 g and aliquoted into serum, plasma, buffy coat and red blood cells. The lipids, glucose, insulin and hs-CRP concentrations were analyzed with the fresh blood by the clinical diagnostic center of Zhongye Hospital. The remaining sample material was stored at −80°C for later analysis.
The primary outcome of the study was difference in DOB between treatments. Based on previous pilot studies we hypothesized that a 30% difference in DOB between treatments after 12 weeks could have clinical relevance. In order to detect a two-side difference between treatments of 30% using a σ = 919 (based on results from a pilot study), α = 0.05, with a statistical power of 80%, 75 participants in each group would be required. A drop-out rate of 20% was assumed and therefore 90 subjects per treatment arm were recruited.
Anthropometric Measurements
At the screening visit, height was measured on a wall mounted stadiometer (Stature meter 2 m, Dongguan Nancheng Chengbida Electronic Equipment Factory, China) with the participant not wearing shoes. Body weight and body fat [determined by bioelectrical impedance analysis (BIA)], were measured on an electric scale [OMRON V-body HBF-701, OMRON Healthcare (China) Co., Ltd., China] with participants wearing light clothing and no shoes at baseline, week 6, 12 and 24.
A subgroup of 54 participants were selected on a volunteer basis to undertake a dual energy x-ray absorptiometry (DEXA) scan at all three occasions, in addition to the BIA, in order to validate the accuracy of the BIA measurements. The DEXA scan was conducted with a Lunar Prodigy Dual Energy X-ray Absorptiometer (GE healthcare, Illinois, USA) at Shanghai University of Sports under the same fasting conditions as the examinations.
Urea Breath Test
A 13C-UBT (Helicobacter pylori tester SN 2 6918, Shenzhen Zhongnuke Headway Biotechnology Co., Ltd., China) was used as a non-invasive diagnostic tool to measure the presence of H. pylori at screening as well as at examination visits. Participants breathed into a gas collection bag before and 30 min after consuming a tablet containing 75 mg 13C labeled urea along with 80–100 ml of water. The carbon dioxide abundance in the exhaled air was detected by the instrument and the difference between the two time points were calculated and expressed as DOB in %0. As per the manufacturer's recommendation, DOB values above 4%0 were set as the cut-off and interpreted as positive indicator of H. pylori infection.
Biochemical Analysis
Plasma glucose, total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), triglycerides and Apolipoprotein A/B/E/(a) were measured by a 7180 Clinical Analyzer (Hitachi, Japan). Serum hemoglobin A1c was measured by the VARIANT™ II TURBO Hemoglobin Testing System (Bio-Rad, USA). Serum insulin and C-peptide were measured by immunoassay using Cobas E601 analyzer (Roche, USA). Serum hs-CRP was measured with IMMAGE® 800 immunochemistry system (Beckman Coulter, USA). HOMA-IR was calculated as follows: fasting glucose (mmol/L) × [fasting insulin (pmol/L)/6]/22.5 (47). Plasma alkylresorcinols (AR) were analyzed by gas chromatography–mass spectrometry as a measure of wholegrain and bran intake from rye and wheat sources (48).
Antibodies directed against virulence factors were measured in serum, as a supportive measure of the H. pylori infection diagnosis made by 13C-UBT. Antibodies against the following virulence factors were measured: cytotoxin-associated gene A (anti-CagA), vacuolating cytotoxin A (anti-VacA), urease A (anti-UreA) and urease B (anti-UreB). Analysis was conducted by Western blot (Typing detection kit for antibody to Helicobacter pylori, Shenzhen blot biotech Co., Ltd., China) according to the instructions provided by the manufacturer.
Statistical Analysis
All statistical analyses followed a per-protocol approach inherent to the design of the study. All outcomes, except serology, were analyzed using an unadjusted ANOVA model (Model 1) and an ANCOVA model (Model 2) adjusted for weight change (from baseline to week 6 and baseline to week 12, respectively), age and sex. Analyses of body weight and fat percentage were not adjusted for weight change. Models were visually inspected using quantile-quantile-plots and residual plots and response variables were log transformed if residuals were non-normally distributed. Estimates are presented as mean ± standard deviation. Median was used when estimates appeared skewed in a histogram. Stratified analysis dividing participants into overweight and overweight (status at baseline) was conducted by the same procedure as the unstratified analyses.
McNemar's test was used to evaluate the change in the number of seropositive participants from baseline to weeks 6, 12, and 24, respectively.
Analyses of hs-CRP was conducted after excluding observations ≥1 mg/L, as concentrations above this could indicate an acute infection (e.g., influenza, common cold, bacterial infections etc.) rather than low grade/chronic inflammation related to metabolic status/risk. Hs-CRP measures below the detection limit (<12.7% of the observations) was imputed the value of the detection limit (0.02 mg/L).
For the validation of BIA against DEXA pearson's correlation coefficient were calculated for each of the three occasions (baseline, weeks 6 and 12).
All data presented in tables are unadjusted data. p-values < 0.05 are considered statistically significant. Complete-case analysis was defined as the primary analysis strategy. The statistical analyses were carried out using R Studio (version 3.3.0 (2016-05-13), for Windows 7), and all estimates were derived using the plyr-package (version 1.8.3). Figures were made with GraphPad Prism 7 (version 7.04 (2017-11-28), for Windows 7).
Results
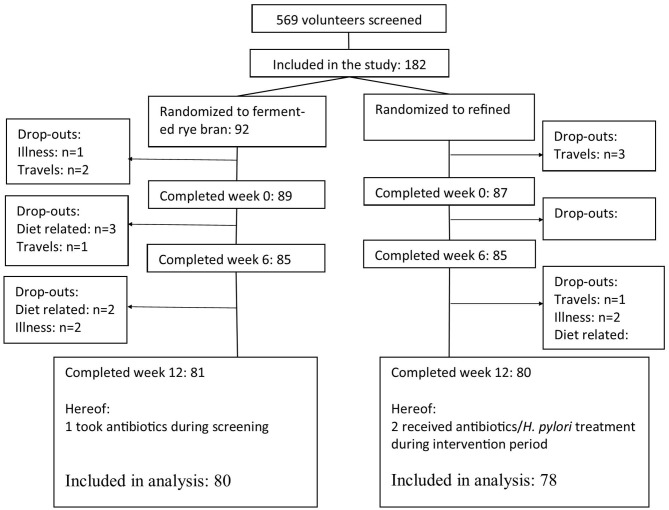
In total, 569 men and women were screened by 13C-UBT to determine whether they were H. pylori positive or not (Figure 1). In total, 316 participants (55%) were found to be H. pylori positive (with DOB > 4%0). After exclusion of participants that did not fulfill additional inclusion/exclusion criteria, 182 H. pylori positive participants, hereof 94 normal weight and 88 overweight, were included in the study and randomized to either FRB (n = 92) or RW (n = 90).
Figure 1.
Flow sheet of participants.
Among 92 participants randomized in the FRB, 81 participants (88%) completed the 12-week intervention. The corresponding figure for the RW was 80 out of 90 randomized participants that completed the study (89%). The reasons for dropout were similar in both groups and included illness, travels, difficulties to adhere to the diet, use of antibiotics and treatment against H. pylori infection (Figure 1). Additionally, three participants (FRB:1, RW:2) were excluded from all data analysis as they reported use of antibiotics during screening and/or intervention period, which was not allowed according to the study protocol.
In total, 158 completed the study and were included in the analysis of the results (Table 2). Some participants were not fasted at the examinations (one in FRB at baseline, 3 in RW at week 6), and their data was therefore not included in the analysis of outcomes that could be sensitive to fasting status. In total, 125 returned for the follow-up visit at week 24, however one without having a blood sample taken. Additionally, 17 participants (FRB: 11, RW: 6) were not fasted at and their data was therefore not included in the reposted results.
Table 2.
Baseline characteristics of the participants who completed the 12-week intervention.
| Intervention groups | ||||||
|---|---|---|---|---|---|---|
| Fermented rye bran (FRB) | Refined wheat (RW) | |||||
| All (n = 80) | Overweightb (n = 41) | Normal weightc (n = 39) | All (n = 78) | Overweightb (n = 34) | Normal weightc (n = 44) | |
| Age (years)a | 44.5 ± 12.5 | 44.9 ± 11.8 | 44.1 ± 13.4 | 45.4 ± 14.0 | 48.9 ± 11.7 | 42.7 ± 15.1 |
| BMI (kg/m2)a | 24.3 ± 3.9 | 27.2 ± 3.1 | 21.4 ± 1.8 | 24.1 ± 4.2 | 27.9 ± 3.2 | 21.1 ± 1.8 |
| Female/male (n) | 62/18 | 28/13 | 34/5 | 64/14 | 27/7 | 37/7 |
Values expressed as mean and standard deviation.
BMI > 24 kg/m2.
BMI ≤ 24 kg/m2.
Compliance, assessed by plasma AR concentrations (Table 3), indicated poor compliance in the FRB group and compliance apparently dropped after 6 weeks of intervention. The compliance in the RW group is difficult to determine using AR, since both the background diet and the refined intervention are expected to lead to low plasma AR concentrations.
Table 3.
Plasma total alkylresorcinol concentration and the C17:0/C21:0 alkylresorcinol ratio.
| Base-line | Week 6 | Week 12 | |
|---|---|---|---|
| Total alkylresorcinol (nmol/L)a | |||
| FRB (n = 77/80/78) | 2.8 (2.7) | 28.0 (81.6) | 16.1 (56.9) |
| RW (n = 79/74/79) | 2.5 (3.7) | 4.1 (4.7) | 3.7 (4.4) |
| C17:0/C21:0a | |||
| FRB (n = 13/72/62)b | 0.10 (0.14) | 0.67 (0.52) | 0.56 (0.53) |
| RW (n = 19/30/17)b | 0.05 (0.03) | 0.11 (0.31) | 0.08 (0.06) |
Values are median (interquartile range).
C17:0 was below the detection limit in many participants, which is indicative of little or no rye intake.
Body weight and body fat remained stable throughout the intervention for all participants, with no differences between groups (Table 4). The assessment of body fat percentage using BIA was validated against body fat percentage measured by DEXA in a subgroup of participants. The fat percentages measured by the two methods were well correlated (Pearson's correlation coefficient ≥ 0.84, p > 0.001, Supplementary Figure 1 in additional file 1), suggesting that use of BIA for body fat was an acceptable tool in this population.
Table 4.
Effects on anthropometrics and clinical outcomes.
| Week 0 | Week 6 | Week 12 | Δ Between groups, week 6 | Δ Between groups, week 12 | |||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | ||||
| Body weight (kg) | |||||||
| FRB | 64.2 ± 13.3 | 64.4 ± 13.4 | 64.4 ± 13.6 | 0.610 | 0.587 | 0.696 | 0.635 |
| RW | 63.0 ± 14.3 | 62.6 ± 14.6 | 62.6 ± 14.4 | ||||
| Body fat (%) | |||||||
| FRB | 30.8 ± 5.9 | 30.3 ± 6.2 | 30.0 ± 5.4 | 0.600 | 0.529 | 0.719 | 0.692 |
| RW | 31.5 ± 5.1 | 30.8 ± 5.9 | 30.5 ± 5.5 | ||||
| Triglycerides (mmol/L) | |||||||
| FRB | 1.14 ± 0.55 (1.06) | 1.25 ± 0.69 (1.08) | 1.49 ± 1.08 (1.25) | 0.685 | 0.821 | 0.207 | 0.162 |
| RW | 1.21 ± 0.61 (1.06) | 1.33 ± 0.71 (1.11) | 1.43 ± 0.85 (1.24) | ||||
| Glucose (mmol/L) | |||||||
| FRB | 5.50 ± 0.86 | 5.54 ± 0.57 | 5.40 ± 0.76 | 0.817 | 0.733 | 0.605 | 0.475 |
| RW | 5.74 ± 0.98 | 5.64 ± 0.83 | 5.58 ± 0.90 | ||||
| Insulin (pmol/L) | |||||||
| FRB | 70.0 ± 38.2 (59.3) | 73.2 ± 46.9 (59.7) | 81.6 ± 55.7 (65.4) | 0.220 | 0.200 | 0.621 | 0.830 |
| RW | 72.2 ± 39.9 (61.0) | 74.9 ± 55.3 (57.3) | 76.1 ± 40.7 (63.6) | ||||
| C-peptide (nmol/L) | |||||||
| FRB | 0.70 ± 0.25 (0.65) | 0.72 ± 0.26 (0.68) | 0.85 ± 0.38 (0.72) | 0.420 | 0.431 | 0.336 | 0.516 |
| RW | 0.74 ± 0.28 (0.70) | 0.76 ± 0.33 (0.65) | 0.82 ± 0.28 (0.77) | ||||
| HOMA-IR | |||||||
| FRB | 2.9 ± 1.8 (2.4) | 3.03 ± 1.99 (2.40) | 3.4 ± 2.6 (2.6) | 0.250 | 0.241 | 0.765 | 0.999 |
| RW | 3.1 ± 2.0 (2.6) | 3.29 ± 2.92 (2.28) | 3.2 ± 2.3 (2.8) | ||||
| HbA1C (%) | |||||||
| FRB | 5.50 ± 0.39 | 5.77 ± 0.39 | 5.78 ± 0.36 | 0.112 | 0.154 | 0.159 | 0.141 |
| RW | 5.57 ± 0.59 | 5.85 ± 0.53 | 5.87 ± 0.53 | ||||
| Apolipoprotein A (g/L) | |||||||
| FRB | 1.38 ± 0.23 | 1.36 ± 0.22 | 1.35 ± 0.20 | 0.619 | 0.463 | 0.377 | 0.518 |
| RW | 1.36 ± 0.22 | 1.34 ± 0.23 | 1.36 ± 0.23 | ||||
| Apolipoprotein B (g/L) | |||||||
| FRB | 1.13 ± 0.28 | 1.17 ± 0.28 | 1.15 ± 0.28 | 0.358 | 0.336 | 0.088 | 0.102 |
| RW | 1.13 ± 0.24 | 1.20 ± 0.20 | 1.22 ± 0.26 | ||||
| Apolipoprotein A/Apolipoprotein B | |||||||
| FRB | 1.29 ± 0.36 | 1.25 ± 0.44 | 1.24 ± 0.37 | 0.175 | 0.109 | 0.244 | 0.203 |
| RW | 1.26 ± 0.31 | 1.15 ± 0.27 | 1.17 ± 0.36 | ||||
| Apolipoprotein E (g/L) | |||||||
| FRB | 33.08 ± 5.75 | 33.18 ± 5.67 | 33.55 ± 5.69 | 0.514 | 0.465 | 0.485 | 0.347 |
| RW | 33.78 ± 5.43 | 33.13 ± 5.47 | 33.6 ± 5.27 | ||||
| Lipoprotein (a) (mg/L) | |||||||
| FRB | 213.9 ± 182.3 (151.0) | 240.8 ± 223.7 (142.0) | 259.8 ± 272.9 (141.5) | 0.457 | 0.371 | 0.643 | 0.499 |
| RW | 180.2 ± 171.7 (121.5) | 208.0 ± 257.4 (113.5) | 211.8 ± 235.3 (116.5) | ||||
| Zonulin (pg/ml) | |||||||
| FRB | 273.30 ± 88.37 (255.36) | 358.10 ± 91.50 (353.77) | 364.24 ± 94.17 (349.53) | 0.954 | 0.847 | 0.639 | 0.727 |
| RW | 286.86 ± 97.00 (280.26) | 356.29 ± 99.00 (329.16) | 360.12 ± 101.63 (356.93) | ||||
Number of participants, when deviating from 80/78 (FRB/RW). Body weight: wk6 = 80/74, wk12 = 79/78, body fat %: wk0 = 78/77, wk12 = 79/78, insulin: wk12 = 79/77, C-peptide: wk12 = 79/76, HOMA-IR: wk12 = 79/77, lipoprotein (a): wk6 = 77/74, wk6 = 77/74, wk12 = 78/78, zonulin: wk6 = 78/75, wk12 = 79/78.
All data is mean ± SD (median).
HOMA-IR, homeostasis model assessment insulin resistance; HbA1C, hemoglobin A1c.
n: FRB = 80, RW = 78 unless otherwise stated.
Statistically significant differences are indicated by *p < 0.05.
Bacterial Load- the Primary Outcome
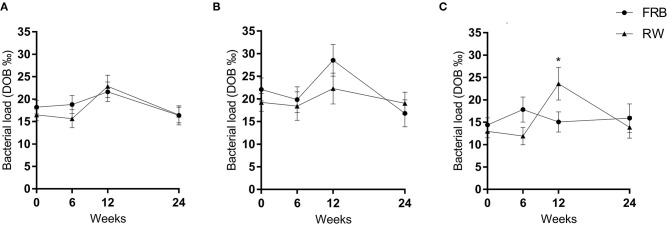
There was no overall difference of bacterial load assessed by 13C-UBT between groups when comparing data from completers after 12 weeks of intervention (Figure 2). When stratifying the analyses for overweight and normal weight participants, we found a higher bacterial load among overweight participants consuming RW, compared to FRB, after 12 weeks of intervention suggesting an adverse effect of RW (Figure 2).
Figure 2.
Bacterial load measured 13C-urea breath test at base-line, after 6 and 12 weeks of intervention and 12 weeks post intervention (week 24) in the fermented rye bran (FRB) and in the refined wheat (RW) group among all participants (A) and across normal weight participants (B) and overweight participants (C). Data is mean ± SEM. DOB, delta-over-baseline. Statistically significant differences are indicated by *p < 0.05.
Bacterial load at baseline differed between overweight and normal weight participants (mean DOB was 14.4%0 in overweight participants and 22.2%0 in normal weight participants, p = 0.01).
Serology
Overall, there were no consistent pattern in changes in the proportion of participants who were measured positive for virulence factor during the intervention across the two treatment groups (Supplementary Table 1 in additional file 1). After 12 weeks of intervention anti-UreA decreased in participants consuming FRB (73 vs. 68%, p < 0.05) but remained stable in participants consuming RW. Anti-CagA increased in a subgroup on normal weight participants consuming RW (59 vs. 79%, p < 0.05) but remained stable across other subgroups. There was no change in the fraction of participants measured positive for anti-VacA and anti-UreB during the 12 week intervention. At follow up (week 24), the proportion of participants measured positive for anti-CagA (65 vs. 46%, p < 0.05), anti-VacA (54 vs. 39%, p < 0.05), anti-UreA (70 vs. 53%, p < 0.05) and anti-UreB (91 vs. 75%, p < 0.05) was reduced in the RW group, compared to baseline, whereas the number of positive subjects in the FRB group had only reduced for anti-UreA (87 vs. 65%, p < 0.05) (Supplementary Table 2 in additional file 1).
Glucose Metabolism and Insulin Sensitivity
We found no statistically significant difference between interventions in any markers of glucose metabolism or insulin sensitivity (Table 4) for all or in any of the subgroups stratified for weight (Supplementary Tables 3, 4 in additional file 1).
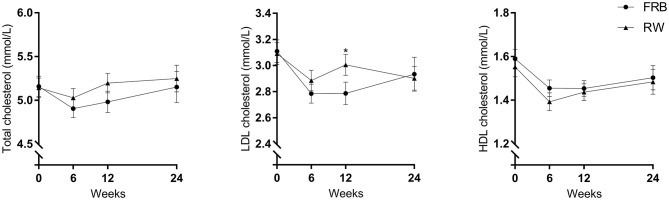
Blood Lipids
No difference in TC or HDL-C was found between the two treatments or within groups, overall or for normal or overweighed participants separately, at base line (Figure 3). However, LDL-C was lower in the group consuming FRB, compared to the RW group after 12 weeks of intervention (2.79 ± 0.77 vs. 3.01 ± 0.71, p = 0.007 in model 1, p = 0.011 in model 2) (Figure 3). The effect had disappeared at the follow-up visit at week 24, when participants consumed their habitual diet (Supplementary Table 5 in additional file 1). Weight stratified analyses showed that the difference in LDL-C was mainly found among normal weight participants, but not among overweight participants, though baseline concentration were similar among the two weight groups (Supplementary Figures 3, 4 in additional file 1).
Figure 3.
Plasma total cholesterol, low density lipoprotein (LDL) cholesterol and high density lipoprotein (HDL) cholesterol at base-line, after 6 and 12 weeks of intervention and 12 weeks post intervention (week 24) in the fermented rye bran (FRB) and in the refined wheat (RW) group among all completed cases. Data is mean ± SEM. Statistically significant differences are indicated by *p < 0.05.
Biomarkers of Inflammation and Leaky Gut
Hs-CRP was significantly lower in participants consuming FRB instead of RW after 12 weeks of intervention (Figure 4). This effect was found among overweight, but not normal weight participants (Supplementary Tables 3, 4 in additional file 1). No differences in zonulin concentrations were found between groups, but the zonulin concentration increased in both groups during the intervention compared to base-line (Table 4). At the week 24 follow-up visit the zonulin concentration was significantly higher in the RW group, compared to FRB group, but the concentration had increased in both groups during the follow-up period (Supplementary Table 5 in additional file 1).
Figure 4.
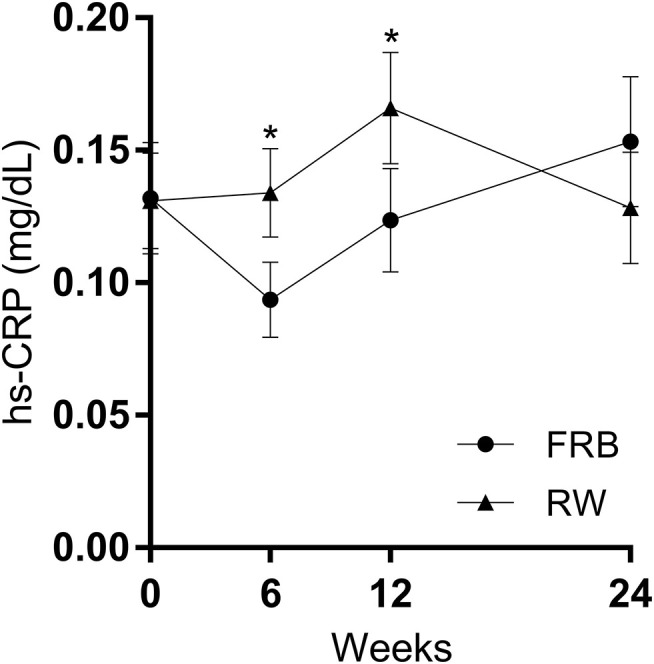
Serum high-sensitive C-reactive protein (hs-CRP) at base-line, after 6 and 12 weeks of intervention and 12 weeks post intervention (week 24) in the fermented rye bran (FRB) and in the refined wheat (RW) group among all completed cases. Observations ≥1 mg/dL were removed (n = 6 in FRB and n = 5 in RW, 1.9% of all observations). Data is mean ± SEM. Statistically significant differences are indicated by *p < 0.05.
Discussion
In the present study we investigated and found no effect of a 12-week dietary intervention with FRB vs. RW on H. pylori bacterial load, measured by 13C-UBT according to per-protocol analysis. This was supported with data showing no difference in measured virulence factors, which remained stable throughout the intervention and showed no difference between diets. In contrast, LDL-C and hs-CRP were lower after 12 weeks of FRB vs. RW consumption suggesting beneficial effects of high fiber rye consumption on important cardiometabolic risk markers.
Effects on H. pylori Bacterial Load
The lack of an apparent effect on bacterial load indicated by 13C-UBT after dietary treatments is difficult to interpret but was supported by the overall no effects on H. pylori virulence factors, which altogether suggest no effect on H. pylori infection. The underlying hypothesis of using UBT was that reduction of bacterial load may have beneficial effects on H. pylori infection and that fermented rye bran could reduce the bacterial load through inhibition of adherence of H. pylori at the stomach mucosa. We assumed that 13C-UBT can be used to assess and monitor the severity of H. pylori infection in a longitudinal study setting rather than just determine whether a subject is positive or negative for H. pylori. The clinical use is primarily aimed toward determine whether a subject is positive or negative for H. pylori, rather than tracking the severity of the infection (49). However, some studies have shown cross-sectional associations between UBT results and severity of infection, assessed by endoscopic examinations and biopsies, which could indicate that UBT can be used to estimate the severity of H. pylori infection (50–52). On the contrary, other studies have not been able to show this association (53, 54), and it remains to be proven whether UBT can be used to detect changes in infections in longitudinal design such as the present study. The absence of the validation of UBT to track infection rate over time is an important weakness of the present study which may explain the lack of difference between the treatment groups. UBT test result has been shown to differ according to factors such as age and gender (55), as well as the use of antibiotics and proton pump inhibitors, gastric pH, bleeding in the gastrointestinal tract, gastric emptying rate and food consumption prior to the test, and the matrix in which the urea tablet is consumed (14, 15). We have attempted to control these factors by excluding participants that did not comply with fasting procedures, had gastric ulcers or reported use of drugs that could have affected the results. Indeed, the bacterial load was found to be higher in a subgroup of overweight participants consuming RW, compared to overweight participants consuming FRB, after 12 weeks of intervention, however there were no difference between groups when assessing the entire study population. Furthermore, the difference between interventions among overweight participants appears to be mainly due to an adverse effect of RW, rather than a positive effect of FRB, on bacterial load and therefore does not support an effect of the tested FRB products on H. pylori infection. Few dietary interventions targeting H. pylori infection have been conducted and it is therefore difficult evaluate whether 12 weeks is a sufficient time-frame to evaluate the potential of a dietary intervention to affect H. pylori infection. Valid evaluation of treatment effect at 4–6 weeks after pharmaceutical eradication of H. pylori infection have been shown for 13C-UBT readouts (49). Treatment with probiotics in time range of 4–6 has likewise been shown to reduce UBT readouts, despite probiotics only showing modest potential for eradication of H. pylori (12). However, it has not been established how fast H. pylori infection will respond to a dietary intervention such as the one in the current study.
Analysis of virulence factor antibodies is less sensitive to the factors mentioned above, however antibodies have been shown to persist more than 1 year after eradication of H. pylori and may not allow distinction between past and present infection in the relatively short timeframe of the present study and is not recommended for monitoring of H. pylori infection (15, 56, 57). Inclusion of endoscopic examinations, either alone or in combination with 13C-UBT, would likely have provided a stronger measure of the bacterial load and the severity of H. pylori infection. However, due to the invasive nature of endoscopic examination it was not possible in the context of this study.
Interestingly, 13C-UBT values at baseline were higher among normal weight participants, compared to overweight participants, which could indicate that the bacterial load was higher among normal weight participants. This is in line with the findings of Eisdorfer et al. who found an inverse association between DOB values and BMI in a cross sectional study of 76,000 H. pylori infected participants (55). Furthermore, increase in BMI following eradication therapy has been observed in some studies (58, 59), as well as an inverse association between the hunger inducing hormone ghrelin and H. pylori infection (60, 61). On the contrary, observational studies have shown that obesity constitutes a risk factor for H. pylori infection (44, 45, 62). A recent cohort study showed that H. pylori infection increased the risk of gastric cancer in participants with BMI <25 kg/m2, whereas infection was not associated with cancer risk in participants with BMI ≥ 25 kg/m2 (63) and it could be speculated that the seemingly protective effect of overweight on gastric cancer risk could be mediated through less severe H. pylori infection. However, the association between H. pylori, body weight and underlying mechanisms warrants further research before conclusions can be made regarding a link between H. pylori, body weight and disease development.
Effects on Cardiometabolic Risk Factors
A significant reduction in LDL-C was found after 12 weeks of FRB compared with RW and the effect size (6%) was in the similar range to what has been achieved in studies investigating the effect of oat β-glucan (64). At the follow up examination in week 24, the difference in LDL-C observed after 12 weeks had disappeared, which strongly support the cholesterol lowering was a result of the FRB treatment. Wholegrain rye has previously been shown to reduce LDL-C (38, 65, 66), however few studies have investigated the effect of wholegrain rye specifically, but rather investigated mixtures of wholegrains or wholegrain wheat, hence more studies investigating the effect of rye is needed to confirm the effect (67). The cholesterol lowering effect of oats has been attributed to the high content of viscous fibers, and it may be that the cholesterol lowering effect of rye is caused by soluble arabinoxylans (37, 64, 68). It has been shown that about 80% of the variation in viscosity of water soluble dietary fiber in wholegrain rye flour is due to water soluble arabinoxylan (69). In contrast to wheat, the water soluble arabinoxylans in rye are more resistant to xylanase activity, and therefore the viscosity is retained to a larger extent throughout different food processes (70). Intake of rye, compared to wheat, has been shown to cause significantly higher viscosity of ileal digesta in pigs (71). Based on this, we hypothesize that wholegrain rye may lower LDL-C due to increased viscosity formed by its water soluble arabinoxylans, in analogy to what has been found for beta-glucans from oats and barley (72).
Wholegrain consumption has been linked to reductions in low-grade systemic inflammation, but the evidence from interventions is limited and has produced conflicting results and few studies have been conducted solely on rye (73–76). One of the mechanisms suggested to be involved in the link between the intake of wholegrain or other fiber- rich foods and inflammation is reductions in gut permeability (77). However, we found no effect of the intervention diet on zonulin, a biomarker of gut permeability. Nevertheless, this is in line with results from a recent study that also found reduced low grade inflammation but no effects on gut permeability following a intervention comparing mixed wholegrain with mixed refined grain (30), suggesting that other mechanisms could be involved. It should be noted that the difference between interventions found in the present study appears to be driven mainly by an increase in hs-CRP concentration in the RW groups, indicating an adverse effect of the RW diet rather than a positive effect of the FRB diet. Consumption of refined cereals has been associated with higher concentration of inflammatory markers in observational studies (78), and it is possible that the introduction of a relatively large amount of refined wheat in a population not accustomed to consumption of refined wheat has caused the increase in hs-CRP.
The increase in systemic zonulin seen in both of the intervention groups might be an effect the protein present in both of the diets. It has previously been shown that intestinal exposure to gliadin upregulates zonulin expression (79).
Previous intervention studies with high-fiber rye has mainly been conducted in northern Europe, where rye is habitually consumed (67). The present study is, to our knowledge, the first study to investigate metabolic effects of high-fiber rye food consumption in a population with little or no rye in the habitual diet. While this has provided the opportunity to test the effect of rye without the influence of a background diet rich in rye, this is likely the reason for the apparently lower compliance than what could have been expected in a population more familiar with the taste of rye, by assessment of plasma AR concentration profiles after intervention (39, 80). The daily amount of intervention products was high, especially considering that the study population were not accustomed to consuming this type of products. However, results from a pilot study indicated that high amounts of intervention foods were required to reach the desired effect, and the participants in the pilot study showed markedly better compliance than the participants of the present study. Nevertheless, the clear trend of a group difference in risk markers during the intervention and absence of this difference at the follow in week 24 clearly supports a causal link between the intervention and the reduction in risk markers, despite a lower than expected compliance. It cannot be ruled out that the use of complete cases analysis, rather than intention to treat analysis, may have overestimated the effect of the intervention and we cannot rule out that some bias was introduced due to this approach. However, as complete cases analysis was the defined analysis strategy for the study based on the design and dimensioning of the study.
Conclusion
In conclusion, the results of the study did not support any effect of the fermented rye bran vs. refined wheat on H. pylori infection. However, we cannot rule out whether this is related to the methodology used to assess the infection rate or the low compliance. Baseline data showed lower breath test values among overweight subjects indicating lower H. pylori bacterial loads, which is in line with a recent cross-sectional study and warrants further research. The FRB diet lowered LDL-C, while the RW diet increased hs-CRP, which adds to the growing evidence of a positive effect of rye consumption vs. refined wheat on important risk markers of CVDs and provides an interesting tool for prevention.
Data Availability Statement
The datasets presented in this article are not readily available because the datasets contains person sensitive information, but the datasets are available from the corresponding author on reasonable request. Requests to access the datasets should be directed to Kia Nøhr Iversen, kia.nohr@chalmers.se.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board at Fudan University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AJ, GHa, PÅ, RL, and TJ designed the study. KX, YL, ZQ, and CD conducted the study. KX and GHe provided essential materials. KI performed statistical analysis. KI and RL wrote the paper. MM contributed to drafting of the introduction. RL had responsibility for the final content. All authors contributed to the article and approved the submitted version.
Conflict of Interest
RL is the scientific leader of Nordic Rye Forum, which is a collaboration platform between academia, institutes and food industry. The platform is funded by industry membership fees. RL has received project grants from industry to conduct specific research, unrelated to the present study. GHa has been working as an adviser for Barilla. GHa, PÅ and AJ have a patent associated with a possible impact of fermented rye on H. pylori binding to gastric mucosa. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by funding from Sweden's Innovation Agency (VINNOVA) and from the National Natural Science Foundation of China (No. 81861138007). Food products were provided by Lantmännen and by Barilla.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2020.608623/full#supplementary-material
References
- 1.Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. (2017) 153:420–9. 10.1053/j.gastro.2017.04.022 [DOI] [PubMed] [Google Scholar]
- 2.Rothenbacher D, Brenner H. Burden of Helicobacter pylori and H. pylori-related diseases in developed countries: recent developments and future implications. Microbes Infect. (2003) 5:693–703. 10.1016/S1286-4579(03)00111-4 [DOI] [PubMed] [Google Scholar]
- 3.Israel DA, Peek RM. The role of persistence in Helicobacter pylori pathogenesis. Curr Opin Gastroenterol. (2006) 22:3–7. 10.1097/01.mog.0000194790.51714.f0 [DOI] [PubMed] [Google Scholar]
- 4.Leung WK, Ng EKW, Lam CCH, Chan K-F, Chan WY, Auyeung ACM, et al. Helicobacter pylori infection in 1st degree relatives of Chinese gastric cancer patients. Scand J Gastroenterol. (2006) 41:274–9. 10.1080/00365520510024269 [DOI] [PubMed] [Google Scholar]
- 5.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. (1991) 325:1127–31. 10.1056/NEJM199110173251603 [DOI] [PubMed] [Google Scholar]
- 6.Zaidi SF, Ahmed K, Saeed SA, Khan U, Sugiyama T. Can diet modulate Helicobacter pylori-associated gastric pathogenesis? An evidence-based analysis. Nutr Cancer. (2017) 69:979–89. 10.1080/01635581.2017.1359310 [DOI] [PubMed] [Google Scholar]
- 7.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. (2001) 345:784–9. 10.1056/NEJMoa001999 [DOI] [PubMed] [Google Scholar]
- 8.Xue FB, Xu YY, Wan Y, Pan BR, Ren J, Fan DM. Association of H. pylori infection with gastric carcinoma: a meta analysis. World J Gastroenterol. (2001) 7:801–4. 10.3748/wjg.v7.i6.801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.IARC Helicobacter Pylori Working Group Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer. Lyon: IARC Helicobacter Pylori Working Group; (2013). [Google Scholar]
- 10.Epplein M, Zheng W, Li H, Peek RM, Correa P, Gao J, et al. Diet, Helicobacter pylori strain-specific infection, and gastric cancer risk among Chinese Men. Nutr Cancer. (2014) 66:550–7. 10.1080/01635581.2014.894096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia Y, Meng G, Zhang Q, Liu L, Wu H, Shi H, et al. Dietary patterns are associated with Helicobacter pylori infection in chinese adults: a cross-sectional study. Sci Rep. (2016) 6:32334. 10.1038/srep32334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Losurdo G, Cubisino R, Barone M, Principi M, Leandro G, Ierardi E, et al. Probiotic monotherapy and Helicobacter pylori eradication: a systematic review with pooled-data analysis. World J Gastroenterol. (2018) 24:139–49. 10.3748/wjg.v24.i1.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaidi SF. Helicobacter pylori associated Asian enigma: does diet deserve distinction? World J Gastrointest Oncol. (2016) 8:341. 10.4251/wjgo.v8.i4.341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gisbert JP, Pajares JM. Review article: 13C-urea breath test in the diagnosis of Helicobacter pylori infection - a critical review. Aliment Pharmacol Ther. (2004) 20:1001–17. 10.1111/j.1365-2036.2004.02203.x [DOI] [PubMed] [Google Scholar]
- 15.Wang Y-K, Kuo F-C, Liu C-J, Wu M-C, Shih H-Y, Wang SSW, et al. Diagnosis of Helicobacter pylori infection: current options and developments. World J Gastroenterol. (2015) 21:11221–35. 10.3748/wjg.v21.i40.11221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization Global Atlas on Cardiovascular Disease Prevention and Control. Geneva: World Health Organization; (2011). [Google Scholar]
- 17.Chen W-W, Gao R-L, Liu L-S, Zhu M-L, Wang W, Wang Y-J, et al. China cardiovascular diseases report 2015: a summary. J Geriatr Cardiol. (2017) 14:1–10. 10.11909/j.issn.1671-5411.2017.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. (2017) 317:2515–23. 10.1001/jama.2017.7596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity. Circulation. (2016) 133:187–225. 10.1161/CIRCULATIONAHA.115.018585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the council on clinical cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation. (2003) 107:3109–16. 10.1161/01.CIR.0000075572.40158.77 [DOI] [PubMed] [Google Scholar]
- 21.Shiroma EJ, Lee I-M. Physical activity and cardiovascular health: lessons learned from epidemiological studies across age, gender, and race/ethnicity. Circulation. (2010) 122:743–52. 10.1161/CIRCULATIONAHA.109.914721 [DOI] [PubMed] [Google Scholar]
- 22.Pearson TA. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update: consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. Circulation. (2002) 106:388–91. 10.1161/01.CIR.0000020190.45892.75 [DOI] [PubMed] [Google Scholar]
- 23.Wei H, Gao Z, Liang R, Li Z, Hao H, Liu X. Whole-grain consumption and the risk of all-cause, CVD and cancer mortality: a meta-analysis of prospective cohort studies. Br J Nutr. (2016) 116:514–25. 10.1017/S0007114516001975 [DOI] [PubMed] [Google Scholar]
- 24.Li B, Zhang G, Tan M, Zhao L, Jin L, Tang X, et al. Consumption of whole grains in relation to mortality from all causes, cardiovascular disease, and diabetes. Medicine. (2016) 95:e4229. 10.1097/MD.0000000000004229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ. (2016) 353:i2716. 10.1136/bmj.i2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. (2019) 393:434–54. 10.1016/S0140-6736(18)31809-9 [DOI] [PubMed] [Google Scholar]
- 27.Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev. (2010) 23:65–134. 10.1017/S0954422410000041 [DOI] [PubMed] [Google Scholar]
- 28.North CJ, Venter CS, Jerling JC. The effects of dietary fibre on C-reactive protein, an inflammation marker predicting cardiovascular disease. Eur J Clin Nutr. (2009) 63:921–33. 10.1038/ejcn.2009.8 [DOI] [PubMed] [Google Scholar]
- 29.Jiao J, Xu J-Y, Zhang W, Han S, Qin L-Q. Effect of dietary fiber on circulating C-reactive protein in overweight and obese adults: a meta-analysis of randomized controlled trials. Int J Food Sci Nutr. (2015) 66:114–9. 10.3109/09637486.2014.959898 [DOI] [PubMed] [Google Scholar]
- 30.Roager HM, Vogt JK, Kristensen M, Hansen LBS, Ibrügger S, Mærkedahl RB, et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: a randomised cross-over trial. Gut. (2019) 68:83–93. 10.1136/gutjnl-2017-314786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vitaglione P, Mennella I, Ferracane R, Rivellese AA, Giacco R, Ercolini D, et al. Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: role of polyphenols bound to cereal dietary fiber. Am J Clin Nutr. (2015) 101:251–61. 10.3945/ajcn.114.088120 [DOI] [PubMed] [Google Scholar]
- 32.Fan J, Watanabe T. Inflammatory reactions in the pathogenesis of atherosclerosis. J Atheroscler Thromb. (2003) 10:63–71. 10.5551/jat.10.63 [DOI] [PubMed] [Google Scholar]
- 33.Pirillo A, Bonacina F, Norata GD, Catapano AL. The interplay of lipids, lipoproteins, and immunity in atherosclerosis. Curr Atheroscler Rep. (2018) 20:12. 10.1007/s11883-018-0715-0 [DOI] [PubMed] [Google Scholar]
- 34.Akbari M, Hassan-Zadeh V. IL-6 signalling pathways and the development of type 2 diabetes. Inflammopharmacology. (2018) 26:685–98. 10.1007/s10787-018-0458-0 [DOI] [PubMed] [Google Scholar]
- 35.Ridker PM. Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: implications for longevity. Nutr Rev. (2007) 65 (12 Pt 2):S253–9. 10.1301/nr.2007.dec.S253-S259 [DOI] [PubMed] [Google Scholar]
- 36.Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. (2004) 109:2–10. 10.1161/01.CIR.0000129535.04194.38 [DOI] [PubMed] [Google Scholar]
- 37.Jonsson K, Andersson R, Bach Knudsen KE, Hallmans G, Hanhineva K, Katina K, et al. Rye and health - where do we stand and where do we go? Trends Food Sci Technol. (2018) 79:78–87. 10.1016/j.tifs.2018.06.018 [DOI] [Google Scholar]
- 38.Leinonen KS, Poutanen KS, Mykkänen HM, Mykkanen HM. Rye bread decreases serum total and LDL cholesterol in men with moderately elevated serum cholesterol. J Nutr. (2000) 130:164–70. 10.1093/jn/130.2.164 [DOI] [PubMed] [Google Scholar]
- 39.Suhr J, Vuholm S, Iversen KN, Landberg R, Kristensen M. Wholegrain rye, but not wholegrain wheat, lowers body weight and fat mass compared with refined wheat: a 6-week randomized study. Eur J Clin Nutr. (2017) 71:959–67. 10.1038/ejcn.2017.12 [DOI] [PubMed] [Google Scholar]
- 40.Rosén LA, Silva LOB, Andersson UK, Holm C, Östman EM, Björck IM. Endosperm and whole grain rye breads are characterized by low post-prandial insulin response and a beneficial blood glucose profile. Nutr J. (2009) 8:42. 10.1186/1475-2891-8-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Theander O, Aman P, Westerlund E, Andersson R, Pettersson D. Total dietary fiber determined as neutral sugar residues, uronic acid residues, and Klason lignin (the Uppsala method): collaborative study. J AOAC Int. (1995) 78:1030–44. 10.1093/jaoac/78.4.1030 [DOI] [PubMed] [Google Scholar]
- 42.McCleary BV, Murphy A, Mugford DC. Measurement of total fructan in foods by enzymatic/spectrophotometric method: collaborative study. J AOAC Int. (2000) 83:356–64. 10.1093/jaoac/83.2.356 [DOI] [PubMed] [Google Scholar]
- 43.McCleary BV, Monaghan DA. Measurement of total starch in cereal products by amyloglucosidase - α-amylase method: collaborative study. J AOAC Int. (1997) 80:571–9. 10.1093/jaoac/80.3.571 [DOI] [Google Scholar]
- 44.Xu C, Yan M, Sun Y, Joo J, Wan X, Yu C, et al. Prevalence of Helicobacter pylori infection and its relation with body mass index in a Chinese Population. Helicobacter. (2014) 19:437–42. 10.1111/hel.12153 [DOI] [PubMed] [Google Scholar]
- 45.Lender N, Talley NJ, Enck P, Haag S, Zipfel S, Morrison M, et al. Review article: associations between Helicobacter pylori and obesity - an ecological study. Aliment Pharmacol Ther. (2014) 40:24–31. 10.1111/apt.12790 [DOI] [PubMed] [Google Scholar]
- 46.Zhou B-F Cooperative Meta-Analysis Group of the Working Group on Obesity in China Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. (2002) 15:83–96. 10.1046/j.1440-6047.11.s8.9.x [DOI] [PubMed] [Google Scholar]
- 47.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. (2004) 27:1487–95. 10.2337/diacare.27.6.1487 [DOI] [PubMed] [Google Scholar]
- 48.Wierzbicka R, Wu H, Franek M, Kamal-Eldin A, Landberg R. Determination of alkylresorcinols and their metabolites in biological samples by gas chromatography–mass spectrometry. J Chromatogr B. (2015) 1000:120–9. 10.1016/j.jchromb.2015.07.009 [DOI] [PubMed] [Google Scholar]
- 49.Garza-González E, Perez-Perez GI, Maldonado-Garza HJ, Bosques-Padilla FJ. A review of Helicobacter pylori diagnosis, treatment, and methods to detect eradication. World J Gastroenterol. (2014) 20:1438–49. 10.3748/wjg.v20.i6.1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perri F, Clemente R, Pastore M, Quitadamo M, Festa V, Bisceglia M, et al. The 13C-urea breath test as a predictor of intragastric bacterial load and severity of Helicobacter pylori gastritis. Scand J Clin Lab Invest. (1998) 58:19–27. 10.1080/00365519850186797 [DOI] [PubMed] [Google Scholar]
- 51.Zagari RM, Pozzato P, Martuzzi C, Fuccio L, Martinelli G, Roda E, et al. 13C-urea breath test to assess Helicobacter pylori bacterial load. Helicobacter. (2005) 10:615–9. 10.1111/j.1523-5378.2005.00358.x [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi D, Eishi Y, Ohkusa T, Ishige I, Suzuki T, Minami J, et al. Gastric mucosal density of Helicobacter pylori estimated by real-time PCR compared with results of urea breath test and histological grading. J Med Microbiol. (2002) 51:305–11. 10.1099/0022-1317-51-4-305 [DOI] [PubMed] [Google Scholar]
- 53.Tummala S, Sheth SG, Goldsmith JD, Goldar-Najafi A, Murphy CK, Osburne MS, et al. Quantifying gastric Helicobacter pylori infection: a comparison of quantitative culture, urease breath testing, and histology. Dig Dis Sci. (2007) 52:396–401. 10.1007/s10620-006-9377-9 [DOI] [PubMed] [Google Scholar]
- 54.Hilker E, Domschke W, Stoll R. 13C-urea breath test for detection of Helicobacter pylori and its correlation with endoscopic and histologic findings. J Physiol Pharmacol. (1996) 47:79–90. [PubMed] [Google Scholar]
- 55.Eisdorfer I, Shalev V, Goren S, Chodick G, Muhsen K. Sex differences in urea breath test results for the diagnosis of Helicobacter pylori infection: a large cross-sectional study. Biol Sex Differ. (2018) 9:1. 10.1186/s13293-017-0161-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter pylori infection—the Maastricht V/Florence Consensus Report. Gut. (2017) 66:6–30. 10.1136/gutjnl-2016-312288 [DOI] [PubMed] [Google Scholar]
- 57.Herbrink P, van Doorn LJ. Serological methods for diagnosis of Helicobacter pylori infection and monitoring of eradication therapy. Eur J Clin Microbiol Infect Dis. (2000) 19:164–73. 10.1007/s100960050454 [DOI] [PubMed] [Google Scholar]
- 58.Azuma T, Suto H, Ito Y, Muramatsu A, Ohtani M, Dojo M, et al. Eradication of Helicobacter pylori infection induces an increase in body mass index. Aliment Pharmacol Ther. (2002) 16 (Suppl. 2):240–4. 10.1046/j.1365-2036.16.s2.31.x [DOI] [PubMed] [Google Scholar]
- 59.Lane JA, Murray LJ, Harvey IM, Donovan JL, Nair P, Harvey RF. Randomised clinical trial: Helicobacter pylori eradication is associated with a significantly increased body mass index in a placebo-controlled study. Aliment Pharmacol Ther. (2011) 33:922–9. 10.1111/j.1365-2036.2011.04610.x [DOI] [PubMed] [Google Scholar]
- 60.Mantero P, Matus GS, Corti RE, Cabanne AM, Zerbetto de Palma GG, Marchesi Olid L, et al. Helicobacter pylori and corpus gastric pathology are associated with lower serum ghrelin. World J Gastroenterol. (2018) 24:397–407. 10.3748/wjg.v24.i3.397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nweneka C V, Prentice AM. Helicobacter pylori infection and circulating ghrelin levels - A systematic review. BMC Gastroenterol. (2011) 11:7. 10.1186/1471-230X-11-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suki M, Leibovici Weissman Y, Boltin D, Itskoviz D, Tsadok Perets T, Comaneshter D, et al. Helicobacter pylori infection is positively associated with an increased BMI, irrespective of socioeconomic status and other confounders. Eur J Gastroenterol Hepatol. (2018) 30:143–8. 10.1097/MEG.0000000000001014 [DOI] [PubMed] [Google Scholar]
- 63.Jang J, Cho E-J, Hwang Y, Weiderpass E, Ahn C, Choi J, et al. Association between body mass index and gastric cancer risk according to effect modification by helicobacter pylori infection. Cancer Res Treat. (2018) 51:1107–16. 10.4143/crt.2018.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ho HVT, Sievenpiper JL, Zurbau A, Blanco Mejia S, Jovanovski E, Au-Yeung F, et al. The effect of oat β-glucan on LDL-cholesterol, non-HDL-cholesterol and apoB for CVD risk reduction: a systematic review and meta-analysis of randomised-controlled trials. Br J Nutr. (2016) 116:1369–82. 10.1017/S000711451600341X [DOI] [PubMed] [Google Scholar]
- 65.Magnusdottir OK, Landberg R, Gunnarsdottir I, Cloetens L, Akesson B, Rosqvist F, et al. Whole grain rye intake, reflected by a biomarker, is associated with favorable blood lipid outcomes in subjects with the metabolic syndrome - a randomized study. PLoS ONE. (2014) 9:e110827. 10.1371/journal.pone.0110827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eriksen AK, Brunius C, Mazidi M, Hellström PM, Risérus U, Iversen KN, et al. Effects of whole-grain wheat, rye, and lignan supplementation on cardiometabolic risk factors in men with metabolic syndrome: a randomized crossover trial. Am J Clin Nutr. (2020) 1:846–76. 10.1093/ajcn/nqaa026 [DOI] [PubMed] [Google Scholar]
- 67.Hollænder PLB, Ross AB, Kristensen M. Whole-grain and blood lipid changes in apparently healthy adults: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. (2015) 102:556–72. 10.3945/ajcn.115.109165 [DOI] [PubMed] [Google Scholar]
- 68.Frølich W, Åman P, Tetens I. Whole grain foods and health - a Scandinavian perspective. Food Nutr Res. (2013) 57:18503. 10.3402/fnr.v57i0.18503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bengtsson S, Andersson R, Westerlund E, Åman P. Content, structure and viscosity of soluble arabinoxylans in rye grain from several countries. J Sci Food Agric. (1992) 58:331–7. 10.1002/jsfa.2740580307 [DOI] [Google Scholar]
- 70.Johansson DP, Gutiérrez JLV, Landberg R, Alminger M, Langton M. Impact of food processing on rye product properties and their in vitro digestion. Eur J Nutr. (2018) 57:1651–66. 10.1007/s00394-017-1450-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lærke HN, Arent S, Dalsgaard S, Bach Knudsen KE. Effect of xylanases on ileal viscosity, intestinal fiber modification, and apparent ileal fiber and nutrient digestibility of rye and wheat in growing pigs. J Anim Sci. (2015) 93:4323–35. 10.2527/jas.2015-9096 [DOI] [PubMed] [Google Scholar]
- 72.Wolever TMS, Tosh SM, Gibbs AL, Brand-Miller J, Duncan AM, Hart V, et al. Physicochemical properties of oat β-glucan influence its ability to reduce serum LDL cholesterol in humans: a randomized clinical trial. Am J Clin Nutr. (2010) 92:723–32. 10.3945/ajcn.2010.29174 [DOI] [PubMed] [Google Scholar]
- 73.Buyken AE, Goletzke J, Joslowski G, Felbick A, Cheng G, Herder C, et al. Association between carbohydrate quality and inflammatory markers: systematic review of observational and interventional studies. Am J Clin Nutr. (2014) 99:813–33. 10.3945/ajcn.113.074252 [DOI] [PubMed] [Google Scholar]
- 74.Zamaratskaia G, Mhd Omar NA, Brunius C, Hallmans G, Johansson J-E, Andersson S-O, et al. Consumption of whole grain/bran rye instead of refined wheat decrease concentrations of TNF-R2, e-selectin, and endostatin in an exploratory study in men with prostate cancer. Clin Nutr. (2019) 39:159–65. 10.1016/j.clnu.2019.01.007 [DOI] [PubMed] [Google Scholar]
- 75.Xu Y, Wan Q, Feng J, Du L, Li K, Zhou Y. Whole grain diet reduces systemic inflammation: a meta-analysis of 9 randomized trials. Medicine. (2018) 97:e12995. 10.1097/MD.0000000000012995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hajihashemi P, Haghighatdoost F. Effects of whole-grain consumption on selected biomarkers of systematic inflammation: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Nutr. (2018) 38:1–11. 10.1080/07315724.2018.1490935 [DOI] [PubMed] [Google Scholar]
- 77.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. (2009) 58:1091–103. 10.1136/gut.2008.165886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Masters RC, Liese AD, Haffner SM, Wagenknecht LE, Hanley AJ. Whole and refined grain intakes are related to inflammatory protein concentrations in human plasma. J Nutr. (2010) 140:587–94. 10.3945/jn.109.116640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hollon J, Puppa EL, Greenwald B, Goldberg E, Guerrerio A, Fasano A. Effect of gliadin on permeability of intestinal biopsy explants from celiac disease patients and patients with non-celiac gluten sensitivity. Nutrients. (2015) 7:1565–76. 10.3390/nu7031565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kristensen M, Toubro S, Jensen MG, Ross AB, Riboldi G, Petronio M, et al. Whole grain compared with refined wheat decreases the percentage of body fat following a 12-week, energy-restricted dietary intervention in postmenopausal women. J Nutr. (2012) 142:710–6. 10.3945/jn.111.142315 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this article are not readily available because the datasets contains person sensitive information, but the datasets are available from the corresponding author on reasonable request. Requests to access the datasets should be directed to Kia Nøhr Iversen, kia.nohr@chalmers.se.