Abstract
Intestinal environments such as microbiota, mucosal barrier function, and cytokine production affect egg production in laying hens. Dextran sodium sulfate (DSS) is an agent that disrupts the intestinal environment. Previously, we reported that the oral administration of dextran sodium sulfate (DSS: 0.9 g/kg BW) for 5 days caused severe intestinal inflammation in laying hens. However, the DSS concentration in the previous study was much higher to induce a milder disruption of the intestinal environment without heavy symptoms. Thus, the goal of this study was to determine the effects of a lower dose of DSS on the intestinal environment and egg production in laying hens. White Leghorn laying hens (330-day old) were oral administered with or without 0.225 g DSS/kg BW for 28 days (DSS and control group: n = 7 and 8, respectively). Weekly we collected all laid eggs and blood plasma samples. Intestinal tissues, liver, ovarian follicles, and the anterior pituitary gland were collected 1 day after the final treatment. Lower concentrations of orally administered DSS caused (1) a decrease in the ratio of villus height/crypt depth, occludin gene expressions in large intestine and cecal microbiota diversity, (2) a decrease in egg yolk weight, (3) an increase in VLDLy in blood plasma, (4), and enhanced the egg yolk precursor accumulation in the gene expression pattern in the follicular granulosa layer, (5) an increase in FSH and IL-1β gene expression in the pituitary gland, and (6) an increase in concentration of plasma lipopolysaccharide binding protein. These results suggested that the administration of the lower concentration of DSS caused a slight disruption in the intestinal environment. This disruption included poor intestinal morphology and decreased cecal microbiome diversity. The change in the intestinal environment decreases egg yolk size without decreasing the VLDLy supply from the liver. The decrease in egg yolk size is likely to be caused by the dysfunction of egg-yolk precursor uptake in ovarian follicles. In conclusion, the oral administration of a lower dose of DSS is an useful method to cause slight disruptions of intestinal environment, and the intestinal condition decreases egg yolk size through disfunction of ovarian follicle.
Keywords: chicken, intestinal environment, egg yolk, dextran sodium sulfate, very-low-density lipoprotein
Introduction
The intestine is the primary organ for digestion and absorption of nutrients. The healthy maintenance of the intestine is important for sustainable meats and egg production in chickens. Intestinal health is closely related to the condition of the intestinal environment, such as the balance of microbiota, mucosal barrier function, and cytokine production. A good intestinal environment contributes to the efficiency of feed digestion and nutrient absorptive function, preventing microbial infection and enhancing chicken egg and meat production (Possemiers et al., 2009; Shang et al., 2018). Therefore, the control of the intestinal environment is important for best practice poultry production.
Intestinal infection by pathogen such as Clostridium and Eimeria disrupts the intestinal environment, namely the induction of pro-inflammatory cytokine production, such as interleukin (IL)-1β and IL-6, and histological inflammatory events in the intestinal mucosa (Hong et al., 2006; Belote et al., 2018; Fasina and Lillehoj, 2019). It has been reported that C. perfringes and E. tenella infection also cause changes in cecal microflora composition in broiler chickens (Stanley et al., 2014; Lin et al., 2017). In addition, pathogenic infections in the intestine of layer and broiler breeder hens reduce their egg and meat production (Klasing, 2007; Lensing et al., 2012; Ritzi et al., 2014). Accordingly, it is suggested that pathogen infection causes disruption in the intestinal environment, including intestinal inflammation and a decline in poultry productivity. However, the mechanism by which egg production is reduced remains unknown. Furthermore, the disruption of the intestinal environment is related to dysbiosis, intestinal barrier function, and intestinal inflammation and has become a common problem since the ban on growth-promoting antibiotics in animal feed (Huyghebaert et al., 2011; Ducatelle et al., 2018). Therefore, understanding the mechanism by which the intestinal environment affects poultry production is highly important.
Dextran sodium sulfate (DSS) is commonly used as an intestinal inflammation inducer. DSS-induced intestinal inflammation in rodents has been used in animal models of human colitis, such as inflammatory bowel disease and colitis-associated cancer (Saleh and Trinchieri, 2011; Heijmans et al., 2014). The mechanism suggested by DSS induced intestinal inflammation is as follows: orally treated DSS directly injures intestinal epithelial cells, enteric bacteria translocate into the lamina propria from the lumen through the lesion, and the translocated bacteria and DSS in the mucosal tissue attract and activate immune cells, resulting in intestinal inflammation (Saleh and Trinchieri, 2011). Mice administered DSS in drinking water showed a higher serum IL-6 concentration and histological intestinal inflammatory events as well as profound changes in colon microflora (Hakansson et al., 2015). Therefore, we suggest that DSS is suitable to use to disrupt the intestinal environment. Recently, the DSS caused intestinal inflammation method had started to be used in chicks for poultry intestinal research (Kuttappan et al., 2015, 2016; Menconi et al., 2015; Simon et al., 2016). We previously reported that the oral administration of DSS (0.9 g/kg BW) for 5 days caused severe intestinal inflammation and reduced egg laying in laying hens through the disruption of the egg yolk precursor production in association with liver inflammation (Nii et al., 2020). This experiment mimics the acute intestinal inflammation caused by pathogenic bacterial infection. DSS caused severe inflammation in the intestinal mucosa with inflammatory symptoms such as bloody stools, anorexia, and body weight loss. Thus, the DSS treatment condition in our previous study was suitable for the mediation of severe acute intestinal inflammation, but the condition was too strong to induce a milder disruption of the intestinal environment without heavy symptoms. Simon et al. (2016) reported a treatment with 2.5% DSS in drinking water caused histological intestinal damage in the colon, but not when administering a lower concentration of DSS. We hypothesize that an oral administration of a lower concentration of DSS when compared with our previous study (0.9 g/kg BW), causes a milder disruption of the intestinal environment without heavy symptoms such as bloody stools, anorexia, and body weight loss.
The goal of this study was to determine the effects of the oral administration of a lower dose of DSS on the intestinal environment and egg production in laying hens. We focused on whether (1) lower concentration DSS disrupted the intestinal environment, (2) the DSS mediated disruption of the intestinal environment affects egg production, (3) egg yolk precursor production in the liver is inhibited, (4) the accumulation of egg yolk precursor into ovarian follicles is affected, and (5) the endocrine regulation related egg production in the pituitary gland is changed.
Materials and Methods
Experimental Birds
White Leghorn hens regularly laying seven or more eggs in a clutch (~330-days old) were housed separating in individual wire cages (W300 × D400 × H600 mm) equipped individual feed cup and water cup with a 14:10-h light-dark cycle. They were provided feed and water ad libitum. The birds were divided into two groups, namely the control (CON) and DSS groups (n = 8 and 7, respectively). Birds were administered a single oral dose of sterilized water with or without 0.225 g DSS/kg body weight (molecular weight: 5,000–6,000, Nacalai Tesque, Inc., Kyoto, Japan) by cannula for 28 days (DSS and CON groups, respectively).
The total number of eggs laid during the experimental period (28 days) was recorded. The egg-laying ratio was calculated for each hen group. Laid eggs were collected once a week, and the whole egg, egg yolk, and eggshell weights were measured. Blood was collected once a week at 11:00–12:00 (~2–3 h after oviposition). The blood samples were inverted with heparin (Mochida Pharmaceutical Co., Ltd., Tokyo, Japan). Plasma samples were separated by centrifugation (1,000 × g, 10 min, room temperature), and stored at −80°C until used for the biochemical analysis of TG, total cholesterol (T-CHO), and enzyme-linked immunosorbent assay (ELISA) analysis of lipopolysaccharide binding protein (LBP) concentrations.
Birds were euthanized under anesthesia with sodium pentobarbital (Somnopentyl; Kyoritsu Seiyaku Corporation, Tokyo, Japan), and the intestinal tissues [jejunum, ileum, cecum, and rectum (we collected one to three centimeter of lower part tissues from ileum-cecum junction, and defined that part as rectum)], liver, follicular granulosa layer (F1 and F5 follicles), and anterior pituitary gland were collected. Intestine and liver tissue samples were processed into paraffin sections. All tissue samples were used for total RNA extraction in same day. In addition, cecal contents were also collected (n = 4, each group) for microbiota analysis.
The experiment was done three independent experimental repetitions, namely 1st: CON (n = 2) and DSS (n = 2), 2nd: CON (n = 2) and DSS (n = 2), 3rd: CON (n = 4) and DSS (n = 3), and we pooled all samples for each analysis. All experiments in this study were approved by the Hiroshima University Animal Research Committee (Approval No. C17-3). We adhered to all animal handling regulations.
Tissue Preparation and Staining for Histology
Intestinal and liver tissues were fixed with 10% (v/v) formalin in PBS and processed into paraffin sections (4 μm thick). Sections were stained with Hansen's hematoxylin and eosin for histological observation. Intestinal tissue sections were examined under a light microscope connected to image analysis software (NIS-Elements; Nikon, Tokyo, Japan). The ratio of the villus height/crypt depth and the height of epithelial cells of the jejunum, ileum, cecum, and rectum were measured. The measurement was performed in triplicate on a section from each sample, and the average was calculated.
Real-Time Polymerase Chain Reaction (PCR) Analysis for the Expression of Inflammation and Lipid Metabolism-Related Genes
Total RNA was extracted from the liver, follicular granulosa layer, and anterior pituitary gland using Sepasol RNA I Super (Nacalai Tesque, Inc.). Total RNA was extracted and purified from intestinal mucosal samples (jejunum, ileum, cecum, and rectum) using the NucleoSpin RNA kit (Macherey-Nagel GmbH & Co. KG., Duren, Germany), which is a column-type RNA extraction kit because any remaining DSS in the intestinal tissues would inhibit the PCR (Kerr et al., 2012). The extracted total RNA samples were dissolved in TE buffer (10 mM Tris-HCl, pH 8.0, with 1 mM EDTA) and stored at −80°C until RT-PCR. The concentration of total RNA in each sample was measured using a Nano Drop Lite (Thermo Fisher Scientific, MA, USA). The RNA samples were reverse transcribed using ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo Co., Ltd., Osaka, Japan) on a PTC-100 programmable thermal controller (MJ Research, Waltham, MA, USA), programmed according to the manufacturer's instructions. Real-time PCR was performed using an AriaMX real-time PCR system (Agilent Technologies, CA, USA) with Brilliant III Ultra-Fast SYBR Green QPCR Master Mix (Agilent Technologies). Table 1 shows the primers used for the PCR. The cycle parameters used for amplification were as follows: denaturation at 95°C for 5 s and annealing at 58°C (for TGFβ-2 and TGFβ-4), 60°C (for IL-6, ApoB, Claudin-1, LDLr, LR8, FSH, LH, and RPS17), 62°C [for very low-density lipoprotein (VLDL)-II, vitellogenin (VTG)-II and Claudin5], or 63°C (for IL-1β) for 10 s, and the denaturation and annealing steps were performed for 50 cycles. The cycle parameters for the melting step were 95°C for 30 s, 65°C for 30 s, and 95°C for 30 s. RNA expression levels were calculated by the relative quantification method using a standard curve generated with serially diluted PCR products of each target gene. The relative expression level of the target mRNA in each sample was normalized to the RPS17 housekeeping gene, and was described as the mean fold change when compared to a standard sample from the control group.
Table 1.
PCR primers used for mRNA expression analysis.
| Target genes | Forward primer | Revers primer | Product size | Accession no. |
|---|---|---|---|---|
| IL-1β | GTGAGGCTCAACATTGCGCTGTA | TGTCCAGGCGGTAGAAGATGAAG | 214 | NM_204524.1 |
| IL-6 | AGAAATCCCTCCTCGCCAAT | AAATAGCGAACGGCCCTCA | 121 | NM_204628.1 |
| TGFβ-2 | AGGAATGTGCAGGATAATT | ATTTTGGGTGTTTTGCCAA | 269 | NM_001031045.3 |
| TGFβ-4 | ATGAGTATTGGGCCAAAG | ACGTTGAACACGAAGAAG | 109 | NM_001318456.1 |
| ApoB | CTGCAAATGCTGGGCTGTTT | CTGGTTGAGCCATCCAGCTT | 106 | NM_001044633.1 |
| VLDL-II | AGGGCTGAACTGGTACCAACAAAC | GGATGACCAGCCAGTCACGA | 140 | NM_205483.2 |
| VTG-II | CAACATATCTTCCGCTTGTAACATTG | TTCACAACAAAGATTTCTCCAGTAGC | 147 | NM_001031276 |
| Claudin1 | GACTCGCTGCTTAAGCTGGA | AAATCTGGTGTTAACGGGTG | 276 | NM_001013611.2 |
| Claudin5 | GTCCCGCTCTGCTGGTTC | CCCTATCTCCCGCTTCTGG | 84 | NM_204201.1 |
| LDLr | GGAGCAGTCACAGCATCAGCT | CTGTGTCACACTCCGCTGTCTC | 109 | NM_204452.1 |
| LR8 | TGCTACAGGAGTGTGCAAGG | TCTCTCAAGGCCAATCTTCC | 88 | NM_205229.1 |
| FSH | TGCTTCACAAGGGATCCAG | CAGCCAGGGATCTTCACTGT | 104 | NM_204257.1 |
| LH | GTGTCGCCCCATAAACGTAA | CAAAGGGCTGCGATACACC | 124 | HQ872606.1 |
| GnRHr | ACCTCGGCCACTCACTGC | CACCTTTGCTGCTTTTGTGAAC | 63 | NM_204653.1 |
| RPS17 | AAGCTGCAGGAGGAGGAGAGG | GGTTGGACAGGCTGCCGAAGT | 136 | NM_204217.1 |
Microbiome Analysis
The cecal contents at day 28 were collected and stored at −80°C until use (n = 4, each group). DNA from these cecal contents was extracted using the NucleoSpin DNA Stool (Macherey-Nagel GmbH & Co., Dürer, Germany) according to the manufacturer's instructions. 2-step tailed PCR was performed to process the amplicon of the V3 and V4 regions of the 16S rRNA genes. Sequencing was conducted using the Miseq system (Illumina, CA, USA) for 300 × 2 bp. Two-step tailed PCR and sequencing were performed by Bioengineering Lab Co., Ltd (Kanagawa, Japan).
The sequenced fast-q data were analyzed using QIIME2 (ver. 2020.2) with Greengenes 13_8 99% of OTUs full-length sequences for reference. The first 22 bases of all reads were trimmed to achieve a length of 250 bases and they were denoised by dada2. The relative abundance of taxa at the family level of the groups was presented as a mean % value. The alpha diversity, as species richness, was measured using the observed OTUs. Beta diversity plots, which suggest changes in species diversity among samples, were constructed to visualize the distance of the samples using unweighted UniFrac.
Biochemical Assays of Blood Plasma
Blood parameters, namely TG and T-CHO, were measured by a Beckman Colter AU480 automatic biochemistry analysis system (Beckman Coulter, Inc., CA, USA) using reagent kits provided by the manufacturer.
The VLDLy (particle size with diameter of 25–44 nm) was separated, and cholesterol (CHO) and TG concentrations contained in the VLDLy fraction were measured by high performance liquid chromatography (HPLC) using LipoSEARCH service in Funakoshi Co., Ltd (n = 4, each group).
ELISA Protocol
Plasma corticosterone and estradiol-17β were analyzed by a competitive enzyme immunoassay as described previously (Isobe and Nakao, 2003). The lipid phase of the blood plasma was extracted with dichloromethane. The microplate wells were precoated with anti-rabbit IgG antibody (Sato et al., 2011) for 4 h. Then we added anti-corticosterone antibody (Cosmo Bio Co., Ltd., Tokyo, Japan) or anti-estradiol-17β antibody (Cosmo Bio Co., Ltd.), and HRP-conjugated corticosterone (FKA 419; Cosmo Bio Co., Ltd) or HRP-conjugated estradiol-17β (FKA 236-E; Cosmo Bio Co., Ltd) for 4 h at 20°C. The plates were washed three times with phosphate-buffered saline (PBS) containing Tween 20, and then 3,3′,5,5′-tetramethylbenzidine substrate solution was added, and the plate was incubated for 30 min. The optical density was read at 450 nm using a microplate reader (Multiskan FC; Thermo Fisher Scientific). The corticosterone and estradiol-17β concentrations were calculated using the absolute quantification method with a standard curve.
The plasma concentration of chicken LBP was analyzed with a sandwich-ELISA principle using a commercially available kit (Chicken LBP ELISA Kit; Catalog No: MBS2503399, MyBioSource, Inc., CA, USA). Plasma samples were centrifuged (15,000 × rpm, 60 min, 4°C) and the top lipid-rich layer was removed before ELISA. The intermediate translucent layer was used for ELISA because the samples with high lipid numbers were not suitable for ELISA assay.
Statistical Analysis
Values are expressed as mean ± SEM. The significant differences between the CON and DSS groups in the ratio of villus heights/crypt depth, mRNA expression levels, and CHO and TG concentrations in VLDLy in each tissue were evaluated by Student's t-test for homoscedastic samples and Welch's t-test for heteroscedastic samples. Egg parameters and all blood parameters in the control and DSS groups on days 0, 7, 14, 21, and 28 were analyzed by two-way repeated measures ANOVA and Tukey's test to assess the significance of the interaction between time and DSS treatment. The significant difference in OTU numbers between CON and DSS groups on alpha-diversity was calculated by Kruskal-Wallis analysis. The pairwise PERMANOVA analysis method in QIIME2 was performed on the unweighted UniFrac distance matrix of 8 samples for beta-diversity. The significance of the PERMANOVA was obtained by a 999-permutation test. Differences were considered significant when the P-value was <0.05.
Results
Body Weight and Feed Intake
Average body weight in the CON group was 1.66, 1.64, 1.62, 1.63, and 1.62 kg on day 0, 7, 14, 21, 28. Average body weight in the DSS group was 1.66, 164, 1.64, 1.64, and 1.63 kg on day 0, 7, 14, 21, 28, respectively. The average feed intake per day in the CON group was 88.4, 94.3, 95.3, 103.1, and 96.3 g on day 0, 7, 14, 21, 28. The average feed intake per day in the DSS group was 94.9, 91.1, 97.4, 97.0, and 96.6 g on day 0, 7, 14, 21, 28, respectively. The body weight and feed intake results between the CON and DSS groups were not significantly different throughout the experiment.
Intestinal Structure, Mucosal Cytokine Expression, and Cecal Microflora
The mucosal tissue of the cecum did not show any histological damage, such as disintegration of epithelial cells and a large amount of red blood cell accumulation in the lamina propria in either the CON or the DSS groups (Figures 1A,B). The rectum did not show any histological damage in either the CON or DSS groups (Figures 1C,D). However, the ratios of villus height/crypt depth in the cecum and rectum were significantly lower in the DSS group than in the CON group, but not in the jejunum and ileum (Figure 1E). In addition, the expression of occludin (a mucosal protein content of tight junctions) was lower in the DSS group than in the CON group in the cecum (Figure 1F).
Figure 1.
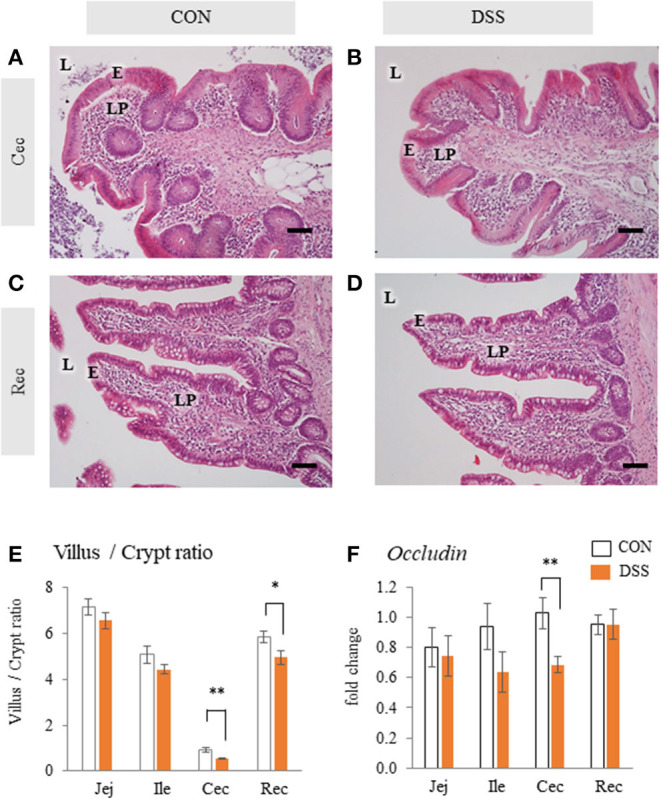
Histology and Occludin mRNA expression in the intestinal mucosa with or without dextran sodium sulfate (DSS). Micrographs demonstrate the HE staining of the cecum [Cec: (A) and (B)] and rectum [Rec: (C) and (D)] in hens orally administered DSS (DSS group) or water (CON group). E = mucosal epithelium, L = lumen, LP = lamina propria. Scale bars = 50 μm. (E) Ratio of villus height/ crypt depth of jejunum (Jej), ileum (Ile), Cec, and Rec. Open bars represent the control group, and orange filled bars represent the DSS group. Values are the ratio of villus height/crypt depth (CON and DSS, n = 8 and 7). (F) Effects of the oral administration of DSS on the mRNA expression of Occludin in the intestinal mucosa. Values are the fold change in target gene expression when compared to a standard sample from the CON group of each segment (CON and DSS, n = 8 and 7). Target gene expression was normalized to the house-keeping gene RPS17. Asterisks (*, **) indicate significant differences between the CON and DSS groups (P < 0.05 and P < 0.01, respectively).
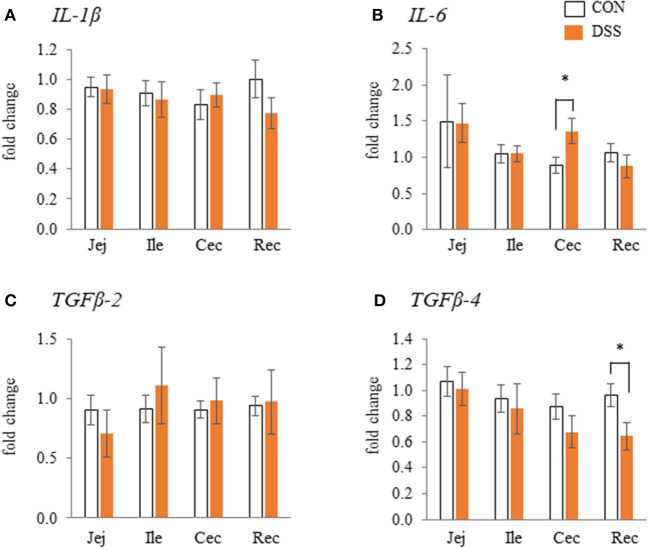
The gene expression levels of IL-6 in the cecum were significantly higher in the DSS group than in the CON group, but not in the jejunum, ileum, and rectum (Figure 2B). The gene expression level of TGF-β4 in the rectum was significantly lower in the DSS group than in the control group (Figure 2D). However, no significant differences in expression were observed in the other segments. The IL-1β and TGFβ-2 gene expression levels in all intestinal segments were not significantly different between the CON and DSS groups (Figures 2A,C).
Figure 2.
The effects of dextran sodium sulphate (DSS) on the mRNA expression of pro- [IL-1β and IL-6: (A) and (B)] and anti- [TGFβ-2 and 4: (C) and (D)] inflammatory cytokines in the mucosal tissues of the jejunum (Jej), ileum (Ile), cecum (Cec), and rectum (Rec). The effects of dextran sodium sulfate (DSS) on the mRNA expression of pro- and anti-inflammatory cytokines in the mucosal tissues of the jejunum (Jej), ileum (Ile), cecum (Cec), and rectum (Rec). Open bars represent the CON group, and orange filled bars represent the DSS group. Values are the fold change in the target gene expression when compared to a standard sample from the CON group of each segment (CON and DSS, n = 8 and 7). The target gene expression was normalized to the house-keeping gene RPS17. Asterisks (*) indicate significant differences between the water (CON) and DSS-treated groups (P < 0.05).
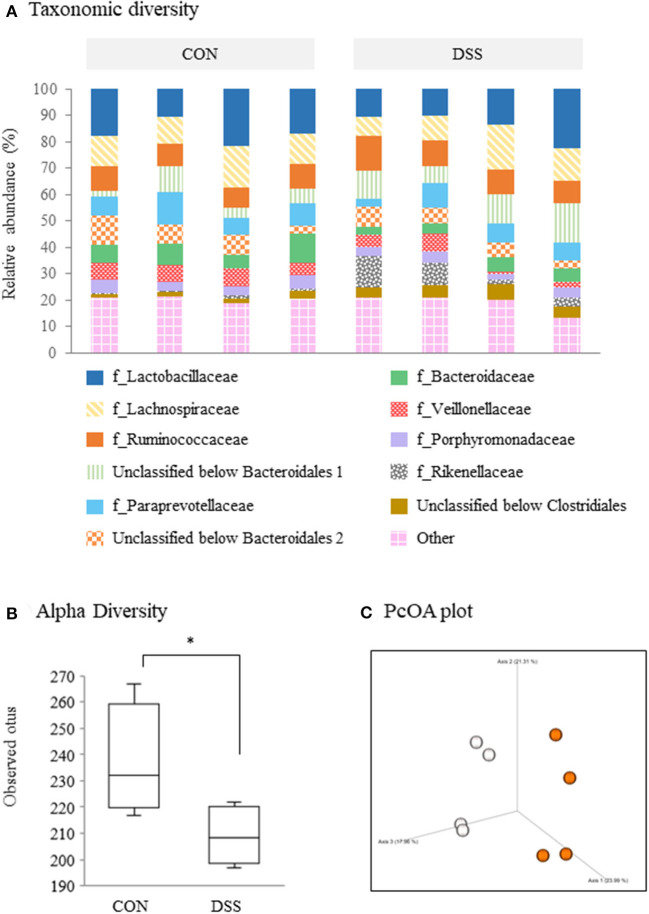
Figure 3 shows the microbiome analysis of the cecal contents using eight samples (four randomly selected samples from the CON and DSS groups). A taxonomic bar plot (to the family level) is shown in Figure 3A. Lactobacillaceae, Lachnospiraceae, and Ruminococcaceae were in higher frequencies in all hens. The frequencies decreased in order from unclassified Bacteroidales 1, Paraprevotellaceae, unclassified Bacteroidales 2, Bacteroidaceae, Veillonellaceae, Porphyromonadaceae, Rikenellaceae, unclassified Clostridiales, and others (Figure 3A). The percentile abundances of Elusimicrobiaceae (including “others”) were significantly lower in the DSS group (0.00%) than in the CON group (0.06%) using ANCOM statistics. In addition, the observed OTUs (representing alpha diversity) were lower in the DSS group than in the CON group (Figure 3B). A 3D principal coordinates analysis (PCoA) plot constructed from the unweighted UniFrac matrix of the cecal microbiota (representing beta diversity) identified a significant difference between the CON and DSS groups (Figure 3C, p = 0.0433, q = 0.0433).
Figure 3.
Microbiome profiles in the cecum contents of laying hens administered dextran sodium sulfate (DSS group) or water (CON group). (A) A taxonomic diversity bar plot showing relative abundance of taxa identified to the family level (n = 4). (B) Alpha-diversity boxplot of observed OTUs. Boxplots show the quartiles, median, and extremities of the value. Asterisk (*) indicate significant differences between the water (CON) and DSS-treated groups (P < 0.05). (C) 3D PCoA plot based on an unweighted UniFrac distance matrix. White circles represent the CON group, and orange circles represent the DSS group.
Egg Laying Rate and Egg Weight
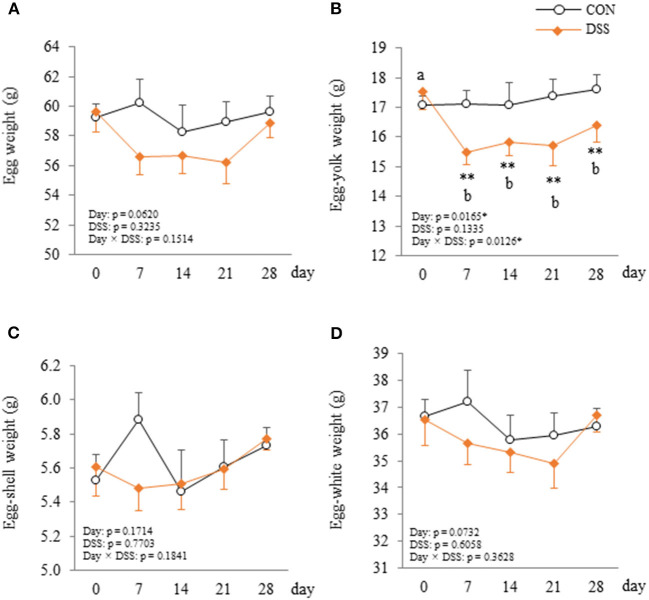
Egg laying rate during the experimental period in the DSS group (92.3 ± 3.7%) was significantly lower than that in the CON group (98.7 ± 0.6%) (P < 0.05). Egg-yolk weight was significantly lower in the DSS group than in the CON group at days 7, 14, 21, and 28, and the yolk weight in DSS group decreased from day 7 to 28 when compared with the first day (Figure 4B). Whole egg, eggshell, and egg-white weights did not show any significant differences in the DSS group during the experimental period (Figures 4A,C,D).
Figure 4.
Changes in the egg (A), egg-yolk (B), egg-shell (C), and egg-white (D) weight in hens administered dextran sodium sulphate (DSS group) or water (CON group). Open circle plots represent the CON group, and the orange rhombus plots represent the DSS group. Values are the mean ± SEM (CON and DSS, n = 8 and 7). Asterisks (**) indicate significant differences between the CON and DSS groups at the same time point (P < 0.01). Lower case letters (a,b) indicate significant differences between the time points (P < 0.05).
Liver Tissue Observation and VLDLy Production
The hepatic tissues of the CON and DSS groups were filled with hepatic cells and sinusoidal capillaries (Figures 5A,B). There was no observed difference between the CON and DSS groups in the liver tissue.
Figure 5.
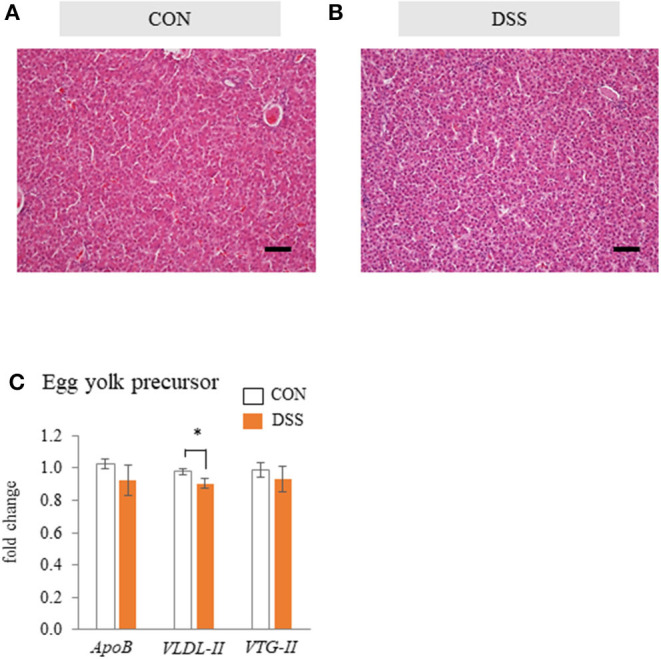
The effects of the oral administration of dextran sodium sulfate (DSS) on hepatic histology and function in hens. Micrographs of the liver tissue of hens treated with water [(A); CON group] and DSS [(B); DSS group]. HE staining. Scale bars = 50 μm. (C) Effects of the oral administration of DSS on the mRNA expression of egg yolk precursor related genes. Open bars represent the CON group, and orange filled bars represent the DSS group. Values demonstrate the fold change in the target gene expression when compared to a standard sample from the CON group of each segment (CON and DSS, n = 8 and 7). Target gene expression was normalized to the house-keeping gene RPS17. Asterisk (*) indicate significant difference between the control and DSS groups (P < 0.05).
Gene expression levels of egg yolk precursor-related factors (ApoB, VLDL-II, and VTG-II) in the liver tissue are shown in Figure 5C. The expression of VLDL-II was significantly lower in the DSS group than in the CON group, but there was no significant difference in ApoB and VTG-II.
Blood Parameter
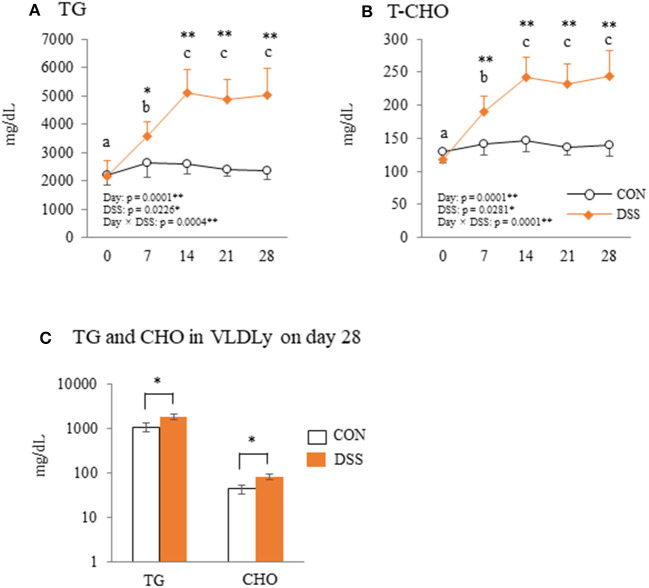
Blood plasma TG and T-CHO in the DSS group increased from day 7, peaked on day 14, then remained stable until day 28 (Figures 6A,B). Both TG and T-CHO were significantly higher in the DSS group than in the CON group from days 7 to 28. Both TG and CHO concentrations in the VLDLy particles in the blood plasma were higher in the DSS group than in the CON group (Figure 6C).
Figure 6.
The plasma parameters of triglyceride (TG) and total cholesterol (T-CHO). Changes in plasma TG (A) and T-CHO (B) during the experimental period in hens administered dextran sodium sulfate (DSS group) or water (CON group). Open circle plots represent the control group, and the orange rhombus plots represent the DSS group. Values are the mean ± SEM (CON and DSS, n = 8 and 7). (C) The concentration of TG and cholesterol (CHO) in VLDLy (particle size with diameter of 25–44 nm) separated by high performance liquid chromatography (HPLC). Open circle plots represent the CON group, and the orange rhombus plots represent the DSS group. Asterisks (*, **) indicate significant differences between the control and DSS groups at the same time point (P < 0.05 and P < 0.01, respectively). Lower case letters (a−c) indicate significant differences between the time points (P < 0.05).
Gene Expression of Tight Junction Proteins and Lipoprotein Receptors in the Follicular Granulosa Layer
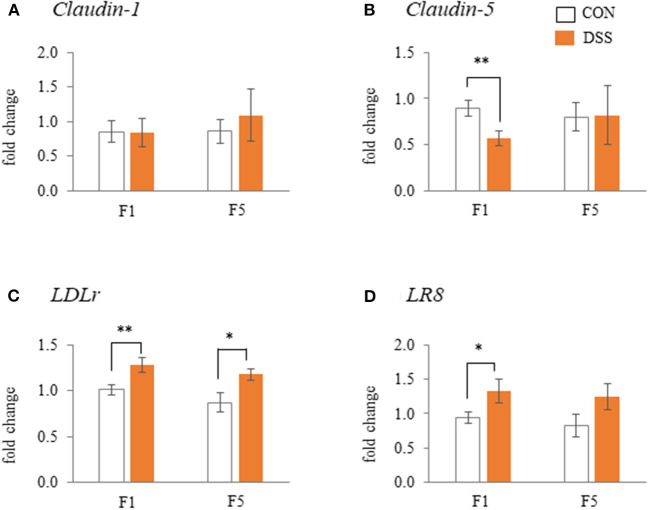
The weight of the F1 follicle was 14.78 ± 0.79 g in CON group and 13.99 ± 0.35 g in DSS group. The F5 follicle weight was 1.78 ± 0.54 g in CON group and 2.12 ± 0.29 g in DSS group. There were no significant differences between the CON and DSS groups in both F1 and F5 follicles. Gene expression of Claudin-5 in the F1 follicles was lower in the DSS group than in the CON group, but not in the F5 follicle (Figure 7B). DSS treatment did not affect Claudin-1 gene expression in either F1 or F5 follicles (Figure 7A). The gene expression of LDLr in F1, F5, and LR8 in F1 was significantly higher in the DSS group than in the CON group, but not in the LR8 of the F5 follicle (Figures 7C,D).
Figure 7.
Effects of dextran sodium sulfate (DSS) on the mRNA expression of the tight-junction [(A) and (B)] and lipoprotein receptors [(C) and (D)] in the granulosa cells of F1 and F5 follicles. Open bars represent the CON group, and orange filled bars represent the DSS group. Values are the fold change in target gene expression when compared to a standard sample from the CON group of each segments (CON and DSS, n = 8 and 7). Target gene expression was normalized to the house-keeping gene RPS17. Asterisks (*, **) indicate significant differences between the water (control) and DSS-treated groups (P < 0.05 and P < 0.01, respectively).
Gene Expression of Gonadotropin and IL-1β in the Anterior Pituitary Gland
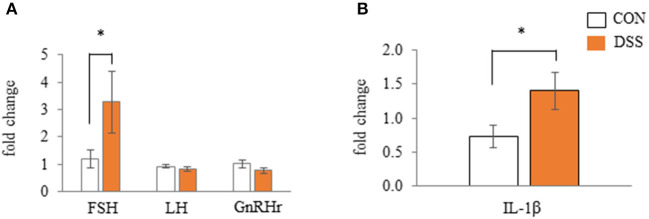
The pituitary gland expressed the FSH, LH, and GnRHr gene. Only the FSH gene expression was significantly higher in the DSS group when compared to the CON group (Figure 8A). IL-1β (a pro-inflammatory cytokine) was higher in the DSS group than in the CON group (Figure 8B).
Figure 8.
Effects of dextran sodium sulfate (DSS) on the mRNA expression of gonadotropin and receptor (A) and interleukin-1β (B) in the anterior pituitary gland. Values are the fold change in target gene expression when compared to a standard sample from the CON group of each segments (CON and DSS, n = 8 and 7). Target gene expression was normalized to the house-keeping gene RPS17. Asterisk (*) indicate significant differences between the water (control) and DSS-treated groups (P < 0.05).
Plasma Corticosterone and LBP Levels During the Experiment
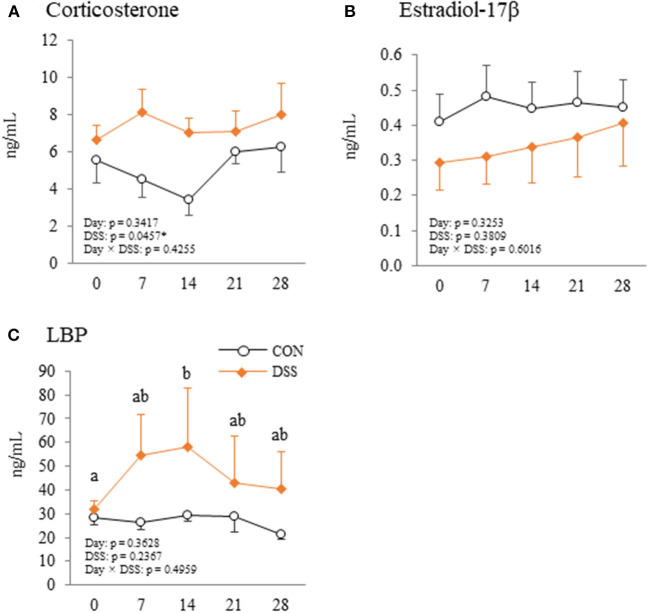
Corticosterone levels were higher in the DSS group than in the CON group, but the level did not vary with time in the DSS group (Figure 9A). Estradiol-17β concentrations did not vary with either DSS administration or time (Figure 9B). Plasma LBP concentration was significantly higher at day 14 than day 0 in DSS group (Figure 9C).
Figure 9.
Changes in plasma corticosterone (A), estradiol-17β (B), and lipopolysaccharide binding protein (LBP) (C) during the experimental period in hens administered dextran sodium sulphate (DSS group) or water (CON group). Open circle plots represent the control group, and the orange rhombus plots represent the DSS group. Values are the mean ± SEM (CON and DSS, n = 8 and 7). Lower case letters (a,b) indicate significant differences between the time points (P < 0.05).
Discussion
This report demonstrates the effects of the oral administration of a low dose of DSS on the intestinal environment and egg production in laying hens. The significant findings in this study identified that a lower concentration of orally administered DSS caused (1) a decrease in the ratio of villus height/crypt depth and occludin gene expression, TGF-β4 expression, and diversity of cecal microbiota and an increase in IL-6, (2) a decrease in egg production and egg yolk weight, (3) no effect on the liver function that is related to yolk precursor production, and an increase in VLDLy in the blood plasma, (4) enhanced egg yolk precursor accumulation in the gene expression pattern in the follicular granulosa layer, and (5) an increase in FSH and IL-1β gene expression in the pituitary gland.
DSS has been used to induce intestinal inflammation. We previously orally administered 0.9 g DSS/kg BW to laying hens for five consecutive days. This caused histological disintegration of the mucosal epithelial tissue in the cecum, as well as an increased frequency of monocytes/macrophages, and increased expression of IL-1β, IL-6, CXCLi2, IL-10, and TGFβ-4 with a decrease in feed intake (Nii et al., 2020). The previous results suggested that the DSS administration caused heavy intestinal inflammation and damage to the intestinal mucosa. In the present study, we chose a lower DSS concentration than in our previous research. Oral administration of 0.225 g DSS/kg BW for 28 days increased IL-6 gene expression in the cecum and decreased TGFβ-4 in the rectum (Figures 2B,C). In addition, DSS treatment decreased the ratio of villus/crypt in the cecum and rectum and Occludin gene expression in the cecum (Figures 1E,F). However, BW and feed intake did not change in the DSS group, and the specific signs of mucosal damage, such as infiltration of the blood cells and detachment of the epithelium, were not observed in any of the intestinal segments in the DSS group (Figures 1A–D). It has been reported that the ratio of villus/crypt in the intestinal mucosa decreases under inflammatory conditions, for example, during an Eimeria infection (Li et al., 2018). The decrease in the villus/crypt ratio has been widely acknowledged as a biomarker of poor intestinal health (Ducatelle et al., 2018). Therefore, the oral administration of a lower concentration of DSS in this study caused poor intestinal morphology without severe symptoms. Furthermore, DSS treatment decreased the alpha-diversity in the cecal contents. The beta-diversity was different between the DSS group and the CON group (Figures 3B,C). The presence of Elusimicrobaceae was eliminated by DSS administration in the present study. The character of Elusimicrobaceae remains unknown, but its population increases with probiotic treatment in the human intestine (Nagpal et al., 2018). It is known that DSS-induced colitis causes dysbiosis, including decreased microbiota diversity (Munyaka et al., 2016; Liu et al., 2020). Our results suggest that low dose DSS treatment may disrupt the intestinal environment, causing poor intestinal morphology and decreased microbiome diversity in the lower parts of the intestinal tract.
Direct-fed microbiotics (containing Lactobacillus acidophilus, Lactobacillus casei, Enterococcus faecium, and Bifidobacterium thermophium) are known to balance microorganisms and increase egg size in laying hens (Davis and Anderson, 2002). This report suggests that the intestinal environment may affect egg size. DSS treatment slightly reduced the ratio of egg production and clearly decreased egg yolk weight in this study (Figure 4B). However, the weight of the whole-egg, eggshell, and egg white in the DSS group did not change during the experiment (Figures 4A,C,D). Our results suggest that DSS induced disruption in the intestinal environment and suppressed egg yolk accumulation into the ovarian follicle, but did not have a strong effect on oviduct function, such as egg white and eggshell formation.
Nutritional conditions are important factors that affect egg quality. In general, intestinal inflammation causes malnutrition through a decrease in feed intake, digestion, and absorption functions (Peuhkuri et al., 2010). Malnutrition caused by feed restriction (60–80% decrease compared with normal feed intake) decreased not only the whole-egg weight, but also egg-shell thickness with a decrease in plasma TG in laying hens in our previous study (Nii et al., 2020). In addition, the plasma TG level of molting hens (caused by feed removal) was significantly decreased during the molting phase (Meng et al., 2013). In the present study, feed intake and BW did not decrease with slight intestinal inflammation. In addition, plasma TG levels increased from day 7 to 28 in the DSS group (Figure 6A). The dynamics of TG concentration in this study are completely different to malnutrition caused by feed restrictions that show low blood TG levels (Landers et al., 2008). Furthermore, in general, malnourished hens produce thin eggshell eggs. The eggshell weight did not change during the experimental period in the DSS group in this study (Figure 4C). Therefore, it is assumed that the nutrient condition is not the sole factor suppressing egg yolk size in the DSS group in this study.
VLDL and VTG are the main components of egg yolk precursors. Specific VLDLs, with a particle size of 25–44 nm, are called “VLDLy” that accumulates in developing egg yolk (Yang et al., 2013). VLDLy is formed with 23 units of VLDL-II (known as ApoVLDL-II) and 1 unit of apolipoprotein (apo) B100 (ApoB), and it is produced in the liver (Bujo et al., 1997; Walzem et al., 1999). We previously reported that rapid severe intestinal inflammation caused by a higher concentration of DSS induced liver tissue inflammation, and the gene expression of lipid synthesis and egg yolk precursor production were significantly decreased in the liver (Nii et al., 2020). Therefore, we hypothesized that the decrease in egg yolk size was also caused by dysfunction of the production of the egg yolk precursor in the liver in the present study. However, liver tissue did not show any apparent damage in the DSS group (Figures 5A,B). In addition, the gene expression of ApoB and VTG-II was not changed in the DSS group. The VLDL-II was decreased in DSS group, but the value did not show a noticeable difference between the CON and DSS groups (Figure 5C). In contrast, TG and CHO levels in the VLDLy fraction directly reflecting the plasma VLDLy quantity was higher in the DSS group than in the CON group (Figure 6C). High concentrations of plasma TG and T-CHO in the DSS group may reflect a high quantity of VLDLy in the blood in the DSS group (Figures 6A,B). These results suggest that the liver maintained the function for yolk precursor production, and a sufficient amount of VLDLy was supplied from the liver into the blood. Nonetheless, VLDLy may not be utilized for follicle development in the ovary.
As mentioned above, we expected the yolk precursor uptake from blood to the follicle might be disrupted by DSS administration. The gene expression of Clauin-5 was decreased. The LDLr in F1 and F5 follicles, and LR8 in F1 follicles were increased in the granulosa layer of the DSS group (Figures 7B–D). During follicular growth, the egg yolk precursor uptake in ovarian follicles is enhanced by an increase in lipoprotein receptor expression and a weakening in the tight junction connection (Schuster et al., 2004; Stephens and Johnson, 2012; Rosewell et al., 2013). Hence, the present results of gene expression of Claudin-5 and lipoprotein receptors suggested that ovarian follicles in the DSS group more actively took up yolk precursor than in the CON group, and the activity may be higher in the F1 than in the F5 follicles. LDLr protein expression in follicular granulosa cells did not change among F1, F2, and F3 follicles (Hummel et al., 2003). LDLr and LR8 gene expression did not change during yellow follicle growth in White Leghorn laying hens (Seol et al., 2007). In addition, the weight of F1 and F5 follicles did not show significant differences between the CON and DSS groups in the present study. This result suggests that the difference in Claudin-5, LDLr, and LR8 gene expression in the DSS group did not depend on follicular size. Moreover, FSH gene expression in the pituitary gland was significantly higher in the DSS group than in the CON group in the present study (Figure 8A). FSH secreted from the anterior pituitary gland enhances follicle growth in the ovary. Therefore, the change in Claudin-5, LDLr, and LR8 gene expression in the granulosa layer may reflect the activation of follicle growth with an increase in FSH expression in the pituitary gland. Thus, it seems that ovarian follicles in the DSS group are activated by yolk precursor uptake through changes in the expression of Claudin-5 and lipoprotein receptors. Conversely, the actual yolk precursor uptake was suppressed. Further research is necessary to determine why follicle growth was suppressed by DSS induced disruption of the intestinal environment.
IL-1β, a pro-inflammatory cytokine, increased with stress conditions in the pituitary glands of mice (Nguyen et al., 2000). Intracerebroventricular injection of IL-1β increases plasma corticosterone and adrenocorticotropic hormone (ACTH) in rats (Brown et al., 1991). High plasma levels of corticosterone mediated by corticosterone pellet transplantation decreased the egg yolk mass in White Leghorn hens (Henriksen et al., 2011). These reports assumed that secreted IL-1β in the pituitary gland may increase blood corticosterone levels, and that corticosterone decreases egg yolk size in laying hens. In the present study, gene expression of IL-1β in the pituitary gland was significantly higher in the DSS group than in the CON group (Figure 8B), but the plasma corticosterone level did not change during the experimental period in both the CON and DSS groups (Figure 9A). Meanwhile, IL-1β injection into the brain caused a total disruption of the estrous cycle with a decrease in mRNA expression of LH and FSH in the pituitary gland as well as their plasma levels in rats (Rivest et al., 1993). However, the mRNA expression of FSH increased, and LH was not changed in the DSS group when compared to the CON group (Figure 8A), and the plasma estradiol-17β concentration in the DSS group did not change over time in the present study (Figure 9B). Therefore, it is unknown whether the increase in IL-1β in the pituitary gland affects the decrease in egg yolk size in the present study.
We previously reported that severe intestinal inflammation caused by DSS may lead to an influx of enteric gram-negative bacteria or LPS from the cecum to the liver through the portal vein (Nii et al., 2020). In addition, destruction of the intestinal mucosal barrier led to the invasion of enteric bacteria into the lamina propria, followed by an influx of bacteria and/or endotoxin to other organs through the blood stream (Jahnel et al., 2009; Qin et al., 2018). LPS stimulation increased the apoptosis level of follicular ovarian granulosa cells in cows (Wang et al., 2020). This report suggests that LPS contaminated blood may disrupt normal follicular growth. In the present study, DSS orally administration caused slight mucosal inflammation in the large intestine and decreased occludin gene expression in the cecum (Figures 1F, 2). In addition, the plasma LBP level, which indirectly indicates plasma LPS level, was increased in the DSS group (Figure 9C). Therefore, the endotoxin originating from the intestine by the bloodstream is likely to be one of the factors that cause dysfunction in egg yolk precursor accumulation in ovarian follicles.
The research showed the importance of the intestinal environment for poultry egg production. Specifically, weakened intestinal mucosal barrier function may result in an increase in endotoxin influx into the blood stream derived from intestinal contents, and it may inhibit ovarian follicular growth and causes decrease in egg-yolk size. Therefore, it is expected that an enhancement of intestinal mucosal barrier function improves egg-yolk size. Some feed additive such as probiotics and organic acid was reported to enhance intestinal mucosal barrier function (Wang et al., 2018; Yang et al., 2019). Thus, these materials are expected to contribute to the improvement of egg-yolk size in poultry farming. In addition, the DSS method used in this study is useful for the studies on poor intestinal environment model in hens. The method is expected to be used for screening of some materials which have ameliorating effects on the intestinal environment and egg production in laying hens.
Therefore, the lower concentration of DSS administered orally caused a slight disruption in the intestinal environment. This disruption included poor intestinal morphology and decreased the cecal microbiome diversity. The change in the intestinal environment decreased the egg yolk size without decreasing the VLDLy supply from the liver. The decrease in egg yolk size is likely to be caused by dysfunctional egg-yolk precursor uptake in the ovarian follicles in association with increase in the influx of LPS. In conclusion, the oral administration of a lower dose of DSS is useful method to causes slight disruption of intestinal environment, and the disrupted intestinal condition decreases egg yolk size through disfunction of ovarian follicle.
Data Availability Statement
The datasets generated for this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ddbj.nig.ac.jp/, DRA010795.
Ethics Statement
The animal study was reviewed and approved by Hiroshima University Animal Research Committee.
Author Contributions
TN: conceived, designed, performed, and analyzed the experiments. NI: ELISA analysis of corticosterone and estradiol-17β. TB and YY: critical discussion and reviewing of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Funding. This work was supported by a Grant-in-Aid for Research Activity Start-up (No. 17H06892) and Grant-in-Aid for Early-Career Scientists (No. 18K14569) from the Japan Society for the Promotion of Science (JSPS) to TN.
References
- Belote B. L., Tujimoto-Silva A., Hummelgen P. H., Sanches A. W. D., Wammes J. C. S., Hayashi R. M., et al. (2018). Histological parameters to evaluate intestinal health on broilers challenged with Eimeria and Clostridium perfringens with or without enramycin as growth promoter. Poult. Sci. 97, 2287–2294. 10.3382/ps/pey064 [DOI] [PubMed] [Google Scholar]
- Brown R., Li Z., Vriend C. Y., Nirula R., Janz L., Falk J., et al. (1991). Suppression of splenic macrophage interleukin-1 secretion following intracerebroventricular injection of interleukin-1β: evidence for pituitary-adrenal and sympathetic control. Cell. Immunol. 132, 84–93. 10.1016/0008-8749(91)90008-Y [DOI] [PubMed] [Google Scholar]
- Bujo H., Hermann M., Lindstedt K. A., Nimpf J., Schneider W. J. (1997). Low density lipoprotein receptor gene family members mediate yolk deposition. J. Nutr. 127(5 Suppl.), 801s–804s. 10.1093/jn/127.5.801S [DOI] [PubMed] [Google Scholar]
- Davis G. S., Anderson K. E. (2002). The effects of feeding the direct-fed microbial, primalac, on growth parameters and egg production in single comb white leghorn hens. Poult. Sci. 81, 755–759. 10.1093/ps/81.6.755 [DOI] [PubMed] [Google Scholar]
- Ducatelle R., Goossens E., De Meyer F., Eeckhaut V., Antonissen G., Haesebrouck F., et al. (2018). Biomarkers for monitoring intestinal health in poultry: present status and future perspectives. Vet. Res. 49:43. 10.1186/s13567-018-0538-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasina Y. O., Lillehoj H. S. (2019). Characterization of intestinal immune response to Clostridium perfringens infection in broiler chickens. Poult. Sci. 98, 188–198. 10.3382/ps/pey390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson A., Tormo-Badia N., Baridi A., Xu J., Molin G., Hagslatt M. L., et al. (2015). Immunological alteration and changes of gut microbiota after dextran sulfate sodium (DSS) administration in mice. Clin. Exp. Med. 15, 107–120. 10.1007/s10238-013-0270-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijmans J., Wielenga M. C., Rosekrans S. L., van Lidth de Jeude J. F., Roelofs J., Groothuis P., et al. (2014). Oestrogens promote tumorigenesis in a mouse model for colitis-associated cancer. Gut 63, 310–316. 10.1136/gutjnl-2012-304216 [DOI] [PubMed] [Google Scholar]
- Henriksen R., Groothuis T. G., Rettenbacher S. (2011). Elevated plasma corticosterone decreases yolk testosterone and progesterone in chickens: linking maternal stress and hormone-mediated maternal effects. PLoS ONE 6:e23824. 10.1371/journal.pone.0023824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y. H., Lillehoj H. S., Lee S. H., Dalloul R. A., Lillehoj E. P. (2006). Analysis of chicken cytokine and chemokine gene expression following Eimeria acervulina and Eimeria tenella infections. Vet. Immunol. Immunopathol. 114, 209–223. 10.1016/j.vetimm.2006.07.007 [DOI] [PubMed] [Google Scholar]
- Hummel S., Lynn E. G., Osanger A., Hirayama S., Nimpf J., Schneider W. J. (2003). Molecular characterization of the first avian LDL receptor: role in sterol metabolism of ovarian follicular cells. J. Lipid Res. 44, 1633–1142. 10.1194/jlr.M300014-JLR200 [DOI] [PubMed] [Google Scholar]
- Huyghebaert G., Ducatelle R., Immerseel F. V. (2011). An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 187, 182–188. 10.1016/j.tvjl.2010.03.003 [DOI] [PubMed] [Google Scholar]
- Isobe N., Nakao T. (2003). Direct enzyme immunoassay of progesterone in bovine plasma. Anim. Sci. J. 74, 369–373. 10.1046/j.1344-3941.2003.00128.x [DOI] [Google Scholar]
- Jahnel J., Fickert P., Langner C., Hogenauer C., Silbert D., Gumhold J., et al. (2009). Impact of experimental colitis on hepatobiliary transporter expression and bile duct injury in mice. Liver Int. 29, 1316–1325. 10.1111/j.1478-3231.2009.02044.x [DOI] [PubMed] [Google Scholar]
- Kerr T. A., Ciorba M. A., Matsumoto H., Davis V. R., Luo J., Kennedy S., et al. (2012). Dextran sodium sulfate inhibition of real-time polymerase chain reaction amplification: a poly-A purification solution. Inflamm. Bowel. Dis. 18, 344–348. 10.1002/ibd.21763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasing K. C. (2007). Nutrition and the immune system. Br. Poult. Sci. 48, 525–537. 10.1080/00071660701671336 [DOI] [PubMed] [Google Scholar]
- Kuttappan V. A., Berghman L. R., Vicuna E. A., Latorre J. D., Menconi A., Wolchok J. D., et al. (2015). Poultry enteric inflammation model with dextran sodium sulfate mediated chemical induction and feed restriction in broilers. Poult. Sci. 94, 1220–1226. 10.3382/ps/pev114 [DOI] [PubMed] [Google Scholar]
- Kuttappan V. A., Vicuna E. A., Faulkner O. B., Huff G. R., Freeman K. A., Latorre J. D., et al. (2016). Evaluation of changes in serum chemistry in association with feed withdrawal or high dose oral gavage with dextran sodium sulfate- (DSS-) induced gut leakage in broiler chickens. Poult. Sci. 95, 2565–2569. 10.3382/ps/pew171 [DOI] [PubMed] [Google Scholar]
- Landers K. L., Moore R. W., Herrera P., Landers D. A., Howard Z. R., McReynolds J. L., et al. (2008). Organ weight and serum triglyceride responses of older (80 week) commercial laying hens fed an alfalfa meal molt diet. Bioresour. Technol. 99, 6692–6696. 10.1016/j.biortech.2007.11.045 [DOI] [PubMed] [Google Scholar]
- Lensing M., van der Klis J. D., Yoon I., Moore D. T. (2012). Efficacy of Saccharomyces cerevisiae fermentation product on intestinal health and productivity of coccidian-challenged laying hens. Poult. Sci. 91, 1590–1597. 10.3382/ps.2011-01508 [DOI] [PubMed] [Google Scholar]
- Li Z., Wang W., Liu D., Guo Y. (2018). Effects of Lactobacillus acidophilus on the growth performance and intestinal health of broilers challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 9:25. 10.1186/s40104-018-0243-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Xu S., Zeng D., Ni X., Zhou M., Zeng Y., et al. (2017). Disruption in the cecal microbiota of chickens challenged with Clostridium perfringens and other factors was alleviated by Bacillus licheniformis supplementation. PLoS ONE 12:e0182426. 10.1371/journal.pone.0182426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G. H., Liu H. M., Chen Y. S., Lee T. Y. (2020). Effect of electroacupuncture in mice with dextran sulfate sodium-induced colitis and the influence of gut microbiota. Evid. Based Complement Altern. Med. 2020:2087903. 10.1155/2020/2087903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menconi A., Hernandez-Velasco X., Vicuna E. A., Kuttappan V. A., Faulkner O. B., Tellez G., et al. (2015). Histopathological and morphometric changes induced by a dextran sodium sulfate (DSS) model in broilers. Poult. Sci. 94, 906–911. 10.3382/ps/pev054 [DOI] [PubMed] [Google Scholar]
- Meng X. T., Hou N. N., Wang X. J., Jiao H. C., Zhao J. P., Song Z. G., et al. (2013). Increased hepatic yolk precursor synthesis, secretion and facilitated uptake by follicles are involved in the rejuvenation of reproductive performance of molted hens (Gallus gallus domesticus). General Comp. Endocrinol. 194, 198–207. 10.1016/j.ygcen.2013.09.007 [DOI] [PubMed] [Google Scholar]
- Munyaka P. M., Rabbi M. F., Khafipour E., Ghia J.-E. (2016). Acute dextran sulfate sodium (DSS)-induced colitis promotes gut microbial dysbiosis in mice. J. Basic Microbiol. 56, 986–998. 10.1002/jobm.201500726 [DOI] [PubMed] [Google Scholar]
- Nagpal R., Wang S., Ahmadi S., Hayes J., Gagliano J., Subashchandrabose S., et al. (2018). Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome. Sci. Rep. 8:12649. 10.1038/s41598-018-30114-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen K. T., Deak T., Will M. J., Hansen M. K., Hunsaker B. N., Fleshner M., et al. (2000). Timecourse and corticosterone sensitivity of the brain, pituitary, and serum interleukin-1β protein response to acute stress. Brain Res. 859, 193–201. 10.1016/S0006-8993(99)02443-9 [DOI] [PubMed] [Google Scholar]
- Nii T., Bungo T., Isobe N., Yoshimura Y. (2020). Intestinal inflammation induced by dextran sodium sulphate causes liver inflammation and lipid metabolism disfunction in laying hens. Poul. Sci. 99, 1663–1677. 10.1016/j.psj.2019.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuhkuri K., Vapaatalo H., Korpela R. (2010). Even low-grade inflammation impacts on small intestinal function. World J. Gastroenterol. 16, 1057–1062. 10.3748/wjg.v16.i9.1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possemiers S., Grootaert C., Vermeiren J., Gross G., Marzorati M., Verstraete W., et al. (2009). The intestinal environment in health and disease - recent insights on the potential of intestinal bacteria to influence human health. Curr. Pharm. Des. 15, 2051–2065. 10.2174/138161209788489159 [DOI] [PubMed] [Google Scholar]
- Qin C., Zhang H., Zhao L., Zeng M., Huang W., Fu G., et al. (2018). Microbiota transplantation reveals beneficial impact of berberine on hepatotoxicity by improving gut homeostasis. Sci. China Life Sci. 61, 1537–1544. 10.1007/s11427-017-9202-0 [DOI] [PubMed] [Google Scholar]
- Ritzi M. M., Abdelrahman W., Mohnl M., Dalloul R. A. (2014). Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poult. Sci. 93, 2772–2778. 10.3382/ps.2014-04207 [DOI] [PubMed] [Google Scholar]
- Rivest S., Lee S., Attardi B., Rivier C. (1993). The chronic intracerebroventricular infusion of interleukin-1 beta alters the activity of the hypothalamic-pituitary-gonadal axis of cycling rats. I. Effect on LHRH and gonadotropin biosynthesis and secretion. Endocrinology 133, 2424–2430. 10.1210/endo.133.6.8243260 [DOI] [PubMed] [Google Scholar]
- Rosewell K. L., Li F., Puttabyatappa M., Akin J. W., Brännström M., Curry T. E., Jr. (2013). Ovarian expression, localization, and function of tissue inhibitor of metalloproteinase 3 (TIMP3) during the periovulatory period of the human menstrual cycle. Biol. Reprod. 89:121. 10.1095/biolreprod.112.106989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh M., Trinchieri G. (2011). Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat. Rev. Immunol. 11, 9–20. 10.1038/nri2891 [DOI] [PubMed] [Google Scholar]
- Sato M., Sugino T., Yoshimura Y., Isobe N. (2011). Follicular persistence induced by adrenocorticotropic hormone administration in goats. J. Reprod. Dev. 57, 212–216. [DOI] [PubMed] [Google Scholar]
- Schuster M. K., Schmierer B., Shkumatava A., Kuchler K. (2004). Activin A and follicle-stimulating hormone control tight junctions in avian granulosa cells by regulating occludin expression. Biol. Reprod. 70, 1493–1499. 10.1095/biolreprod.103.024331 [DOI] [PubMed] [Google Scholar]
- Seol H. S., Sato K., Matsubara Y., Schneider W. J., Akiba Y. (2007). Modulation of sterol regulatory element binding protein-2 in response to rapid follicle development in chickens. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 147, 698–703. 10.1016/j.cbpb.2007.04.012 [DOI] [PubMed] [Google Scholar]
- Shang Y., Kumar S., Oakley B., Kim W. K. (2018). Chicken gut microbiota: importance and detection technology. Front. Vet. Sci. 5:254. 10.3389/fvets.2018.00254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon K., Arts J. A., de Vries Reilingh G., Kemp B., Lammers A. (2016). Effects of early life dextran sulfate sodium administration on pathology and immune response in broilers and layers. Poult. Sci. 95, 1529–1542. 10.3382/ps/pew074 [DOI] [PubMed] [Google Scholar]
- Stanley D., Wu S. B., Rodgers N., Swick R. A., Moore R. J. (2014). Differential responses of cecal microbiota to fishmeal, Eimeria and Clostridium perfringens in a necrotic enteritis challenge model in chickens. PLoS ONE 9:e104739 10.1371/journal.pone.0104739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens C. S., Johnson P. A. (2012). Gene expression and regulation of the tight junction protein occludin in the growing follicle of the domestic hen. Biol. Reprod. 87(Suppl. 1), 510–510. 10.1093/biolreprod/87.s1.510 [DOI] [Google Scholar]
- Walzem R. L., Hansen R. J., Williams D. L., Hamilton R. L. (1999). Estrogen induction of VLDLy assembly in egg-laying hens. J. Nutr. 129(2S Suppl.), 467s–472s. 10.1093/jn/129.2.467S [DOI] [PubMed] [Google Scholar]
- Wang L., Li L., Lv Y., Chen Q., Feng J., Zhao X. (2018). Lactobacillus plantarum restores intestinal permeability disrupted by salmonella infection in newly-hatched chicks. Sci. Rep. 8:2229 10.1038/s41598-018-20752-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Li C., Wang Y., Li L., Han Z., Wang G. (2020). UFL1 alleviates LPS-induced apoptosis by regulating the NF-κB signaling pathway in bovine ovarian granulosa cells. Biomolecules 10:260 10.3390/biom10020260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Suh Y., Choi Y. M., Shin S., Han J. Y., Lee K. (2013). Loss of fat with increased adipose triglyceride lipase-mediated lipolysis in adipose tissue during laying stages in quail. Lipids 48, 13–21. 10.1007/s11745-012-3742-6 [DOI] [PubMed] [Google Scholar]
- Yang X., Liu Y., Yan F., Yang C. (2019). Effects of encapsulated organic acids and essential oils on intestinal barrier, microbial count, and bacterial metabolites in broiler chickens. Poult. Sci. 98, 2858–2865. 10.3382/ps/pez031 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ddbj.nig.ac.jp/, DRA010795.