Abstract
Purpose
Swallowing is a complex process, mediated by a broad bilateral neural network that spans from the brainstem to subcortical and cortical brain structures. Although the cortex's role in swallowing was historically neglected, we now understand, especially through clinical observations and research of patients with stroke, that it substantially contributes to swallowing control. Neuroimaging techniques (e.g., magnetic resonance imaging) have helped significantly to elucidate the role of cortical and subcortical brain areas, in general, and the importance of specific areas in swallowing control in healthy individuals and patients with stroke. We will review recent discoveries in cortical and subcortical neuroimaging research studies and their generalizability across patients to discuss their potential implications and translation to dysphagia diagnosis and treatment in clinical practice.
Conclusions
Stroke lesion locations have been identified that are commonly associated across patients with the occurrence and recovery of dysphagia, suggesting that clinical brain scans provide useful information for improving the diagnosis and treatment of patients with stroke. However, individual differences in brain structure and function limit the generalizability of these relationships and emphasize that the extent of the motor and sensory pathology in swallowing, and how the patient recovers, also depends on a patient's individual brain constitution. The involvement of the damaged brain tissue in swallowing control before the stroke and the health of the residual, undamaged brain tissue are crucial factors that can differ between individuals.
Every 40 s, an individual has a stroke, with 795,000 people experiencing a new or recurrent stroke per year in the United States (Mozaffarian et al., 2015). Fortunately, there has been a remarkable decline in stroke mortality with a decrease of at least 35% since 2001 (Mozaffarian et al., 2015; Wilmskoetter et al., 2016). Nevertheless, stroke remains a leading cause of death and disability worldwide (Centers for Disease Control and Prevention, 2009; Mozaffarian et al., 2015).
A common and disabling condition after stroke is dysphagia. Dysphagia affects between 55% and 78% of all stroke survivors (Daniels & Foundas, 1999; Martino et al., 2005), with studies using instrumental imaging techniques (such as the videofluoroscopic swallowing study [VFSS]) identifying a higher prevalence than noninstrumental techniques. An investigation of swallowing using VFSS found that 70% of all patients with acute stroke aspirated food or liquids into the lower airway indicating severe dysphagia (Osawa et al., 2013). Dysphagia is directly associated with stroke mortality (Sharma et al., 2001) and can lead to serious poststroke complications such as malnutrition, dehydration (Crary et al., 2013), and pneumonia (Marik & Kaplan, 2003; Martino et al., 2005). Generally, dysphagia can have severe social and psychological impact on patients with adverse effects on self-esteem, socialization, and enjoyment of life (Ekberg et al., 2002). With this broad range of complications and adverse effects from dysphagia, it is essential to understand the occurrence and recovery of poststroke dysphagia in order to provide the best health care for patients with stroke.
Why Is Dysphagia Common After Stroke?
The main reasons for the common occurrence of dysphagia after stroke are likely (a) the physiological and biomechanical complexity of swallowing that can easily be interrupted and (b) the number of brain regions involved in the control of swallowing that can be damaged by a stroke. Swallowing is a highly frequent, fast, and complex process. Healthy individuals swallow about 600 times a day (Lear et al., 1965). Transporting a bolus from the mouth to esophagus is as fast as 1–2 s and involves several cranial and spinal nerves and more than 30 muscle pairs. Swallowing can be divided into different components that occur—synergistically—during the swallow. These components can refer to the movement of structures involved in the swallow, such as the lips, tongue, velum, pharynx, epiglottis, larynx, and upper esophageal sphincter. Because of the complexity of the swallow process, even minor disruptions of the sensory and motor control of components involved in swallowing can lead to serious swallow impairments.
Due to the complexity of swallowing, it is mediated by numerous brain regions. Swallowing depends on the concerted activity between brainstem nuclei and neocortical control. Historically, it is well known that the brainstem is a main contributor to swallowing (Jean, 2001; Jean & Dallaporta, 2013). The brainstem includes parts of the corticobulbar tracts with the cranial nerve nuclei that directly send and receive projections from muscles of the face, mouth, larynx, and pharynx. However, swallowing impairments often arise from damage to the isolated neocortex (Robbins et al., 1993; Smithard, O'Neill, Martin, & England, 1997) or subcortical regions (Cola et al., 2010; Levine et al., 1992) with complete sparing of the brainstem.
Why Do Many but Not All Patients With Stroke Recover From Dysphagia?
Patients with stroke are at a high risk to develop dysphagia, but fortunately, their chances to recover from poststroke dysphagia are also high. Up to 50% of patients with stroke-related dysphagia spontaneously recover within the first 7 days (Langdon et al., 2007; Smithard, O'Neill, England, et al., 1997).
Those patients who do not improve spontaneously may recover completely or, in part, with dysphagia therapy (Huckabee & Cannito, 1999; Robbins et al., 2007; Shaker et al., 2002). Nevertheless, a large number of patients (10%–50%) with persistent stroke-related dysphagia may never recover, even with therapy (Broadley et al., 2003; Langdon et al., 2007; Mann et al., 1999; Smithard, O'Neill, England, et al., 1997).
One major factor that generally contributes to recovery after stroke is “brain plasticity.” Brain plasticity after stroke depends on multiple factors, such as age, genetic factors (Jayasekeran et al., 2011), complications following the stroke (e.g., hemorrhagic transformation; Bustamante et al., 2016), patient health status, and rehabilitation therapy (Kleim & Jones, 2008; Warraich & Kleim, 2010). In turn, the success of rehabilitation therapy depends on a variety of factors, for example, the treatment's specificity, intensity, and repetition (Kleim & Jones, 2008; Robbins et al., 2008); the patient's lifestyle; and time after stroke (Warraich & Kleim, 2010). Furthermore, brain-related factors, such as the preservation of structural brain networks (Bonilha et al., 2015) and the location of the lesion, have an impact on stroke recovery and rehabilitation success (Hope et al., 2013; Kim et al., 2019; Plowman et al., 2012). The reason for the association between lesion locations and stroke recovery might be that the potential for compensatory neural recovery, such as recruitment of new residual brain areas, depends on the brain region that is damaged.
Little is known about how lesion locations affect swallowing recovery. Interestingly, the high recovery rate within the first weeks after stroke is not typically seen in other sensorimotor or cognitive functions. It is speculated that bilateral representations of the swallowing network and alternative routes of innervations facilitate rapid recovery in dysphagia. For example, it has been proposed that recovery from cortical stroke–induced dysphagia depends on the cortical reorganization in the unaffected hemisphere (Hamdy et al., 1998, 2000; Li et al., 2009). However, engaging the unaffected hemisphere or alternative routes within the swallowing network does not apply to all individuals with poststroke dysphagia because not everyone recovers. One reason might be patients' individual premorbid swallowing topography. Not every brain is built and functions in the same way. Thus, besides differences in factors contributing to brain plasticity and rehabilitation, differences in the premorbid neural control of swallowing can explain why some but not all patients with stroke recover from dysphagia.
What Is the Clinical Importance of Understanding Relationships Between Lesion Locations and Dysphagia?
Understanding the detailed relationship between lesion locations and dysphagia occurrence would allow an early identification of patients at risk for dysphagia because information on lesion location is usually available within minutes or hours after hospital admission. Moreover, if lesion locations were predictive not only of dysphagia occurrence but of the underlying detailed swallowing impairment, clinicians could use this information as an adjunct to their swallowing assessments to estimate and/or verify their diagnosis of a patient's underlying swallowing impairment. Knowing a patient's swallowing impairment is essential for clinicians to prescribe optimized dysphagia treatment because the treatment of dysphagia targets the physiology of the swallow (Daniels et al., 2019; Logemann, 1998; Martin-Harris et al., 2000).
Furthermore, lesion locations may be one of many determinants of swallowing recovery after stroke as well as responsiveness to different treatment approaches (Kim et al., 2019; Suntrup-Krueger et al., 2018). Depending on which regions of the neural swallowing network are lesioned, patients may be more or less likely to recover and to respond to certain types of dysphagia treatment. However, evidence is scarce, and further investigations of the relationship between lesion locations and swallowing recovery are needed to support translation into clinical practice.
Cortical and Subcortical Control of Swallowing Impairment After Stroke
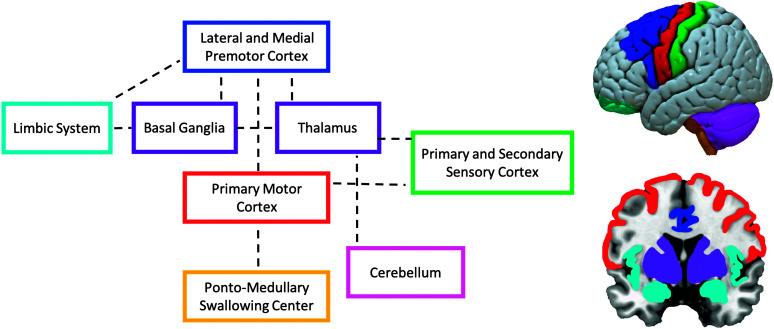
The neuroanatomical model of swallowing developed by Daniels et al. exemplifies the complexity of the neural control of swallowing (Cola et al., 2010; Daniels & Foundas, 1999; Leopold & Daniels, 2010). This model is based on evidence from functional imaging, lesion–symptom mapping, and animal studies (see Figure 1). The model emphasizes that swallowing is mediated by a complex distributed, hierarchically organized, neural network consisting of bilateral sensory and motor cortical regions that communicate through ascending and descending white matter tracts with various subcortical and brainstem regions. Previous research has identified a cerebral network that controls swallowing, which involves the somatosensory cortex, supplementary motor area, operculum, prefrontal and inferior frontal cortex, cingulate cortex, insular cortex, thalamus, basal ganglia, cerebellum, pons, and medulla (Babaei et al., 2013; Hamdy, 2006; Lowell et al., 2012; Martin et al., 2001; Mosier & Bereznaya, 2001; Yuan et al., 2015). Determining which of these brain regions are activated during swallowing depends on the stimuli that trigger swallowing initiation (such as cognitive, emotional, and intero- or exteroceptive stimuli; Leopold & Daniels, 2010). In corticospinal motor systems such as locomotion, it is believed that the motor areas in the cerebral cortex are responsible for skilled movements, the basal ganglia is responsible for the initiation and selection of motor programs, the cerebellum is responsible for motor coordination and correction, and the thalamus serves as a relay station for primarily afferent but also efferent signals traveling between the cortex and those brain regions (Grillner, 2008). Thus, for swallowing, the orchestration of supranuclear brain regions that project directly or indirectly to the brainstem—where the final swallowing command is elicited—results in an accurately timed and precisely executed swallow.
Figure 1.
Neuroanatomical model of swallowing by Leopold and Daniels (2010, p. 251). Colored boxes and brain figures were added. Adapted with permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Dysphagia, “Supranuclear Control of Swallowing,” Norman A. Leopold and Stephanie K. Daniels (2010), Copyright © 2009, Springer Nature.
Although the model by Daniels et al. highlights the involvement of many cortical brain regions in swallowing control, recent studies suggest that several other cortical regions are also involved in swallowing control and, more importantly, when lesioned, lead to swallowing impairment (Suntrup et al., 2015; Suntrup-Krueger et al., 2017; Wilmskoetter, Bonilha, et al., 2019). The differentiation between regions being involved in swallowing and regions being crucial for swallowing is important because not every region that is active during a task (e.g., swallowing) leads to impairment when a stroke damages this region (Rorden et al., 2007). For example, regions that show activation during activation studies (such as functional magnetic resonance imaging [MRI], electroencephalography, magnetoencephalography) might not be crucial in case of a brain injury because their role can be compensated for by other regions or the activation reflects connections to other brain regions or a different aspect of the task (such as taste). Thus, to understand which lesion locations can lead to swallowing impairment, a review of studies conducting lesion–symptom mapping (studies investigating which lesion locations are associated with swallowing impairment) might be most informative. We will review factors such as the side, site, and size of the lesion in terms of their impact on dysphagia occurrence.
Does the Side of the Lesion Matter?
Swallowing is a midline function and is bilaterally represented; thus, regions in the left and right hemisphere are involved in swallowing control. Although bilateral lesions are strong predictors for dysphagia after stroke (Kumar et al., 2014), unilateral lesions in either hemisphere can lead to dysphagia (Daniels & Foundas, 1999; Mistry et al., 2007; Wilmskoetter et al., 2018). Moreover, swallowing is asymmetrically represented, which explains why the side of the stroke lesion seems to matter whether or to what degree a patient develops swallowing impairment after a stroke. Which hemisphere has stronger swallowing representations (the swallowing dominant hemisphere) is not related to an individual's hand dominance and varies between individuals and even within individuals in regard to swallowing musculature and task (Hamdy et al., 1996, 1997; Mistry et al., 2007). These variations in the lateralization of swallowing may at least, in part, explain why studies report inconsistent findings regarding which hemisphere leads to dysphagia (or more severe dysphagia) when lesioned. For example, some studies report that lesions in the left hemisphere are more strongly associated with dysphagia (Cola et al., 2010), others report the right hemisphere (Suntrup et al., 2015; Wilmskoetter et al., 2018), and even other studies report no differences between the hemispheres (Daniels et al., 1999; Daniels & Foundas, 1999; Daniels et al., 2017).
Does the Size of the Lesion Matter?
Lesion size is one of many factors contributing to worse outcome and recovery after stroke. The more that crucial brain areas are damaged, the higher the likelihood that dysphagia (González-Fernández et al., 2008; Galovic et al., 2013) and other sequelae (e.g., aphasia [Benghanem et al., 2019], infections [Hug et al., 2009]) occur and persist because less residual brain areas are intact to compensate for the damage. However, lesion size and dysphagia severity are not in a perfect linear relationship. If a crucial region that is essential for swallowing is lesioned, it does not matter if the overall lesion size is large or small. For example, small lesions in the internal capsule (less than 5 ml) can lead to severe dysphagia (Wilmskoetter et al., 2018). Thus, recent evidence suggests that overall lesion volume is less important in regard to functional outcomes and recovery after stroke than lesion size to specific, crucial brain areas (Chen et al., 2000; Zhu et al., 2010).
Cortical Regions Crucial for Swallowing
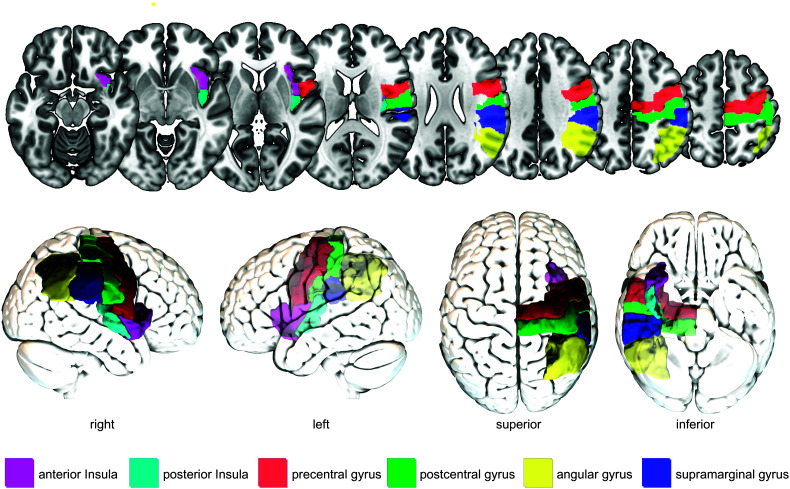
Several cortical regions have been identified in different studies with dysphagia after stroke. Here, we will discuss the sensorimotor, parieto-temporal, and insular cortices because of their common identification as crucial regions for poststroke swallowing (see Figure 2). Importantly, this review does not cover all brain regions possibly involved in swallowing control after stroke but focuses on commonly reported regions.
Figure 2.
Commonly identified cortical brain regions crucial for swallowing after stroke. For illustration purposes, brain regions are shown only in the right hemisphere and not in both hemispheres. Colors were chosen arbitrarily.
Sensorimotor Cortices
The primary motor and primary sensory cortices (the sensorimotor cortex) are located in the precentral and postcentral gyrus, respectively, and are both known for their topographic organization called the motor and sensory homunculus. The homunculus contains motor or sensory representations of certain body parts, including swallowing relevant body parts such as the face, lips, teeth, gums, jaw, tongue, larynx, pharynx (e.g., Brown et al., 2008), and the upper part of the esophagus (Aziz et al., 1996; Dziewas et al., 2005). The primary somatosensory cortex regulates and executes movements by controlling and providing feedback to the brainstem through the corticobulbar tract that directly connects the swallow relevant areas in the primary motor cortex with the brainstem (González-Fernández et al., 2013; Teismann et al., 2007). With lost or diminished control of the somatosensory cortex, the brainstem will still likely generate a swallow, but the swallow is less coordinated (Teismann et al., 2007). Lesion–symptom mapping studies on patients with acute stroke demonstrated a link between lesions in the somatosensory cortices and dysphagia occurrence, in general (Suntrup et al., 2015; Suntrup-Krueger et al., 2017), and impairments in laryngeal elevation and vestibular closure, in particular (Wilmskoetter, Bonilha, et al., 2019). Because the fact that these impairments are related to less coordinated swallowing movements is difficult to tease apart in lesion–symptom mapping studies on patients with stroke, thus Mistry et al. (2007) sought to investigate the impact of “artificial” lesions to the primary motor cortex for swallowing performance in 13 healthy individuals (age range: 24–58 years). Participants were instructed to swallow on command 3-ml boluses of water delivered through a catheter in their mouth. The primary motor cortex was suppressed with repetitive transcranial magnetic stimulation creating an artificial, focal lesion. If applied to the dominant hemisphere (hemisphere with larger corticobulbar projections to the pharynx), swallowing reaction times decreased significantly as measured with a pressure transducer positioned in the pharynx. Furthermore, primary motor cortex suppression of either hemisphere decreased the performance in a highly skilled swallowing task where participants had to precisely time their swallow in a specific window of time. Participants still executed their swallowing tasks with no reports on decreased swallowing safety. Nevertheless, swallowing performance seemed less coordinated and less skilled after primary motor cortex suppression. Mistry et al. concluded that (a) unilateral lesions in the dominant primary motor cortex can lead to dysphagia and (b) the primary motor cortex is involved in the initiation of the swallow and, in contrast to other brain regions that are involved in swallowing initiation (e.g., insula, supplementary motor area, cingulate cortex), might have an inhibitory role on the brainstem swallowing regulation (observed by faster swallowing reaction times and speculative less-controlled swallowing after primary motor cortex suppression).
Parietal-Temporal Cortices
Besides lesions in the somatosensory cortices, lesions in the posterior part of the parietal and temporal lobes have also been linked to dysphagia after stroke. The role of these brain areas in swallowing control is unclear. Likely, these posterior parietal areas (i.e., the supramarginal and angular gyri) are involved in sensory–motor integration of afferent, sensory signals from swallowing musculature and structures to the cortex and efferent, motor signals from the cortex to swallowing musculature and structures. Rich connections of the temporal lobe to the frontal, parietal, and occipital lobes and the thalamus underline the potentially integrative role of these regions (Kiernan, 2012).
Oropharyngeal residue is commonly reported in association with lesions in the parietal–temporal cortical regions (Im Moon et al., 2012; Suntrup-Krueger et al., 2017; Wilmskoetter, Bonilha, et al., 2019). Furthermore, delayed or absent swallowing responses, impaired laryngeal vestibular closure, and reduced hyolaryngeal elevation have been reported in patients with parietal–temporal lesions compared to patients without lesions in these regions.
Insular Cortex
The insula is involved in multiple functions that are directly or indirectly related to swallowing, such as taste, touch, somatosensory and motor information (Augustine, 1996; Rolls, 2016), language (Fridriksson et al., 2018), and emotions (Phan et al., 2002). The multifaceted involvement of the insula in swallowing likely explains why the insula is one of the most consistently activated areas during swallowing and considered to be a central hub of the “swallowing network” (Babaei et al., 2013; Hamdy, 2006; Leopold & Daniels, 2010; Mosier & Bereznaya, 2001; Riecker et al., 2009; Yuan et al., 2015). The functional engagement of the insula is reflected by rich structural connections to brain areas crucial for swallowing. For example, the insula is closely connected to the primary and secondary somatosensory cortex, premotor area, supplementary motor area, frontal operculum, thalamus, anterior cingulate, and the nucleus tractus solitarius in the brainstem (Augustine, 1996; Daniels & Foundas, 1997; Leopold & Daniels, 2010).
Lesion–symptom mapping studies found that patients with lesions involving the insula compared to patients without lesions involving the insula were more often feeding tube–dependent within the first 48 hr after stroke (Galovic et al., 2016) and more likely to have persistent oral intake restrictions for more than 4 weeks (Galovic et al., 2017). In terms of poststroke swallowing physiology, lesions to the insula were associated with a later initiation of the pharyngeal swallow (Daniels & Foundas, 1997; Riecker et al., 2009; Stickler et al., 2003) and impaired laryngeal elevation and laryngeal vestibular closure (Wilmskoetter, Bonilha, et al., 2019). Likely, these relationships are consequences of the insula's contribution to the timing and synchronization of swallowing motor events through sensory–motor integration (Mosier & Bereznaya, 2001).
Subcortical Regions Crucial for Swallowing
Lesion–symptom mapping studies have revealed that the basal ganglia, internal capsule, periventricular white matter (PVWM), and thalamus are the main subcortical areas involved in dysphagia after stroke (Cola et al., 2010; Daniels et al., 2019; Leopold & Daniels, 2010). Cola et al. (2010) found that patients with subcortical stroke had a significantly slower oral transfer in comparison to healthy adults. The authors assessed seven patients with subcortical stroke (including PVWM, thalamus, deep white matter, internal capsule, putamen, external capsule) who presented with dysphagia. Based on their observations, “Swallowing deficits involving oral control and transfer may be a marker of subcortical neural axis involvement” (p. 482). Although this study aimed to identify swallow characteristics of patients with subcortical lesions in general, other studies focused on lesions to more specific subcortical regions as described in the following.
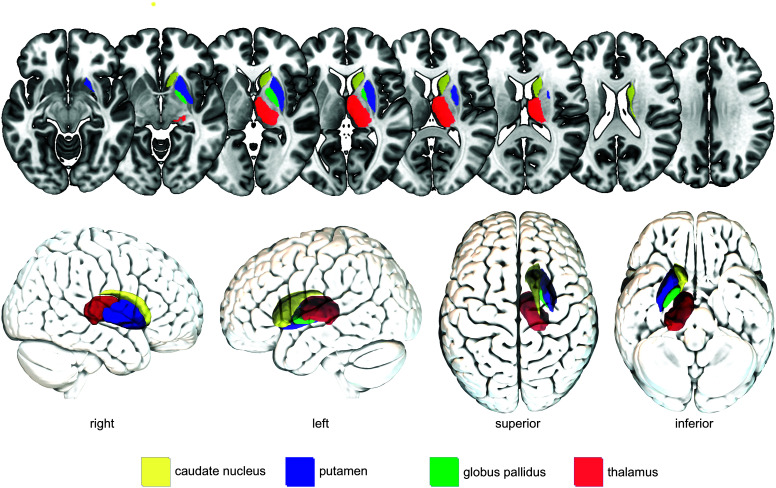
Subcortical Gray Matter Regions
Figure 3 shows the anatomical location of the subcortical gray matter regions discussed in this review.
Figure 3.
Commonly identified subcortical gray matter brain regions crucial for swallowing after stroke. For illustration purposes, brain regions are shown only in the right hemisphere and not in both hemispheres. Colors were chosen arbitrarily.
Basal ganglia. Studies on striatocapsular lesions (usually involving the putamen, caudate nucleus, globus pallidus, internal capsule, and subinsular area) show a high incidence of dysphagia in the first days after stroke (e.g., > 75% of patients with striatocapsular hemorrhage; Suntrup et al., 2012). Logemann et al. (1993) assessed swallowing using VFSS of eight patients with a focal infarct in the left basal ganglia and internal capsule compared to healthy age-matched controls. They found that the group of patients with stroke swallowed slower and less efficiently. This observation fits to the role of the basal ganglia in functionally connecting the cortex and the thalamus and thus likely playing a role in gaiting sensory input during swallowing (González-Fernández et al., 2013; Suntrup et al., 2012).
Thalamus. The thalamus is a central gray matter structure primarily associated with relaying sensory and motor information between subcortical and cortical areas, such as the globus pallidus, primary motor cortex, and supplementary motor area. It is believed that the thalamus plays a crucial role in the sensory–motor integration during swallowing by processing and relaying afferent and efferent signals (Mosier et al., 1999). The importance of the thalamus has been verified by lesion–symptom mapping studies in patients with stroke demonstrating associations between strokes in the thalamus and impaired anterior hyoid movement (Wilmskoetter, Bonilha, et al., 2019), and oral intake restrictions (Galovic et al., 2017; Maeshima et al., 2014).
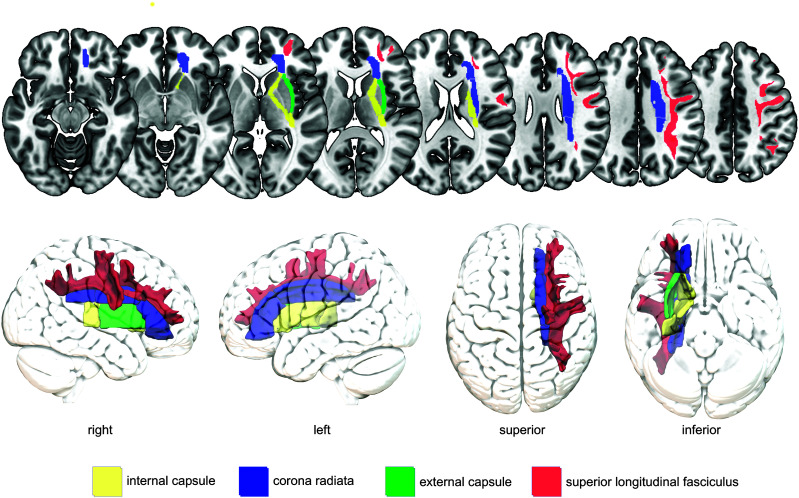
Subcortical White Matter Regions
Besides gray matter regions, white matter tracts connecting gray matter regions have also been identified as crucial regions for the occurrence and recovery of dysphagia after stroke (see Figure 4). We will review subcortical, white matter tracts commonly identified in lesion–symptom mapping studies.
Figure 4.
Commonly identified white matter brain tracts crucial for swallowing after stroke. Periventricular white matter has been identified as crucial for poststroke dysphagia but is not labeled in this figure because of controversies about which and to what extent regions belong to these white matter tracts. For illustration purposes, brain regions are shown only in the right hemisphere and not in both hemispheres. Colors were chosen arbitrarily.
Corona radiata. The corona radiata is a “radiating,” subcortical, white matter mass that carries ascending and descending projection fibers between the cortex and the brainstem. Fibers from the frontal lobe including the motor cortex and parietal lobe enter the corona radiata and continue as the internal capsule. The corticobulbar, corticopontine, and corticospinal tracts that connect the primary motor cortex with the brainstem and spinal cord travel through the corona radiata. Similar to the internal capsule, the corona radiata is somatotopically organized with separately localized fibers projecting to the face/head and upper and lower extremities. Although there is some degree of variability between individuals, the corticobulbar tracts (leading to muscles of the face, tongue, and throat) are typically localized anterior-lateral to the corticospinal tracts (leading to muscles of the hands and feet; Hazzaa et al., 2019). Lesions in the corona radiata have been strongly associated with persistent oral intake restrictions after stroke (Galovic et al., 2016, 2017). On a swallowing impairment level, lesions in the corona radiata were linked to impaired laryngeal elevation, laryngeal vestibular closure, and more pharyngeal residue compared to patients without lesions in the corona radiata (Wilmskoetter, Bonilha, et al., 2019).
Internal capsule. The internal capsule contains ascending and descending fibers, such as the corticobulbar and corticospinal tracts that travel caudally from the corona radiata to become the internal capsule. Same as the corona radiata, the internal capsule is somatotopically organized with corticobulbar tracts typically located in the middle (genu) to posterior (posterior limb) part of the internal capsule (Hazzaa et al., 2019). Studies have commonly identified the internal capsule as a crucial region for swallowing performance after stroke ranging from observations of aspiration and pharyngeal residue to dysphagia in general (Daniels & Foundas, 1999; Galovic et al., 2013; González-Fernández et al., 2008; Wilmskoetter, Bonilha, et al., 2019). A lesion to these white matter tracts likely disrupts connections between the cortex, subcortical areas, and brainstem, which are critical for swallowing.
External capsule. The external capsule lies between the putamen and claustrum and is a collection of white matter fibers whose structural organization and functional role is not yet fully understood. The external capsule includes striatal fibers that, for example, connect the primary sensorimotor cortex with the putamen and the supplementary motor area with the caudate nucleus (Schmahmann et al., 2008). Thus, it is believed that the external capsule is a critical link between cortical motor regions and the basal ganglia and that it contributes to the engagement of the basal ganglia in motor control. Lesion–symptom mapping studies showed that lesions to the external capsule are related to impaired laryngeal elevation and vestibular closure (Wilmskoetter, Bonilha, et al., 2019) and oral intake restrictions after stroke (Galovic et al., 2016, 2017).
Superior longitudinal fasciculus. The superior longitudinal fasciculus is a long-range white matter tract connecting all four (frontal, temporal, parietal, and occipital) cortical lobes, thus being an excellent anatomical structure to transmit cortical neural signals across long and short distances. For swallowing, the importance of the superior longitudinal fasciculus might be in the connection of different cortical sensory–motor regions, as this tract connects, for example, the supramarginal gyrus with premotor and prefrontal regions. In lesion–symptom mapping studies, lesions to the superior longitudinal fasciculus were associated with impaired laryngeal elevation, pharyngeal residue (Wilmskoetter, Bonilha, et al., 2019), and oral intake restrictions (Galovic et al., 2016). It remains speculative if lesions to the superior longitudinal fasciculus might disrupt signal transmissions from the temporal–parietal swallowing regions to the frontal primary and secondary motor areas, thus impeding swallow initiation and coordination.
PVWM. The PVWM are fiber tracts adjacent to the lateral ventricles of the brain. Lesions to the PVWM have been related to the occurrence of dysphagia (Cola et al., 2010; Im Moon et al., 2017). The physiological mechanisms that are disrupted by lesions in the PVWM remain to be explored. It is possible that projection fibers traveling between the cortex and brainstem are damaged when the PVWM is lesioned, thus interrupting efferent and afferent neural signals.
Cortical and Subcortical Control of Swallowing Recovery After Stroke
Little is known about how lesion locations affect dysphagia recovery. It has been proposed that recovery from cortical stroke–induced dysphagia depends on the cortical reorganization in the unaffected hemisphere (Hamdy et al., 1998, 2000; Li et al., 2009). If the unaffected hemisphere can successfully engage the homolog, swallowing areas depends on multiple factors. One factor is the hemisphere that is lesioned. This is an important consideration because both hemispheres do not seem to equally compensate for damage after a lesion. Furthermore, the unaffected hemisphere can compensate for some brain regions better than for other regions. Lowell et al. (2012) discussed that the potential for neural recovery depends on the brain region that is damaged and its degree of lateralization. A higher degree of lateralization might diminish the potential of reorganization in the other hemisphere with little or no swallow representation. For example, unilateral damage to the insula may show only little compensatory mechanisms in recovery compared to damage to the somatosensory cortex because of the insula's greater swallowing functional lateralization to the left hemispheres (Lowell et al., 2012). Lesion–symptom mapping studies support the hypothesis that the insula might have low recovery potential because lesions to the insula were associated with prolonged dysphagia, defined as greater than 14 days (Broadley et al., 2003; Galovic et al., 2017).
In addition to the factors of the side of the lesion and the degree of lateralization of the lesioned brain region, the combination of lesioned regions might also determine recovery success. For example, Galovic et al. (2013) suggest that combined, not isolated, lesioning of the insula and frontal operculum is an independent predictor for prolonged (≥ 7 days) dysphagia. Reasons for this observation might be that both areas are responsible for similar swallowing functions, and damage to both areas might cause increased and longer-lasting swallowing impairment. Furthermore, it might be possible that the frontal operculum is an important area for recruitment of peri-infarct tissue after insula stroke (Galovic et al., 2017). If the frontal operculum was damaged in concordance with the insula or if white matter connections were damaged, then such a recruitment would be compromised.
Whether gray matter or white matter lesions have a worse impact on swallowing recovery after stroke is controversial. Some research suggests that lesions to the white matter may be associated with acute but not prolonged swallowing impairment (Galovic et al., 2013; Suntrup et al., 2012). White matter pathways connecting the cortex with swallowing centers in the brainstem might improve rapidly by bypassing the lesioned segments (Galovic et al., 2013; González-Fernández et al., 2008). Conversely, research by Wu et al. (2015) suggests that white matter lesions are associated with poor (general) long-term recovery after stroke (Wu et al., 2015).
Importantly, any relationships between lesion locations and recovery potential are not generalizable across all individuals with a stroke. Lowell et al. (2012) pointed out that the insula's left lateralization was not observed in all participants (in eight and nine of the 14 participants for the anterior and posterior insula, respectively). Thus, patients who did have swallowing functions lateralized to one side of the insula before their stroke might have the same recovery potential as patients with lesions to the somatosensory cortex. Additionally, intra- and interindividual differences in the lateralization of muscle groups and functions involved in swallowing (Hamdy et al., 1996) can explain differences in recovery. Patients with the same lesion location in the motor cortex might show a different recovery based on the patients' individual premorbid swallowing topography in the motor cortex.
Neuroanatomy of Poststroke Dysphagia: Clinical Observations
BB is a 72-year-old man who presented to the emergency department with an acute onset of left-sided weakness and slurred speech. Previous medical history was significant for hypertension, hyperlipidemia, and coronary artery disease. He was a retired lawyer, married, and completely independent prior to admission. BB's initial National Institutes of Health Stroke Scale score was 21, characterized by decreased alertness, confusion, right gaze preference, left visual field deficit, left facial weakness, dysarthria, complete left hemiplegia, and dense left sensory loss with left hemineglect. A computed tomography scan confirmed an acute right middle cerebral artery ischemic stroke. As BB presented to the emergency department within 2 hr of symptom onset, tissue plasminogen activator was administered. Tissue plasminogen activator helps to break up or dissolve blood clots and may limit stroke damage and the severity of disability.
Swallowing was not screened by nursing on admission due to BB's decreased level of alertness; he received no intake by mouth (NPO), including medication. A clinical swallowing examination was completed by the speech-language pathologist on Day 2 of BB's acute care hospitalization. He was fully oriented but lethargic. Oral hygiene was adequate. Both at rest and extension, left central facial weakness and left tongue deviation were evident indicating unilateral upper motor neuron lesioning. Mild-to-moderate unilateral upper motor neuron dysarthria was identified. Swallowing was evaluated using ice chips, cup- and straw-sips of water, and teaspoons of puree. Decreased labial seal, decreased lingual control, delayed onset in the palpation of hyolaryngeal movement, multiple swallows to clear, and coughing with straw-sips of water were evident. The speech-language pathologist recommended continued NPO status; a small-bore feeding tube was placed for nutrition.
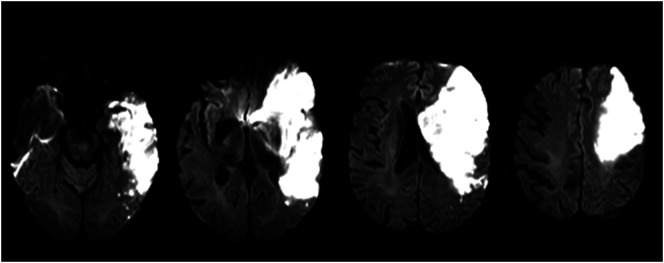
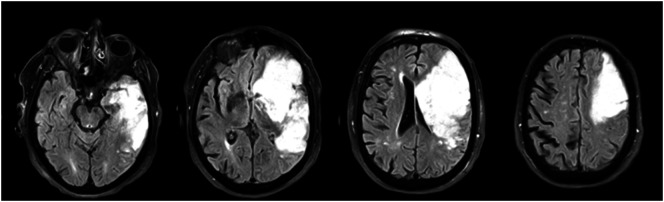
On Hospital Day 3, BB became somnolent. A repeat computed tomography scan revealed right cerebral edema and midline shift for which he was administered mannitol and hypertonic saline. Following this, he remained neurologically stable but intermittently lethargic during his acute care hospitalization. An MRI scan was completed on Hospital Day 5 and revealed a large infarct (> 300-ml infarct volume) with lesions involving more than 90% of the right insula, operculum, globus pallidus, putamen, caudate, superior longitudinal fasciculus, internal and external capsules, and greater than 50% but less than 90% of the corona radiata, somatosensory, supramarginal, and angular cortices (see Figures 5 and 6). The left hemisphere, brainstem, and cerebellum were spared by the lesion, and no old large vessel infarcts were visible. However, the MRI showed bilateral subcortical and PVWM hyperintensities, which were likely related to chronic microangiopathy (small vessel brain disease) already existent before the stroke.
Figure 5.
Diffusion-weighted imaging, magnetic resonance imaging sequence.
Figure 6.
Fluid-attenuated inversion recovery magnetic resonance imaging sequence.
Due to the change in BB's level of consciousness on Day 3, a VFSS was not completed until consistent alertness was evident, which was Day 8 following admission. The Modified Barium Swallow Impairment Profile protocol was utilized with administration of thin, nectar, and honey-thick liquids and purees (Martin-Harris et al., 2008). A solid bolus was not provided due to safety concerns. The highest Modified Barium Swallow Impairment Profile component scores and highest Penetration–Aspiration Scale scores (Rosenbek et al., 1996) for each consistency are listed in Table 1. Reduced orolingual control yielding valleculae and pyriform sinus pooling of thin liquids with intermittent preswallow aspiration (Penetration–Aspiration Scale 6) was evident. Airway invasion was prevented with nectar-thick liquids as well as with a chin tuck posture with ingestion of thin liquids; however, the client required continual tactile and verbal cues to maintain the chin tuck posture during swallowing. Oropharyngeal residue was not evident with nectar-thick liquid but was evident with honey-thick liquids. Decreased base of tongue to posterior pharyngeal wall contact was also identified and resulted in moderate valleculae residue with barium pudding; however, airway invasion was not observed. Effortful swallowing was tested during the VFSS to ensure no immediate negative effects as it was going to be implemented as a rehabilitation technique to improve base of tongue retraction. No increase in residue or entry of material into the nasal cavity was observed during effortful swallowing (Garcia et al., 2004; Molfenter et al., 2018).
Table 1.
Modified Barium Swallow Impairment Profile (MBSImP) component scores. Scores are overall impression scores: the highest (most severe) score across all swallowing tasks.
| OI |
PI |
PAS score (highest) |
||||||
|---|---|---|---|---|---|---|---|---|
| Component | VFSS 1 | VFSS 2 | Component | VFSS 1 | VFSS 2 | Consistency | VFSS 1 | VFSS 2 |
| Lip closure | 2 | 1 | Soft palate elevation | 0 | 0 | Thin liquid | 6 | 2 |
| Tongue control–bolus hold | 3 | 0 | Laryngeal elevation | 1 | 0 | Nectar liquid | 1 | 1 |
| Bolus preparation/mastication | 3 | 1 | Anterior hyoid excursion | 0 | 0 | Honey liquid and puree | 1 | 1 |
| Bolus transport | 3 | 0 | Epiglottic movement | 0 | 0 | |||
| Oral residue | 2 | 1 | Laryngeal vestibular closure | 0 | 0 | |||
| Initiation of pharyngeal swallow | 1 | 1 | Pharyngeal stripping wave | 1 | 0 | |||
| Total OI a | 14 | 2 | Pharyngeal contraction | 0 | 0 | |||
| PES opening | 0 | 0 | ||||||
| Tongue base retraction | 3 | 1 | ||||||
| Pharyngeal residue | 2 | 1 | ||||||
| Total PI b | 7 | 0 | ||||||
Note. OI = oral impairment; PI = pharyngeal impairment; PAS = penetration-aspiration scale; VFSS = videofluoroscopic swallowing study; PES = pharyngoesophageal segment.
According to the MBSImP protocol, scores of 1 for components “lip closure” and “oral residue” do not comprise the total OI score.
According to the MBSImP protocol, scores of 1 for components “tongue base retraction” and “pharyngeal residue” do not comprise the total PI score.
Following the VFSS, oral intake was initiated: nectar-thick liquids (International Dysphagia Diet Standardization Initiative [IDDSI] Level 2; www.iddsi.org) and purees (IDDSI Level 4). Thin liquids (IDDSI Level 0) were also allowed with supervision. BB's alertness level fluctuated daily and affected oral intake. A percutaneous endoscopic gastrostomy (PEG) tube was placed on Day 15 of hospitalization due to decreased intake; however, oral intake continued. BB was seen daily for swallowing and cognitive therapy; however, lethargy negatively impacted participation. Swallowing therapy included lingual resistance exercises using the Iowa Oral Performance Instrument, Masako maneuver, and effortful swallowing. BB was discharged to acute rehabilitation on Hospital Day 20. His National Institutes of Health Stroke Scale score was 17 and modified Rankin score was 5 (severe disability, bedridden, incontinent, requiring continual nursing care and attention) at the time of discharge.
BB was in acute rehabilitation for 6 weeks. Alertness slowly increased, as did his participation in therapy. Using visual feedback, the Iowa Oral Performance Instrument for lingual resistance exercises and surface electromyography for effortful swallowing appeared to facilitate his attention to task. A repeat VFSS during Week 4 of his acute rehabilitation stay revealed notable improvement in swallowing (see Table 1); however, impulsiveness was evident characterized by rapid rate and large, uncontrolled ingestion of liquid volumes. His diet was advanced to IDDSI Level 0 for liquids and Level 6 for solids; however, monitoring of impulsiveness during meals continued. By Week 5, the PEG tube was removed, and he was discharged to home on Week 6. His modified Rankin score was 4 (moderately severe disability, unable to walk and attend to bodily needs without assistance). He continued to receive weekly outpatient language therapy for 3 months to improve cognition as well as twice-weekly occupational and physical therapy.
Can the Understanding of Lesion Locations Assist in BB's Dysphagia Diagnosis, Treatment, and Prognosis?
BB suffered a large unilateral hemisphere stroke involving the majority of the right cortical and subcortical regions. Almost all regions previously discussed in this article to be involved in swallowing control were at least 50%, if not 90%, lesioned in the right hemisphere.
The likelihood for BB to present significant swallowing impairment as a result of his large right hemisphere stroke is high. Although there is no one-to-one relationship of large lesions and severe dysphagia, larger lesions tend to be associated with more severe overall conditions after stroke. Right hemisphere strokes in particular have been associated with pronounced pharyngeal swallowing deficits and worse prognosis as compared to left hemisphere strokes that have been associated more often with oral swallowing deficits (Wilmskoetter et al., 2018). This generalization, however, does not necessarily hold true for every single patient because the functional swallowing organization can vary between patients (Hamdy et al., 1996, 1997; Mistry et al., 2007). In BB's case, his presentation of severe oral and mild pharyngeal swallowing impairment as visualized in the initial VFSS on Hospital Day 8 suggests that the left, and not the right, hemisphere was his dominant pharyngeal swallowing hemisphere before the stroke. If the hemisphere with the weaker swallowing representation is lesioned, dysphagia can still occur, but the prognosis for the speed and extent of recovery is usually better compared to a lesion to the nondominant hemisphere because the unaffected, dominant hemisphere can compensate more easily. Indeed, BB showed nearly complete swallowing recovery within 6 weeks after his stroke as visualized by the follow-up VFSS during inpatient rehabilitation.
Although BB initially presented with oropharyngeal swallowing impairments, his swallowing function was diagnosed safe and efficient enough to prescribe a full oral diet with specific food and liquid restrictions. However, BB did not achieve his nutritional needs orally and required a PEG tube. Throughout the entire hospital stay, BB showed significant deficits in cognition and alertness that were the main reasons for his slow oral diet progression. Oral swallowing impairment (e.g., impairments in lip closure, tongue movement, bolus hold) in patients poststroke has been linked to cognitive deficits (Im Moon et al., 2012). BB's presentation of severe oral swallowing impairments accompanied by cognitive deficits support this association. Thus, BB's main challenge was his decreased alertness and cognition as a result of the stroke, which likely impacted his swallowing function, leading to deficits in oral intake. Again, this association supports the speculation that the right hemisphere was not BB's dominant swallowing hemisphere before his stroke.
Besides the stroke lesion, many other factors probably contributed to the occurrence and recovery of BB's swallowing impairments. For example, BB showed white matter hyperintensities in the deep white matter and around the ventricles. White matter hyperintensities are commonly observed in patients with small vessel brain disease even in the absence of a (large vessel) stroke. White matter hyperintensities (or leukoaraiosis) are associated with worse outcomes after stroke, likely because white matter hyperintensities predispose patients to decompensate more severely following a stroke compared to individuals without white matter hyperintensities (Wilmskoetter, Marebwa, et al., 2019). BB presented mild-to-moderate white matter hyperintensities (Fazekas et al., 1987); thus, his residual reserve was likely decreased poststroke, but it was not a major factor for his recovery potential.
Conclusions
Swallowing is mediated by a broad, bilateral neural network encompassing cortical, subcortical, and bulbar gray matter brain regions, as well as white matter tracts connecting these regions. Our current knowledge that lesions in many different areas of the brain can be associated with dysphagia falsifies statements such as “this patient cannot have dysphagia because the lesion is only unilateral/is too small/is not in the brain stem.” Lesions involving many different locations, of varied sizes, and in either hemisphere can result in swallowing impairment.
Although some relationships between stroke lesion patterns and swallowing impairment seem consistent across patients, interindividual differences exist. Relationships that hold true at a group level may not be translatable to an individual patient. Two patients can have the same lesion in terms of site, side, and size, but only one may have dysphagia (or recovers), and the other one does not. These differences may result from structural and functional brain differences or from factors other than the stroke lesion. For example, the overall health condition, comorbidities, vigilance, attention, cognition, or perceptual deficits may be associated with dysphagia occurrence and recovery independent of lesion location and size. Clinicians can use information about the stroke lesion as an adjunct to their clinical and instrumental assessments to identify swallowing impairments and prescribe optimized treatment by estimating what patterns of neural swallowing control may be disordered.
Acknowledgment
Funding for this study was provided by National Institutes of Health grant P20GM109040, awarded to Steven Kautz.
Funding Statement
Funding for this study was provided by National Institutes of Health grant P20GM109040, awarded to Steven Kautz.
References
- Augustine J. R. (1996). Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research Reviews, 22(3), 229–244. [DOI] [PubMed] [Google Scholar]
- Aziz Q., Rothwell J. C., Hamdy S., Barlow J., & Thompson D. G. (1996). The topographic representation of esophageal motor function on the human cerebral cortex. Gastroenterology, 111(4), 855–862. https://doi.org/10.1016/S0016-5085(96)70053-7 [DOI] [PubMed] [Google Scholar]
- Babaei A., Ward B. D., Siwiec R. M., Ahmad S., Kern M., Nencka A., Li S.-J., & Shaker R. (2013). Functional connectivity of the cortical swallowing network in humans. NeuroImage, 76, 33–44. https://doi.org/10.1016/j.neuroimage.2013.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benghanem S., Rosso C., Arbizu C., Moulton E., Dormont D., Leger A., Pires C., & Samson Y. (2019). Aphasia outcome: The interactions between initial severity, lesion size and location. Journal of Neurology, 266(6), 1303–1309. https://doi.org/10.1007/s00415-019-09259-3 [DOI] [PubMed] [Google Scholar]
- Bonilha L., Gleichgerrcht E., Nesland T., Rorden C., & Fridriksson J. (2015). Success of anomia treatment in aphasia is associated with preserved architecture of global and left temporal lobe structural networks. Neurorehabilitation and Neural Repair, 30(3), 266–279. https://doi.org/10.1177/1545968315593808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadley S., Croser D., Cottrell J., Creevy M., Teo E., Yiu D., Pathi R., Taylor J., & Thompson P. D. (2003). Predictors of prolonged dysphagia following acute stroke. Journal of Clinical Neuroscience, 10(3), 300–305. [DOI] [PubMed] [Google Scholar]
- Brown S., Ngan E., & Liotti M. (2008). A larynx area in the human motor cortex. Cerebral Cortex, 18(4), 837–845. https://doi.org/10.1093/cercor/bhm131 [DOI] [PubMed] [Google Scholar]
- Bustamante A., Garcia-Berrocoso T., Rodriguez N., Llombart V., Ribó M., Molina C., & Montaner J. (2016). Ischemic stroke outcome: A review of the influence of post-stroke complications within the different scenarios of stroke care. European Journal of Internal Medicine, 29, 9–21. https://doi.org/10.1016/j.ejim.2015.11.030 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2009). Prevalence and most common causes of disability among adults—United States, 2005. MMWR: Morbidity and Mortality Weekly Report, 58(16), 421–426. [PubMed] [Google Scholar]
- Chen C. L., Tang F. T., Chen H. C., Chung C. Y., & Wong M. K. (2000). Brain lesion size and location: Effects on motor recovery and functional outcome in stroke patients. Archives of Physical Medicine and Rehabilitation, 81(4), 447–452. https://doi.org10.1053/mr.2000.3837 [DOI] [PubMed] [Google Scholar]
- Cola M. G., Daniels S. K., Corey D. M., Lemen L. C., Romero M., & Foundas A. L. (2010). Relevance of subcortical stroke in dysphagia. Stroke, 41(3), 482–486. https://doi.org/10.1161/STROKEAHA.109.566133 [DOI] [PubMed] [Google Scholar]
- Crary M. A., Humphrey J. L., Carnaby-Mann G., Sambandam R., Miller L., & Silliman S. (2013). Dysphagia, nutrition, and hydration in ischemic stroke patients at admission and discharge from acute care. Dysphagia, 28(1), 69–76. https://doi.org/10.1007/s00455-012-9414-0 [DOI] [PubMed] [Google Scholar]
- Daniels S. K., Brailey K., & Foundas A. L. (1999). Lingual discoordination and dysphagia following acute stroke: Analyses of lesion localization. Dysphagia, 14(2), 85–92. [DOI] [PubMed] [Google Scholar]
- Daniels S. K., & Foundas A. L. (1997). The role of the insular cortex in dysphagia. Dysphagia, 12(3), 146–156. [DOI] [PubMed] [Google Scholar]
- Daniels S. K., & Foundas A. L. (1999). Lesion localization in acute stroke patients with risk of aspiration. Journal of NeuroImaging, 9(2), 91–98. [DOI] [PubMed] [Google Scholar]
- Daniels S. K., Huckabee M. L., & Gozdzikowska K. (2019). Dysphagia following stroke (3rd ed.). San Diego, CA: Plural. [Google Scholar]
- Daniels S. K., Pathak S., Mukhi S. V., Stach C. B., Morgan R. O., & Anderson J. A. (2017). The relationship between lesion localization and dysphagia in acute stroke. Dysphagia, 32(6), 777–784. [DOI] [PubMed] [Google Scholar]
- Dziewas R., Sörös P., Ishii R., Chau W., Henningsen H., Ringelstein E. B., Knecht S., & Pantev C. (2005). Cortical processing of esophageal sensation is related to the representation of swallowing. NeuroReport, 16(5), 439–443. [DOI] [PubMed] [Google Scholar]
- Ekberg O., Hamdy S., Woisard V., Wuttge-Hannig A., & Ortega P. (2002). Social and psychological burden of dysphagia: Its impact on diagnosis and treatment. Dysphagia, 17(2), 139–146. https://doi.org/10.1007/s00455-001-0113-5 [DOI] [PubMed] [Google Scholar]
- Fazekas F., Chawluk J. B., Alavi A., Hurtig H. I., & Zimmerman R. A. (1987). MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. American Journal of Roentgenology, 149(2), 351–356. [DOI] [PubMed] [Google Scholar]
- Fridriksson J., den Ouden D.-B., Hillis A. E., Hickok G., Rorden C., Basilakos A., Yourganov G., & Bonilha L. (2018). Anatomy of aphasia revisited. Brain, 141(3), 848–862. https://doi.org/10.1093/brain/awx363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galovic M., Leisi N., Müller M., Weber J., Abela E., Kägi G., & Weder B. (2013). Lesion location predicts transient and extended risk of aspiration after supratentorial ischemic stroke. Stroke, 44(10), 2760–2767. https://doi.org/10.1161/STROKEAHA.113.001690 [DOI] [PubMed] [Google Scholar]
- Galovic M., Leisi N., Müller M., Weber J., Tettenborn B., Brugger F., Abela E. Weder B. & Kägi G. (2016). Neuroanatomical correlates of tube dependency and impaired oral intake after hemispheric stroke. European Journal of Neurology, 23(5), 926–934. https://doi.org/10.1111/ene.12964 [DOI] [PubMed] [Google Scholar]
- Galovic M., Leisi N., Pastore-Wapp M., Zbinden M., Vos S. B., Mueller M., Weber J., Brugger F., Kägi G., & Weder B. J. (2017). Diverging lesion and connectivity patterns influence early and late swallowing recovery after hemispheric stroke. Human Brain Mapping, 38(4), 2165–2176. https://doi.org/10.1002/hbm.23511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J. M., Hakel M., & Lazarus C. (2004). Unexpected consequence of effortful swallowing: Case study report. Journal of Medical Speech-Language Pathology, 12(2), 59–67. [Google Scholar]
- González-Fernández M., Kleinman J. T., Ky P. K., Palmer J. B., & Hillis A. E. (2008). Supratentorial regions of acute ischemia associated with clinically important swallowing disorders: A pilot study. Stroke, 39(11), 3022–3028. https://doi.org/10.1161/STROKEAHA.108.518969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Fernández M., Ottenstein L., Atanelov L., & Christian A. B. (2013). Dysphagia after stroke: An overview. Current Physical Medicine and Rehabilitation Reports, 1(3), 187–196. https://doi.org/10.1007/s40141-013-0017-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. (2008). Fundamentals of motor systems. In Squire L., Bloom F. E., Spitzer N. C., Squire L. R., Berg D., du Lac S., & Ghosh A. (Eds.), Fundamental neuroscience (3rd ed., pp. 663–676). San Diego, CA: Academic Press. [Google Scholar]
- Hamdy S. (2006). Role of cerebral cortex in the control of swallowing. GI Motility Online. https://www.nature.com/gimo/contents/pt1/full/gimo8.html [Google Scholar]
- Hamdy S., Aziz Q., Rothwell J. C., Crone R., Hughes D., Tallis R. C., & Thompson D. G. (1997). Explaining oropharyngeal dysphagia after unilateral hemispheric stroke. The Lancet, 350(9079), 686–692. https://doi.org/10.1016/s0140-6736(97)02068-0 [DOI] [PubMed] [Google Scholar]
- Hamdy S., Aziz Q., Rothwell J. C., Power M., Singh K. D., Nicholson D. A., Tallis R. C., & Thompson D. G. (1998). Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology, 115(5), 1104–1112. [DOI] [PubMed] [Google Scholar]
- Hamdy S., Aziz Q., Rothwell J. C., Singh K. D., Barlow J., Hughes D. G., Tallis R. C., & Thompson D. G. (1996). The cortical topography of human swallowing musculature in health and disease. Nature Medicine, 2(11), 1217–1224. [DOI] [PubMed] [Google Scholar]
- Hamdy S., Rothwell J. C., Aziz Q., & Thompson D. G. (2000). Organization and reorganization of human swallowing motor cortex: Implications for recovery after stroke. Clinical Science, 99(2), 151–157. [PubMed] [Google Scholar]
- Hazzaa N. M., Mancini L., Thornton J., & Yousry T. A. (2019). Somatotopic organization of corticospinal/corticobulbar motor tracts in controls and patients with tumours: A combined fMRI–DTI study. NeuroImage: Clinical, 23, 101910 https://doi.org/10.1016/j.nicl.2019.101910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope T. M. H., Seghier M. L., Leff A. P., & Price C. J. (2013). Predicting outcome and recovery after stroke with lesions extracted from MRI images. NeuroImage: Clinical, 2, 424–433. https://doi.org/10.1016/j.nicl.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckabee M. L., & Cannito M. P. (1999). Outcomes of swallowing rehabilitation in chronic brainstem dysphagia: A retrospective evaluation. Dysphagia, 14(2), 93–109. https://doi.org/10.1007/pl00009593 [DOI] [PubMed] [Google Scholar]
- Hug A., Dalpke A., Wieczorek N., Giese T., Lorenz A., Auffarth G., Liesz A., & Veltkamp R. (2009). Infarct volume is a major determiner of post-stroke immune cell function and susceptibility to infection. Stroke, 40(10), 3226–3232. https://doi.org/10.1161/STROKEAHA.109.557967 [DOI] [PubMed] [Google Scholar]
- Im Moon H., Nam J. S., Leem M. J., & Kim K. H. (2017). Periventricular white matter lesions as a prognostic factor of swallowing function in older patients with mild stroke. Dysphagia, 32(4), 480–486. https://doi.org/10.1007/s00455-017-9788-0 [DOI] [PubMed] [Google Scholar]
- Im Moon H., Pyun S. B., & Kwon H. K. (2012). Correlation between location of brain lesion and cognitive function and findings of videofluoroscopic swallowing study. Annals of Rehabilitation Medicine, 36(3), 347–355. https://doi.org/10.5535/arm.2012.36.3.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasekeran V., Pendleton N., Holland G., Payton A., Jefferson S., Michou E., Vasant D., Ollier B., Horan M., Rothwell J., & Hamdy S. (2011). Val66Met in brain-derived neurotrophic factor affects stimulus-induced plasticity in the human pharyngeal motor cortex. Gastroenterology, 141(3), 827–836.e3. https://doi.org/10.1053/j.gastro.2011.05.047 [DOI] [PubMed] [Google Scholar]
- Jean A. (2001). Brain stem control of swallowing: Neuronal network and cellular mechanisms. Physiological Reviews, 81(2), 929–969. [DOI] [PubMed] [Google Scholar]
- Jean A., & Dallaporta M. (2013). Brainstem control of deglutition: Swallowing pattern generator. In Shaker R., Postma G., Belafsky P., & Easterling C. (Eds.), Principles of deglutition: A multidisciplinary text for swallowing and its disorders (pp. 67–87). San Diego, CA: Springer. [Google Scholar]
- Kiernan J. A. (2012). Anatomy of the temporal lobe. Epilepsy Research and Treatment, 2012, 176157 https://doi.org/10.1155/2012/176157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H., Oh S. H., Jeong H. J., Sim Y. J., Kim D. G., & Kim G. C. (2019). Association between duration of dysphagia recovery and lesion location on magnetic resonance imaging in patients with middle cerebral artery infarction. Annals of Rehabilitation Medicine, 43(2), 142–148. https://doi.org/10.5535/arm.2019.43.2.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim J. A., & Jones T. A. (2008). Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. Journal of Speech, Language, and Hearing Research, 51(1), S225–S239. https://doi.org/10.1044/1092-4388(2008/018) [DOI] [PubMed] [Google Scholar]
- Kumar S., Doughty C., Doros G., Selim M., Lahoti S., Gokhale S., & Schlaug G. (2014). Recovery of swallowing after dysphagic stroke: An analysis of prognostic factors. Journal of Stroke and Cerebrovascular Diseases, 23(1), 56–62. https://doi.org/10.1016/j.jstrokecerebrovasdis.2012.09.005 [DOI] [PubMed] [Google Scholar]
- Langdon P. C., Lee A. H., & Binns C. W. (2007). Dysphagia in acute ischaemic stroke: Severity, recovery and relationship to stroke subtype. Journal of Clinical Neuroscience, 14(7), 630–634. https://doi.org/10.1016/j.jocn.2006.04.009 [DOI] [PubMed] [Google Scholar]
- Lear C. S., Flanagan J. B. Jr., & Moorrees C. F. (1965). The frequency of deglutition in man. Archives of Oral Biology, 10(1), 83–99. [DOI] [PubMed] [Google Scholar]
- Leopold N. A., & Daniels S. K. (2010). Supranuclear control of swallowing. Dysphagia, 25(3), 250–257. https://doi.org/10.1007/s00455-009-9249-5 [DOI] [PubMed] [Google Scholar]
- Levine R., Robbins J. A., & Maser A. (1992). Periventricular white matter changes and oropharyngeal swallowing in normal individuals. Dysphagia, 7(3), 142–147. [DOI] [PubMed] [Google Scholar]
- Li S., Luo C., Yu B., Yan B., Gong Q., He C., He L., Huang X., Yao D., Lui S., Tang H., Chen Q., Zeng Y., & Zhou D. (2009). Functional magnetic resonance imaging study on dysphagia after unilateral hemispheric stroke: A preliminary study. Journal of Neurology, Neurosurgery and Psychiatry, 80(12), 1320–1329. https://doi.org/10.1136/jnnp.2009.176214 [DOI] [PubMed] [Google Scholar]
- Logemann J. A. (1998). Evaluation and treatment of swallowing disorders (2nd ed.). Austin, TX: Pro-Ed. [Google Scholar]
- Logemann J. A., Shanahan T., Rademaker A. W., Kahrilas P. J., Lazar R., & Halper A. (1993). Oropharyngeal swallowing after stroke in the left basal ganglion/internal capsule. Dysphagia, 8(3), 230–234. [DOI] [PubMed] [Google Scholar]
- Lowell S. Y., Reynolds R. C., Chen G., Horwitz B., & Ludlow C. L. (2012). Functional connectivity and laterality of the motor and sensory components in the volitional swallowing network. Experimental Brain Research, 219(1), 85–96. https://doi.org/10.1007/s00221-012-3069-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima S., Osawa A., Yamane F., Ishihara S., & Tanahashi N. (2014). Dysphagia following acute thalamic haemorrhage: Clinical correlates and outcomes. European Neurology, 71(3–4), 165–172. https://doi.org/10.1159/000355477 [DOI] [PubMed] [Google Scholar]
- Mann G., Hankey G. J., & Cameron D. (1999). Swallowing function after stroke: Prognosis and prognostic factors at 6 months. Stroke, 30(4), 744–748. https://doi.org/10.1161/01.str.30.4.744 [DOI] [PubMed] [Google Scholar]
- Marik P. E., & Kaplan D. (2003). Aspiration pneumonia and dysphagia in the elderly. Chest, 124(1), 328–336. https://doi.org/10.1378/chest.124.1.328 [DOI] [PubMed] [Google Scholar]
- Martin R., Goodyear B. G., Gati J. S., & Menon R. S. (2001). Cerebral cortical representation of automatic and volitional swallowing in humans. Journal of Neurophysiology, 85(2), 938–950. [DOI] [PubMed] [Google Scholar]
- Martin-Harris B., Brodsky M. B., Michel Y., Castell D. O., Schleicher M., Sandidge J., Maxwell R. & Blair J. (2008). MBS measurement tool for swallow impairment—MBSImp: Establishing a standard. Dysphagia, 23(4), 392–405. https://doi.org/10.1007/s00455-008-9185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Harris B., Logemann J. A., McMahon S., Schleicher M., & Sandidge J. (2000). Clinical utility of the modified barium swallow. Dysphagia, 15(3), 136–141. [DOI] [PubMed] [Google Scholar]
- Martino R., Foley N., Bhogal S., Diamant N., Speechley M., & Teasell R. (2005). Dysphagia after stroke: Incidence, diagnosis, and pulmonary complications. Stroke, 36(12), 2756–2763. https://doi.org/10.1161/01.STR.0000190056.76543.eb [DOI] [PubMed] [Google Scholar]
- Mistry S., Verin E., Singh S., Jefferson S., Rothwell J. C., Thompson D. G., & Hamdy S. (2007). Unilateral suppression of pharyngeal motor cortex to repetitive transcranial magnetic stimulation reveals functional asymmetry in the hemispheric projections to human swallowing. Journal of Physiology, 585(2), 525–538. https://doi.org/10.1113/jphysiol.2007.144592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfenter S. M., Hsu C. Y., Lu Y., & Lazarus C. L. (2018). Alterations to swallowing physiology as the result of effortful swallowing in healthy seniors. Dysphagia, 33(3), 380–388. https://doi.org/10.1007/s00455-017-9863-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier K., & Bereznaya I. (2001). Parallel cortical networks for volitional control of swallowing in humans. Experimental Brain Research, 140(3), 280–289. [DOI] [PubMed] [Google Scholar]
- Mosier K. M., Liu W. C., Maldjian J. A., Shah R., & Modi B. (1999). Lateralization of cortical function in swallowing: A functional MR imaging study. American Journal of Neuroradiology, 20(8), 1520–1526. [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D., Benjamin E. J., Go A. S., Arnett D. K., Blaha M. J., Cushman M., de Ferranti S., Després J.-P., Fullerton H. J., Howard V. J., Huffman M. D., Judd S. E., Kissela B. M., Lackland D. T., Lichtman J. H., Lisabeth L. D., Liu S., Mackey R. H., Matchar D. B., McGuire D. K., … American Heart Association Statistics Committee and Stroke Statistics Subcommittee. (2015). Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation, 131(4), e29–e322. https://doi.org/10.1161/cir.0000000000000152 [DOI] [PubMed] [Google Scholar]
- Osawa A., Maeshima S., Matsuda H., & Tanahashi N. (2013). Functional lesions in dysphagia due to acute stroke: Discordance between abnormal findings of bedside swallowing assessment and aspiration on videofluorography. Neuroradiology, 55(4), 413–421. https://doi.org/10.1007/s00234-012-1117-6 [DOI] [PubMed] [Google Scholar]
- Phan K. L., Wager T., Taylor S. F., & Liberzon I. (2002). Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. NeuroImage, 16(2), 331–348. http://doi.org/10.1006/nimg.2002.1087 [DOI] [PubMed] [Google Scholar]
- Plowman E., Hentz B., & Ellis C. Jr. (2012). Post-stroke aphasia prognosis: A review of patient-related and stroke-related factors. Journal of Evaluation in Clinical Practice, 18(3), 689–694. https://doi.org/10.1111/j.1365-2753.2011.01650.x [DOI] [PubMed] [Google Scholar]
- Riecker A., Gastl R., Kuhnlein P., Kassubek J., & Prosiegel M. (2009). Dysphagia due to unilateral infarction in the vascular territory of the anterior insula. Dysphagia, 24(1), 114–118. https://doi.org/10.1007/s00455-008-9164-1 [DOI] [PubMed] [Google Scholar]
- Robbins J., Butler S. G., Daniels S. K., Diez Gross R., Langmore S., Lazarus C. L., Martin-Harris B., McCabe D., Musson N., & Rosenbek J. (2008). Swallowing and dysphagia rehabilitation: Translating principles of neural plasticity into clinically oriented evidence. Journal of Speech, Language, and Hearing Research, 51(1), S276–S300. https://doi.org/10.1044/1092-4388(2008/021) [DOI] [PubMed] [Google Scholar]
- Robbins J., Kays S. A., Gangnon R. E., Hind J. A., Hewitt A. L., Gentry L. R., & Taylor A. J. (2007). The effects of lingual exercise in stroke patients with dysphagia. Archives of Physical Medicine and Rehabilitation, 88(2), 150–158. https://doi.org/10.1016/j.apmr.2006.11.002 [DOI] [PubMed] [Google Scholar]
- Robbins J., Levine R. L., Maser A., Rosenbek J. C., & Kempster G. B. (1993). Swallowing after unilateral stroke of the cerebral cortex. Archives of Physical Medicine and Rehabilitation, 74(12), 1295–1300. [DOI] [PubMed] [Google Scholar]
- Rolls E. T. (2016). Functions of the anterior insula in taste, autonomic, and related functions. Brain and Cognition, 110, 4–19. https://doi.org/10.1016/j.bandc.2015.07.002 [DOI] [PubMed] [Google Scholar]
- Rorden C., Karnath H. O., & Bonilha L. (2007). Improving lesion-symptom mapping. Journal of Cognitive Neuroscience, 19(7), 1081–1088. https://doi.org/10.1162/jocn.2007.19.7.1081 [DOI] [PubMed] [Google Scholar]
- Rosenbek J. C., Robbins J. A., Roecker E. B., Coyle J. L., & Wood J. L. (1996). A penetration-aspiration scale. Dysphagia, 11(2), 93–98. [DOI] [PubMed] [Google Scholar]
- Schmahmann J. D., Smith E. E., Eichler F. S., & Filley C. M. (2008). Cerebral white matter: Neuroanatomy, clinical neurology, and neurobehavioral correlates. Annals of the New York Academy of Sciences, 1142(1), 266–309. https://doi.org/10.1196/annals.1444.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaker R., Easterling C., Kern M., Nitschke T., Massey B., Daniels S., Grande B., Kazandjian M., & Dikeman K. (2002). Rehabilitation of swallowing by exercise in tube-fed patients with pharyngeal dysphagia secondary to abnormal UES opening. Gastroenterology, 122(5), 1314–1321. [DOI] [PubMed] [Google Scholar]
- Sharma J. C., Fletcher S., Vassallo M., & Ross I. (2001). What influences outcome of stroke—Pyrexia or dysphagia? International Journal of Clinical Practice, 55(1), 17–20. [PubMed] [Google Scholar]
- Smithard D. G., O'Neill P. A., England R. E., Park C. L., Wyatt R., Martin D. F., & Morris J. (1997). The natural history of dysphagia following a stroke. Dysphagia, 12(4), 188–193. https://doi.org/10.1007/pl00009535 [DOI] [PubMed] [Google Scholar]
- Smithard D. G., O'Neill P. A., Martin D. F., & England R. (1997). Aspiration following stroke: Is it related to the side of the stroke? Clinical Rehabilitation, 11(1), 73–76. [DOI] [PubMed] [Google Scholar]
- Stickler D., Gilmore R., Rosenbek J. C., & Donovan N. J. (2003). Dysphagia with bilateral lesions of the insular cortex. Dysphagia, 18(3), 179–181. [DOI] [PubMed] [Google Scholar]
- Suntrup S., Kemmling A., Warnecke T., Hamacher C., Oelenberg S., Niederstadt T., Heindel W., Wiendl H., & Dziewas R. (2015). The impact of lesion location on dysphagia incidence, pattern and complications in acute stroke. Part 1: Dysphagia incidence, severity and aspiration. European Journal of Neurology, 22(5), 832–838. https://doi.org/10.1111/ene.12670 [DOI] [PubMed] [Google Scholar]
- Suntrup S., Warnecke T., Kemmling A., Teismann I. K., Hamacher C., Oelenberg S., & Dziewas R. (2012). Dysphagia in patients with acute striatocapsular hemorrhage. Journal of Neurology, 259(1), 93–99. https://doi.org/10.1007/s00415-011-6129-3 [DOI] [PubMed] [Google Scholar]
- Suntrup-Krueger S., Kemmling A., Warnecke T., Hamacher C., Oelenberg S., Niederstadt T., Heindel W., Wiendl H., & Dziewas R. (2017). The impact of lesion location on dysphagia incidence, pattern and complications in acute stroke. Part 2: Oropharyngeal residue, swallow and cough response, and pneumonia. European Journal of Neurology, 24(6), 867–874. https://doi.org/10.1111/ene.13307 [DOI] [PubMed] [Google Scholar]
- Suntrup-Krueger S., Ringmaier C., Muhle P., Wollbrink A., Kemmling A., Hanning U., Claus I., Warnecke T., Teismann I., Pantev C., & Dziewas R. (2018). Randomized trial of transcranial direct current stimulation for poststroke dysphagia. Annals of Neurology, 83(2), 328–340. https://doi.org/10.1002/ana.25151 [DOI] [PubMed] [Google Scholar]
- Teismann I. K., Steinstraeter O., Stoeckigt K., Suntrup S., Wollbrink A., Pantev C., & Dziewas R. (2007). Functional oropharyngeal sensory disruption interferes with the cortical control of swallowing. BMC Neuroscience, 8(1), 62 https://doi.org/10.1186/1471-2202-8-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warraich Z., & Kleim J. A. (2010). Neural plasticity: The biological substrate for neurorehabilitation. PM&R, 2(12S), S208–S219. https://doi.org/10.1016/j.pmrj.2010.10.016 [DOI] [PubMed] [Google Scholar]
- Wilmskoetter J., Bonilha L., Martin-Harris B., Elm J. J., Horn J., & Bonilha H. S. (2019). Mapping acute lesion locations to physiological swallow impairments after stroke. NeuroImage: Clinical, 22, 101685 https://doi.org/10.1016/j.nicl.2019.101685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmskoetter J., Marebwa B., Basilakos A., Fridriksson J., Rorden C., Stark B. C., Johnson L., Hickok G., Hillis A. E., & Bonilha L. (2019). Long-range fibre damage in small vessel brain disease affects aphasia severity. Brain, 142(10), 3190–3201. https://doi.org/10.1093/brain/awz251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmskoetter J., Martin-Harris B., Pearson W. G. Jr., Bonilha L., Elm J. J., Horn J., & Bonilha H. S. (2018). Differences in swallow physiology in patients with left and right hemispheric strokes. Physiology and Behavior, 194, 144–152. https://doi.org/10.1016/j.physbeh.2018.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmskoetter J., Simpson A. N., Simpson K. N., & Bonilha H. S. (2016). Practice patterns of percutaneous endoscopic gastrostomy tube placement in acute stroke: Are the guidelines achievable? Journal of Stroke and Cerebrovascular Diseases, 25(11), 2694–2700. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu O., Cloonan L., Mocking S. J. T., Bouts M. J., Copen W. A., Cougo-Pinto P. T., Fitzpatrick K., Kanakis A., Schaefer P. W., Rosand J., Furie K. L., & Rost N. S. (2015). Role of acute lesion topography in initial ischemic stroke severity and long-term functional outcomes. Stroke, 46(9), 2438–2444. https://doi.org/10.1161/STROKEAHA.115.009643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X.-D., Zhou L.-F., Wang S.-J., Zhao Y.-S., Wang X.-J., Zhang L.-L., Wang S.-H., Zhang Y.-J., & Chen L. (2015). Compensatory recombination phenomena of neurological functions in central dysphagia patients. Neural Regeneration Research, 10(3), 490–497. https://doi.org/10.4103/1673-5374.153701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L. L., Lindenberg R., Alexander M. P., & Schlaug G. (2010). Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke, 41(5), 910–915. https://doi.org/10.1161/STROKEAHA.109.577023 [DOI] [PMC free article] [PubMed] [Google Scholar]