Abstract
Background
High ventilatory frequencies increase static lung strain and possibly lung stress by shortening expiratory time, increasing intrathoracic pressure, and causing dynamic hyperinflation. We hypothesised that high intraoperative ventilatory frequencies were associated with postoperative respiratory complications.
Methods
In this retrospective hospital registry study, we analysed data from adult non-cardiothoracic surgical cases performed under general anaesthesia with mechanical ventilation at a single centre between 2005 and 2017. We assessed the association between intraoperative ventilatory frequency (categorised into four groups) and postoperative respiratory complications, defined as composite of invasive mechanical ventilation within 7 days after surgery or peripheral oxygen desaturation after extubation, using multivariable logistic regression. In a subgroup, we adjusted analyses for arterial blood gas parameters.
Results
A total of 102 632 cases were analysed. Intraoperative ventilatory frequencies ranged from a median (inter-quartile range [IQR]) of 8 (8–9) breaths min−1 (Group 1) to 15 (14–18) breaths min−1 (Group 4). High ventilatory frequencies were associated with higher odds of postoperative respiratory complications (adjusted odds ratio=1.26; 95% confidence interval, 1.14–1.38; P<0.001), which was confirmed in a subgroup after adjusting for arterial partial pressure of carbon dioxide and the ratio of arterial oxygen partial pressure to fractional inspired oxygen. We identified considerable variability in the use of high ventilatory frequencies attributable to individual provider preference (ranging from 22% to 88%) and temporal change; however, the association with postoperative respiratory complications remained unaffected.
Conclusions
High intraoperative ventilatory frequency was associated with increased risk of postoperative respiratory complications, and increased postoperative healthcare utilisation.
Keywords: desaturation, intraoperative mechanical ventilation, perioperative care, postoperative respiratory complications, reintubation, ventilatory frequency
Editor's key points.
-
•
Mechanical ventilation strategies in the ICU are aimed at reducing lung injury, but it is unclear whether this is relevant in the intraoperative period.
-
•
Low tidal volumes are often used with high ventilatory frequencies aiming to reduce lung injury, but high ventilatory frequencies, of themselves, may cause lung injury.
-
•
This extensive retrospective analysis of intraoperative ventilator settings found an association between high ventilatory frequencies (even 14–18 breaths min−1) and postoperative respiratory complications.
Mechanical ventilation can lead to ventilator-induced lung injury: high pressure or volume, shear stress during cyclic alveolar collapse and expansion, and inflammatory responses are known contributing factors.1 Lung protective ventilation strategies can lower morbidity and mortality during anaesthesia and intensive care.2, 3, 4 Observational studies suggested an association of high tidal volumes, low positive end-expiratory pressure (PEEP), high driving pressure, and high fraction of inspired oxygen (Fio2) with postoperative respiratory complications.5,6 However, the effects of ventilatory frequency on postoperative pulmonary complications during short-term intraoperative mechanical ventilation remain undefined.
High ventilatory frequencies during mechanical ventilation are often needed to reduce hypercapnia in low-tidal-volume ventilation, but also lead to disruptions in respiratory physiology.7 Increased ventilatory frequencies alter the inspiratory/expiratory ratio; shorten the expiratory time; increase inspiratory flow, intrinsic PEEP, and intrathoracic pressures; and may lead to dynamic hyperinflation,8 all of which increase cyclic lung strain and lung stress. Furthermore, high ventilatory frequencies increase dead space ventilation and may cause lung damage because of cyclic stress. In primary rat alveolar epithelial cells, cell damage occurred more often after cyclic than after tonically held cell deformations, with frequency being a major determinant of cell injury.9 Further experimental evidence confirmed the harmful effects of high-respiratory-rate ventilation: More oedema and perivascular haemorrhage occurred at higher ventilatory frequencies in isolated, perfused rabbit lungs,10 whereas lower ventilatory frequencies ameliorated ventilator-induced lung injury, lung inflammation, and molecular stress signalling in rodents.11, 12, 13 Similarly, lung injury caused by excessive mechanical power as a result of high-respiratory-rate ventilation was observed in a porcine model.14
In this study, we aimed to investigate the association between intraoperative ventilatory frequency and postoperative pulmonary complications. We hypothesised that high intraoperative ventilatory frequencies increase the risk of postoperative respiratory complications.
Methods
Study design
In this retrospective hospital registry study, we analysed surgical cases performed between October 2005 and September 2017 at an academic tertiary care centre in Massachusetts, USA (Beth Israel Deaconess Medical Center). This study was reviewed by the local institutional review board (Committee on Clinical Investigations), which determined that the study meets the criteria for exempt status (protocol number: 2019P000362). The requirement for informed written consent was waived. Data sources are described in Supplementary Appendix S1.
Patient selection
Surgical cases undergoing general anaesthesia with mechanical ventilation and documented primary endpoint were included in the analysis. Patients younger than 18 yr, with an ASA physical status classification of 6, more than one surgical procedure within the preceding month, or admission to an ICU before surgery were excluded. Cardiac and thoracic surgical cases were excluded because of the perturbations to pulmonary physiology associated with thoracotomy and single-lung ventilation. We excluded cases with missing data for exposure or confounder variables using the complete case method for initial analyses.5
Exposure
The exposure was defined as the median ventilatory frequency recorded during the time of intraoperative mechanical ventilation. Ventilatory frequency measurements were categorised into four approximately equally sized groups, with the lowest ventilatory frequencies (Group 1) serving as the reference group.
Outcomes
The primary outcome was defined a priori as a composite of postoperative respiratory complications, which included invasive mechanical ventilation requirement within 7 days after surgery, or post-extubation desaturation, defined as the occurrence of at least one peripheral oxygen saturation (Spo2) measurement <90% within 10 min of extubation. Secondary outcomes included PACU length of stay and ICU admission rates within 7 days after surgery.
Covariate model
Based on available literature, and biological and clinical plausibility, analyses were adjusted for the following patient-specific confounders: age, sex, body mass index, ASA physical status classification, Charlson comorbidity index,15 history of smoking, chronic obstructive pulmonary disease, or heart failure within 1 yr before surgery (defined by the International Classification of Disease 9/10 codes), and history of home oxygen use or respiratory support at home. Case-specific confounders included surgical specialty, emergency surgery, high-risk surgery (i.e. neurosurgery, general, transplant, vascular, or burn surgery),16 duration of surgery, and work relative value units. Analyses were further adjusted for high risk of postoperative pulmonary complications (i.e. Score for Prediction of Postoperative Respiratory Complications [SPORC] ≥7)16 and the following intraoperative factors: age-adjusted minimum alveolar concentration of volatile anaesthetics and nitrous oxide,17 morphine-equivalent doses of short- and long-acting opioids,18 neuromuscular blocking agent dose,19 neostigmine dose,20 norepinephrine-equivalent vasopressor dose, intravenous fluid volume,21 packed red blood cell units, intraoperative hypotension (i.e. time with mean arterial pressure <55 mm Hg),22 and airway device (i.e. tracheal tube, laryngeal mask airway, or combination). The following ventilatory parameters were included: median Fio2,6 median PEEP, median peak inspiratory pressure (PIP), weight-adjusted tidal volume, median end-tidal carbon dioxide (etCO2), Spo2/Fio2 ratio, and use of non-protective ventilation (i.e. no PEEP or median PEEP >15 cm H2O, median PIP >35 cm H2O, or median tidal volume >10 ml kg−1).5
Analyses of effect modification
To evaluate whether the association between high-respiratory-rate ventilation and postoperative respiratory complications was modified by other ventilatory parameters, we examined interaction terms between high ventilatory frequency (Group 4) and the following dichotomised variables: median etCO2 (≤45 vs >45 mm Hg), tidal volume (≤10 vs >10 ml kg−1), PEEP (≤5 vs >5 cm H2O), driving pressure (<15 vs ≥15 cm H2O), and median Fio2 (<60% vs ≥60%). Analogously, we analysed effect modification using ASA physical status (<3 vs ≥3), BMI (<35 vs ≥35 kg m−2), emergency surgery status, smoking history, duration of surgery (cut-off at median), non-protective ventilation, or high preoperative risk of postoperative respiratory complications (SPORC <7 vs ≥7, and SPORC-223 <14 vs ≥14 in cases with available confounder information). Lastly, we estimated the propensity for undergoing high-respiratory-rate ventilation (Supplementary Appendix S1) and evaluated whether a high baseline propensity for high-respiratory-rate ventilation modified the association between exposure and primary outcome.
Analyses of variability
As mechanical ventilation practice may have changed over the study period, we described temporal trends in the use of ventilatory frequencies and analysed whether these changes affected the association between high ventilatory frequencies and postoperative respiratory complications by adjusting for year of surgery and deriving annual predicted probabilities for the primary outcome. To account for variability in the use of high ventilatory frequencies across different anaesthesia providers, we analysed provider variability using mixed-effects models (Supplementary Appendix S1).
Sensitivity analyses
We evaluated the association of ventilatory frequency with the individual components of the primary outcome: invasive mechanical ventilation requirement and post-extubation desaturation.
In a subgroup of patients with available intraoperative arterial blood gas analyses, we assessed the effect of ventilatory frequency on the primary outcome and adjusted analyses for arterial partial pressure of carbon dioxide (Paco2), as a marker of hypercarbia, and the ratio of arterial partial pressure of oxygen (Pao2) and Fio2 (Pao2/Fio2 ratio), as a clinical indicator of hypoxaemia, in addition to predetermined covariates. We further assessed effect modification by these parameters (Supplementary Appendix S3).
To address potential bias arising from the use of specific airway devices, we analysed subgroups of cases performed with tracheal tubes or laryngeal mask airways, respectively, and included interaction terms into the primary model to assess effect modification by airway device.
Additional sensitivity analyses included recategorisation of the exposure, change of reference group, alternative outcome definitions, additional confounder adjustments, propensity-score matching and multiple imputation (Supplementary Appendix S3). With an exploratory intent, we analysed the association between ventilatory frequency and in-hospital mortality (Supplementary Appendix S4).
Statistical analyses
Multivariable logistic regression was used to analyse associations between intraoperative ventilatory frequency and binary outcomes, and negative binomial regression to assess the association with PACU length of stay. Results from logistic and negative binomial regression analyses are presented as odds ratios (ORs) and incidence rate ratios (IRRs) with 95% confidence intervals (CIs), respectively. Continuous confounder variables were divided into quintiles; ordinal variables into clinically relevant categories. For effect modification analyses, interaction terms were included separately into the primary regression model. Linear combinations of the respective main effect and interaction term were performed to assess the association between exposure and outcome across different subgroups. Assuming a 5% incidence of postoperative respiratory complications in the reference group and defining a 20% increase as clinically meaningful difference, a sample size of 10 266 cases was required to achieve 99% power (Supplementary Appendix S1). A two-tailed P-value <0.05 was considered statistically significant. Statistical analyses were performed using Stata (version 15; StataCorp LLC, College Station, TX, USA).
Results
Study cohort and characteristics
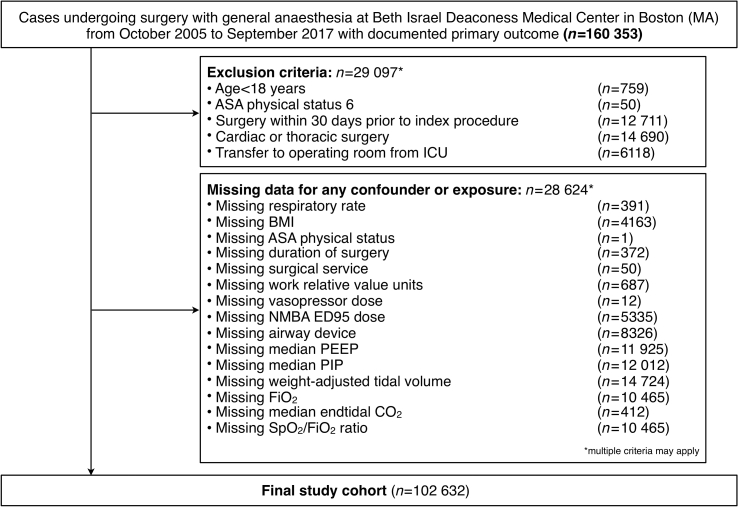
A total of 160 353 cases undergoing surgery with general anaesthesia between October 2005 and September 2017 were considered for analysis. After excluding ineligible cases and cases with missing information for exposure or confounder variables, the final study cohort included 102 632 cases (Fig. 1). Ventilatory frequencies ranged from a median of 8 (range 3–9.75) breaths min−1 in Group 1 to 15 (range 12.5–43) breaths min−1 in Group 4. The distribution of intraoperative ventilatory frequencies is shown in Fig. 2a. Characteristics of the study population are shown in Table 1.
Fig 1.
Study flow diagram. Study flow diagram indicating numbers of cases in the final study cohort. NMBA, neuromuscular blocking agent; PEEP, positive end-expiratory pressure; PIP, positive inspiratory pressure; Fio2, fraction of inspired oxygen; Spo2, peripheral oxygen saturation.
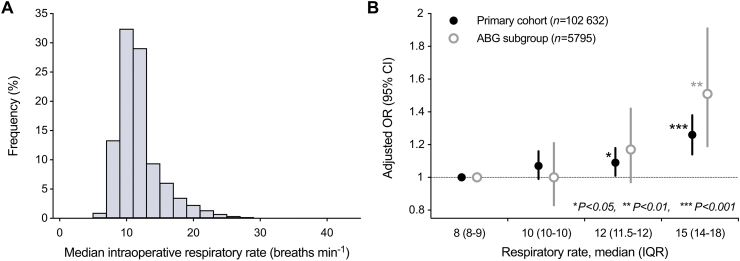
Fig 2.
Median intraoperative ventilatory frequency and its association with postoperative respiratory complications. (a) The histogram depicts the distribution of median intraoperative ventilatory frequencies in the study cohort. (b) Increasing intraoperative ventilatory frequencies are associated with an increased risk of postoperative respiratory complications in the primary cohort and a subgroup of cases with available arterial blood gas (ABG) analyses. Data are shown as adjusted ORs; error bars indicate 95% CIs. Significance levels: ∗P<0.05, ∗∗P<0.01, ∗∗∗P<0.001. CI, confidence intervals; OR, odds ratio; IQR, inter-quartile range.
Table 1.
Characteristics of study population by intraoperative ventilatory frequency. Data are expressed as n (%), mean (standard deviation), or median (inter-quartile range [25th–75th percentile], values separated by comma). ∗Score for Prediction of Postoperative Respiratory Complications. †ED95 dose of neuromuscular blocking agents (NMBA): median effective dose required to achieve a 95% reduction in maximal twitch response from baseline. Fio2, fraction of inspired oxygen; PEEP, positive end-expiratory pressure; PIP, peak inspiratory pressure; Spo2, peripheral oxygen saturation.
| Characteristics | Group 1 (n=24 392) |
Group 2 (n=23 574) |
Group 3 (n=28 125) |
Group 4 (n=26 541) |
|---|---|---|---|---|
| Intraoperative ventilatory frequency, median (IQR) | 8 (8, 9) | 10 (10, 10) | 12 (11.5, 12) | 15 (14, 18) |
| Intraoperative ventilatory frequency, range | 3–9.75 | 10–10.75 | 11–12.25 | 12.5–43 |
| Age, yr (range) | 55.9 (16.8) (18–104) | 53.9 (16.3) (18–102) | 52.2 (15.9) (18–102) | 50.6 (16.2) (18–102) |
| Sex, male | 7312 (30.0) | 8966 (38.0) | 13 477 (47.9) | 12 739 (48.0) |
| BMI, kg m−2 | 26.87 (5.7) | 28.33 (6.6) | 29.58 (7.4) | 28.98 (7.2) |
| ASA physical status classification | 2 (2, 3) | 2 (2, 3) | 2 (2, 3) | 2 (2, 3) |
| ASA ≥3 | 7826 (32.1) | 7933 (33.7) | 9954 (35.4) | 6928 (26.1) |
| Emergency surgery | 1433 (5.9) | 1703 (7.2) | 2234 (7.9) | 1428 (5.4) |
| Comorbidities | ||||
| Charlson comorbidity index | 1 (0, 2) | 1 (0, 2) | 1 (0, 2) | 0 (0, 2) |
| History of chronic obstructive pulmonary disease | 1093 (4.5) | 1153 (4.9) | 1379 (4.9) | 983 (3.7) |
| History of heart failure | 948 (3.9) | 993 (4.2) | 1059 (3.8) | 794 (3.0) |
| History of smoking | 2140 (8.8) | 2932 (12.4) | 4627 (16.5) | 3427 (12.9) |
| SPORC score,∗ ≥7 | 695 (2.8) | 702 (3.0) | 778 (2.8) | 535 (2.0) |
| Home oxygen therapy | 95 (0.4) | 122 (0.5) | 151 (0.5) | 141 (0.5) |
| Respiratory support at home | 105 (0.4) | 145 (0.6) | 173 (0.6) | 156 (0.6) |
| Intraoperative factors | ||||
| High-risk surgery | 10 548 (43.2) | 9803 (41.6) | 10 926 (38.8) | 7088 (26.7) |
| Work relative value units (RVU) | 13.99 (7.96, 18.23) | 13.99 (8.16, 18.88) | 13.18 (7.96, 19.02) | 8.57 (5.96, 15.26) |
| Duration of surgery, min | 124 (83, 180) | 127 (86, 186) | 129 (85, 195) | 87 (52, 144) |
| Fluids administered, ml | 2500 (2000, 4250) | 2500 (2000, 4250) | 2500 (2000, 4250) | 2125 (1500, 3000) |
| Total long-acting opioid dose (oral morphine equivalents), mg | 0.00 (0.00, 17.00) | 6.80 (0.00, 17.00) | 9.00 (0.00, 20.40) | 0.00 (0.00, 12.00) |
| Total short-acting opioid dose (oral morphine equivalents), mg | 37.50 (25.00, 62.50) | 37.50 (25.00, 62.50) | 31.25 (25.00, 62.50) | 25.00 (12.50, 37.50) |
| Minimum alveolar concentration of volatile anaesthetics and nitrous oxide | 0.99 (0.85, 1.14) | 1.00 (0.86, 1.14) | 1.00 (0.87, 1.14) | 1.01 (0.87, 1.15) |
| NMBA ED95 dose,† mg | 1.76 (0.00, 2.70) | 1.83 (0.27, 2.76) | 1.79 (0.00, 2.84) | 0.00 (0.00, 1.49) |
| Neostigmine dose, mg | 2.00 (0.00, 3.00) | 2.00 (0.00, 3.00) | 2.00 (0.00, 3.50) | 0.00 (0.00, 0.00) |
| Mean arterial pressure <55 mm Hg, min | 1 (0, 3) | 1 (0, 3) | 1 (0, 3) | 0 (0, 2) |
| Total vasopressor dose, mg | 0.00 (0.00, 0.04) | 0.00 (0.00, 0.05) | 0.00 (0.00, 0.06) | 0.00 (0.00, 0.02) |
| Packed red blood cell units | ||||
| 0 | 23 693 (97.1) | 22 975 (97.5) | 27 577 (98.1) | 26 302 (99.1) |
| 1 | 330 (1.4) | 264 (1.1) | 206 (0.7) | 76 (0.3) |
| 2 | 251 (1.0) | 202 (0.9) | 201 (0.7) | 81 (0.3) |
| ≥ 3 | 118 (0.5) | 133 (0.6) | 141 (0.5) | 82 (0.3) |
| Respiratory parameters | ||||
| Airway device | ||||
| Tracheal tube | 21 994 (90.2) | 21 847 (92.7) | 24 875 (88.4) | 9650 (36.4) |
| Laryngeal mask airway | 2323 (9.5) | 1625 (6.9) | 3110 (11.1) | 16 791 (63.3) |
| Combination | 75 (0.3) | 102 (0.4) | 140 (0.5) | 100 (0.4) |
| Median Fio2, % | 54.1 (14.7) | 55.4 (14.1) | 56.6 (14.0) | 55.6 (15.7) |
| Median PEEP, cm H2O | 2 (2, 5) | 3 (2, 5) | 4 (2, 5) | 2 (2, 5) |
| Median PIP, cm H2O | 20.5 (5.9) | 21.2 (5.9) | 21.8 (6.7) | 16.2 (8.8) |
| Median tidal volume, ml | 575 (115) | 566 (106) | 553 (111) | 394 (143) |
| Median tidal volume per kg, ml kg−1 | 9.9 (2.2) | 9.5 (2.0) | 8.9 (2.0) | 6.3 (2.2) |
| Median minute volume, L min−1 | 4.8 (1.0) | 5.7 (1.1) | 6.5 (1.3) | 6.2 (1.9) |
| Median end-tidal CO2, mm Hg | 34.6 (4.7) | 34.8 (4.1) | 36.1 (4.7) | 40.6 (6.6) |
| Spo2/Fio2 ratio | 195 (52) | 189 (48) | 184 (45) | 190 (51) |
| Non-protective ventilation | 11 640 (47.7) | 8583 (36.4) | 7857 (27.9) | 2967 (11.2) |
Primary outcome
In total, 6659 (6.49%) cases experienced postoperative respiratory complications: 1448 (5.94%) in Group 1, 1607 (6.82%) in Group 2, 2079 (7.39%) in Group 3, and 1525 (5.75%) in Group 4 (Table 2). After adjusting for pre-specified confounders, patients ventilated with the highest ventilatory frequencies (Group 4) had higher odds of postoperative respiratory complications compared with patients in Group 1 (adjusted OR [aOR]=1.26; 95% CI, 1.14–1.38; P<0.001; Table 2 and Fig. 2b).
Table 2.
Primary and secondary outcomes. Data are expressed as n (%), or median (inter-quartile range [25th–75th percentile], values separated by comma). Results are reported as odds ratios (ORs), or incidence rate ratios (IRRs). ∗Analysis performed only in cases with available PACU admission and discharge times (n=85 970). CI, confidence interval; IQR, inter-quartile range.
| Outcomes | Group 1 (n=24 392) |
Group 2 (n=23 574) |
Group 3 (n=28 125) |
Group 4 (n=26 541) |
|---|---|---|---|---|
| Primary outcome | ||||
| Postoperative respiratory complications | 1448 (5.94) | 1607 (6.82) | 2079 (7.39) | 1525 (5.75) |
| Unadjusted OR (95% CI) | Reference | 1.16 (1.08, 1.25) P<0.001 |
1.26 (1.18, 1.36) P<0.001 |
0.97 (0.90, 1.04) P=0.360 |
| Adjusted OR (95% CI) | Reference | 1.07 (0.99, 1.16) P=0.085 |
1.09 (1.01, 1.18) P=0.028 |
1.26 (1.14, 1.38) P<0.001 |
| Secondary outcomes | ||||
| PACU length of stay (min) ∗, median (IQR) | 179 (136, 251) | 183 (139, 256) | 183 (137, 256) | 154 (117, 210) |
| Unadjusted IRR (95% CI) | Reference | 1.01 (1.00, 1.02) P=0.039 |
1.02 (1.01, 1.03) P<0.001 |
0.84 (0.83, 0.84) P<0.001 |
| Adjusted IRR (95% CI) | Reference | 1.01 (1.00, 1.02) P=0.017 |
1.04 (1.03, 1.05) P<0.001 |
1.04 (1.02, 1.05) P<0.001 |
| ICU admission within 7 days | 758 (3.11%) | 855 (3.63) | 1321 (4.70) | 816 (3.07) |
| Unadjusted OR (95% CI) | Reference | 1.17 (1.06, 1.30) P=0.002 |
1.54 (1.40, 1.68) P<0.001 |
0.99 (0.89, 1.09) P=0.829 |
| Adjusted OR (95% CI) | Reference | 1.11 (0.98, 1.25) P=0.089 |
1.47 (1.31, 1.65) P<0.001 |
1.75 (1.51, 2.02) P<0.001 |
Secondary outcomes
Use of higher ventilatory frequencies showed significant association with PACU and ICU utilisation (Table 2): higher ventilatory frequencies were associated with increased PACU length of stay (Group 2: adjusted IRR (aIRR)=1.01; 95% CI, 1.00–1.02; P=0.017; Group 3: aIRR=1.04; 95% CI, 1.03–1.05; P<0.001; Group 4: aIRR=1.04; 95% CI, 1.02–1.05; P<0.001) and higher odds of ICU admission within 7 days (Group 3: aOR=1.47; 95% CI, 1.31–1.65; P<0.001; Group 4: aOR=1.75; 95% CI, 1.51–2.02; P<0.001).
Assessment of effect modification
The association between ventilatory frequency and postoperative respiratory complications was not modified by other ventilatory parameters. Interaction terms between high ventilatory frequency and high tidal volume, high etCO2, high PEEP, high driving pressure, and high Fio2 were not significant (P for interaction=0.25, 0.52, 0.11, 0.38, and 0.69, respectively). Similarly, ASA physical status ≥3, obesity, smoking history, duration of surgery, and non-protective ventilation showed no effect modification (P for interaction=0.40, 0.52, 0.53, 0.55, and 0.81, respectively). The association between high ventilatory frequency and postoperative respiratory complications was significantly modified by emergency status (P for interaction=0.043). Linear combinations of the main effect of high ventilatory frequency and the interaction term (high ventilatory frequency × emergency status) derived from the primary cohort revealed a significant association between high ventilatory frequency and the primary outcome among non-emergent cases (aOR=1.22; 95% CI, 1.10–1.35; P<0.001) and, more pronounced, among emergent cases (aOR=1.57; 95% CI, 1.24–1.98; P<0.001). The interaction of high ventilatory frequencies with high SPORC was not significant (P for interaction=0.35). Similarly, in 86 992 cases with available information for SPORC-2 calculation, a high SPORC-2 did not modify the association between high ventilatory frequencies and the primary outcome (P for interaction=0.43). No effect modification by high baseline propensity to undergo high-respiratory-rate ventilation was observed (P for interaction=0.13). Results from subgroup analyses are shown in Supplementary Table S1.
Assessment of variability in the use of high ventilatory frequency
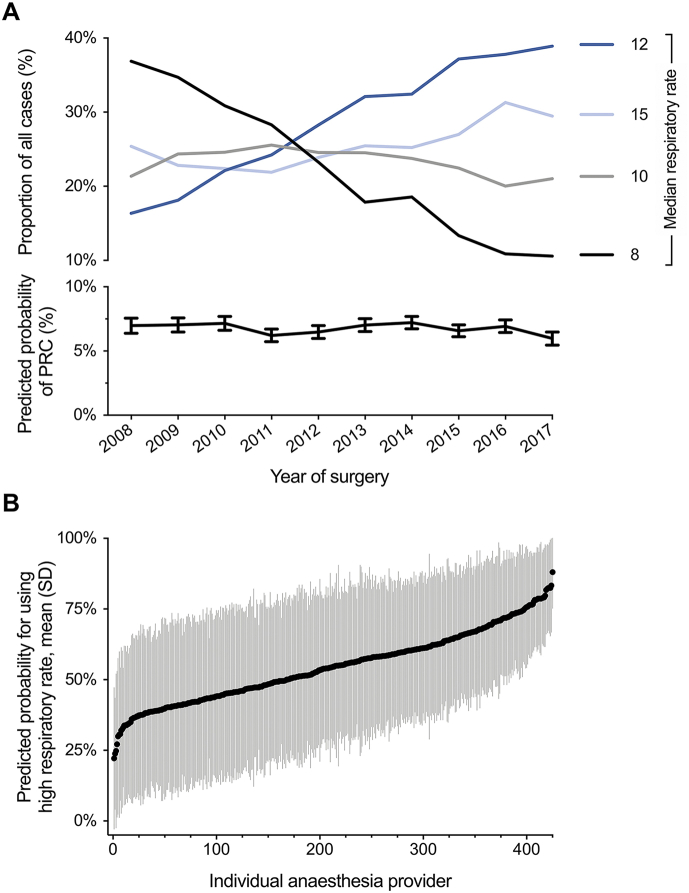
The proportion of cases ventilated with higher ventilatory frequencies increased over time, whereas the annual predicted probability of postoperative respiratory complications remained constant (Fig. 3a). When adjusting analyses for year of surgery, high ventilatory frequency remained significantly associated with postoperative respiratory complications (aOR=1.22; 95% CI, 1.11–1.35; P<0.001; Supplementary Appendix S2).
Fig 3.
Variability in the use of high ventilatory frequencies over time and across individual providers. (a) Changes in the proportion of cases in each ventilatory frequency group (represented by the respective group median) and the predicted probability of postoperative respiratory complications (PRC) are plotted against time (year of surgery) over a 10-yr period. The fraction of cases ventilated with higher ventilatory frequencies increased over time. Despite this change in practice, the rate of postoperative respiratory complications remained unchanged over time. Predicted probabilities for the outcome of PRC were derived from the primary regression model; error bars indicate 95% confidence intervals. (b) The predicted preference for an individual anaesthesia provider to use high instead of low ventilatory frequency (cut-off at median) ranged from 22.2% to 88.0%, indicating a high variability across providers. Individual provider preference for using high ventilatory frequencies was derived from a mixed-effects logistic regression model with ventilatory frequency as the outcome, individual anaesthesia providers as the random effect and all covariates of the primary analysis as fixed effects. Data are expressed as mean (standard deviation, sd) of the adjusted preference of using high ventilatory frequencies across all cases performed by an individual provider.
Across 425 individual anaesthesia providers represented in the study cohort, the predicted preference for individual providers to use high instead of low ventilatory frequencies ranged from 22.2% to 88.0% (Fig. 3b). The high variability had no impact on the association of high ventilatory frequency and primary outcome, when adjusted for in a mixed-effects model with the individual provider as random effect (aOR=1.25; 95% CI, 1.14–1.38; P<0.001; Supplementary Appendix S2).
Sensitivity analyses
When considering the individual components of the composite outcome (Supplementary Table S2), high ventilatory frequencies were associated with increased odds of invasive mechanical ventilation within 7 days (aOR=1.24; 95% CI, 1.04–1.49; P=0.016) and post-extubation desaturation within 10 min (aOR=1.26; 95% CI, 1.12–1.41; P<0.001).
Intraoperative arterial blood gas analyses were performed in 5795 cases (Table 3). In this subgroup, postoperative respiratory complications occurred in 1510 (26.1%) cases: 365 (21.2%) in Group 1, 357 (23.8%) in Group 2, 463 (27.5%) in Group 3, and 325 (36.6%) in Group 4. High ventilatory frequency was associated with postoperative respiratory complications in unadjusted analysis (OR=2.14; 95% CI, 1.79–2.56; P<0.001; Table 3) and after adjusting for Paco2 and Pao2/Fio2 ratio (aOR=1.51; 95% CI, 1.19–1.91; P=0.001; Table 3 and Fig. 2b). No effect modification by arterial blood gas parameters was observed (Supplementary Appendix S3).
Table 3.
Subgroup analyses in cases with available intraoperative arterial blood gas analyses (n=5795). Data are expressed as mean (standard deviation), or n (%). Results are reported as odds ratios (ORs). ∗If multiple blood gas analyses were performed during one case, the mean of all measurement was considered for a given case. †Logistic regression analysis was adjusted for Paco2 and Pao2/Fio2 ratio in addition to age, sex, BMI, ASA status, emergency surgery, duration of surgery, work relative value units, history of smoking, chronic obstructive pulmonary disease, or heart failure, Charlson comorbidity index, high Score for Prediction of Postoperative Respiratory Complications (SPORC), packed red blood cell units, short and long-acting opioid doses, neuromuscular blocking agent dose, intraoperative hypotension, airway device, median positive end-expiratory pressure (PEEP), median peak inspiratory pressure (PIP), tidal volume, and non-protective ventilation.
| Group 1 (n=1723) | Group 2 (n=1501) | Group 3 (n=1682) | Group 4 (n=889) | |
|---|---|---|---|---|
| Arterial blood gas results ∗ | ||||
| pH | 7.40 (0.06) | 7.39 (0.06) | 7.39 (0.06) | 7.38 (0.08) |
| Partial pressure of oxygen (Pao2), mm Hg | 210.6 (84.8) | 210.2 (83.3) | 205.3 (80.2) | 201.0 (79.0) |
| Partial pressure of carbon dioxide (Paco2), mm Hg | 40.48 (5.30) | 40.50 (5.64) | 40.44 (6.53) | 41.38 (8.25) |
| Pao2/Fio2 ratio | 390.9 (146.7) | 391.6 (144.7) | 378.5 (147.7) | 364.3 (141.8) |
| Paco2−etCO2 gradient, mm Hg | 7.81 (5.09) | 7.49 (5.46) | 6.84 (6.02) | 6.78 (6.73) |
| Postoperative respiratory complications | 365 (21.18) | 357 (23.78) | 463 (27.53) | 325 (36.56) |
| Unadjusted OR (95% CI) | Reference | 1.16 (0.98, 1.37) P=0.077 |
1.41 (1.21, 1.65) P<0.001 |
2.14 (1.79, 2.56) P<0.001 |
| Adjusted OR (95% CI) † | Reference | 1.00 (0.83, 1.21) P=0.997 |
1.17 (0.97, 1.42) P=0.106 |
1.51 (1.19, 1.91) P=0.001 |
Given the imbalance in the use of tracheal tubes and laryngeal mask airways between groups, we evaluated the primary outcome separately for cases performed using each airway device (Supplementary Table S2). Among 78 783 cases with tracheal intubation, high ventilatory frequencies were associated with postoperative respiratory complications (aOR=1.22; 95% CI, 1.10–1.35; P<0.001). Laryngeal mask airway devices were used in 24 266 cases, with similar odds of postoperative respiratory complications (aOR=1.27; 95% CI, 0.92–1.76; P=0.15). A lack of effect modification by airway device was further confirmed by non-significant interaction terms between high ventilatory frequency and use of tracheal tube or laryngeal mask airway (P for interaction=0.41 and 0.80, respectively).
Results remained robust when using different exposure categories (Supplementary Tables S3 and S4) or alternative outcome definitions (Supplementary Tables S5), in a propensity score-matched cohort (Supplementary Tables S6 and S7), and after imputing missing confounder data (Supplementary Table S8). In exploratory analyses, we further found high ventilatory frequencies to be associated with in-hospital mortality (aOR=1.55; 95% CI, 1.04–2.31; P=0.030; Supplementary Appendix S4).
Discussion
In this study, we found high ventilatory frequencies to be significantly associated with postoperative respiratory complications in patients undergoing intraoperative mechanical ventilation for non-cardiac and non-thoracic surgical procedures. We also identified considerable variability in the use of high ventilatory frequencies, which could not be explained by patient characteristics alone, but was attributable to individual providers and practice change over time. Low ventilatory frequency may represent an important variable to consider when reporting data from studies on protective mechanical ventilation.
We defined postoperative respiratory complications as the occurrence of either oxygen saturations below 90% after extubation, or the requirement of invasive mechanical ventilation within 7 days after surgery. Both outcomes are clinically relevant indicators of postoperative respiratory failure and have been studied before.24,25 Mechanical ventilation and reintubation are important quality and safety indicators defined by the Agency for Healthcare Research and Quality (AHRQ).26 Immediate post-extubation desaturation was further independently associated with adverse postoperative outcomes, including reintubation.24 This may in part be explained by atelectasis, which impairs gas exchange because of pulmonary shunt and increases the vulnerability for more severe respiratory complications.27
Several experimental studies have shown harmful effects of high-respiratory-rate ventilation,9, 10, 11, 12, 13, 14 but data on the effects of mechanical ventilation with high ventilatory frequencies in humans are limited, especially concerning the perioperative period. In a secondary analysis of the LUNG SAFE (Large observational study to UNderstand the Global impact of Severe Acute respiratory FailurE) cohort study, increased ventilatory frequency, low PEEP, and high peak inspiratory and driving pressures were associated with higher mortality in patients with acute respiratory distress syndrome.28 Our study supports the significance of these findings, and adds evidence of an association of short-term mechanical ventilation with high ventilatory frequencies and postoperative complications in patients with normal lungs.
Pathophysiologically, a higher respiratory frequency shortens inspiratory and expiratory times, which results in higher flow rates and pressures to maintain a pre-set tidal volume, causes more cumulative trauma with each delivered breath, and increases the total cycles of injury to the lung. High inspiratory peak flows augment parenchymal shear stress,29 worsen oxygenation,30 and contribute to higher pressure gradients on epithelial cells.31 Consequently, limiting inspiratory flow rates protects against the development of lung injury.32 Shortened expiration times may contribute to dynamic hyperinflation and the development of intrinsic PEEP, which can lead to reduced compliance and increased transpulmonary pressure.7 Intrinsic PEEP further contributes to adverse extrapulmonary manifestations, such as cardiovascular impairment or diaphragmatic dysfunction.7 Higher ventilatory frequencies also increase the mechanical power transferred from the ventilator to the respiratory system.14 Taken together, these changes occurring during non-physiological positive-pressure ventilation augment lung stress and strain and ultimately increase the risk of ventilator-induced lung injury.14,33
Lung-protective ventilation commonly involves low-tidal-volume strategies,34 which increase the likelihood of using higher ventilatory frequencies to maintain minute ventilation and avoid hypercapnia. Coinciding with the advent of lung-protective ventilation, we observed a shift towards the use of higher ventilatory frequencies over time. Although intraoperative hypocapnia by higher-than-necessary minute ventilation has been widely accepted in the past,35 a growing body of evidence suggests that permissive hypercapnia may be pneumoprotective.36,37 Furthermore, hypercapnia may improve upper airway patency in anaesthetised patients.38 To rule out hypercapnia or low tidal volumes as the driving factors of the observed association between high ventilatory frequency and pulmonary complications, we included end-tidal or, if available, arterial CO2 values and tidal volumes as confounders in the analyses. We also demonstrated lack of effect modification by expiratory or arterial CO2. Furthermore, the increased use of higher ventilatory frequencies over time did not affect the association with respiratory complications. We speculate that reductions of tidal volumes may have led to improved outcomes, but the associated practice change towards the use of higher ventilatory frequencies may have counterbalanced this benefit. In combination with the potential benefits of permissive hypercapnia, these data support the approach of avoiding high ventilatory frequencies, even in low-tidal-volume ventilation. By adjusting analyses for the Pao2/Fio2 ratio as a marker of hypoxaemia, we further ruled out the possibility that the observed worse outcomes with high ventilatory frequencies were an epiphenomenon of impaired gas exchange requiring invasive mechanical ventilation. We examined possible interactions of high ventilatory frequency and indicators of more severe underlying illness and high preoperative risk, including ASA status, body mass index, baseline risk for postoperative pulmonary complications (measured using SPORC16 and SPORC-223), or emergency surgery. The association between exposure and primary outcome remained robust in these analyses. Taken together, these findings suggest an independent effect of high-respiratory-rate ventilation on the association with postoperative respiratory complications. The absence of effect modification also suggests that ventilatory frequency is often not selected because of objective patient needs, but based on individual provider preferences. This is supported by our respective analyses and previous evidence of provider preference driving variability in the use of protective ventilation.39
High ventilatory frequency was associated with prolonged PACU length of stay and increased risk of ICU admission. Although tachypnoea is a well-established clinical sign of deterioration and a predictor for the need of ICU care if present on admission,40 the effects of a pre-set high ventilatory frequency during intraoperative mechanical ventilation on these endpoints have not been studied before. Our findings are clinically plausible: even mild postoperative pulmonary complications prolong length of stay, increase ICU admissions, and contribute to early postoperative mortality.41
The use of laryngeal mask airways often coincides with spontaneously breathing patients at our institution and elsewhere.42 In our analyses, lack of effect modification by airway device suggests an independent effect of high ventilatory frequency in increasing the vulnerability to lung injury, both in patients breathing spontaneously and those receiving positive-pressure ventilation. This is clinically plausible,7 and harmful effects of high ventilatory frequencies have been reported in patients undergoing spontaneous, noninvasive ventilation.43 We further speculate that spontaneously breathing patients with laryngeal mask airways partly account for the absence of significance in unadjusted analyses. Although these patients had higher ventilatory frequencies, they were also at lower risk and underwent minor surgery of shorter duration when compared with cases undergoing tracheal intubation. Spontaneous breathing in patients with laryngeal mask airways also explains the extremes of high and low ventilatory frequencies observed in our cohort (Supplementary Appendix S5).
Several limitations arise from the retrospective design of the presented study. Although the large cohort size permitted extensive confounder adjustment, residual unidentified confounding cannot be completely ruled out. Analyses were further limited to parameters available from electronic medical records, which prevented us from including potentially important confounders. For example, intraoperative ventilation mode, which affects postoperative pulmonary complications,44 is not routinely captured in our institution's electronic anaesthesia records. Because we analysed data from a single, tertiary referral centre, results may lack generalisability. However, our study cohort is comprised of different service locations performing a large variety of procedures, ranging from ambulatory care to complex surgery in hospitalised patients requiring critical care therapy. We therefore believe our findings still to be relevant for a widespread spectrum of patients.
In conclusion, short-term intraoperative ventilation with high ventilatory frequencies was associated with postoperative respiratory complications, prolonged PACU lengths of stay, and ICU admissions after surgery. These findings were independent of ventilatory, respiratory, and patient-specific factors, as well as temporal and provider-specific variability. The use of low ventilatory frequency as a—so far overlooked—component of protective ventilation strategies warrants further investigation.
Authors' contributions
Study conception: ME
Study design: PS, SZ, ME
Data acquisition: PS, SZ, MH, SN
Data analysis: PS, SZ, MH, SN
Supervision of data analysis: ME
Data interpretation: PS, SZ, MH, AP, YL, SKR, MFVM, ME
Manuscript drafting; PS, SZ, MH, SN, ME
Discussion of results and revision for important intellectual content: AP, YL, SKR, MFVM, ME
Guarantor of the study taking responsibility for the integrity of the work as a whole: ME
All authors reviewed the manuscript and approved the final version.
Declarations of interest
ME has received unrestricted funds from philanthropic donors Jeffrey and Judy Buzen during the conduct of the study, has received grants for investigator-initiated trials not related to this manuscript from Merck Sharpe & Dohme Corp., a subsidiary of Merck & Co., and serves as a consultant on the advisory board of Merck & Co. SKR reports personal fees from Fresenius Kabi USA (scientific advisor), outside the submitted work. MFVM has received grants from the US National Institutes of Health for work not related to this study. All other authors declare that they have no conflicts of interest.
Funding
Philanthropic donations from Jeffrey and Judy Buzen to ME.
Acknowledgements
The authors acknowledge Lea Albrecht and Friederike Althoff (Department of Anesthesia, Critical Care and Pain Medicine, Beth Israel Deaconess Medical Center) for making a non-author contribution by helping with data acquisition and analyses for the revision.
Handling editor: Paul Myles
Footnotes
A conference abstract highlighting preliminary results of this research was presented at the annual meeting of the American Society of Anesthesiologists in Orlando, FL (October 19–23, 2019).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.02.018.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Slutsky A.S., Ranieri V.M. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 2.Amato M.B., Barbas C.S., Medeiros D.M. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 3.Acute Respiratory Distress Syndrome Network. Brower R.G., Matthay M.A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 4.Futier E., Constantin J.M., Paugam-Burtz C. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369:428–437. doi: 10.1056/NEJMoa1301082. [DOI] [PubMed] [Google Scholar]
- 5.Ladha K., Vidal Melo M.F., McLean D.J. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: hospital based registry study. BMJ. 2015;351:h3646. doi: 10.1136/bmj.h3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staehr-Rye A.K., Meyhoff C.S., Scheffenbichler F.T. High intraoperative inspiratory oxygen fraction and risk of major respiratory complications. Br J Anaesth. 2017;119:140–149. doi: 10.1093/bja/aex128. [DOI] [PubMed] [Google Scholar]
- 7.Akoumianaki E., Vaporidi K., Georgopoulos D. The injurious effects of elevated or nonelevated respiratory rate during mechanical ventilation. Am J Respir Crit Care Med. 2019;199:149–157. doi: 10.1164/rccm.201804-0726CI. [DOI] [PubMed] [Google Scholar]
- 8.Vieillard-Baron A., Prin S., Augarde R. Increasing respiratory rate to improve CO2 clearance during mechanical ventilation is not a panacea in acute respiratory failure. Crit Care Med. 2002;30:1407–1412. doi: 10.1097/00003246-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Tschumperlin D.J., Oswari J., Margulies A.S. Deformation-induced injury of alveolar epithelial cells. Effect of frequency, duration, and amplitude. Am J Respir Crit Care Med. 2000;162:357–362. doi: 10.1164/ajrccm.162.2.9807003. [DOI] [PubMed] [Google Scholar]
- 10.Hotchkiss J.R., Jr., Blanch L., Murias G. Effects of decreased respiratory frequency on ventilator-induced lung injury. Am J Respir Crit Care Med. 2000;161:463–468. doi: 10.1164/ajrccm.161.2.9811008. [DOI] [PubMed] [Google Scholar]
- 11.Conrad S.A., Zhang S., Arnold T.C., Scott L.K., Carden D.L. Protective effects of low respiratory frequency in experimental ventilator-associated lung injury. Crit Care Med. 2005;33:835–840. doi: 10.1097/01.ccm.0000159532.56865.8a. [DOI] [PubMed] [Google Scholar]
- 12.Vaporidi K., Voloudakis G., Priniannakis G. Effects of respiratory rate on ventilator-induced lung injury at a constant PaCO2 in a mouse model of normal lung. Crit Care Med. 2008;36:1277–1283. doi: 10.1097/CCM.0b013e318169f30e. [DOI] [PubMed] [Google Scholar]
- 13.Rich P.B., Douillet C.D., Hurd H., Boucher R.C. Effect of ventilatory rate on airway cytokine levels and lung injury. J Surg Res. 2003;113:139–145. doi: 10.1016/s0022-4804(03)00195-1. [DOI] [PubMed] [Google Scholar]
- 14.Cressoni M., Gotti M., Chiurazzi C. Mechanical power and development of ventilator-induced lung injury. Anesthesiology. 2016;124:1100–1108. doi: 10.1097/ALN.0000000000001056. [DOI] [PubMed] [Google Scholar]
- 15.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Brueckmann B., Villa-Uribe J.L., Bateman B.T. Development and validation of a score for prediction of postoperative respiratory complications. Anesthesiology. 2013;118:1276–1285. doi: 10.1097/ALN.0b013e318293065c. [DOI] [PubMed] [Google Scholar]
- 17.Grabitz S.D., Farhan H.N., Ruscic K.J. Dose-dependent protective effect of inhalational anesthetics against postoperative respiratory complications: a prospective analysis of data on file from three hospitals in New England. Crit Care Med. 2017;45:e30–e39. doi: 10.1097/CCM.0000000000002015. [DOI] [PubMed] [Google Scholar]
- 18.Long D.R., Lihn A.L., Friedrich S. Association between intraoperative opioid administration and 30-day readmission: a pre-specified analysis of registry data from a healthcare network in New England. Br J Anaesth. 2018;120:1090–1102. doi: 10.1016/j.bja.2017.12.044. [DOI] [PubMed] [Google Scholar]
- 19.McLean D.J., Diaz-Gil D., Farhan H.N., Ladha K.S., Kurth T., Eikermann M. Dose-dependent association between intermediate-acting neuromuscular-blocking agents and postoperative respiratory complications. Anesthesiology. 2015;122:1201–1213. doi: 10.1097/ALN.0000000000000674. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki N., Meyer M.J., Malviya S.A. Effects of neostigmine reversal of nondepolarizing neuromuscular blocking agents on postoperative respiratory outcomes: a prospective study. Anesthesiology. 2014;121:959–968. doi: 10.1097/ALN.0000000000000440. [DOI] [PubMed] [Google Scholar]
- 21.Shin C.H., Long D.R., McLean D. Effects of intraoperative fluid management on postoperative outcomes: a hospital registry study. Ann Surg. 2018;267:1084–1092. doi: 10.1097/SLA.0000000000002220. [DOI] [PubMed] [Google Scholar]
- 22.Mascha E.J., Yang D., Weiss S., Sessler D.I. Intraoperative mean arterial pressure variability and 30-day mortality in patients having noncardiac surgery. Anesthesiology. 2015;123:79–91. doi: 10.1097/ALN.0000000000000686. [DOI] [PubMed] [Google Scholar]
- 23.Lukannek C., Shaefi S., Platzbecker K. The development and validation of the Score for the Prediction of Postoperative Respiratory Complications (SPORC-2) to predict the requirement for early postoperative tracheal re-intubation: a hospital registry study. Anaesthesia. 2019;74:1165–1174. doi: 10.1111/anae.14742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rostin P., Teja B.J., Friedrich S. The association of early postoperative desaturation in the operating theatre with hospital discharge to a skilled nursing or long-term care facility. Anaesthesia. 2019;74:457–467. doi: 10.1111/anae.14517. [DOI] [PubMed] [Google Scholar]
- 25.Raub D, Santer P, Nabel S, et al. BOSTN bundle intervention for perioperative screening and management of patients with suspected obstructive sleep apnea: a hospital registry study. Anesth Analg Adv Access published on July 2, 2019, doi:10.1213/ANE.0000000000004294. [DOI] [PubMed]
- 26.Agency for Healthcare Research and Quality Toolkit for using the AHRQ quality indicators. http://www.ahrq.gov/professionals/systems/hospital/qitoolkit/index.html Available from.
- 27.Duggan M., Kavanagh B.P. Pulmonary atelectasis: a pathogenic perioperative entity. Anesthesiology. 2005;102:838–854. doi: 10.1097/00000542-200504000-00021. [DOI] [PubMed] [Google Scholar]
- 28.Laffey J.G., Bellani G., Pham T. Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Med. 2016;42:1865–1876. doi: 10.1007/s00134-016-4571-5. [DOI] [PubMed] [Google Scholar]
- 29.Garcia C.S., Abreu S.C., Soares R.M. Pulmonary morphofunctional effects of mechanical ventilation with high inspiratory air flow. Crit Care Med. 2008;36:232–239. doi: 10.1097/01.CCM.0000295309.69123.AE. [DOI] [PubMed] [Google Scholar]
- 30.Fujita Y., Fujino Y., Uchiyama A., Mashimo T., Nishimura M. High peak inspiratory flow can aggravate ventilator-induced lung injury in rabbits. Med Sci Monit. 2007;13:BR95–BR100. [PubMed] [Google Scholar]
- 31.Kay S.S., Bilek A.M., Dee K.C., Gaver D.P., 3rd Pressure gradient, not exposure duration, determines the extent of epithelial cell damage in a model of pulmonary airway reopening. J Appl Physiol (1985) 2004;97:269–276. doi: 10.1152/japplphysiol.01288.2003. [DOI] [PubMed] [Google Scholar]
- 32.Rich P.B., Reickert C.A., Sawada S. Effect of rate and inspiratory flow on ventilator-induced lung injury. J Trauma. 2000;49:903–911. doi: 10.1097/00005373-200011000-00019. [DOI] [PubMed] [Google Scholar]
- 33.Chiumello D., Carlesso E., Cadringher P. Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med. 2008;178:346–355. doi: 10.1164/rccm.200710-1589OC. [DOI] [PubMed] [Google Scholar]
- 34.O'Gara B., Talmor D. Perioperative lung protective ventilation. BMJ. 2018;362:k3030. doi: 10.1136/bmj.k3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laffey J.G., Kavanagh B.P. Hypocapnia. N Engl J Med. 2002;347:43–53. doi: 10.1056/NEJMra012457. [DOI] [PubMed] [Google Scholar]
- 36.Contreras M., Masterson C., Laffey J.G. Permissive hypercapnia: what to remember. Curr Opin Anaesthesiol. 2015;28:26–37. doi: 10.1097/ACO.0000000000000151. [DOI] [PubMed] [Google Scholar]
- 37.Roth J.V. Is permissive hypercarbia pneumoprotective? Anesthesiology. 2015;122:1179. doi: 10.1097/ALN.0000000000000622. [DOI] [PubMed] [Google Scholar]
- 38.Ruscic K.J., Bogh Stokholm J., Patlak J. Supplemental carbon dioxide stabilizes the upper airway in volunteers anesthetized with propofol. Anesthesiology. 2018;129:37–46. doi: 10.1097/ALN.0000000000002239. [DOI] [PubMed] [Google Scholar]
- 39.Ladha K.S., Bateman B.T., Houle T.T. Variability in the use of protective mechanical ventilation during general anesthesia. Anesth Analg. 2018;126:503–512. doi: 10.1213/ANE.0000000000002343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farley H., Zubrow M.T., Gies J. Emergency department tachypnea predicts transfer to a higher level of care in the first 24 hours after ED admission. Acad Emerg Med. 2010;17:718–722. doi: 10.1111/j.1553-2712.2010.00796.x. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez-Bustamante A., Frendl G., Sprung J. Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: a multicenter study by the Perioperative Research Network Investigators. JAMA Surg. 2017;152:157–166. doi: 10.1001/jamasurg.2016.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verghese C., Brimacombe J.R. Survey of laryngeal mask airway usage in 11,910 patients: safety and efficacy for conventional and nonconventional usage. Anesth Analg. 1996;82:129–133. doi: 10.1097/00000539-199601000-00023. [DOI] [PubMed] [Google Scholar]
- 43.Bellani G., Laffey J.G., Pham T. Noninvasive ventilation of patients with acute respiratory distress syndrome. Insights from the LUNG SAFE study. Am J Respir Crit Care Med. 2017;195:67–77. doi: 10.1164/rccm.201606-1306OC. [DOI] [PubMed] [Google Scholar]
- 44.Bagchi A., Rudolph M.I., Ng P.Y. The association of postoperative pulmonary complications in 109,360 patients with pressure-controlled or volume-controlled ventilation. Anaesthesia. 2017;72:1334–1343. doi: 10.1111/anae.14039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.