Summary
In order to understand general anaesthesia and certain seizures, a fundamental understanding of the neurobiology of unconsciousness is needed. This review article explores similarities in neuronal and network changes during general anaesthesia and seizure-induced unconsciousness. Both seizures and anaesthetics cause disruption in similar anatomical structures that presumably lead to impaired consciousness. Despite differences in behaviour and mechanisms, both of these conditions are associated with disruption of the functionality of subcortical structures that mediate neuronal activity in the frontoparietal cortex. These areas are all likely to be involved in maintaining normal consciousness. An assessment of the similarities in the brain network disruptions with certain seizures and general anaesthesia might provide fresh insights into the mechanisms of the alterations of consciousness seen in these particular unconscious states, allowing for innovative therapies for seizures and the development of anaesthetic approaches targeting specific networks.
Keywords: awareness, general anaesthesia, mechanism, seizure, unconsciousness
Editor's key points.
-
•
When explaining general anaesthesia to patients and families, anaesthetists often state that we are putting people to sleep, which is fundamentally inaccurate.
-
•
General anaesthesia, unlike sleep, is not a restorative and easily disrupted state of unconsciousness, but is rather a dangerous neurophysiological perturbation more akin to coma; although with highly trained anaesthetists, modern drugs, and sophisticated monitors it has become much safer.
-
•
Both the cortical and subcortical brain regions and networks that are affected with generalised seizures appear to be similarly targeted and disrupted with general anaesthesia.
-
•
Studying the unconscious states associated with seizures might shed light on states of general anaesthesia, and might also improve our understanding of the neurobiological and dynamic underpinnings of both consciousness and unconsciousness.
The study of consciousness has important scientific and philosophical implications. Despite difficulties with definitions, most people inherently understand the concept of being conscious or aware of one's experience. In clinical practice, unconsciousness with general anaesthesia is casually equated with the term ‘asleep’ and contrasted with the term ‘awake’, as patients losing consciousness after receiving anaesthetic agents appear to be falling asleep.1, 2, 3 Anaesthesiologists often tell patients to ‘pick out a good dream’ and colloquially refer to general anaesthesia as ‘putting patients to sleep,’ a soothing turn of phrase for anxious patients and families who worry about relinquishing control over their minds and bodies.4,5 Sleep is a universally understandable and familiar state popularly associated with the positive connotations of restfulness, relaxation, and rejuvenation. The concept of sleep as an unconscious state was popularised by Steriade and colleagues and others, and more recently the similarities to coma have been noted.3,6, 7, 8, 9 While sleep shares several behavioural and neuronal mechanisms with loss of consciousness under anaesthesia, they are different brain states with distinct neurobiological processes.3,10, 11, 12, 13, 14, 15
An alternative model for general anaesthesia is seizures associated with loss of consciousness, although a comparison between seizures and anaesthesia would probably not calm nervous patients preoperatively. Seizures are divided into two main categories, generalised and focal, which refer, respectively, to the seizure originating from the whole brain or from a localised region. Three types of both generalised and focal seizures impair consciousness: 1) focal onset impaired awareness seizures (previously called complex partial seizures), 2) absence seizures, and 3) generalised tonic-clonic seizures.16,17 In focal onset impaired awareness seizures, seizures originate most often in the temporal lobe.18 These seizures produce automaton-like behaviour, with sudden impaired consciousness.18 Absence seizures are generalised non-convulsive seizures that consist of characteristic brief episodes of unconsciousness, with transient staring spells.19 Generalised tonic-clonic seizures typically involve profound unresponsiveness, resembling coma during the seizure and postictal periods.16 Electroconvulsive therapy electrically induces generalised tonic-clonic seizures to treat patients with certain mental health disorders.20,21
Despite differences in behaviour and physiology, these three seizure types associated with unconsciousness involve disruptions of a common set of brain structures, including the frontoparietal association cortex and the subcortical arousal systems in the thalamus and upper brainstem.16,17 The cortex, thalamus, and other subcortical structures appear to be involved in changes of arousal during general anaesthesia as well.22, 23, 24, 25, 26, 27, 28, 29 This common involvement of anatomical structures allows for a useful comparison relating the state of unconsciousness under general anaesthesia to the loss of consciousness caused by seizures. Both states consist of a short and often reversible loss of consciousness with similar side-effects, which are represented by comparable electrophysiological features and directed by the corticothalamic network. In this review, a thorough analysis of the mechanisms of both seizure and anaesthesia-induced unconsciousness reveals neurophysiological similarities that elucidate fundamental new insights into the conscious mind.
Thalamocortical structures and mechanisms of consciousness
Beyond a colloquial understanding of awareness, a more precise definition of consciousness consists of two underlying aspects: ‘level’, described by wakefulness or arousal and alertness, and ‘content’, described by awareness to stimuli.30, 31, 32 These two functions are mediated by certain anatomical structures of the brain that are important for changes in consciousness, referred to as the ‘consciousness system’, including the reticular formation of the brainstem, hypothalamus, basal forebrain, thalamus, and cerebral cortex.16,26,30,33
Recent progress in consciousness research has determined the involvement of the thalamocortical network on several key features of consciousness.6,34, 35, 36, 37, 38, 39, 40, 41 It is possible that an interference in the higher order association cortex and related subcortical regions, including the thalamus, causes loss of consciousness through either aberrant excitation or inhibition.42,43 The relationship between cortical and thalamic regions to loss of consciousness is complex, but recent literature has suggested various ways that those regions are modulated.
The role of the frontoparietal cortex
The frontoparietal cortex serves an important function in unconscious states such as general anaesthesia and seizures.44 It has been established that the dorsolateral prefrontal and superior/posterior parietal association cortex are implicit in various cognitive functions such as attention, perception, working memory, and consciousness.33,45,46 In various models of epilepsy and anaesthesia, cortical changes observed in the regions of the frontoparietal cortex are crucial in regulating consciousness.33,47
Despite a global reduction in neuronal activity under general anaesthesia, specific regions in the cortex, including the parietal and frontal association cortex, have been shown to have markedly decreased activity.48, 49, 50, 51, 52 This cortical decrease is associated with characteristic slower-wave oscillatory changes on EEG.53,54 Evidence in the literature has not conclusively established whether anaesthetics target the cortex directly,55, 56, 57, 58 or indirectly by thalamocortical disruption,3,7,59, 60, 61, 62 hypothalamic inhibition,63,64 microtubule interaction,65 or a combination of all three.
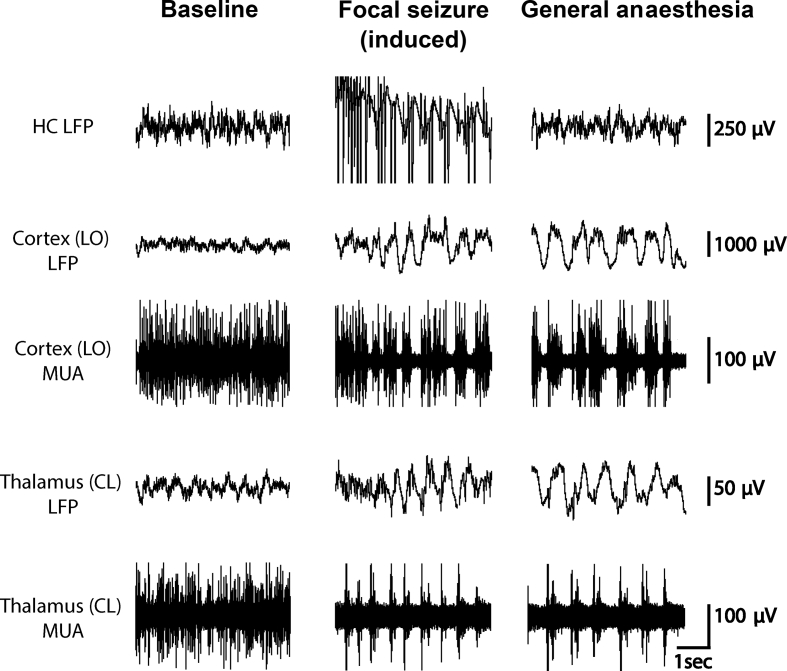
Electrophysiological monitoring of the frontoparietal cortex during focal seizures and under general anaesthesia shows similar slow-wave formations (1–3 Hz delta or slower, see Fig 1). Rodent models have demonstrated similar patterns of cortical slow waves and decreased cortical neuronal activity during focal seizures and under general anaesthesia administered by ketamine and xylazine (Fig 1).66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78 In general, slow wave oscillation is characterised on EEG by rhythmic fluctuations in membrane potentials with cycles of synaptically-driven depolarisation and action potentials (Up states), followed by a reduction in synaptic inputs, membrane hyperpolarisation, and cessation of activity (Down states).76 During Up states, recurrent network activity is initiated and moderated by an exact balance between excitatory and inhibitory inputs that likely influences the neuronal response.76,79, 80, 81, 82, 83 During focal onset impaired awareness seizures, neocortical slow waves demonstrate increases and decreases in neuronal firing that resemble Up and Down states observed under general anaesthesia.73 The electrophysiological measurements of Up and Down states during these types of seizures and under general anaesthesia, specifically membrane potential, firing rate, and input resistance, are significantly similar.84 Thus, though the electrical activity in the region of origin in focal onset impaired awareness seizures does not resemble activity under general anaesthesia, the neuronal activity looks very similar in areas outside of origin, such as in the frontal or parietal cortex.66,73, 74, 75,84
Fig 1.
Comparison of neuronal changes seen in the unconscious states of general anaesthesia and focal onset seizures in a rodent model. Note similar electrophysiologic patterns in the cortex and thalamus in the deeply anaesthetised and seizure states. Data used to produce this figure were obtained by permission from Feng and colleagues.67 Seizures were induced by hippocampal stimulation with a 2 s current injection of 200 micA in the hippocampus with 60 Hz frequency. General anaesthesia was induced with ketamine-xylazine (90/15 mg kg−1) and doses were reduced to establish baseline periods. Local field potential (LFP) filter 0.1–100 Hz. Multi-unit activity (MUA) filter 400 Hz–10 KHz. CL, central lateral nucleus of the thalamus; HC, hippocampus; LO, lateral orbital frontal cortex.
Despite the classification of both absence seizures and generalised tonic-clonic seizures as generalised, recent evidence has suggested that these seizures do not necessarily occur throughout the whole brain and instead spare some brain regions.85,86 EEG and neuroimaging techniques have demonstrated that these seizures preferentially affect focal bilateral regions in the brain involved in the conscious system.16 Certain anaesthetics have also been demonstrated to selectively target comparable brain regions.87, 88, 89, 90 During absence seizures, decreases in cortical activity appear in the medial frontal, medial parietal, anterior and posterior cingulate, and lateral parietal cortices, and both increases and decreases in cortical activity are observed in the lateral frontal cortex.16,91,92 Just as uncertainty persists about the predominant brain region of anaesthetic action, it is debated whether absence seizures are generated in the thalamus or cortex.16 However, animal studies favour a cortical origin, suggesting that populations of hyperexcitable deep somatosensory cortical neurones initiate the spike-and-wave discharge.93,94 During generalised tonic-clonic seizures, increases in cortical activity have been demonstrated in the lateral, frontal, and medial parietal cortices, with postictal decreases in the medial and lateral frontoparietal association cortices.21,95,96 Thus, these types of seizures (absence, generalised tonic-clonic, and focal) and general anaesthesia have all been associated with analogous cortical changes.91,92,95,96
Subcortical involvement
There is strong evidence to suggest that subcortical areas, such as the thalamus and the brainstem, are critical to maintaining consciousness. Neurones within the central thalamus have been identified for restoring function at the network level for patients with consciousness disorders.97 Consequently, stimulation of subcortical structures in the brain, such as the thalamus, has been shown to reverse unconsciousness in both seizures and anaesthesia.16,34 Modulation of thalamic activity decreases arousal and may contribute to the mechanisms underlying the changes in consciousness seen under general anaesthesia and seizures, and in other states including sleep and coma.98, 99, 100
It has been shown that many anaesthetics, including propofol, dexmedetomidine, halothane, and isoflurane, target subcortical structures such as the thalamus.7,48,60 A subsequent theory of the thalamic switch of consciousness was developed, which proposes that the thalamocortical neurones become hyperpolarised and therefore interrupt the normal function of the thalamocortical circuits required for consciousness.7,33,101 During anaesthesia, microfusion of nicotine and infusion of antibodies into the central medial thalamus have been shown to reverse the effects of sevoflurane and desflurane anaesthesia, even when the anaesthetics are continually administered.43,102, 103, 104, 105
It is not clear if the thalamus itself constitutes the primary focus of anaesthetic modulation, or if changes that appear within it are secondary effects from other brain regions.33 Anaesthetic-induced regulation of cortical activity may precede suppression in the thalamus, which would imply a secondary or consequential effect in the subcortical region.26,56 But the close relationship between the thalamus and the cortex suggests that it may not be possible to separate thalamic activity entirely.33
In a rat model of focal onset seizures, population firing of neurones in arousal nuclei of the thalamus, such as the central lateral nuclei, has been shown to decrease during seizures while the cortex exhibited slow waves (Fig 1).67 There is evidence that stimulating certain subcortical areas in the brain with cholinergic agents during focal seizures and under general anaesthesia leads to arousal.34,102,103 Gummadavelli and colleagues34 showed that stimulating the central lateral nucleus of the thalamus under deep anaesthesia and during electrically-induced focal onset seizures led to reduced cortical slow-wave activity and increased arousal. A follow-up study by Kundishora and colleagues66 showed that simultaneous stimulation of the central lateral nucleus of the intralaminar thalamus and the pontine nucleus oralis restored awake-like cortical activity, reversing the decreased arousal associated with focal onset seizures.
Most functional MRI studies describe increases in the thalamus during absence seizures and generalised tonic-clonic seizures.91,92 Several studies suggest that the thalamus plays a critical role inpropagating absence seizures.93,106 As during focal seizures,107 stimulation to the thalamus reticular nucleus appears to suppress absence seizure activity.108 While the exact mechanism is unclear, it is possible that abnormal activation of subcortical structures, such as the thalamus, during generalised tonic-clonic seizures plays an important role in the disruption of ascending arousal regions.95,109
In both anaesthesia and seizures, areas of the brainstem are likely involved with the disruption of thalamocortical function, causing unconsciousness through direct engagement with the cerebral cortex or via the thalamus and basal forebrain.110 The nuclei that primarily affect cerebral activity are located in the upper pons and in the midbrain, with some possible influence from lower brainstem structures, and rely on a network of nuclei families, rather than a single nucleus or family.110,111
The mesopontine has been identified as an activator for the cortex for shifting between sleep and wakeful states through a network described as the reticular activating system.112 It was subsequently suggested that many anaesthetics, specifically those that target gamma aminobutyric acid (GABA) type A receptors, work by suppressing the activation system in the mesopontine.111,113,114 Recent studies have shown that microinjections of pentobarbital into the mesopontine tegmentum in a rat model induce an anaesthesia-like state.115,116 However, because this region does not induce a coma, it has been suggested that it may act as a ‘gatekeeper’ to switch between states of awareness.115 An alternative theory suggests that anaesthetics target the mesopontine primarily, with cortical inhibition as a secondary effect.116 Regions of the upper brainstem have been shown to be responsible for emergence from general anaesthesia. In the ventral tegmental area, dopaminergic neurones induce arousal under propofol and isoflurane.117, 118, 119
Areas of the brainstem have also been associated with focal and generalised convulsive seizures. One theory proposes that generalised tonic-clonic seizures and focal seizures result from primary or secondary disruption of the reticular activating system.120,121 Anticonvulsant therapy has been shown to reverse the effects of reticular neurones in animal models of brainstem-triggered generalised tonic-clonic seizures.122,123 During in vivo focal seizures, cholinergic neurones in the pedunculopontine tegmental nucleus have been observed with decreased neuronal activity.124,125 Moreover, stimulation of the pons and central thalamus simultaneously during focal seizures led to a return of baseline cortical electrophysiology and behaviour, although stimulation of each area separately was not sufficient to produce this same effect.66 Similarly, stimulation of the pontine reticular nucleus increased connectivity preferentially in the basal forebrain-paralimbic networks, likely reflecting a disruption in awareness.126
The basal ganglia, hypothalamus, and cerebellum are other subcortical structures that may be involved in loss of consciousness in seizure and under anaesthesia,127, 128, 129, 130 though some of these theories are controversial.29,131,132 The basal ganglia may be involved in modulation of neocortical generalised absence seizures and other types of generalised seizures.133, 134, 135 Stimulation of the internal globus pallidus region of the basal ganglia has induced wakeful unawareness under propofol.128 The claustrum may have a role in arousal as well.136 One study suggests an association between unresponsiveness produced by propofol and disruption of functionality in the putamen (a subcortical structure in the basal ganglia) more than in the thalamus,129 putting into question the wide acceptance of the thalamus' importance in consciousness.137 Animal studies, for example, show some limited behavioural functions that are present even with thalamic ablation.138
Functional connectivity and network systems
The importance of thalamocortical interactions in mediating consciousness has been well-established. One theory suggests that conscious function relies on the complex interactions within neuronal networks that integrate information.22, 23, 24, 25 Specifically, changes in consciousness may occur as a result of disruption in or unbinding of these networks.26, 27, 28,33
In anaesthesia, two popular models define our understanding of how anaesthetics interfere with the level and content of consciousness through network changes: a ‘bottom-up’ and a ‘top-down’ approach.31,139, 140, 141 The bottom-up approach explains the disruption that anaesthetics cause to nuclei and neuronal circuits in the brainstem and thalamus that lead to changes in the primary sensory and ultimately higher-order association cortices.141 The second, top-down approach describes the function of anaesthetics in interrupting information flow from higher-order association to early sensory cortices.31 The bottom-up approach may be correlated with level of consciousness, and the top-down approach with content.31 Successful anaesthetic-induced unconsciousness likely works by targeting both the level and the content of consciousness through a combination of top-down and bottom-up approaches.142
Seizures with associated loss of consciousness also affect content and level of consciousness.16,143,144 The Ictal Consciousness Inventory,143 the Consciousness Seizure Scale,35 the Seizure Perception Survey,145 and the Responsiveness in Epilepsy Scale146, 147, 148 are posited techniques to measure level and content during episodes of impaired consciousness during seizure to understand more fully the mechanisms of awareness and arousal.149 Although the mechanisms of seizure-induced unconsciousness have not been described using top-down and bottom-up approaches specifically, it is likely that a combined approach may also be applied to understand the mechanisms of ictal loss of consciousness.
The puzzling impairment in consciousness seen during focal temporal lobe seizures, which originate in an area of the brain not typically associated with consciousness, may be explained by the network inhibition hypothesis.16 The network inhibition hypothesis proposes that focal seizures that initiate in areas of the brain not part of the consciousness system, such as the temporal lobe, lead to unconsciousness that is secondary to seizure spread through activation of inhibitory subcortical structures.29 These subcortical structures deactivate frontal and parietal association cortical regions required to maintain normal consciousness.29
Cortical sensory processing
There are limited cortical processes that still function during loss of consciousness in both seizure and anaesthesia.16,92,150 Neuronal responses to sound in the primary sensory areas appear to be unaffected under light anaesthesia,51,151, 152, 153, 154 while higher-order cortical areas reveal impaired responses.40,153,155, 156, 157 Thus, network activity in higher-order cortical regions could be primarily affected by anaesthesia and seizure.
Cerebral blood flow and metabolic activity
Changes in unconsciousness are accompanied by fluctuations in cerebral blood flow (CBF) and metabolic activity. Anaesthesia is a hypometabolic state, producing large-scale, global reductions in those two processes.3,33,48,49,61,158, 159, 160 CBF during anaesthesia is mediated through the cortex, and the largest reductions in CBF by propofol can be seen in the cuneus, precuneus, and posterior cingulate and retrosplenial cortex regions, and in the frontal cortex.61,161 High concentrations of sevoflurane also produce a reduction in frontal CBF, at 1.0–2.0 MAC.50,162
During seizures, it is generally understood that there is an increase in CBF and metabolic demand ictally.163,164 However, as noted above, differences in neuronal activity are observed between types of seizures and regionally within a single type. During focal seizures that impair consciousness, there is evidence that CBF and metabolic activity are increased in the area of origin, but reduced regionally in areas such as the frontoparietal cortex.77,165 Moreover, regions in the brain during absence seizures show decreases in CBF and metabolism.166
It is unclear if the anaesthetic reductions in CBF and metabolic activity cause unconsciousness or if they are a result of alterations in network interactions.33 Patients recovering from a vegetative state who regained consciousness, for example, continued to show substantially decreased global cerebral metabolism.167,168 There is great variation in cerebral metabolic rate among individuals even at baseline,48 which disputes the idea of a consistent correlation between metabolic activity and consciousness.33
Associated consequences
Many of the consequences associated with loss of consciousness are shared between certain seizures and anaesthetics. For example, changes of consciousness during seizure and anaesthesia are transient. Patients also experience a recovery period of variable duration postictally or postoperatively, with delirium, generally defined as a disturbance of consciousness, a common side-effect of both conditions.169, 170, 171, 172 Older adults are especially prone to delirium after anaesthesia and after seizures.173,174
Limitations
There are several limitations to this comparative methodology between seizures and anaesthesia-induced unconsciousness. Despite disruptions in similar anatomical structures, types of anaesthetics or seizures may act by different mechanisms.16,17,175 At first glance, ketamine appears to be an exception to the comparison between changes of consciousness under anaesthesia and seizure because of its unique mechanisms. Ketamine, unlike most anaesthetic agents, is an antagonist of the n-methyl-d-aspartate subtype of glutamate receptors (similar to nitrous oxide and xenon).33 It has previously been described that cats administered ketamine anaesthesia produced bursts of hypersynchronous slow waves on EEG.176 These slow waves were rhythmic, synchronous, and frequently accompanied by spikes, forming a spike-and-wave complex.176, 177, 178, 179 Moreover, in many neurones, neuronal activity was modified during the slow wave bursts that mimic patterns observed during experimental seizures.176,177 It has thus been suggested that the functional state of the brain produced by ketamine resembles absence epilepsy, because of similar EEG spike-and-wave complexes and non-convulsive loss of consciousness.176,180 This is supported by the fact that the absence seizure therapy trimethadione also suppresses ketamine-induced seizure-like EEG activity.180 While there is evidence of both convulsant and anticonvulsant properties of ketamine,181, 182, 183 the exact relationship between ketamine and seizures is still unclear.
While advances in techniques that record brain activity in human patients and animal models have provided important insights into the mechanisms of unconsciousness, the difficulty of understanding these mechanisms rests in the complexity of consciousness and its effects. When we study consciousness, we are limited to studying conditions such as anaesthesia and seizure that have changes in consciousness as a secondary effect. All of these states have effects on cognition, blood flow, and metabolism, making it difficult to define loss of consciousness exclusively. Once we establish the exact interplay of regions of the brain involved in these conditions and mechanisms of network changes, it will be easier to identify and clarify consciousness. Until then, direct comparisons between changes in neuronal activity in the anaesthetic- and seizure-induced states of unconsciousness are an ideal strategy for the advancement of consciousness research.
Conclusions
The past decade has explored new frontiers of research into the effects of anaesthesia. At this point, however, it is only possible to study the downstream effects of anaesthesia, as we do not yet understand the fundamentals of the complicated unconscious state that anaesthesia induces. To clarify these effects, the unconscious state under anaesthesia should be compared with other states associated with unconsciousness. Both seizures and anaesthetics cause disruption in similar anatomical structures that lead to impaired consciousness. Although they differ in behaviour and mechanism, both of these conditions disrupt the functionality of subcortical structures that mediate normal activity in the frontoparietal cortex, which represent the consciousness system.16,33,42 Understanding which brain networks are shared between these conditions will provide new insights into the mechanisms of alterations in consciousness seen in seizures and anaesthesia, and can lead to novel therapies and anaesthetics targeting these networks.
Author's Contributions
BFG is the sole author of this paper, accountable for its accuracy and integrity.
Declarations of interest
The author declares that they have no conflict of interest.
Funding
This work was supported in part by a grant from the Foundation for Anesthesia Education and Research. BFG is a PhD student in the Investigative Medicine Program at Yale which is supported by Clinical and Translational Science Awards (UL1 TR001863) from the National Center for Advancing Translational Science, a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the author and do not necessarily represent the official view of the NIH.
Acknowledgements
The author gratefully acknowledges Li Feng, Department of Neurology, Xiangya Hospital, Central South University, Changsha, China, and Lim-Anna Sieu, Department of Neurology, Yale School of Medicine, New Haven, CT, USA, who were instrumental in providing and obtaining the data to produce Figure 1; Hal Blumenfeld, Department of Neurology, Yale School of Medicine, New Haven, CT, USA, for his insightful comments and suggestions; and Shaun Gruenbaum, Department of Anesthesiology and Perioperative Medicine, Mayo Clinic, Jacksonville, FL, USA, for his editorial assistance.
Handling editor: Michael Avidan
References
- 1.Gawande A., Denno D.W., Truog R.D., Waisel D. Physicians and execution--highlights from a discussion of lethal injection. N Engl J Med. 2008;358:448–451. doi: 10.1056/NEJMp0800378. [DOI] [PubMed] [Google Scholar]
- 2.Dershwitz M., Henthorn T.K. The pharmacokinetics and pharmacodynamics of thiopental as used in lethal injection. Fordham Urb LJ. 2008;35:931. [Google Scholar]
- 3.Brown E.N., Lydic R., Schiff N.D. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–2650. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shillcutt S.K. Rounds. JAMA. 2018;319:1863. doi: 10.1001/jama.2018.4307. [DOI] [PubMed] [Google Scholar]
- 5.Przybylo H.J. WW Norton & Company; New York: 2017,. Counting backwards: a doctor's notes on Anesthesia. [Google Scholar]
- 6.Steriade M., McCormick D.A., Sejnowski T.J. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 7.Alkire M.T., Haier R.J., Fallon J.H. Toward a unified theory of narcosis: brain imaging evidence for a thalamocortical switch as the neurophysiologic basis of anesthetic-induced unconsciousness. Conscious Cogn. 2000;9:370–386. doi: 10.1006/ccog.1999.0423. [DOI] [PubMed] [Google Scholar]
- 8.Steriade M. Basic mechanisms of sleep generation. Neurology. 1992;42:9–17. discussion 8. [PubMed] [Google Scholar]
- 9.Baars B.J. Global workspace theory of consciousness: toward a cognitive neuroscience of human experience. Prog Brain Res. 2005;150:45–53. doi: 10.1016/S0079-6123(05)50004-9. [DOI] [PubMed] [Google Scholar]
- 10.Steriade M., Contreras D., Amzica F. Synchronized sleep oscillations and their paroxysmal developments. Trends Neurosci. 1994;17:199–208. doi: 10.1016/0166-2236(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 11.Chamberlin N.L., Eikermann M. This is no humbug: anesthetic agent-induced unconsciousness and sleep are visibly different. Anesthesiology. 2010;113:1007–1009. doi: 10.1097/ALN.0b013e3181f69825. [DOI] [PubMed] [Google Scholar]
- 12.van der Meij J., Martinez-Gonzalez D., Beckers G.J.L., Rattenborg N.C. Neurophysiology of avian sleep: comparing natural sleep and isoflurane anesthesia. Front Neurosci. 2019;13:262. doi: 10.3389/fnins.2019.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lydic R., Baghdoyan H.A. Sleep, anesthesiology, and the neurobiology of arousal state control. Anesthesiology. 2005;103:1268–1295. doi: 10.1097/00000542-200512000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Massimini M., Ferrarelli F., Huber R., Esser S.K., Singh H., Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- 15.Tononi G., Koch C. The neural correlates of consciousness: an update. Ann N Y Acad Sci. 2008;1124:239–261. doi: 10.1196/annals.1440.004. [DOI] [PubMed] [Google Scholar]
- 16.Blumenfeld H. Impaired consciousness in epilepsy. Lancet Neurol. 2012;11:814–826. doi: 10.1016/S1474-4422(12)70188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blumenfeld H., Meador K.J. Consciousness as a useful concept in epilepsy classification. Epilepsia. 2014;55:1145–1150. doi: 10.1111/epi.12588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quesney L. Clinical and EEG features of complex partial seizures of temporal lobe origin. Epilepsia. 1986;27:S27–S45. doi: 10.1111/j.1528-1157.1986.tb05738.x. [DOI] [PubMed] [Google Scholar]
- 19.Hughes J.R. Absence seizures: a review of recent reports with new concepts. Epilepsy Behav. 2009;15:404–412. doi: 10.1016/j.yebeh.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 20.RD Weiner . American Psychiatric Pub; 2008. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging (a task force report of the American Psychiatric Association) [Google Scholar]
- 21.Enev M., McNally K.A., Varghese G., Zubal I.G., Ostroff R.B., Blumenfeld H. Imaging onset and propagation of ECT-induced seizures. Epilepsia. 2007;48:238–244. doi: 10.1111/j.1528-1167.2007.00919.x. [DOI] [PubMed] [Google Scholar]
- 22.Tononi G. An information integration theory of consciousness. BMC Neurosci. 2004;5:42. doi: 10.1186/1471-2202-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tononi G., Edelman G.M. Consciousness and complexity. Science. 1998;282:1846–1851. doi: 10.1126/science.282.5395.1846. [DOI] [PubMed] [Google Scholar]
- 24.Tononi G. Consciousness, information integration, and the brain. Prog Brain Res. 2005;150:109–126. doi: 10.1016/S0079-6123(05)50009-8. [DOI] [PubMed] [Google Scholar]
- 25.Tononi G. Consciousness as integrated information: a provisional manifesto. Biol Bull. 2008;215:216–242. doi: 10.2307/25470707. [DOI] [PubMed] [Google Scholar]
- 26.Alkire M.T. Probing the mind: anesthesia and neuroimaging. Clin Pharmacol Ther. 2008;84:149–152. doi: 10.1038/clpt.2008.75. [DOI] [PubMed] [Google Scholar]
- 27.Hudetz A.G. Suppressing consciousness: mechanisms of general anesthesia. Semin Anesth Periop Med Pain. 2006;25:196–204. [Google Scholar]
- 28.Mashour G.A. Cognitive unbinding in sleep and anesthesia. Science. 2005;310:1768–1769. doi: 10.1126/science.310.5755.1768b. author reply -9. [DOI] [PubMed] [Google Scholar]
- 29.Norden A.D., Blumenfeld H. The role of subcortical structures in human epilepsy. Epilepsy Behav. 2002;3:219–231. doi: 10.1016/s1525-5050(02)00029-x. [DOI] [PubMed] [Google Scholar]
- 30.Benarroch E.E., Cutsforth-Gregory J.K., Flemming K.D. Oxford University Press; Oxford, England: 2017. Mayo clinic medical neurosciences: organized by neurologic system and level. [Google Scholar]
- 31.Mashour G.A., Hudetz A.G. Bottom-up and top-down mechanisms of general anesthetics modulate different dimensions of consciousness. Front Neural Circ. 2017;11:44. doi: 10.3389/fncir.2017.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plum F., Posner J.B. The diagnosis of stupor and coma. Contemp Neurol Ser. 1972;10:1–286. [PubMed] [Google Scholar]
- 33.Hudetz A.G. General anesthesia and human brain connectivity. Brain Connect. 2012;2:291–302. doi: 10.1089/brain.2012.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gummadavelli A., Motelow J.E., Smith N., Zhan Q., Schiff N.D., Blumenfeld H. Thalamic stimulation to improve level of consciousness after seizures: evaluation of electrophysiology and behavior. Epilepsia. 2015;56:114–124. doi: 10.1111/epi.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arthuis M., Valton L., Regis J. Impaired consciousness during temporal lobe seizures is related to increased long-distance cortical-subcortical synchronization. Brain. 2009;132:2091–2101. doi: 10.1093/brain/awp086. [DOI] [PubMed] [Google Scholar]
- 36.Van der Werf Y.D., Witter M.P., Groenewegen H.J. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- 37.Jones E.G. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 2001;24:595–601. doi: 10.1016/s0166-2236(00)01922-6. [DOI] [PubMed] [Google Scholar]
- 38.Kinomura S., Larsson J., Gulyas B., Roland P.E. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science. 1996;271:512–515. doi: 10.1126/science.271.5248.512. [DOI] [PubMed] [Google Scholar]
- 39.Steriade M., Contreras D., Amzica F., Timofeev I. Synchronization of fast (30-40 Hz) spontaneous oscillations in intrathalamic and thalamocortical networks. J Neurosci. 1996;16:2788–2808. doi: 10.1523/JNEUROSCI.16-08-02788.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boveroux P., Vanhaudenhuyse A., Bruno M.A. Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology. 2010;113:1038–1053. doi: 10.1097/ALN.0b013e3181f697f5. [DOI] [PubMed] [Google Scholar]
- 41.Liu X., Lauer K.K., Ward B.D., Li S.J., Hudetz A.G. Differential effects of deep sedation with propofol on the specific and nonspecific thalamocortical systems: a functional magnetic resonance imaging study. Anesthesiology. 2013;118:59–69. doi: 10.1097/ALN.0b013e318277a801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blumenfeld H., Taylor J. Why do seizures cause loss of consciousness? Neuroscientist. 2003;9:301–310. doi: 10.1177/1073858403255624. [DOI] [PubMed] [Google Scholar]
- 43.Langsjo J.W., Alkire M.T., Kaskinoro K. Returning from oblivion: imaging the neural core of consciousness. J Neurosci. 2012;32:4935–4943. doi: 10.1523/JNEUROSCI.4962-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baars B.J., Ramsoy T.Z., Laureys S. Brain, conscious experience and the observing self. Trends Neurosci. 2003;26:671–675. doi: 10.1016/j.tins.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 45.Naghavi H.R., Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: shared demands on integration? Conscious Cogn. 2005;14:390–425. doi: 10.1016/j.concog.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Rees G., Kreiman G., Koch C. Neural correlates of consciousness in humans. Nat Rev Neurosci. 2002;3:261–270. doi: 10.1038/nrn783. [DOI] [PubMed] [Google Scholar]
- 47.Vogt B.A., Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Prog Brain Res. 2005;150:205–217. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alkire M.T., Haier R.J., Barker S.J., Shah N.K., Wu J.C., Kao Y.J. Cerebral metabolism during propofol anesthesia in humans studied with positron emission tomography. Anesthesiology. 1995;82:393–403. doi: 10.1097/00000542-199502000-00010. discussion 27A. [DOI] [PubMed] [Google Scholar]
- 49.Alkire M.T., Haier R.J., Shah N.K., Anderson C.T. Positron emission tomography study of regional cerebral metabolism in humans during isoflurane anesthesia. Anesthesiology. 1997;86:549–557. doi: 10.1097/00000542-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Kaisti K.K., Langsjo J.W., Aalto S. Effects of sevoflurane, propofol, and adjunct nitrous oxide on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology. 2003;99:603–613. doi: 10.1097/00000542-200309000-00015. [DOI] [PubMed] [Google Scholar]
- 51.Heinke W., Fiebach C.J., Schwarzbauer C., Meyer M., Olthoff D., Alter K. Sequential effects of propofol on functional brain activation induced by auditory language processing: an event-related functional magnetic resonance imaging study. Br J Anaesth. 2004;92:641–650. doi: 10.1093/bja/aeh133. [DOI] [PubMed] [Google Scholar]
- 52.Heinke W., Kenntner R., Gunter T.C., Sammler D., Olthoff D., Koelsch S. Sequential effects of increasing propofol sedation on frontal and temporal cortices as indexed by auditory event-related potentials. Anesthesiology. 2004;100:617–625. doi: 10.1097/00000542-200403000-00023. [DOI] [PubMed] [Google Scholar]
- 53.Hashemi M., Hutt A., Hight D., Sleigh J. Anesthetic action on the transmission delay between cortex and thalamus explains the beta-buzz observed under propofol anesthesia. PLoS One. 2017;12 doi: 10.1371/journal.pone.0179286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hagihira S. Changes in the electroencephalogram during anaesthesia and their physiological basis. BJA: Br J Anaesth. 2015;115:i27–i31. doi: 10.1093/bja/aev212. [DOI] [PubMed] [Google Scholar]
- 55.Alkire M.T., Hudetz A.G., Tononi G. Consciousness and anesthesia. Science. 2008;322:876–880. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Velly L.J., Rey M.F., Bruder N.J. Differential dynamic of action on cortical and subcortical structures of anesthetic agents during induction of anesthesia. Anesthesiology. 2007;107:202–212. doi: 10.1097/01.anes.0000270734.99298.b4. [DOI] [PubMed] [Google Scholar]
- 57.Hentschke H., Schwarz C., Antkowiak B. Neocortex is the major target of sedative concentrations of volatile anaesthetics: strong depression of firing rates and increase of GABAA receptor-mediated inhibition. Eur J Neurosci. 2005;21:93–102. doi: 10.1111/j.1460-9568.2004.03843.x. [DOI] [PubMed] [Google Scholar]
- 58.Antkowiak B. Different actions of general anesthetics on the firing patterns of neocortical neurones mediated by the GABA(A) receptor. Anesthesiology. 1999;91:500–511. doi: 10.1097/00000542-199908000-00025. [DOI] [PubMed] [Google Scholar]
- 59.Akeju O., Loggia M.L., Catana C. Disruption of thalamic functional connectivity is a neural correlate of dexmedetomidine-induced unconsciousness. Elife. 2014;3 doi: 10.7554/eLife.04499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baker R., Gent T.C., Yang Q. Altered activity in the central medial thalamus precedes changes in the neocortex during transitions into both sleep and propofol anesthesia. J Neurosci. 2014;34:13326–13335. doi: 10.1523/JNEUROSCI.1519-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fiset P., Paus T., Daloze T. Brain mechanisms of propofol-induced loss of consciousness in humans: a positron emission tomographic study. J Neurosci. 1999;19:5506–5513. doi: 10.1523/JNEUROSCI.19-13-05506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nelson L.E., Guo T.Z., Lu J., Saper C.B., Franks N.P., Maze M. The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci. 2002;5:979–984. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- 63.Zecharia A.Y., Nelson L.E., Gent T.C. The involvement of hypothalamic sleep pathways in general anesthesia: testing the hypothesis using the GABAA receptor β3N265M knock-in mouse. J Neurosci. 2009;29:2177–2187. doi: 10.1523/JNEUROSCI.4997-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uygun D.S., Ye Z., Zecharia A.Y. Bottom-up versus top-down induction of sleep by zolpidem acting on histaminergic and neocortex neurones. J Neurosci. 2016;36:11171–11184. doi: 10.1523/JNEUROSCI.3714-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Craddock T.J.A., Hameroff S.R., Ayoub A.T., Klobukowski M., Tuszynski J.A. Anesthetics act in quantum channels in brain microtubules to prevent consciousness. Curr Top Med Chem. 2015;15:523–533. doi: 10.2174/1568026615666150225104543. [DOI] [PubMed] [Google Scholar]
- 66.Kundishora A.J., Gummadavelli A., Ma C. Restoring conscious arousal during focal limbic seizures with deep brain stimulation. Cereb Cortex. 2017;27:1964–1975. doi: 10.1093/cercor/bhw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feng L., Motelow J.E., Ma C. Seizures and sleep in the thalamus: focal limbic seizures show divergent activity patterns in different thalamic nuclei. J Neurosci. 2017;37:11441–11454. doi: 10.1523/JNEUROSCI.1011-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Metherate R., Ashe J.H. Ionic flux contributions to neocortical slow waves and nucleus basalis-mediated activation: whole-cell recordings in vivo. J Neurosci. 1993;13:5312–5323. doi: 10.1523/JNEUROSCI.13-12-05312.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steriade M., Nunez A., Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurones in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cowan R.L., Wilson C.J. Spontaneous firing patterns and axonal projections of single corticostriatal neurones in the rat medial agranular cortex. J Neurophysiol. 1994;71:17–32. doi: 10.1152/jn.1994.71.1.17. [DOI] [PubMed] [Google Scholar]
- 71.Timofeev I., Grenier F., Bazhenov M., Sejnowski T.J., Steriade M. Origin of slow cortical oscillations in deafferented cortical slabs. Cereb Cortex. 2000;10:1185–1199. doi: 10.1093/cercor/10.12.1185. [DOI] [PubMed] [Google Scholar]
- 72.Waters J., Helmchen F. Boosting of action potential backpropagation by neocortical network activity in vivo. J Neurosci. 2004;24:11127–11136. doi: 10.1523/JNEUROSCI.2933-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Englot D.J., Mishra A.M., Mansuripur P.K., Herman P., Hyder F., Blumenfeld H. Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci. 2008;28:9066–9081. doi: 10.1523/JNEUROSCI.2014-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Englot D.J., Modi B., Mishra A.M., DeSalvo M., Hyder F., Blumenfeld H. Cortical deactivation induced by subcortical network dysfunction in limbic seizures. J Neurosci. 2009;29:13006–13018. doi: 10.1523/JNEUROSCI.3846-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Englot D.J., Yang L., Hamid H. Impaired consciousness in temporal lobe seizures: role of cortical slow activity. Brain. 2010;133:3764–3777. doi: 10.1093/brain/awq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haider B., Duque A., Hasenstaub A.R., McCormick D.A. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci. 2006;26:4535–4545. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blumenfeld H., McNally K.A., Vanderhill S.D. Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex. 2004;14:892–902. doi: 10.1093/cercor/bhh048. [DOI] [PubMed] [Google Scholar]
- 78.Blumenfeld H., Rivera M., McNally K.A., Davis K., Spencer D.D., Spencer S.S. Ictal neocortical slowing in temporal lobe epilepsy. Neurology. 2004;63:1015–1021. doi: 10.1212/01.wnl.0000141086.91077.cd. [DOI] [PubMed] [Google Scholar]
- 79.Sanchez-Vives M.V., McCormick D.A. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- 80.Shu Y., Hasenstaub A., McCormick D.A. Turning on and off recurrent balanced cortical activity. Nature. 2003;423:288–293. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- 81.McCormick D.A., Shu Y., Hasenstaub A., Sanchez-Vives M., Badoual M., Bal T. Persistent cortical activity: mechanisms of generation and effects on neuronal excitability. Cereb Cortex. 2003;13:1219–1231. doi: 10.1093/cercor/bhg104. [DOI] [PubMed] [Google Scholar]
- 82.Shu Y., Hasenstaub A., Badoual M., Bal T., McCormick D.A. Barrages of synaptic activity control the gain and sensitivity of cortical neurones. J Neurosci. 2003;23:10388–10401. doi: 10.1523/JNEUROSCI.23-32-10388.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hasenstaub A., Shu Y., Haider B., Kraushaar U., Duque A., McCormick D.A. Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron. 2005;47:423–435. doi: 10.1016/j.neuron.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 84.Yue Z., Freedman I.G., Vincent P. Up and down states of cortical neurones in focal limbic seizures. Cereb Cortex. 2020;30:3074–3086. doi: 10.1093/cercor/bhz295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blumenfeld H., Westerveld M., Ostroff R.B. Selective frontal, parietal, and temporal networks in generalized seizures. Neuroimage. 2003;19:1556–1566. doi: 10.1016/s1053-8119(03)00204-0. [DOI] [PubMed] [Google Scholar]
- 86.Blumenfeld H. Cellular and network mechanisms of spike-wave seizures. Epilepsia. 2005;46(Suppl 9):21–33. doi: 10.1111/j.1528-1167.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- 87.Schroter M.S., Spoormaker V.I., Schorer A. Spatiotemporal reconfiguration of large-scale brain functional networks during propofol-induced loss of consciousness. J Neurosci. 2012;32:12832–12840. doi: 10.1523/JNEUROSCI.6046-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bonhomme V., Boveroux P., Brichant J.F., Laureys S., Boly M. Neural correlates of consciousness during general anesthesia using functional magnetic resonance imaging (fMRI) Arch Ital Biol. 2012;150:155–163. doi: 10.4449/aib.v150i2.1242. [DOI] [PubMed] [Google Scholar]
- 89.Leung L.S., Luo T., Ma J., Herrick I. Brain areas that influence general anesthesia. Prog Neurobiol. 2014;122:24–44. doi: 10.1016/j.pneurobio.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 90.Alkire M.T.M., Haier R.J.P., Shah N.K.M., Anderson C.T.M. Positron emission tomography study of regional cerebral metabolism in humans during isoflurane anesthesia. Anesthesiology. 1997;86:549–557. doi: 10.1097/00000542-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 91.Berman R., Negishi M., Vestal M. Simultaneous EEG, fMRI, and behavior in typical childhood absence seizures. Epilepsia. 2010;51:2011–2022. doi: 10.1111/j.1528-1167.2010.02652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guo J.N., Kim R., Chen Y. Impaired consciousness in patients with absence seizures investigated by functional MRI, EEG, and behavioural measures: a cross-sectional study. Lancet Neurol. 2016;15:1336–1345. doi: 10.1016/S1474-4422(16)30295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McCafferty C., David F., Venzi M. Cortical drive and thalamic feed-forward inhibition control thalamic output synchrony during absence seizures. Nat Neurosci. 2018;21:744–756. doi: 10.1038/s41593-018-0130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Polack P.O., Guillemain I., Hu E., Deransart C., Depaulis A., Charpier S. Deep layer somatosensory cortical neurones initiate spike-and-wave discharges in a genetic model of absence seizures. J Neurosci. 2007;27:6590–6599. doi: 10.1523/JNEUROSCI.0753-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blumenfeld H., Varghese G.I., Purcaro M.J. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain. 2009;132:999–1012. doi: 10.1093/brain/awp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takano H., Motohashi N., Uema T. Changes in regional cerebral blood flow during acute electroconvulsive therapy in patients with depression: positron emission tomographic study. Br J Psychiatr. 2007;190:63–68. doi: 10.1192/bjp.bp.106.023036. [DOI] [PubMed] [Google Scholar]
- 97.Schiff N.D. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann N Y Acad Sci. 2008;1129:105–118. doi: 10.1196/annals.1417.029. [DOI] [PubMed] [Google Scholar]
- 98.Lewis L.D., Voigts J., Flores F.J. Thalamic reticular nucleus induces fast and local modulation of arousal state. Elife. 2015;4 doi: 10.7554/eLife.08760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lutkenhoff E.S., McArthur D.L., Hua X., Thompson P.M., Vespa P.M., Monti M.M. Thalamic atrophy in antero-medial and dorsal nuclei correlates with six-month outcome after severe brain injury. Neuroimage Clin. 2013;3:396–404. doi: 10.1016/j.nicl.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Edlow B.L., Haynes R.L., Takahashi E. Disconnection of the ascending arousal system in traumatic coma. J Neuropathol Exp Neurol. 2013;72:505–523. doi: 10.1097/NEN.0b013e3182945bf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mashour G.A. Integrating the science of consciousness and anesthesia. Anesth Analg. 2006;103:975–982. doi: 10.1213/01.ane.0000232442.69757.4a. [DOI] [PubMed] [Google Scholar]
- 102.Alkire M.T., McReynolds J.R., Hahn E.L., Trivedi A.N. Thalamic microinjection of nicotine reverses sevoflurane-induced loss of righting reflex in the rat. Anesthesiology. 2007;107:264–272. doi: 10.1097/01.anes.0000270741.33766.24. [DOI] [PubMed] [Google Scholar]
- 103.Alkire M.T., Asher C.D., Franciscus A.M., Hahn E.L. Thalamic microinfusion of antibody to a voltage-gated potassium channel restores consciousness during anesthesia. Anesthesiology. 2009;110:766–773. doi: 10.1097/aln.0b013e31819c461c. [DOI] [PubMed] [Google Scholar]
- 104.Lioudyno M.I., Birch A.M., Tanaka B.S. Shaker-related potassium channels in the central medial nucleus of the thalamus are important molecular targets for arousal suppression by volatile general anesthetics. J Neurosci. 2013;33:16310–16322. doi: 10.1523/JNEUROSCI.0344-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Scharrer I. Current status of a recombinant antihemophilic factor VIII clinical trial organized by Baxter. Ann Hematol. 1991;63:172–176. doi: 10.1007/BF01703250. [DOI] [PubMed] [Google Scholar]
- 106.Blumenfeld H., McCormick D.A. Corticothalamic inputs control the pattern of activity generated in thalamocortical networks. J Neurosci. 2000;20:5153–5162. doi: 10.1523/JNEUROSCI.20-13-05153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nanobashvili Z., Chachua T., Nanobashvili A., Bilanishvili I., Lindvall O., Kokaia Z. Suppression of limbic motor seizures by electrical stimulation in thalamic reticular nucleus. Exp Neurol. 2003;181:224–230. doi: 10.1016/s0014-4886(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 108.Wang Z., Wang Q. Eliminating absence seizures through the deep brain stimulation to thalamus reticular nucleus. Front Comput Neurosci. 2017;11:22. doi: 10.3389/fncom.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miller J.W. Are generalized tonic-clonic seizures really "generalized"? Epilepsy Curr. 2010;10:80–81. doi: 10.1111/j.1535-7511.2010.01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Parvizi J., Damasio A. Consciousness and the brainstem. Cognition. 2001;79:135–160. doi: 10.1016/s0010-0277(00)00127-x. [DOI] [PubMed] [Google Scholar]
- 111.Minert A., Yatziv S.L., Devor M. Location of the mesopontine neurones responsible for maintenance of anesthetic loss of consciousness. J Neurosci. 2017;37:9320–9331. doi: 10.1523/JNEUROSCI.0544-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moruzzi G., Magoun H.W. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- 113.French J., Verzeano M., Magoun H. A neural basis of the anesthetic state. AMA Arch Neurol Psychiatr. 1953;69:519–529. doi: 10.1001/archneurpsyc.1953.02320280107010. [DOI] [PubMed] [Google Scholar]
- 114.Magni F., Moruzzi G., Rossi G., Zanchetti A. EEG arousal following inactivation of the lower brain stem by selective injection of barbiturate into the vertebral circulation. Arch Ital Biol. 1959;97:33–46. [Google Scholar]
- 115.Sukhotinsky I., Minert A., Soja P., Devor M. Mesopontine switch for the induction of general anesthesia by dedicated neural pathways. Anesth Analg. 2016;123:1274–1285. doi: 10.1213/ANE.0000000000001489. [DOI] [PubMed] [Google Scholar]
- 116.Abulafia R., Zalkind V., Devor M. Cerebral activity during the anesthesia-like state induced by mesopontine microinjection of pentobarbital. J Neurosci. 2009;29:7053–7064. doi: 10.1523/JNEUROSCI.1357-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhou X., Wang Y., Zhang C. The role of dopaminergic VTA neurones in general anesthesia. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Taylor N.E., Van Dort C.J., Kenny J.D. Optogenetic activation of dopamine neurones in the ventral tegmental area induces reanimation from general anesthesia. Proc Natl Acad Sci U S A. 2016;113:12826–12831. doi: 10.1073/pnas.1614340113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Solt K., Van Dort C.J., Chemali J.J., Taylor N.E., Kenny J.D., Brown E.N. Electrical stimulation of the ventral tegmental area induces reanimation from general anesthesia. Anesthesiology. 2014;121:311–319. doi: 10.1097/ALN.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chiu P., Burnham W.M. The effect of anticonvulsant drugs on convulsions triggered by direct stimulation of the brainstem. Neuropharmacology. 1982;21:355–359. doi: 10.1016/0028-3908(82)90100-9. [DOI] [PubMed] [Google Scholar]
- 121.Englot D.J., D'Haese P.F., Konrad P.E. Functional connectivity disturbances of the ascending reticular activating system in temporal lobe epilepsy. J Neurol Neurosurg Psychiatr. 2017;88:925–932. doi: 10.1136/jnnp-2017-315732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Czapinski P., Blaszczyk B., Czuczwar S.J. Mechanisms of action of antiepileptic drugs. Curr Top Med Chem. 2005;5:3–14. doi: 10.2174/1568026053386962. [DOI] [PubMed] [Google Scholar]
- 123.Faingold C.L., Hoffmann W.E., Caspary D.M. Mechanisms of sensory seizures: brain-stem neuronal response changes and convulsant drugs. Fed Proc. 1985;44:2436–2441. [PubMed] [Google Scholar]
- 124.Andrews J.P., Yue Z., Ryu J.H., Neske G., McCormick D.A., Blumenfeld H. Mechanisms of decreased cholinergic arousal in focal seizures: in vivo whole-cell recordings from the pedunculopontine tegmental nucleus. Exp Neurol. 2019;314:74–81. doi: 10.1016/j.expneurol.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Motelow J.E., Li W., Zhan Q. Decreased subcortical cholinergic arousal in focal seizures. Neuron. 2015;85:561–572. doi: 10.1016/j.neuron.2014.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pillay S., Liu X., Baracskay P., Hudetz A.G. Brainstem stimulation increases functional connectivity of basal forebrain-paralimbic network in isoflurane-anesthetized rats. Brain Connect. 2014;4:523–534. doi: 10.1089/brain.2014.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ring H.A., Serra-Mestres J. Neuropsychiatry of the basal ganglia. J Neurol Neurosurg Psychiatr. 2002;72:12–21. doi: 10.1136/jnnp.72.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Moll C.K., Sharott A., Hamel W. Waking up the brain: a case study of stimulation-induced wakeful unawareness during anaesthesia. Prog Brain Res. 2009;177:125–145. doi: 10.1016/S0079-6123(09)17710-5. [DOI] [PubMed] [Google Scholar]
- 129.Mhuircheartaigh R.N., Rosenorn-Lanng D., Wise R., Jbabdi S., Rogers R., Tracey I. Cortical and subcortical connectivity changes during decreasing levels of consciousness in humans: a functional magnetic resonance imaging study using propofol. J Neurosci. 2010;30:9095–9102. doi: 10.1523/JNEUROSCI.5516-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gummadavelli A., Kundishora A.J., Willie J.T. Neurostimulation to improve level of consciousness in patients with epilepsy. Neurosurg Focus. 2015;38:E10. doi: 10.3171/2015.3.FOCUS1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bischoff-Grethe A., Ivry R.B., Grafton S.T. Cerebellar involvement in response reassignment rather than attention. J Neurosci. 2002;22:546–553. doi: 10.1523/JNEUROSCI.22-02-00546.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dreher J.C., Grafman J. The roles of the cerebellum and basal ganglia in timing and error prediction. Eur J Neurosci. 2002;16:1609–1619. doi: 10.1046/j.1460-9568.2002.02212.x. [DOI] [PubMed] [Google Scholar]
- 133.Vuong J., Devergnas A. The role of the basal ganglia in the control of seizure. J Neural Transm (Vienna) 2018;125:531–545. doi: 10.1007/s00702-017-1768-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Deransart C., Vercueil L., Marescaux C., Depaulis A. The role of basal ganglia in the control of generalized absence seizures. Epilepsy Res. 1998;32:213–223. doi: 10.1016/s0920-1211(98)00053-9. [DOI] [PubMed] [Google Scholar]
- 135.Halasz P., Rasonyi G. The network or system-oriented approach in understanding human epilepsy (comments on the article of Susan S. Spencer: "Neural networks in human epilepsy: evidence of and implications for treatment") Epilepsia. 2003;44:625. doi: 10.1046/j.1528-1157.2003.55102.x. author reply 6. [DOI] [PubMed] [Google Scholar]
- 136.Stiefel K.M., Merrifield A., Holcombe A.O. The claustrum's proposed role in consciousness is supported by the effect and target localization of Salvia divinorum. Front Integr Neurosci. 2014;8:20. doi: 10.3389/fnint.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mashour G.A., Alkire M.T. Consciousness, anesthesia, and the thalamocortical system. Anesthesiology. 2013;118:13–15. doi: 10.1097/ALN.0b013e318277a9c6. [DOI] [PubMed] [Google Scholar]
- 138.Villablanca J., Marcus R. Sleep-wakefulness, EEG and behavioral studies of chronic cats without neocortex and striatum: the 'diencephalic' cat. Arch Ital Biol. 1972;110:348–382. [PubMed] [Google Scholar]
- 139.Pal D., Dean J.G., Liu T. Differential role of prefrontal and parietal cortices in controlling level of consciousness. Curr Biol. 2018;28:2145–2152. doi: 10.1016/j.cub.2018.05.025. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Baars B.J. Cambridge University Press; Cambridge, England: 1993. A cognitive theory of consciousness. [Google Scholar]
- 141.Mashour G.A. Top-down mechanisms of anesthetic-induced unconsciousness. Front Syst Neurosci. 2014;8:115. doi: 10.3389/fnsys.2014.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Laureys S. The neural correlate of (un)awareness: lessons from the vegetative state. Trends Cogn Sci. 2005;9:556–559. doi: 10.1016/j.tics.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 143.Cavanna A.E., Mula M., Servo S. Measuring the level and content of consciousness during epileptic seizures: the Ictal Consciousness Inventory. Epilepsy Behav. 2008;13:184–188. doi: 10.1016/j.yebeh.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 144.Gloor P. Experiential phenomena of temporal lobe epilepsy. Facts and hypotheses. Brain. 1990;113(Pt 6):1673–1694. doi: 10.1093/brain/113.6.1673. [DOI] [PubMed] [Google Scholar]
- 145.Campora N., Kochen S. Subjective and objective characteristics of altered consciousness during epileptic seizures. Epilepsy Behav. 2016;55:128–132. doi: 10.1016/j.yebeh.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 146.Bauerschmidt A., Koshkelashvili N., Ezeani C.C. Prospective assessment of ictal behavior using the revised Responsiveness in Epilepsy Scale (RES-II) Epilepsy Behav. 2013;26:25–28. doi: 10.1016/j.yebeh.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yang L., Shklyar I., Lee H.W. Impaired consciousness in epilepsy investigated by a prospective responsiveness in epilepsy scale (RES) Epilepsia. 2012;53:437–447. doi: 10.1111/j.1528-1167.2011.03341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McPherson A., Rojas L., Bauerschmidt A. Testing for minimal consciousness in complex partial and generalized tonic-clonic seizures. Epilepsia. 2012;53:e180–e183. doi: 10.1111/j.1528-1167.2012.03657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nani A., Cavanna A.E. The quantitative measurement of consciousness during epileptic seizures. Epilepsy Behav. 2014;30:2–5. doi: 10.1016/j.yebeh.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 150.Chen W.C., Chen E.Y., Gebre R.Z. Epilepsy and driving: potential impact of transient impaired consciousness. Epilepsy Behav. 2014;30:50–57. doi: 10.1016/j.yebeh.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chipaux M., Vercueil L., Kaminska A., Mahon S., Charpier S. Persistence of cortical sensory processing during absence seizures in human and an animal model: evidence from EEG and intracellular recordings. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gaese B.H., Ostwald J. Anesthesia changes frequency tuning of neurones in the rat primary auditory cortex. J Neurophysiol. 2001;86:1062–1066. doi: 10.1152/jn.2001.86.2.1062. [DOI] [PubMed] [Google Scholar]
- 153.Plourde G., Belin P., Chartrand D. Cortical processing of complex auditory stimuli during alterations of consciousness with the general anesthetic propofol. Anesthesiology. 2006;104:448–457. doi: 10.1097/00000542-200603000-00011. [DOI] [PubMed] [Google Scholar]
- 154.Meeren H.K., van Cappellen van Walsum A.M., van Luijtelaar E.L., Coenen A.M. Auditory evoked potentials from auditory cortex, medial geniculate nucleus, and inferior colliculus during sleep-wake states and spike-wave discharges in the WAG/Rij rat. Brain Res. 2001;898:321–331. doi: 10.1016/s0006-8993(01)02209-0. [DOI] [PubMed] [Google Scholar]
- 155.Gross W.L., Lauer K.K., Liu X. Propofol sedation alters perceptual and cognitive functions in healthy volunteers as revealed by functional magnetic resonance imaging. Anesthesiology. 2019;131:254–265. doi: 10.1097/ALN.0000000000002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dueck M.H., Petzke F., Gerbershagen H.J. Propofol attenuates responses of the auditory cortex to acoustic stimulation in a dose-dependent manner: a FMRI study. Acta Anaesthesiol Scand. 2005;49:784–791. doi: 10.1111/j.1399-6576.2005.00703.x. [DOI] [PubMed] [Google Scholar]
- 157.Kerssens C., Hamann S., Peltier S., Hu X.P., Byas-Smith M.G., Sebel P.S. Attenuated brain response to auditory word stimulation with sevoflurane: a functional magnetic resonance imaging study in humans. Anesthesiology. 2005;103:11–19. doi: 10.1097/00000542-200507000-00006. [DOI] [PubMed] [Google Scholar]
- 158.Bonhomme V., Fiset P., Meuret P. Propofol anesthesia and cerebral blood flow changes elicited by vibrotactile stimulation: a positron emission tomography study. J Neurophysiol. 2001;85:1299–1308. doi: 10.1152/jn.2001.85.3.1299. [DOI] [PubMed] [Google Scholar]
- 159.Veselis R.A., Reinsel R.A., Beattie B.J. Midazolam changes cerebral blood flow in discrete brain regions: an H2(15)O positron emission tomography study. Anesthesiology. 1997;87:1106–1117. doi: 10.1097/00000542-199711000-00015. [DOI] [PubMed] [Google Scholar]
- 160.Shulman R.G., Hyder F., Rothman D.L. Cerebral metabolism and consciousness. C R Biol. 2003;326:253–273. doi: 10.1016/s1631-0691(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 161.Veselis R.A., Feshchenko V.A., Reinsel R.A., Dnistrian A.M., Beattie B., Akhurst T.J. Thiopental and propofol affect different regions of the brain at similar pharmacologic effects. Anesth Analg. 2004;99:399–408. doi: 10.1213/01.ANE.0000131971.92180.DF. table of contents. [DOI] [PubMed] [Google Scholar]
- 162.Kaisti K.K., Metsahonkala L., Teras M. Effects of surgical levels of propofol and sevoflurane anesthesia on cerebral blood flow in healthy subjects studied with positron emission tomography. Anesthesiology. 2002;96:1358–1370. doi: 10.1097/00000542-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 163.Duncan R. Epilepsy, cerebral blood flow, and cerebral metabolic rate. Cerebrovasc Brain Metab Rev. 1992;4:105–121. [PubMed] [Google Scholar]
- 164.Ingvar M. Cerebral blood flow and metabolic rate during seizures. Relationship to epileptic brain damage. Ann N Y Acad Sci. 1986;462:194–206. doi: 10.1111/j.1749-6632.1986.tb51254.x. [DOI] [PubMed] [Google Scholar]
- 165.Avery R.A., Zubal I.G., Stokking R. Decreased cerebral blood flow during seizures with ictal SPECT injections. Epilepsy Res. 2000;40:53–61. doi: 10.1016/s0920-1211(00)00109-1. [DOI] [PubMed] [Google Scholar]
- 166.Williams M.S., Lecas S., Charpier S., Mahon S. Phase-dependent modulation of cortical and thalamic sensory responses during spike-and-wave discharges. Epilepsia. 2020 doi: 10.1111/epi.16422. [DOI] [PubMed] [Google Scholar]
- 167.Laureys S., Goldman S., Phillips C. Impaired effective cortical connectivity in vegetative state: preliminary investigation using PET. Neuroimage. 1999;9:377–382. doi: 10.1006/nimg.1998.0414. [DOI] [PubMed] [Google Scholar]
- 168.Chatelle C., Laureys S., Schnakers C. Disorders of consciousness: what do we know? In: Dehaene S., Christen Y., editors. Characterizing consciousness: from cognition to the clinic? Springer; Berlin, Heidelberg: 2011. pp. 85–98. [Google Scholar]
- 169.Krauss G., Theodore W.H. Treatment strategies in the postictal state. Epilepsy Behav. 2010;19:188–190. doi: 10.1016/j.yebeh.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.European Delirium Association, American Delirium Society The DSM-5 criteria, level of arousal and delirium diagnosis: inclusiveness is safer. BMC Med. 2014;12:141. doi: 10.1186/s12916-014-0141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Abbott T.E.F., Pearse R.M. Depth of anesthesia and postoperative delirium. JAMA. 2019;321:459–460. doi: 10.1001/jama.2019.0164. [DOI] [PubMed] [Google Scholar]
- 172.Association A.P. American Psychiatric Pub; Washington, DC.: 2013. Diagnostic and statistical manual of mental disorders (DSM-5®) [Google Scholar]
- 173.Sheth R.D., Drazkowski J.F., Sirven J.I., Gidal B.E., Hermann B.P. Protracted ictal confusion in elderly patients. Arch Neurol. 2006;63:529–532. doi: 10.1001/archneur.63.4.529. [DOI] [PubMed] [Google Scholar]
- 174.Safavynia S.A., Arora S., Pryor K.O., Garcia P.S. An update on postoperative delirium: clinical features, neuropathogenesis, and perioperative management. Curr Anesthesiol Rep. 2018;8:252–262. [PMC free article] [PubMed] [Google Scholar]
- 175.Steriade M., Nunez A., Amzica F. Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993;13:3266–3283. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kayama Y., Iwama K. The EEG, evoked potentials, and single-unit activity during ketamine anesthesia in cats. Anesthesiology. 1972;36:316–328. doi: 10.1097/00000542-197204000-00004. [DOI] [PubMed] [Google Scholar]
- 177.Mori K., Kawamata M., Mitani H., Yamazaki Y., Fujita M. A neurophysiologic study of ketamine anesthesia in the cat. Anesthesiology. 1971;35:373–382. doi: 10.1097/00000542-197110000-00012. [DOI] [PubMed] [Google Scholar]
- 178.Winters W., Ferrar-Allado T., Guzman-Flores C., Alcaraz M. The cataleptic state induced by ketamine: a review of the neuropharmacology of anesthesia. Neuropharmacology. 1972;11:303–315. doi: 10.1016/0028-3908(72)90016-0. [DOI] [PubMed] [Google Scholar]
- 179.Wong D.H., Jenkins L.C. An experimental study of the mechanism of action of ketamine on the central nervous system. Can Anaesth Soc J. 1974;21:57–67. doi: 10.1007/BF03004579. [DOI] [PubMed] [Google Scholar]
- 180.Kayama Y. Ketamine and EEG seizure waves: interaction with anti-epileptic drugs. Br J Anaesth. 1982;54:879–883. doi: 10.1093/bja/54.8.879. [DOI] [PubMed] [Google Scholar]
- 181.Kurdi M.S., Sushma K.S., Ranjana R., Kiran P.B. Ketamine: a convulsant? Anesth Essays Res. 2017;11:272–273. doi: 10.4103/0259-1162.200241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Khandrani J., Rajput A., Dahake S., Verma N. Ketamine induced seizures. Internet J Anesthesiol. 2009:19. [Google Scholar]
- 183.Myslobodsky M.S., Golovchinsky V., Mintz M. Ketamine: convulsant or anti-convulsant? Pharmacol Biochem Behav. 1981;14:27–33. doi: 10.1016/0091-3057(81)90099-x. [DOI] [PubMed] [Google Scholar]