Abstract
Background
The mechanisms underlying the role of T-type calcium channels (T-channels) in thalamocortical excitability and oscillations in vivo during neurosteroid-induced hypnosis are largely unknown.
Methods
We used patch-clamp electrophysiological recordings from acute brain slices ex vivo, recordings of local field potentials (LFPs) from the central medial thalamic nucleus in vivo, and wild-type (WT) and Cav3.1 knock-out mice to investigate the molecular mechanisms of hypnosis induced by the neurosteroid analogue (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile (3β-OH).
Results
Patch-clamp recordings showed that 3β-OH inhibited isolated T-currents but had no effect on phasic or tonic γ-aminobutyric acid A currents. Also in acute brain slices, 3β-OH inhibited the spike firing mode more profoundly in WT than in Cav3.1 knockout mice. Furthermore, 3β-OH significantly hyperpolarised neurones, reduced the amplitudes of low threshold spikes, and diminished rebound burst firing only in WT mice. We found that 80 mg kg−1 i.p. injections of 3β-OH induced hypnosis in >60% of WT mice but failed to induce hypnosis in the majority of mutant mice. A subhypnotic dose of 3β-OH (20 mg kg−1 i.p.) accelerated induction of hypnosis by isoflurane only in WT mice, but had similar effects on the maintenance of isoflurane-induced hypnosis in both WT and Cav3.1 knockout mice. In vivo recordings of LFPs showed that a hypnotic dose of 3β-OH increased δ, θ, α, and β oscillations in WT mice in comparison with Cav3.1 knock-out mice.
Conclusions
The Cav3.1 T-channel isoform is critical for diminished thalamocortical excitability and oscillations that underlie neurosteroid-induced hypnosis.
Keywords: calcium channel, electrophysiology, hypnosis, mechanisms of anaesthesia, neurosteroid, thalamus
Editor's key points.
-
•
The anaesthetic neurosteroid analogue 3β-OH induces hypnosis in rat pups without triggering neuronal apoptosis, but its mechanisms of action are largely unknown.
-
•
The authors investigated the role of T-type calcium channels (T-channels) in thalamocortical excitability and oscillations in vivo during neurosteroid-induced hypnosis.
-
•
Effects of 3β-OH on the Cav3.1 T-channel isoform were essential for the diminished thalamocortical excitability and oscillations that underlie neurosteroid-induced hypnosis.
-
•
These studies may enable novel and potentially safer approaches in clinical anaesthesia.
The neurosteroid analogue (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile (3β-OH) induces hypnosis in rat pups without triggering neuronal apoptosis.1 However, the specific mechanisms underlying 3β–OH–induced hypnosis are largely unknown. Many neuroactive steroids (neurosteroids) act as γ-aminobutyric acid A (GABAA) receptor potentiators,2, 3, 4, 5 but we reported that 3β-OH blocks low voltage-gated calcium channels or T-type calcium channels (T-channels) in the thalamic reticular nucleus and in sensory neurones of the dorsal root ganglion.6,7
The central medial nucleus (CeM) of the thalamus is part of the non-specific thalamus, with diffuse projections to the anterior and posterior regions of the cerebral cortex and many subcortical structures.8 It has been proposed that the CeM acts as a key hub through which general anaesthesia and natural sleep are induced.9 We have shown that T-channels can modulate excitability of rat CeM neurones10 and that the Cav3.1 isoform of T-channels in CeM neurones is important for isoflurane-induced oscillations in vivo during anaesthesia.11
Given the essential role of T-channels in excitability and oscillations in thalamocortical neurones, we hypothesised that Cav3.1 channels are important for neurosteroid-induced hypnosis and thalamocortical oscillations. We investigated the effects of 3β-OH on excitability of CeM neurones in an ex vivo slice preparation; we also used mouse genetics in vivo to correlate the hypnotic effects of 3β-OH with thalamocortical oscillations using thalamic local field potentials (LFPs) and cortical EEG recordings from wild-type (WT) and Cav3.1 knock-out (KO) mutant mice.
Methods
Animals
Experimental procedures with animals were performed according to a protocol approved by the Institutional Animal Care and Use Committee of the University of Colorado Anschutz Medical Campus, Aurora, CO, USA. Treatments of animals adhered to guidelines set forth in the NIH Guide for the Care and Use of Laboratory Animals. All efforts were made to minimise animal suffering and to use only the necessary number of animals to produce reliable scientific data. Male juvenile C57BL/6J (WT) and Cav3.1 KO mice (postnatal days 30–35 [P30–P35]) were used for ex vivo electrophysiological recordings whereas older male mice (3 months) were used for behavioural and in vivo electrophysiological experiments. C57BL/6 mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) whereas Cav3.1 KO mice (α1G null) (Riken BioResources Centre, Tsukuba, Ibaraki, Japan) were generated on the C57BL/6 background as described.12 All animals were maintained on a 14/10 h light–dark cycle with food and water ad libitum. Behavioural assessments for in vivo experiments were done in a blinded fashion. Details of specific experimental procedures can be found in the Supplementary Material.
Results
Voltage-dependent inhibition of T-currents by 3β-OH in WT mice
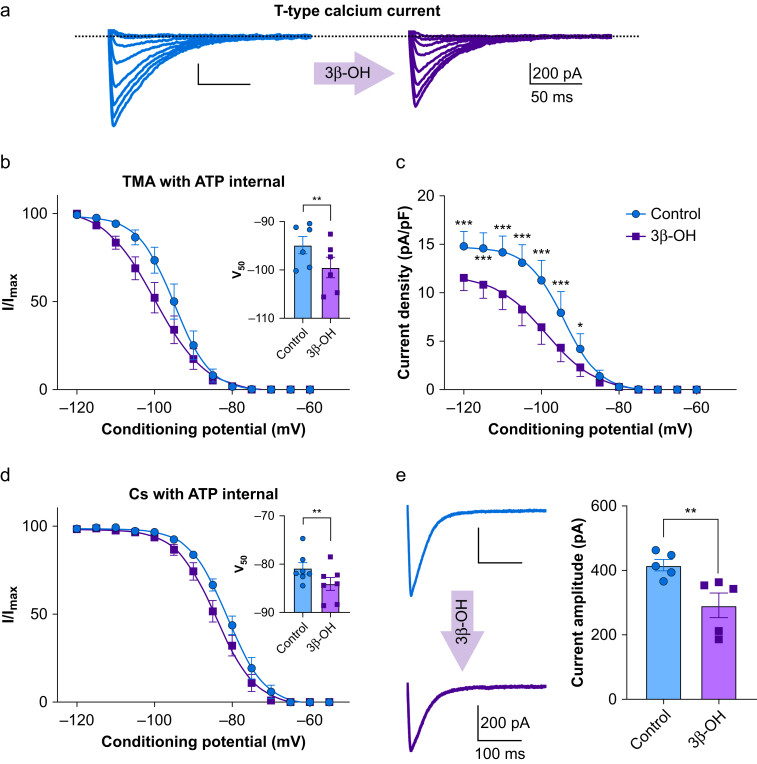
We first examined the effects of 3 μM 3β-OH on well-isolated T-currents in CeM neurones from adolescent WT mice using acute brain slices as we have previously determined that this is a relevant brain concentration during hypnosis.1 Representative current traces from a steady-state inactivation protocol are presented in Fig 1a. The left panel shows traces of inward T-current of a CeM neurone under control conditions, and the right panel shows traces from the same neurone in the presence of 3β-OH, both recorded using a tetramethylammonium with adenosine triphosphate (TMA with ATP) internal solution. 3β-OH stabilised inactive states of the channel and shifted inactivation V50 values of about –5 mV (Fig 1b). Furthermore, 3β-OH reduced current density up to 45% over a wide range of membrane potentials (from –120 to –90 mV) when compared with control conditions (Fig 1c). Similarly, with Cs with ATP internal solution, 3β-OH produced a smaller but significant hyperpolarising shift in V50 values of about 3 mV (Fig 1d), and the current amplitude decreased by about 30% (Fig 1e).
Figure 1.
Inhibition of T-currents by 3β-OH in CeM neurones. (a) Original traces of T currents in a representative CeM neurone in control conditions recorded using a double-pulse protocol with 3.6 s long prepulses to variable voltages (from –115 to –80 mV in 5 mV increment; blue); traces from the same cell using the identical voltage protocol during an apparent steady-state inhibition of T-current by 3 μM 3β-OH (purple) recorded with TMA with ATP internal solution. (b) Average normalised steady-state inactivation (I/Imax) curves in control conditions and after application of 3β-OH in the same cells. 3β-OH induced a hyperpolarising shift in V50 of 4.6 mV; the average V50 value for steady-state inactivation was –94.9 (1.8) mV in control conditions (blue; six cells, three animals). After 3β-OH perfusion of the same cells, the V50 value was –99.5 (2.1) mV (purple). In the inset of panel (b), the V50 was significantly different after 3β-OH perfusion (two tailed paired t-test: t(5)=4.08, P<0.01). (c) Average current density calculated from the steady-state inactivation protocol. Application of 3β-OH (purple line and data points; six cells, three animals) decreased current density when compared with control conditions (blue line and data points). Data were analysed with two-way RM analysis of variance (anova): interaction F(12,60)=13.91, P<0.001; voltage F(12.60)=46.66, P<0.001; 3β-OH F(1,5)=15.81, P=0.011; Sidak's post hoc presented in figure). (d) Average normalised steady-state inactivation (I/Imax) curves in control conditions and after application of 3β-OH in the same cells recorded with Cs with ATP internal solution. 3β-OH induced a hyperpolarising shift in V50 of 3.2 mV because the average V50 value for steady-state inactivation was –80.9 (1.2) mV under control conditions (blue; seven cells, four animals), and after 3β-OH perfusion in the same cells, the V50 value was –84.1 (1.3) mV (purple). In the inset of panel (d), the V50 was significantly different after 3β-OH perfusion (two tailed paired t-test: t(6)=3.92, P=0.008). (e) Averaged representative traces recorded under control conditions (blue) and after application of 3β-OH (purple) using a protocol Vt=−50 mV, Vh=−90 mV, 3β-OH decreased T-current amplitudes from 416.9 (17.5) pA to 291.9 (38.2) pA (two-tailed paired t-test: t(4)=4.67, P<0.01; five cells, three animals) ∗P<0.05, ∗∗P<0.01, ∗∗∗P<0.001. 3β-OH, (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile; ATP, adenosine triphosphate; CeM, central medial nucleus; RM, repeated measure; TMA, tetramethylammonium.
In contrast, perfusion with the vehicle (0.1% dimethylsulphoxide [DMSO]) did not significantly alter inactivation V50 and T-current density (Supplementary Fig. S1a and b, respectively).
Different effects of alphaxalone and 3β-OH on GABAA currents
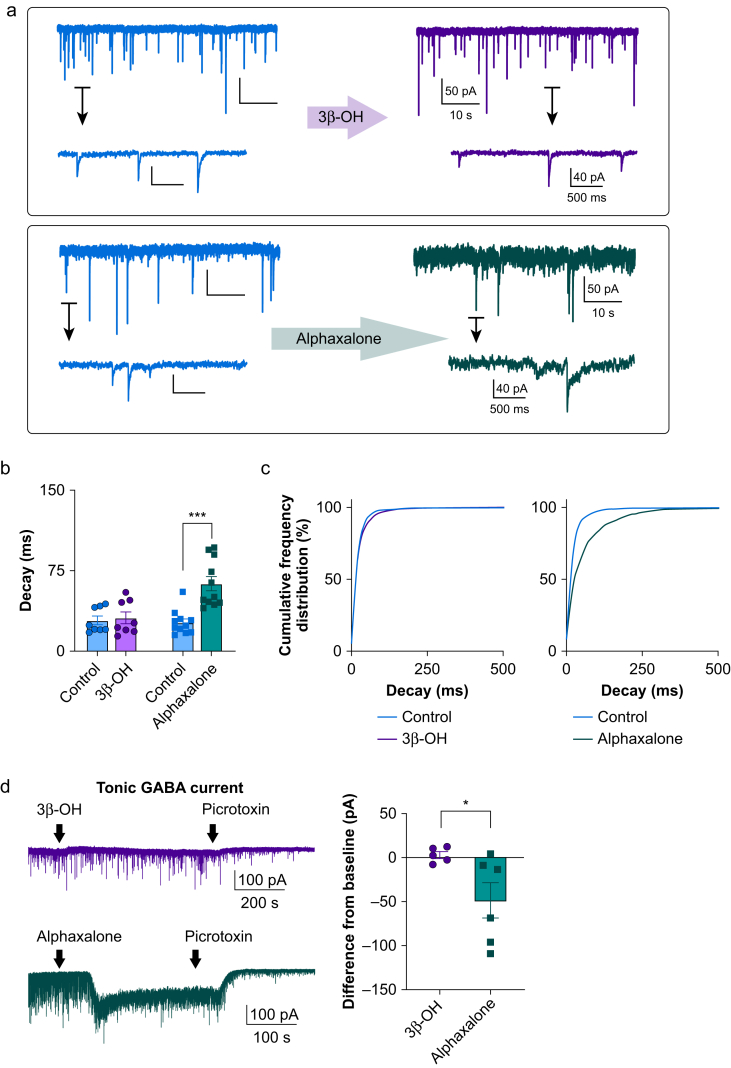
Most neurosteroids prolong the decay of miniature inhibitory postsynaptic currents (mIPSCs).13 We recorded mIPSCs in CeM neurones to compare the effects of 3β-OH and alphaxalone (5α-pregnan-3α-ol-11,20-dione), a GABAergic neurosteroid. Representative traces from these recordings are shown in Fig 2a. Only alphaxalone significantly increased decay tau of mIPSCs by about two-fold compared with control conditions (Fig 2b and c). The amplitudes of mIPSCs were not different from baseline for either neurosteroid (Supplementary Fig. S2a and b), whereas mIPSC frequency was significantly decreased by alphaxalone but not by 3β-OH (Supplementary Fig. S2c and d). These data confirm that, like in the subiculum of rat pups,14 3β-OH inhibits T-currents but does not affect synaptic GABAA receptor currents in the CeM. In addition, we recorded picrotoxin-sensitive tonic GABAA current and found that alphaxalone potently enhanced it, whereas 3β-OH had no significant effect (Fig 2d).
Figure 2.
Potentiation of miniature inhibitory postsynaptic currents by alphaxalone but not by 3β-OH. (a) Representative traces from CeM neurones before and after perfusion with 3β-OH (top panel) or alphaxalone (bottom panel). (b) Decay tau was increased only after perfusion of alphaxalone but not 3β-OH; alphaxalone increased decay tau from 26.7 (3.4) to 62.9 (6.6) ms (two-tailed paired t-test: t(10)=6.82, P<0.001). (c) Left panel shows that cumulative frequency distribution for decay tau confirms no change after application of 3β-OH (control 1479 events, 3β-OH 1497 events); right panel shows longer decay taus after alphaxalone perfusion (1523 events for alphaxalone, 2134 events for the control). (d) Representative traces showing tonic GABA current after 3 μM 3β-OH (purple trace) or 3 μM alphaxalone (green trace) perfusion. (b) Difference from baseline showed a minimal effect of 3β-OH on tonic current in comparison with alphaxalone (3β-OH five cells, alphaxalone six cells; unpaired two-tailed t-test: t(9)=2.31, P=0.046). ∗P<0.05, ∗∗∗P<0.001. 3β-OH, (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile; CeM, central medial nucleus; GABA, γ-aminobutyric acid A.
3β-OH reduces spike firing in CeM neurones
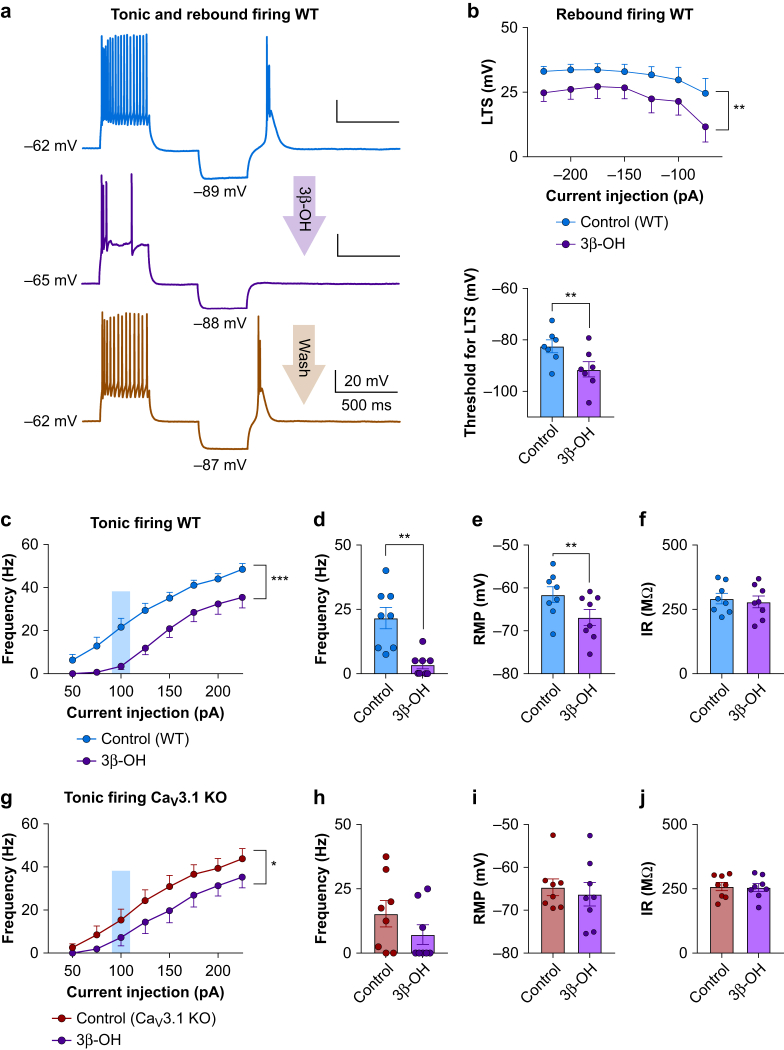
We examined the effects of 3β-OH on the output of CeM neurones using current-clamp recordings from acute brain slices ex vivo. Representative traces after depolarising (125 pA) and hyperpolarising (–75 pA) current injection under baseline conditions in WT mice are presented in Fig 3a. Note that 3β-OH completely abolished T-channel-dependent low threshold spikes (LTSs) and post-inhibitory rebound bursting in CeM neurones. We next studied the effects of 3β-OH on LTSs and rebound burst firing in WT mice elicited by a series of escalating hyperpolarising current injections that served to recover T-channels from inactivation. Summary data from these experiments showed an average reduction of LTS amplitude of 25–50% and increased threshold for LTS by about 30% after 3β-OH application (Fig. 3b top and bottom, respectively).
Figure 3.
3β-OH influence on the excitability of CeM neurons. (a) Representative traces from a WT CeM neurone before application of 3β-OH (blue), after application of 3 μM 3β-OH (purple) and after wash (orange trace) with active membrane responses to a depolarising (125 pA) and hyperpolarising (–75 pA) current injection. (b) Top: averaged LTS amplitude was reduced by application of 3β-OH across all hyperpolarising current pulses from –50 to –225 pA in WT mice (eight cells, four animals; two-way RM analysis of variance [anova]: interaction F(6,42)=0.41, P=0.865; current injection F(6,42)=5.44, P<0.001; 3β-OH F(1,7)=22.63, P=0.002). Bottom: bar graph shows 3β-OH increased the threshold for the occurrence of LTS (seven cells, four animals; paired two-tailed t-test t(6)=4.07, P=0.007). (c) 3β-OH reduced tonic action potential firing frequency across all current pulses in WT mice (eight cells, four animals; from 50 to 225 pA, two-way RM anova: interaction F(7,49)=1.66, P=0.140; current injection F(7,49)=132.70, P<0.001; 3β-OH effect F(1,7)=29.52, P=0.001). (d) 3β-OH decreased tonic frequency at 100 pA current injection in WT mice (eight cells, four animals; paired two-tailed t-test: t(7)=4.80, P=0.002). (e) 3β-OH hyperpolarised RMP in WT mice (eight cells, four animals; paired two-tailed t-test: t(7)=4.00, P=0.005). (f) 3β-OH did not affect IR measured with a hyperpolarising current injection of 100 pA in WT mice. (g) 3β-OH diminished tonic action potential firing frequency in Cav3.1 KO mice (eight cells, three animals; from 50 to 225 pA, two-way RM anova: interaction F(7,49)=1.23, P=0.302; current injection F(7,49)=63.29, P<0.001; 3β-OH effect F(1,7)=8.26, P=0.024). (h) 3β-OH did not significantly affect tonic firing frequency at 100 pA current injection in Cav3.1 KO mice. (i) 3β-OH did not significantly affect RMP in Cav3.1 KO mice. (j) 3β-OH did not significantly affect IR measured with a hyperpolarising current injection of 100 pA in Cav3.1 KO mice. ∗P<0.05, ∗∗P<0.01, ∗∗∗P<0.001. 3β-OH, (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile; CeM, central medial nucleus; GABA, γ-aminobutyric acid A; KO, knock-out; LTS, low threshold spikes; RM, definition; IR, input resistance; RMP, resting membrane potential; WT, wild-type.
We also found that 3β-OH reduced firing frequency over the range of current injections during input–output protocols that we used to interrogate tonic firing mode in WT mice (Fig 3c). For example 3β-OH inhibited spike firing during current injection of 100 pA by about 90% (Fig 3d). Similar to the volatile anaesthetic isoflurane,11 we found that 3β-OH hyperpolarised resting membrane potential (RMP) by about 6 mV but did not significantly affect input resistance (IR) in the CeM of WT mice (Fig 3e and f, respectively).
In Cav3.1 KO mice, 3β-OH reduced tonic firing frequency to a lesser extent (Fig 3g and h) and did not significantly affect RMP or IR of CeM neurones (Fig 3i and j, respectively). We did not observe LTS and rebound burst firing using the identical current injection protocols in the mutant mice.11 These data indicate that Cav3.1 channels are required for LTS and provide constant calcium influx that contributes to RMP likely via the ‘T-type window current’.11 Thus, inhibition of Cav3.1 channels by 3β-OH may diminish both tonic and burst firing modes and hyperpolarise CeM neurones.
Cav3.1 channels are essential for neurosteroid-induced hypnosis
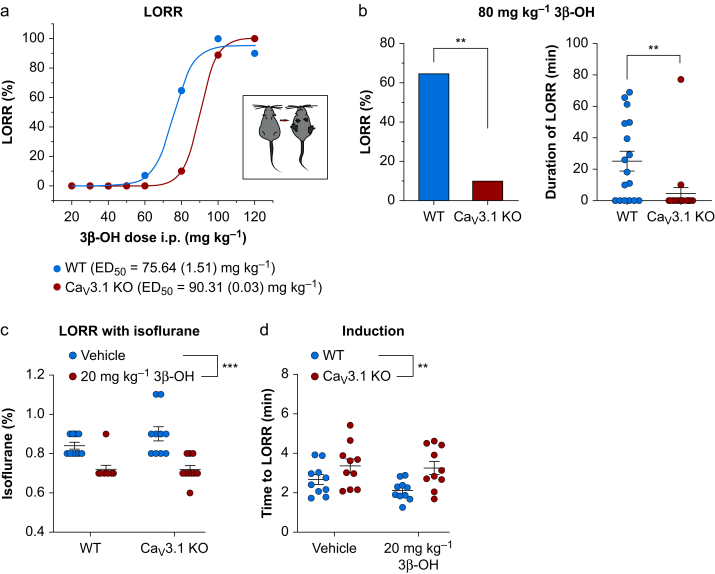
Our current-clamp experiments suggest that the Cav3.1 isoform largely underlies the inhibitory effects of 3β-OH on the excitability of CeM neurones. We next asked if Cav3.1 channels are important for the hypnotic effect of this neurosteroid in vivo by measuring loss of righting reflex (LORR) to compare hypnotic potency in WT and mutant mice. The calculated median effective dose (ED50) for the LORR was 75.6 (1.5) mg kg−1 in WT mice and 90.3 (0.1) mg kg−1 in mutant mice (Fig 4a). Importantly, 3β-OH at 80 mg kg−1 induced LORR in only 10% of mutant mice and >60% of WT mice (Fig 4b, left panel). Duration of hypnosis was several-fold shorter in KO mice than in WT mice (Fig 4b, right panel).
Figure 4.
Effect of 3β-OH on anaesthetic endpoints. (a) Dose–response curve for loss of righting reflex (LORR) in WT and Cav3.1 KO mice with ED50 indicated on the top of the panel. (b) Left, percentage of animals that lost righting reflex under 80 mg kg−1 i.p. 3β-OH in WT (17 animals) and Cav3.1 KO (20 animals) cohorts (80 mg kg−1: χ2 = 9.79, P=0.002). (b) Right: duration of LORR under 80 mg kg−1 i.p. 3β-OH in WT (17 animals) and Cav3.1 KO (20 animals) mice (unpaired two-tailed t-test: t(35)=2.94, P=0.006). (c) 20 mg kg−1 3β-OH lowered the percent of isoflurane needed for LORR in WT and Cav3.1 KO mice (10 mice in each group, two-way RM analysis of variance [anova]: interaction F(1,18)=1.67, P=0.210; mutation effect F(1,18)=1.35, P=0.260; 3β-OH effect F(1,18)=42.19, P<0.001). (d) WT animals pretreated with 3β-OH needed significantly less time for induction with isoflurane compared with mutant animals (10 mice in each group, two-way RM anova: interaction F(1,18)=0.71, P=0.410; mutation effect F(1,18)=9.05, P=0.007; 3β-OH effect F(1,18)=1.59, P=0.223). ∗∗P<0.01, ∗∗∗P<0.001. 3β-OH, (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile; KO, knock-out; WT, wild-type; ED50, median effective dose; RM, definition.
We hypothesised that potency for hypnosis of the common volatile anaesthetic isoflurane would be reduced with concurrent injections of 3β-OH. A sub-hypnotic dose of 3β-OH (20 mg kg−1 i.p.) injected 15 min before administration of escalating concentrations of inhaled isoflurane lowered the final concentration of isoflurane required to maintain LORR similarly in both WT and Cav3.1 KO animals (Fig 4c). The analysis of time to LORR (TTLORR) with a fixed concentration of isoflurane of 1.2 vol% showed that 3β-OH significantly decreased TTLORR by about 20% only in WT mice (Fig 4d). These data suggest that the Cav3.1 isoform of T-channels is essential for 3β–OH–induced hypnosis when administered alone, and for its facilitation of induction but not maintenance of hypnosis with isoflurane.
Oscillations in CeM during 3β-OH induced hypnosis
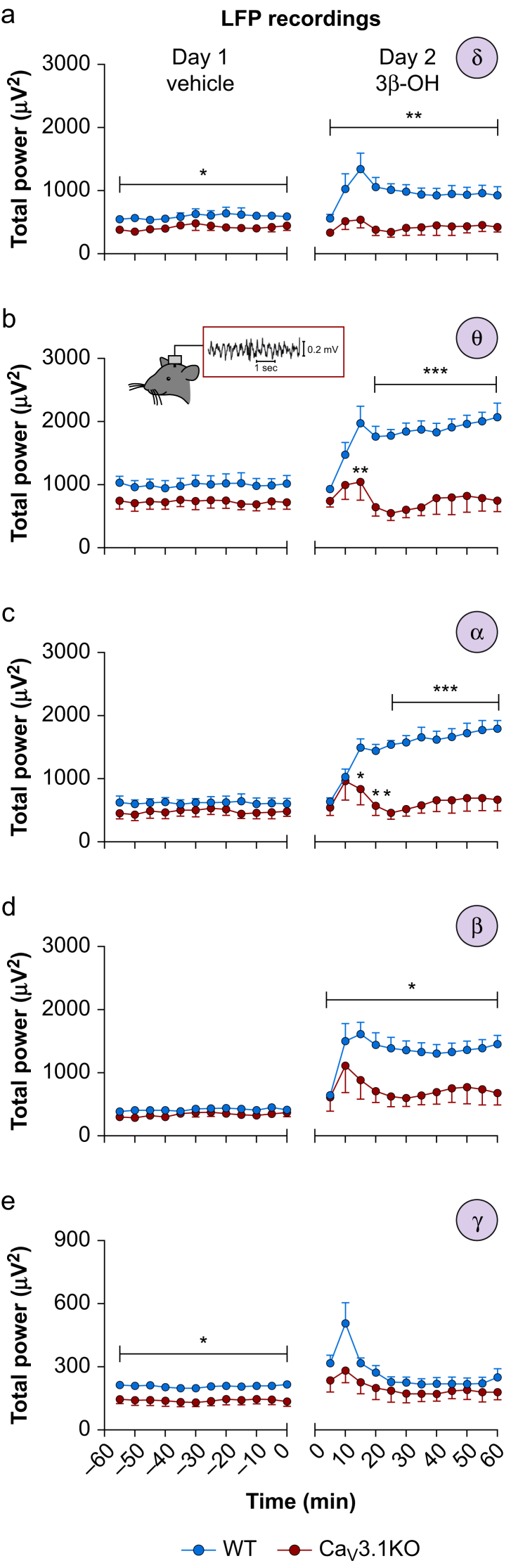
We next recorded LFPs from CeM of WT and mutant mice in order to test the hypothesis that Cav3.1 channels play a significant role in the effects of 3β-OH at 80 mg kg−1 on the rhythmic oscillations of intact thalamocortical circuits. We analysed the effects of the vehicle (25% 2-hydroxypropyl-β-cyclodextrin) and 3β-OH on total power of LFPs during 60 min after injection at 5 min intervals (Fig 5). There was less δ and γ frequency power in the mutant mice with vehicle injections (left panels of Fig. 5a and e), and no statistically significant differences between genotypes in other frequency bands (left panels of Fig. 5b–d). However, there was a profound increase of up to two-fold in total power in WT mice in δ, θ, α, and β oscillations after injections of 3β-OH that was not observed in the mutant mice (right panels of Fig. 5a–d).
Figure 5.
Oscillations in CeM neurones after injections of 3β-OH. (a) Total δ power after injections of the vehicle 2-hydroxypropyl-β-cyclodextrin (Day 1) and 3β-OH (Day 2) in WT and Cav3.1 KO mice. Mutant mice had less δ power on Day 1 (two-way RM analysis of variance [anova]: interaction F(11,121)=0.27, P=0.990; time F(11,121)=1.15, P=0.330; mutation effect F(1,11)=5.93, P=0.033). 3β-OH enhanced δ power in WT animals (two-way RM anova: interaction F(11,121)=1.51, P=0.137; time F(11,121)=3.62, P<0.001; mutation effect F(1,11)=11.99, P=0.005). (b) Total θ power after vehicle (Day 1) or 3β-OH (Day 2) in WT and Cav3.1 KO mice. 3β-OH enhanced θ power in WT animals (two-way RM anova: interaction F(11,121)=4.07, P<0.001; time F(11,121)=4.03, P<0.001; mutation effect F(1,11)=26.60, P<0.001; Sidak's post hoc presented in figure). (c) Total α power for vehicle (Day 1) or 3β-OH (Day 2) in WT and Cav3.1 KO mice. 3β-OH enhanced α power in WT animals (two-way RM anova: interaction F(11,121)=5.87, P<0.001; time F(11,121)=5.10, P<0.001; mutation effect F(1,11)=27.74, P<0.001; Sidak's post hoc presented in figure). (d) Total β power for vehicle (Day 1) or 3β-OH (Day 2) in WT and Cav3.1 KO mice. 3β-OH enhanced β power in WT animals (two-way RM anova: interaction F(11,121)=1.16, P=0.324; time F(11,121)=2.82, P=0.003; mutation effect F(1,11)=8.62, P=0.013; Sidak's post hoc presented in figure). (e) Total γ power for vehicle (Day 1) or 3β-OH (Day 2) in WT and Cav3.1 KO mice. Mutant mice had less γ power on Day 1 (two-way RM anova: interaction F(11,121)=0.53, P=0.879; time F(11,121)=1.77, P=0.067; mutation effect F(1,11)=6.73, P=0.025). 3β-OH did not statistically change γ power between WT and Cav3.1 KO animals. WT, seven animals, Cav3.1 KO, six animals; ∗P<0.05, ∗∗P<0.01, ∗∗∗P<0.001. 3β-OH, (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile; CeM, central medial nucleus; KO, knock-out; LFP, local field potentials; WT, wild-type.
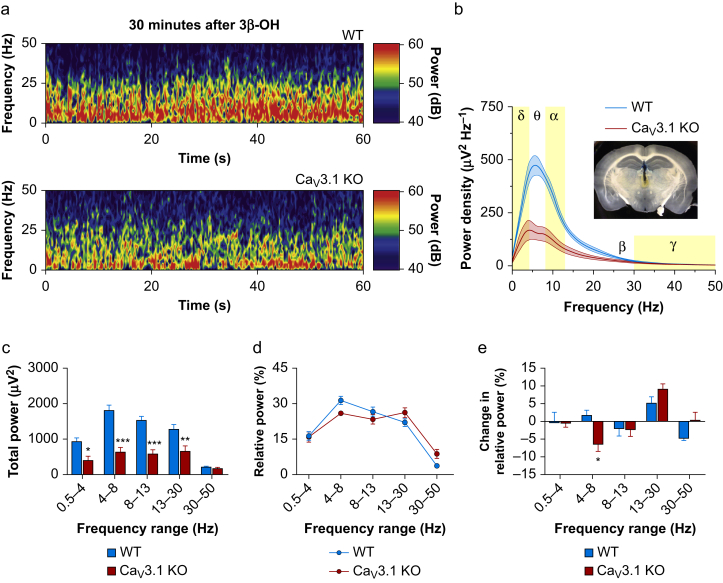
In addition, we performed a detailed analysis of LFPs in CeM in vivo between WT and mutant mice at 30 min after i.p. injection of 3β-OH (Fig 6a). Power density under 3β-OH revealed diminished values in the slower frequencies and in the α frequency range (2–14 Hz) of about three-fold in the mutant mice (Fig 6b). Mutant mice exhibited diminished total power in comparison with WT animals in all frequency ranges except γ (Fig 6c). Analysis of relative power did not reveal statistically significant differences between the effects of 3β-OH (Fig 6d). Nonetheless, change in relative power (differences between relative power under 3β-OH and baseline) showed a significant drop in the θ frequency range in mutant mice that was not seen in WT animals (Fig 6e).
Figure 6.
Oscillatory differences in CeM between WT and Cav3.1 KO mice after 3β-OH. (a) Representative spectrograms from the CeM at 30 min after i.p. injections of 3β-OH recorded from WT (upper image) and Cav3.1 KO mice (lower image). (b) Power density with electrode confirmation (inset in figure). Power density revealed decreased low frequency oscillations in Cav3.1 KO mice under 3β-OH. (c) Total power revealed less power in δ, θ, α, and β frequency range in Cav3.1 KO mice (two-way RM analysis of variance [anova]: interaction F(4,44)=10.76, P<0.001; oscillations F(4,44)=37.80, P<0.001; mutation effect F(1,11)=32.50, P<0.001; Sidak's post hoc presented in figure). (d) Analysis of relative power after neurosteroid injection did not find significant differences between WT and Cav3.1 KO mice. (e) Change in relative power (relative power under 3β-OH minus relative power during baseline in CeM) revealed differences between WT and Cav3.1 KO mice in the θ frequency range (two-way RM anova: interaction F(4,44)=3.30, P=0.019; oscillations F(4,44)=8.08, P<0.001; mutation effect F(1,11)=0.31, P=0.591; Sidak's post hoc presented in figure). WT, seven animals; Cav3.1 KO, six animals; ∗P<0.05, ∗∗P<0.01, ∗∗∗P<0.001. 3β-OH, (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile; CeM, central medial nucleus; KO, knock-out; RM, repeated measure; WT, wild-type.
We also analysed simultaneously recorded EEG oscillations from the barrel cortex. Baseline relative power from the CeM and cortex during the awake state showed significant difference mostly at slower frequencies between WT and mutant mice in the CeM but not in the cortex (Supplementary Fig. S3a). Similar to our findings in the CeM, we noticed a large reduction in power density and total power in the δ, θ, α, and β range in the mutant animals compared with the WT group after injections of 3β-OH (Supplementary Fig. S3b). Relative EEG power showed a significant increase in γ frequencies in mutant mice (Supplementary Fig. S3c), although the change in relative power from baseline was not different (Supplementary Fig. S3d). We propose that the observed differences in oscillations of intact thalamocortical circuits reflect resistance of the mutant mice to the hypnotic effects of 3β-OH.
Discussion
Neurosteroids have sedative/hypnotic properties, with the introduction of alphaxalone and its related 3α,5α analogues15 in the 1970s, which were withdrawn from clinical use because of a relatively high incidence of anaphylactic reactions to the vehicle.16,17 However, alphaxalone has been reformulated recently and is used in veterinary medicine.18 The ongoing use of alphaxalone continues to encourage future development of synthetic neurosteroids with hypnotic/anaesthetic properties.
Interestingly, 3β-OH, as a 3β,5β-reduced steroid, does not act as a positive modulator of GABAA receptors; however, it induces hypnosis in rat pups1 and adult mice (presented herein). This suggests that 3β-reduced neurosteroids produce their hypnotic effects through alternate mechanisms. Here, we report that 3β-OH stabilised inactive states and reduced T-current density but did not affect phasic or tonic GABAA currents in CeM neurones. We further found that 3β-OH reduced rebound burst firing in WT animals and decreased tonic firing in both WT and Cav3.1 KO mice. However, inhibition of tonic firing was less prominent in the mutant mice, particularly at lower amplitude current injections. The modest blocking effect of 3β-OH on tonic firing observed in Cav3.1 KO mice could be a result of homeostatic compensatory changes in the mutant mice. As 3β-OH also inhibits the native Cav3.2 and Cav3.3 isoforms of T-channels,6 7 contributions of other isoforms of T-channels to the hypnotic effect of 3β-OH may not be excluded. Likewise, we cannot exclude the possibility that 3β-OH also inhibits voltage-gated sodium channels, as it is generally accepted that most hypnotics/anaesthetics affect multiple ion channels.19, 20, 21
Event-related rhythmic thalamocortical oscillations are usually categorised into six frequency bands: slow (<1 Hz), δ (0.5–4 Hz), θ (4–8 Hz), α (8–13 Hz), β (13–30 Hz), and γ (>30 Hz).22 Mice lacking the Cav3.1 isoform of T-channels had reduced thalamic δ oscillations and sleep spindles during urethane- and barbiturate-induced hypnosis.23,24 In addition, Cav3.1 KO mice displayed attenuated cortical and thalamic low frequency oscillations (in the 1–4 Hz range) after ketamine and ethanol administration compared with WT mice.25 We observed reduced total power of thalamic and cortical δ, θ, α, and β oscillations in Cav3.1 KO mice after injections of 3β-OH. We propose that the differences in slow frequency oscillations are related to the lack of rebound burst firing in Cav3.1 KO animals and inability of 3β-OH to hyperpolarise neurones in the mutant mice. It is well known that T-channels need to be deinactivated by hyperpolarisation in order to be opened to induce repetitive burst firing.26,27 As observed with ketamine and ethanol,25 hyperpolarisation induced by 3β-OH can explain not only differences in low frequency oscillations between WT and Cav3.1 KO mice but also diminished hypnosis in Cav3.1 null mice. We propose that 3β-OH hyperpolarised CeM neurones in WT mice because of voltage-dependent inhibition of the T-type ‘window current’ as we recently showed for isoflurane and another T channel blocker TTA-P2.11 A previous study found that Cav3.1 KO had delayed TTLORR with volatile anaesthetics.12 However, the same study reported that TTLORR by the intravenous anaesthetic propofol (a GABAA potentiator) was not different between WT and Cav3.1 KO mice. This suggests that different classes of anaesthetics have different mechanisms of interaction with Cav3.1 channels and other molecular targets in the thalamocortical circuitry.
Injection of 3β-OH at 80 mg kg−1 induced hypnosis in WT animals but was insufficient to do so in Cav3.1 KO mice. Although we used a global KO mice, the lack of effect of 3β-OH on lowering isoflurane concentration needed for hypnotic induction in the mutant mice may implicate Cav3.1 channels in CeM because this thalamic nucleus has been proposed to be important for induction but not for maintenance of anaesthetic hypnosis.9 Focal genetic deletion of Cav3.1 channels from the midline thalamus may lead to a fragmented and reduced sleep state.28 This suggests that inhibition of Cav3.1 channels in CeM contributes to the hypnotic effects of 3β-OH. However, future studies with focal deletion of Cav3.1 channels specifically in the CeM may conclusively address this issue. Finally, studies with another structurally unrelated selective T-channel blocker, TTA-A2, revealed that in vivo T-channel inhibition promotes slow-wave sleep in WT mice but not in mice lacking Cav3.1/Cav3.3 T-channels.29
Consistent with expression of Cav3.1 channels in both cortex and the thalamus,28 LFP and EEG recordings revealed similar effects of 3β-OH in WT and Cav3.1 KO mice – namely the mutant mice exhibited diminished total power in comparison with WT animals in all frequency ranges except in the γ band. In the cortex there was an increase in the relative power of γ frequency oscillations after 3β-OH administration compared with vehicle in mutant mice. Considering the role of high frequency oscillations in arousal and memory processes,30 augmentation in the range of γ frequency oscillations could contribute to the observed resistance to hypnosis in mutant mice after 3β-OH. Although interpretation of behavioural studies using global KO mice can be complicated by compensatory changes (as with any KO paradigm), our results with Cav3.1 KO mice strongly implicate a role for this T-channel isoform in neurosteroid-induced hypnosis and thalamocortical oscillations.
Conclusions
A previous study showed that 3β-OH is an effective hypnotic in rat pups1 without causing neuroapoptosis as is frequently observed with other anaesthetics that target neuronal GABAA (propofol) and N-methyl-d-aspartate (ketamine) receptors.31, 32, 33 Here we demonstrate the importance of the Cav3.1 isoform of T-channels in thalamocortical excitability and oscillations that underlie 3β–OH–induced hypnosis. This work has the potential to overturn existing dogma about the anaesthetic mechanisms of neurosteroids and to shift the focus to underappreciated targets, such as thalamic T-channels. These studies may be used as a starting point to develop novel and potentially safer approaches in clinical anaesthesia.
Authors' contributions
Performed experiments: TTS, SF, FMM, DW, DW, TC, HF, PD
Synthetised the neurosteroids: KK
Analysed the data: TTS, SMT
Designed the studies, supervised the overall project, and performed final manuscript preparation: TTS, YHR, DFC, VJT, SMT
Acknowledgements
We thank the University of Colorado Anschutz Medical Campus In Vivo Neurophysiology Core, which is part of NeuroTechnology Centre, for providing facilities to acquire and review video-EEG data. We thank Charles Adrian Handforth for donating breeding pairs of Cav3.1 knockout mice.
Handling editor: Hugh C Hemmings Jr
Footnotes
This article is accompanied by an editorial: Anaesthetic-induced developmental neurotoxicity on (neuro)steroids by A. Evers, Br J Anaesth 2021:126:34–37, doi: 10.1016/j.bja.2020.08.008
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.07.022.
Declarations of interest
The authors declare that they have no conflicts of interest.
Funding
US National Institutes of Health (# R01GM 123746 to SMT and VJT); Department of Anesthesiology and School of Medicine at the Anschutz Medical Campus.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Atluri N., Joksimovic S.M., Oklopcic A. A neurosteroid analogue with T-type calcium channel blocking properties is an effective hypnotic, but is not harmful to neonatal rat brain. Br J Anaesth. 2018;120:768–778. doi: 10.1016/j.bja.2017.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rupprecht R. Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology. 2003;28:139–168. doi: 10.1016/s0306-4530(02)00064-1. [DOI] [PubMed] [Google Scholar]
- 3.Stell B.M., Brickley S.G., Tang C.Y., Farrant M., Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by subunit-containing GABAA receptors. Proc Natl Acad Sci. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambert J.J., Belelli D., Hill-Venning C., Peters J.A. Neurosteroids and GABAA receptor function. Trends Pharmacol Sci. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- 5.Tuem K.B., Atey T.M. Neuroactive steroids: receptor interactions and responses. Front Neurol. 2017;8:1–10. doi: 10.3389/fneur.2017.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Todorovic S.M., Pathirathna S., Brimelow B.C. 5β-reduced neuroactive steroids are novel voltage-dependent blockers of T-Type Ca2+ channels in rat sensory neurons in vitro and potent peripheral analgesics in vivo. Mol Pharmacol. 2004;66:1223–1235. doi: 10.1124/mol.104.002402. [DOI] [PubMed] [Google Scholar]
- 7.Joksovic P.M., Covey D.F., Todorovic S.M. Inhibition of T-type calcium current in the reticular thalamic nucleus by a novel neuroactive steroid. Ann N Y Acad Sci. 2007;1122:83–94. doi: 10.1196/annals.1403.006. [DOI] [PubMed] [Google Scholar]
- 8.Vertes R.P., Hoover W.B., Rodriguez J.J. Projections of the central medial nucleus of the thalamus in the rat: node in cortical, striatal and limbic forebrain circuitry. Neuroscience. 2012;219:120–136. doi: 10.1016/j.neuroscience.2012.04.067. [DOI] [PubMed] [Google Scholar]
- 9.Baker R., Gent T.C., Yang Q. Altered activity in the central medial thalamus precedes changes in the neocortex during transitions into both sleep and propofol anesthesia. J Neurosci. 2014;34:13326–13335. doi: 10.1523/JNEUROSCI.1519-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stamenic T.T., Todorovic S.M. Cytosolic ATP relieves voltage-dependent inactivation of T-type calcium channels and facilitates excitability of neurons in the rat central medial thalamus. Eneuro. 2018;102525:1–16. doi: 10.1523/ENEURO.0016-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Timic Stamenic T., Feseha S., Valdez R., Zhao W., Klawitter J., Todorovic S.M. Alterations in oscillatory behavior of central medial thalamic neurons demonstrate a key role of CaV3.1 isoform of T-channels during isoflurane-induced anesthesia. Cereb Cortex. 2019;29:1–18. doi: 10.1093/cercor/bhz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrenko A.B., Tsujita M., Kohno T., Sakimura K., Baba H. Mutation of α1G T-type calcium channels in mice does not change anesthetic requirements for loss of the righting reflex and minimum alveolar concentration but delays the onset of anesthetic induction. Anesthesiology. 2007;106:1177–1185. doi: 10.1097/01.anes.0000267601.09764.e6. [DOI] [PubMed] [Google Scholar]
- 13.Belelli D., Lambert J.J. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 14.Joksimovic S.M., Izumi Y., Joksimovic S.L. Novel neurosteroid hypnotic blocks T-type calcium channel-dependent rebound burst firing and suppresses long-term potentiation in the rat subiculum. Br J Anaesth. 2019;122:643–651. doi: 10.1016/j.bja.2019.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joksimovic S.L., Covey D.F., Jevtovic-Todorovic V., Todorovic S.M. Neurosteroids in pain management: a new perspective on an old player. Front Pharmacol. 2018;9:1–10. doi: 10.3389/fphar.2018.01127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laszlo G., Lester S. Steroid anesthetics. Anesthesiology. 1975;42:331–343. doi: 10.1097/00000542-197503000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Reddy D.S., Estes W. Clinical potential of neurosteroids for CNS disorders. Trends Pharmacol Sci. 2016;37:543–561. doi: 10.1016/j.tips.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warne L.N., Beths T., Whittem T., Carter J.E., Bauquier S.H. A review of the pharmacology and clinical application of alfaxalone in cats. Vet J. 2015;203:141–148. doi: 10.1016/j.tvjl.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Denomme N., Hull J.M., Mashour G.A. Role of voltage-gated sodium channels in the mechanism of ether-induced unconsciousness. Pharmacol Rev. 2019;71:450–466. doi: 10.1124/pr.118.016592. [DOI] [PubMed] [Google Scholar]
- 20.Purtell K., Gingrich K.J., Ouyang W., Herold K.F., Hemmings H.C. Activity-dependent depression of neuronal sodium channels by the general anaesthetic isoflurane. Br J Anaesth. 2015;115:112–121. doi: 10.1093/bja/aev203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou C., Johnson K.W., Herold K.F., Hemmings H.C. Differential inhibition of neuronal sodium channel subtypes by the general anesthetic isoflurane. J Pharmacol Exp Ther. 2019;369:200–211. doi: 10.1124/jpet.118.254938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crunelli V., Lőrincz M.L., Connelly W.M. Dual function of thalamic low-vigilance state oscillations: rhythm-regulation and plasticity. Nat Rev Neurosci. 2018;19:107–118. doi: 10.1038/nrn.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J., Kim D., Shin H.S. Lack of δ waves and sleep disturbances during non-rapid eye movement sleep in mice lacking α1G-subunit of T-type calcium channels. Proc Natl Acad Sci U S A. 2004;101:18195–18199. doi: 10.1073/pnas.0408089101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J., Song K., Lee K. Sleep spindles are generated in the absence of T-type calcium channel-mediated low-threshold burst firing of thalamocortical neurons. Proc Natl Acad Sci. 2013;110:20266–20271. doi: 10.1073/pnas.1320572110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi S., Yu E., Lee S., Llinás R.R. Altered thalamocortical rhythmicity and connectivity in mice lacking CaV3.1 T-type Ca2+ channels in unconsciousness. Proc Natl Acad Sci. 2015;112:7839–7844. doi: 10.1073/pnas.1420983112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iftinca M.C., Zamponi G.W. Regulation of neuronal T-type calcium channels. Trends Pharmacol Sci. 2009;30:32–40. doi: 10.1016/j.tips.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Connelly W.M., Crunelli V., Errington A.C. The global spike: conserved dendritic properties enable unique Ca2+ spike generation in low-threshold spiking neurons. J Neurosci. 2015;35:15505–15522. doi: 10.1523/JNEUROSCI.2740-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson M.P., Mochizuki T., Xie J. Thalamic CaV3.1 T-type Ca2+ channel plays a crucial role in stabilizing sleep. Proc Natl Acad Sci U S A. 2005;102:1743–1748. doi: 10.1073/pnas.0409644102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraus R.L., Li Y., Gregan Y. In vitro characterization of T-type calcium channel antagonist TTA-A2 and in vivo effects on arousal in mice. J Pharmacol Exp Ther. 2010;335:409–417. doi: 10.1124/jpet.110.171058. [DOI] [PubMed] [Google Scholar]
- 30.Jensen O., Kaiser J., Lachaux J.P. Human γ-frequency oscillations associated with attention and memory. Trends Neurosci. 2007;30:317–324. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Hudson A.E., Hemmings H.C. Are anaesthetics toxic to the brain? Br J Anaesth. 2011;107:30–37. doi: 10.1093/bja/aer122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Creeley C., Dikranian K., Dissen G., Martin L., Olney J., Brambrink A. Propofol-induced apoptosis of neurones and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br J Anaesth. 2013;110:i29–i38. doi: 10.1093/bja/aet173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang C., Liu F., Patterson T.A., Paule M.G., Slikker W. Relationship between ketamine-induced developmental neurotoxicity and NMDA receptor-mediated calcium influx in neural stem cell-derived neurons. Neurotoxicology. 2017;60:254–259. doi: 10.1016/j.neuro.2016.04.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.