To the Editor:
COVID-19-associated respiratory illness may lead to ARDS.1 In intubated patients with severe ARDS, early, prolonged, and repeated sessions of prone positioning (PP) decrease mortality rates.2 , 3 Awake PP is feasible, improves oxygenation in some patients, and may prevent respiratory worsening,4, 5, 6 The main objective of the present study was to evaluate the effect of PP on the outcome of spontaneously breathing patients with COVID-19 with acute respiratory failure.
Methods
We designed an exposed/nonexposed bicentric retrospective matched cohort study to assess the effectiveness of PP for patients hospitalized outside ICU with COVID-19 whose condition required oxygen.
All consecutive patients hospitalized from March 20 to April 20, 2020, in Aix-en-Provence Hospital and Marseille University Hospital, France, were screened for eligibility. Inclusion criteria were age >18 years, hypoxemic respiratory failure that required oxygen supplementation by either conventional oxygen therapy or high-flow nasal cannula (HFNC), and polymerase chain reaction-confirmed severe acute respiratory syndrome coronavirus 2. The study received approval by the appropriate institutional review board (MR 3514070520).
Two treatment strategies were compared: (1) awake PP for at least 3 hours each day during 3 consecutive days (PP group) and (2) no instruction regarding positioning or no tolerance of PP during hospitalization (no-PP group). Each PP session had a minimum duration of 1 hour and a maximum duration of 12 hours, for a minimum total PP duration of 3 hours per day. Position of the patient and duration of PP sessions were monitored by the medical and paramedical team every 3 hours. The oxygen supplementation strategy was to initiate first oxygen therapy, second HFNC, third pressure support with noninvasive ventilation, and finally invasive mechanical ventilation.
It was a retrospective analysis of patient medical records from admission to day 14.
The primary outcome of the study was the “upgrading of oxygen delivery method” evaluated on day 14 and defined by reaching at least 6 L/min with a doubling of the initial oxygen flow for usual oxygen supplementation or the initiation of HFNC, noninvasive ventilation, or invasive mechanical ventilation. The secondary outcome was death at day 14.
Difference testing between groups was performed with the use of the two-tailed t test, Mann-Whitney U test, or chi-square tests, as appropriate. To determine the relative risk of upgrading the oxygen delivery method due to PP, we performed a propensity score analysis to adjust for imbalances in baseline characteristics between patients with and without PP. Using the overall population (n = 168), we developed a logistic regression model to derive a propensity score for PP (used as a binary value). The variables relevant to the model were selected from the univariate analyses (P < .20) or from their clinical relevance: (1) center; (2) age; (3) sex; (4) smoking history; (5) chronic respiratory disease; (6) heart failure; (7) systemic hypertension; (8) BMI; (9) time since symptom onset; (10) severity of condition at admission with the Spo 2/Fio 2 ratio at baseline; (11) percentage of lung affected on thoracic computerized tomography (<25%, 25-50%; ≥50%)7; (12) systemic corticosteroid treatment; and (13) therapeutic or prophylactic anticoagulation. This logistic regression model was used to estimate a propensity score for each patient that corresponded to the probability of PP, given the patient’s characteristics. The matching was performed with the use of greedy matching (1:1 nearest neighbor). Using the matched cohort (48 vs 48 patients in the PP and the no-PP groups), we compared the rate of upgrading the oxygen delivery method, intubation, and day-14 death. We performed a Cox model to calculate the adjusted hazard ratio for the upgrading of oxygen delivery method. All statistics were two-tailed; a probability value of <.05 was considered to be significant. All statistical analyses were performed with IBM SPSS Statistics software (version 20; IBM Inc, Chicago, IL).
Results
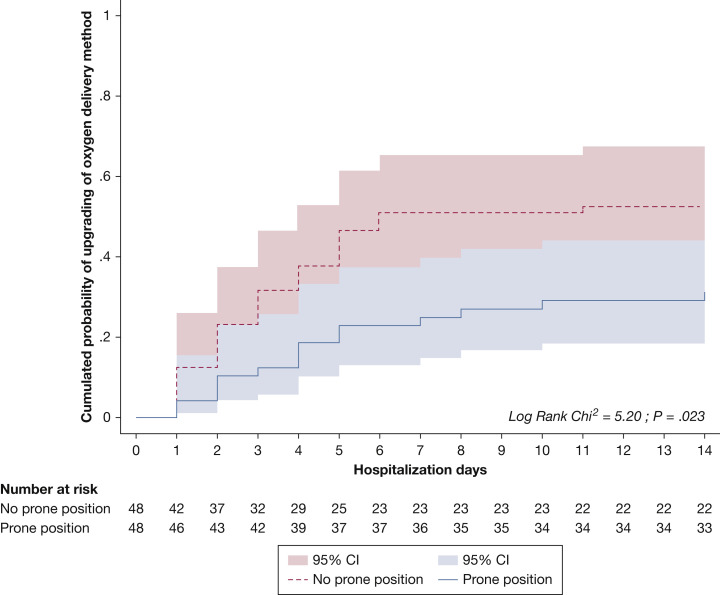
A total of 414 confirmed patients with COVID-19 were admitted during the study period. Two hundred forty-six patients did not meet inclusion criteria (192 patients did not require oxygen supplementation; 31 patients refused the use of their data, and 23 patients had incomplete files). Among the 168 patients who were eligible for analysis, 48 patients received PP for at least 3 hours each day for 3 days, and 120 patients did not. After a propensity score matching was performed, the conditions of 96 patients were analyzed (48 patients in each group). All patients who received PP were matched successfully with a patient without PP (Table 1 ). Patients underwent PP for 6.9 ±5.2 days. Among the 48 patients who received PP, 32 patients (67%) underwent PP for 3 to 8 hours each day, and 16 patients (32%) underwent PP for >8 hours each day. For the primary outcome, 15 patients (31.2 %) in the PP group had an upgrading of oxygen delivery method at day 14, compared with 25 patients (52.1%) in the no-PP group (P = .038) with a hazard ratio of 2.03 (95% CI, 1.07-3.86; P = .003). Kaplan-Meier curve that compared the cumulative probability of upgrading the oxygen delivery method is available in Figure 1 . For the secondary outcome, 4 patients (8.3 %) in the PP group and 6 patients (12.5%) of the non-PP group died at day 14 (P = .74) (Table 1). No patients in the PP-group died or required invasive mechanical ventilation within the first 3 days. No major adverse event was observed.
Table 1.
Patient Characteristics and Outcomes
| Patient Characteristics | Group |
Standardized Difference, (95% CI) | |
|---|---|---|---|
| No Prone Position (n = 48) | Prone Position (n = 48) | ||
| Sex, male, No. (%) | 31 (64.6) | 37 (77.1) | 27.8 (-12.4 to 68.0) |
| Age, y, mean ± SD | 61 ± 18 | 62 ± 11 | 0.07 (-0.32 to 0.48) |
| BMI, mean ± SD | 28 ± 5 | 27 ± 5 | 0.09 (-0.31 to 0.49) |
| Hypertension, No. (%) | 18 (37.5) | 15 (31.2) | 13.2 (-26.9 to 53.2) |
| Diabetes mellitus, No. (%) | 12 (25) | 7 (14.6) | 26.4 (-13.8 to 66.6) |
| Smoking history, No. (%) | 9 (18.8) | 8 (16.7) | 5.5 (-34.6 to 45.5) |
| Respiratory history, No. (%) | 8 (16.7) | 7 (14.6) | 5.7 (-34.3 to 45.8) |
| Immunodepression, No. (%) | 3 (6.2) | 4 (8.3) | 8.0 (-32.0 to 48.0) |
| Delay from the onset of symptoms to hospitalization, d, mean±SD | 9.7±6.9 | 9.6±6.4 | 0.6 (-0.39 to 0.41) |
| Systemic corticosteroid treatment, No. (%) | 28 (58.3) | 28 (58.3) | 0.0 (-40.0 to 40.0) |
| Therapeutic anticoagulation, No. (%) | 16 (33.3) | 15 (31.2) | 4.5 (-35.6 to 44.5) |
| Spo2/Fio2 at admission, mean±SD | 299±45 | 279±84 | 0.30 (-0.10 to 0.70) |
| CT scan finding, % of ground glass of lung parenchyma, No. (%) | |||
| <25% | 13 (27.1) | 14 (29.2) | 4.6 (-35.4 to 44.6) |
| 25-50% | 20 (41.7) | 20 (41.7) | 0.0 (-40.0 to 40.0) |
| >50% | 15 (31.2) | 14 (29.2) | 4.5 (-35.5 to 44.6) |
| Lymphocytes, g/L, mean±SD | 1.03±0.59 | 0.90±0.53 | 0.23 (-0.17 to 0.64) |
| C-reactive protein , mg/L, mean±SD | 116±94 | 112±84 | 0.04 (-0.36 to 0.44) |
| Blood eosinophilia , g/L, mean±SD | 0.03±0.06 | 0.02±0.05 | 0.12 (-0.28 to 0.52) |
| P value | |||
| Upgrading of oxygen delivery method,a No. (%) | 25 (52.1) | 15 (31.2) | .038 |
| Invasive mechanical ventilation, No. (%) | 8 (16.7) | 7 (14.6) | .779 |
| Day 14 death, No. (%) | 6 (12.5) | 4 (8.3) | .740 |
Defined as reaching at least 6 L/min with a doubling of the initial oxygen flow for usual oxygen supplementation or the initiation of high-flow nasal canula, noninvasive ventilation, or invasive mechanical ventilation.
Figure 1.
Cumulated probability of upgrading of oxygen delivery method: hospitalization and risk.
Discussion
This retrospective, bicentric study done on 96 awake, nonintubated, spontaneously breathing patients with COVID-19 with acute hypoxemic respiratory failure that required oxygen supplementation showed that PP for at least 3 hours each day during 3 consecutive days prevented the upgrading of oxygen delivery method on day 14 after hospital admission compared with no instruction regarding PP or no tolerance of PP during hospitalization.
These results are consistent with findings from previous small studies of PP in patients who were not intubated with improvement in oxygenation and a trend to improve clinical outcomes.4 , 5 , 8, 9, 10
The study has several limitations. First, although we used robust statistical techniques for adjustment, treatment was not assigned randomly, and patients were instructed for PP according to the physician in charge. Second, the reasons for the lack of a significant effect on death or intubation remain uncertain; however, the sampling size was suboptimal to answer these questions, and the study was not powered or planned to assess the effect on mortality rates. Third, it was a retrospective collection of data from patients who were hospitalized in a medical ward who were not monitored. That is the reason that we did not evaluate oxygenation response or tolerance during PP sessions.
PP for at least 3 hours each day during 3 consecutive days may be associated with a clinical benefit by preventing the upgrading of oxygen delivery method. These results must be confirmed with further randomized prospective studies.
Acknowledgments
Author contributions: E. Prud’homme had full access to all the data in the study and takes responsibility for the integrity of the data and conflict of interest disclosures. The following is a list of each author’s contribution: concept and design: E. Prud’homme, J-M. Forel, X. Elharrar, Y. Trigui, and S. Lehingue; acquisition, analysis, or interpretation of data: all authors; drafting of the manuscript: E. Prud’homme, J-M. Forel, X. Elharrar, and Y. Trigui; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: A. Loundou; administrative, technical, or material support: J-M. Forel and L. Papazian; and supervision: J-M. Forel and L. Papazian.
Other contributions: We thank Ines Gragueb, MD; Claire Stein, MD; Gabriel Parzy, MD; Aude Sylvestre, MD; Lucie Fages, MD; Florence Daviet, MD; Estelle Pilarczyk, MD; Melanie Adda, MD; Christophe Guervilly, MD; Romain Rambaud, MD; Saida Salmi, MD; Delphine Bastian, MD, and Sami Hraiech, MD, PhD, from Assistance Publique-Hôpitaux de Marseille, Médecine Intensive Réanimation, Marseille, France, for their advice in designing the project. Jean-Baptiste Cornic, LLM, provided English editing service. None of these individuals received compensation for their contributions.
Footnotes
FINANCIAL/NONFINANCIAL DISCLOSURES: None declared.
References
- 1.Wang Y., Lu X., Li Y., et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 2020;201(11):1430–1434. doi: 10.1164/rccm.202003-0736LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guérin C., Reignier J., Richard J.-C., et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 3.Munshi L., Del Sorbo L., Adhikari N.K.J., et al. Prone position for acute respiratory distress syndrome. a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(suppl4):S280–S288. doi: 10.1513/AnnalsATS.201704-343OT. [DOI] [PubMed] [Google Scholar]
- 4.Elharrar X., Trigui Y., Dols A.-M., et al. Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMA. 2020;323(22):2338–2340. doi: 10.1001/jama.2020.8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sartini C., Tresoldi M., Scarpellini P., et al. Respiratory parameters in patients with COVID-19 after using noninvasive ventilation in the prone position outside the intensive care unit. JAMA. 2020;323(22):2336–2338. doi: 10.1001/jama.2020.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Q., Qiu H., Huang M., Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. doi: 10.1186/s13613-020-00650-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li K., Fang Y., Li W., et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur Radiol. 2020;30(8):4407–4416. doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding L., Wang L., Ma W., He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. doi: 10.1186/s13054-020-2738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coppo A., Bellani G., Winterton D., et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med. 2020;8(8):765–774. doi: 10.1016/S2213-2600(20)30268-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Retucci M., Aliberti S., Ceruti C., et al. Prone and lateral positioning in spontaneously breathing patients with COVID-19 pneumonia undergoing noninvasive helmet CPAP treatment. Chest. 2020;158(6):2431–2435. doi: 10.1016/j.chest.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]