Abstract
Muscle weakness and fatigue represent frequent disabling symptoms for Multiple Sclerosis (MS) patients. We evaluated the effects of an intensive task-oriented circuit training (TOCT) on perceived fatigue, muscle strength and changes in motor performance fatigability in mildly impaired MS patients. Fifteen MS patients performed different functional scales, self-reported questionnaires and instrumental evaluations before (T0) and after (T1) TOCT. Strength and performance fatigability were analyzed during isometric knee extension and ankle dorsiflexion through an isokinetic dinamometer, recording surface EMG signals of Vastus Medialis and Tibialis Anterior. The Dinamic Gait Index, Multiple Sclerosis Impact Scale-29, Modified Fatigue Impact Scale and Multiple Sclerosis Walking Scale–12 significantly improved after training. An increase of exerted force during isometric knee extension was observed, whereas no significant changes were revealed on mechanical and electrical fatigue. Moreover, the improvement in perceived disability after treatment was related to strength increase in knee mechanical force output. The TOCT positively modifies perceived fatigue, perceived ambulatory function and knee force output in mildly impaired MS subjects, suggesting a virtuous circle between strength levels, recovery of functional skills and improved quality of life.
Key Words: Circuit-based exercise, Isometric contractions, Muscle Fatigue, Quadriceps muscle
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Muscle weakness and excessive skeletal muscle fatigue represent two frequent disabling symptoms experienced by patients affected by Multiple Sclerosis (MS).1 Reductions in lower-extremity muscle mechanical function, such as muscle strength, muscle power and explosive muscle strength, appear to have critical implications for lower-limb functional capacity, with negative influence on walking performance, balance and mobility.2 Alongside this aspect, fatigue is a subjective symptom that severely affects task performance in daily activities and reduces quality of life in MS people.3 Up to now, the pathophysiology of muscle weakness and fatigue remains uncertain. Beside major theories supporting a role of an altered “central neural drive”, accounting for the inability to promote a sufficient motoneuronal recruitment and a delayed neural transmission,4 the presence of impaired metabolic function of skeletal muscle fibers may represent a key factor in the development and worsening of these two symptoms.5 Indeed, the demyelination of upper motor neurons seems likely to induce chronically reduced maximum discharge rates and altered or incomplete motor unit activation,6 which can also lead to changes in myosin heavy chain (MHC) distribution and fibers’ type representation.5 As regards peripheral modifications, intra-muscular changes comprehend lower tetanic and twitch tension, greater fatigue during electrically stimulated contractions and lower muscle oxidative capacity.6 Finally, changes in fiber-type distribution to faster, more glycolytic fibers, evidenced in weight-bearing muscles (gastrocnemius, soleus, and quadriceps) may promote modifications in contractile characteristics of the muscle and finally worse motor performance and functional abilities.7 The multidimensional character of fatigue makes it difficult to be managed in clinical settings and different pharmacological treatment have demonstrated unable to resolve the symptom, pointing out the importance of an alternative approach to this problem.8 In this light, exercise training may be an alternative behavioral strategy for reducing fatigue in persons with MS.9 In the years, there have been growing efforts to develop and evaluate rehabilitation interventions and build an evidence base to guide practitioners. Actually, the rehabilitative investigations reveal the benefit of different training interventions: a recent meta-analysis of randomized controlled trials (RCTs) of exercise interventions,10 and two systematic reviews of RCTs,11,12 suggest that a planned, structured, repetitive and undertaken over a prolonged period intervention has a positive impact on MS fatigue. Effective rehabilitation treatments on self-reported fatigue comprehend endurance training (ET), resistance training (RT) and combined training (ET + RT). Anyway, previous studies mainly provided clinical outcome measures concerning the perceived fatigue, but only few focused on the neurophysiological mechanisms underlying performance fatigability.13-16 On this panorama, the primary aim of this pilot study is to evaluate whether functional gains expected after a 2-week intensive rehabilitation program are associated with improvements in perceived fatigue, increase in muscle strength and/or changes in motor performance fatigability in MS patients with mild-moderate disability.17 To this purpose, considering the evidence that isometric muscle strength and force production are impaired in lower limb in MS patients,18 we analyzed the strength of knee extensor and ankle dorsiflexors with an isokinetic dynamometer. Moreover, as far as surface electromyography (sEMG) has been widely used to observe changes in neuromuscular activation associated with muscle fatigue,19 we evaluated the performance fatigability during a fatiguing task while sEMG signals of Vastus Medialis (VM) and Tibialis Anterior (TA) were simultaneously recorded. Finally, we administered to patients different functional scales and self-reported questionnaires, in order to verify potential correlations between motor and clinical improvements and modifications in force output and motor fatigue.
Materials and Methods
Participants
We recruited 15 Multiple Sclerosis patients (10 females and 5 males, mean age 51 ± 11.76 years), with either the relapsing-remitting, primary progressive or secondary progressive subtype.
Inclusion and exclusion criteria
Inclusion criteria were:
males and females;
age > 18;
diagnosis of MS according to McDonald Revised Criteria (relapsing-remitting, primary or secondary progressive);20
clinical stability for at least three months (no relapses, no disability worsening and no other medical complications);
motor impairment assessed with the Expanded Disability Status Scale (EDSS) score between 4 and 5.5;21
preserved walking ability for a short distance (10 m) without use of devices.
Exclusion criteria were:
rehabilitation since 1 month before the beginning of the study;
impaired cognitive functioning (Mini Mental Status Examination score less than 24);
other causes than MS which may affect motor function (orthopedic or other neurological diseases);
unstable cardiovascular conditions;
severe lower limb spasticity or contractures that may limit ranges of motion (Ashworth score >4 for hip, knee or ankle flexors/extensors).
Table 1.
Characteristics of study sample
| Patient | Age (years) | Gender (M/F) | EDSS score | Disease Phenotype | Disease Duration (years) | Disease Modifying treatment |
|---|---|---|---|---|---|---|
| p1 | 56 | M | 4,5 | RR | 15 | Glatiramer Acetate |
| p2 | 49 | F | 4,5 | RR | 15 | Natalizumab |
| p3 | 67 | F | 4,5 | RR | 47 | - |
| p4 | 27 | F | 4 | RR | 4 | IFN-β |
| p5 | 46 | F | 4,5 | RR | 20 | Fingolimod Cloridrate |
| p6 | 54 | M | 4,5 | RR | 13 | Fingolimod Cloridrate |
| p7 | 47 | M | 4,5 | PP | 4 | - |
| p8 | 67 | F | 5 | RR | 16 | Glatiramer Acetate |
| p9 | 65 | F | 4,5 | RR | 17 | IFN-β |
| p10 | 44 | M | 4 | RR | 22 | IFN-β |
| p11 | 38 | F | 5,5 | RR | 20 | Natalizumab |
| p12 | 56 | F | 4,5 | RR | 26 | - |
| p13 | 64 | F | 4,5 | PP | 7 | Azathioprine |
| p14 | 43 | M | 4 | RR | 22 | Natalizumab |
| p15 | 41 | F | 5 | RR | 12 | Fingolimod Cloridrate |
Clinical characteristics
The main clinical characteristics of patients are described in Table 1. Written informed consent was obtained before any evaluation or treatment, and subjects received a copy of the consent form during the initial interview. Our study project was designed in accordance with the Declaration of Helsinki and was approved by the local Ethics Committee of Pisa (study protocol - Nr. 484, 2015-52984, approval number 52984). Outcome measures, comprehending functional scales, self-reported questionnaires and instrumental evaluations were assessed the week prior the treatment initiation (T0) and at the end of treatment (T1).
Rehabilitation interventions
All patients underwent a rehabilitative treatment accounting for 10 sessions, 5 sessions per week. Each session lasted 120 minutes overall and was organized as a circuit: 30 minutes of gait training with treadmill, 10 minutes of stretching, 30 minutes of task-oriented circuit, 10 minutes of rest, 30 minutes of task-oriented circuit and 10 minutes of muscle strengthening.17 The task-oriented circuit was organized in several stations in which patients were asked to: i) overcome an obstacles course, made up with sticks placed on the ground, in a frontal and then lateral direction; ii) achieve various targets placed at different heights sights on a mirror with the feet; iii) walk along a 10 meter long line drawn on the ground; iv) climb and descent stairs.
For further details on methodology, please refer to our previous report.17
Motor performance assessment and self-reported questionnaires
All patients underwent a comprehensive examination including four different clinical tests to assess mobility, walking endurance, speed and gait performance: Timed Up & Go (TUG), 6 Minutes Walking Test (6MWT), 10 meter Walk Test (10mWT) and Dynamic Gait Index (DGI).22Moreover, different self-reported questionnaires evaluating health-related quality of life, perceived fatigue and walking ability were administered to subjects: Multiple Sclerosis Impact Scale- 29 (MSIS-29) is an health-related quality of life questionnaire that assesses the impact of MS on physical and psychological functions;23 Functional Assessment of Multiple Sclerosis (FAMS), is a quality of life instrument for use in people with MS;23 perceived fatigue was monitored through Modified Fatigue Impact Scale (MFIS);24 Multiple Sclerosis Walking Scale–12 (MSWS-12), assesses the impact of MS on walking ability.25
Force and fatigue evaluations and analysis
Patients performed different trials to assess muscle force and fatigue on an isokinetic dynamometer (PrimusRS Multi-Joint System dynamometer, BTE Technologies).
Mechanical muscle strength was assessed measuring the maximum voluntary contraction (MVC) during knee extension and ankle dorsiflexion in isometric condition.26 Before starting the test sections, subjects were instructed about the correct procedure to perform a maximal exertion, as hard as possible without moving the rest of the body, and to maintain it for 3 s. Three consecutive repetitions for both sides were taken, with a 10-seconds rest between consecutive trials. The three tests were accepted when the coefficient of variation (CV) was less than 10.0%; the average of the tree consecutive trials was considered the MVC. Subsequently, mechanical muscle fatigue was evaluated measuring force output during sustained contractions in isometric tasks in lower limbs (knee extension and ankle dorsiflexion),26 while recording surface EMG signals from VM and TA to test electrical fatigue (EF). Subjects were instructed to exert MVC for knee extension and for ankle dorsiflexion, and then to maintain it for 60 s without any further encourage during the performance. Both sides were evaluated. Force output was calculated on 1-second time intervals, and, than, normalized by values obtained at MVC, because the absolute level of each parameter differed among the subjects. Final data were expressed in percentage terms, as the average value of each patient, as a function of time. An index of mechanical muscle fatigue was extracted as the slope of linear regression of contraction trends, that is, the normalized force (NF).19 Analysis were performed using MATLAB software.
sEMG recordings and analysis
Surface electromyographic activity (sEMG) of the VM and TA were recorded during sustained contractions in isometric tasks (knee extension and ankle dorsiflexion respectively). Due to changes in contractile properties and involvement in walking abilities of such muscle groups, the VM and TA were chosen to be studied. sEMG signals were sampled with the use of electromyographic devices that use wireless technology (FreeEMG300, BTS S.r.l.). Following skin abrasion with an alcohol soaked cotton pad, electrodes were placed on the muscle bellies according to SENIAM guidelines.27 Signals were recorded with pairs of bipolar silver-silver chloride surface electrodes. Then, probes were placed on surface electrodes: these devices present active electrodes for recording, amplification and transmitting the signal to the receiver connected directly to the PC. sEMG signals were subsequently processed with the BTS EMG –Analyzer software package. EMG data processing consisted of a preliminary zero-lag band pass filtering with cut-off frequencies at 20 and 500 Hz. Then, a sampling rate of 1024 Hz was applied on all sEMG signals, and the Root Mean Square (RMS) was calculated over 1-second epochs. The RMS, along with the Average Rectified Value (ARV), defines the amplitude of an electromyographic signal. The RMS value is the square root of the average signal power in a given period, and therefore reflects the electrical power of the signal. RMS values were normalized by values obtained at MVC. Final data were expressed in percentage terms, as the average value of each patient, as a function of time. An index of electrical muscle fatigue was extracted as the slope of linear regression of contraction trends (normalized RMS).19 Analysis were performed using MATLAB software.
Fig 1.

Clinical assessment (a) and self-reported questionnaires (b) before (T0) and after (T1) the TOCT training.
Fig 2.
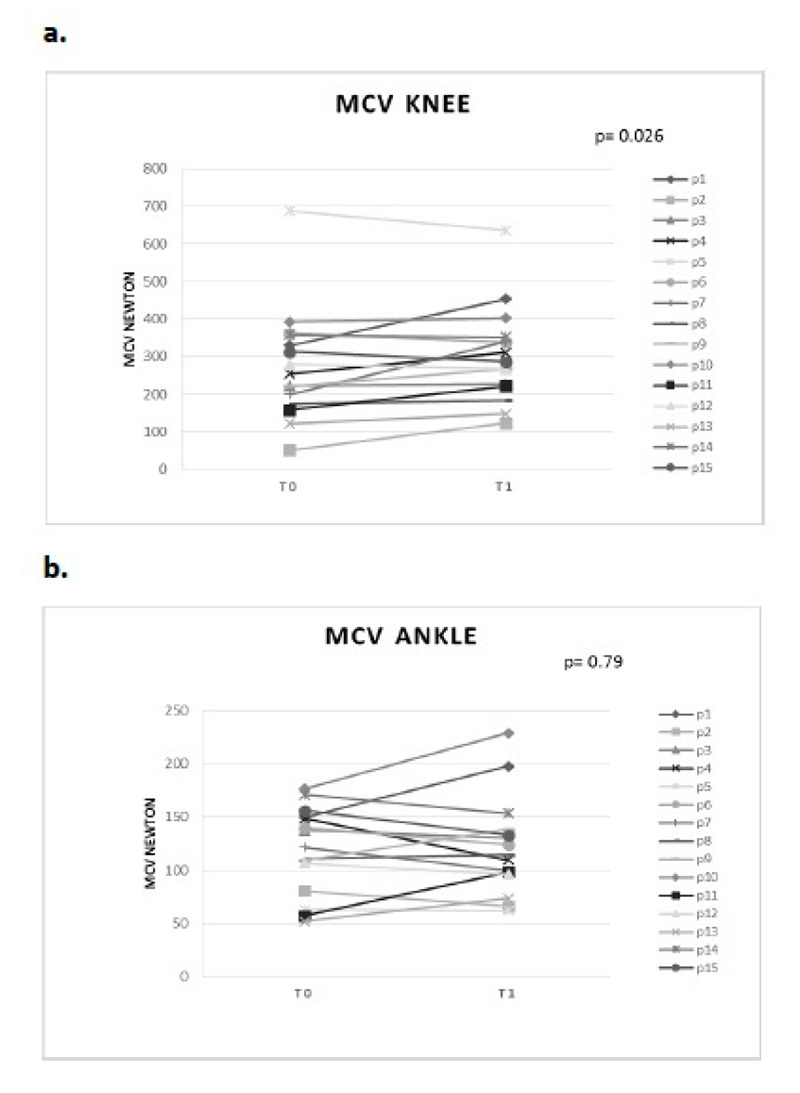
Mechanical muscle force during 3-sec isometric contractions before (T0) and after (T1) TOCT. a. MCV knee extension, b. MCV ankle dorsiflexion.
Statistical Analysis
Descriptive statistic (mean, median, standard deviation, percentiles and confidence interval) were used to describe sample at T0 and T1. Normality of data distribution was assessed through Shapiro-Wilk test. The non-parametric Wilcoxon signed-rank tests were used to determine if changes in outcome measures, such as functional scales, self-reported questionnaires and instrumental evaluations, occurred before (T0) and after (T1) the TOCT intervention. For mechanical and electrical fatigue, the non-parametric Wilcoxon signed-rank test was applied to verify any difference in the linear regression line coefficient before (T0) and after (T1) the TOCT. Paired t-test was used whenever samples accomplished a normality distribution of data. For clinical outcome measures, self-reported questionnaires and mechanical muscle evaluations, changes were calculated by means of the score difference measured after and before the two-weeks training (Δ = T1 - T0). To evaluate the relationship between self-reported improvements as measured by changes in DGI, MSIS-29, MFIS and MSWS-12 (ΔDGI, ΔMSIS-29, ΔMFIS, ΔMSWS-12) and force output variation (ΔMCV Gin), partial correlation analysis using Spearman’s coefficient was employed. All reported p - values are results of two - tailed tests. Results are described in relation to the significance of p < 0.05. All statistical procedures were performed with SPSS/PC (version 20.0) software program.
Results
Clinical assessment
Patients improved significantly their performance in the DGI (16.07 ± 5 a T0 vs 17.93 ± 3.89 a T1, p = 0.012) after training intervention (Fig. 1a). Concerning mobility, endurance and walking speed, our data showed an improvement in the TUG (13.93 ± 8.11 s at T0 vs 13.68 ± 8.75 s at T1, p = 0.8), 6MWT (340.5 ± 120.5 m at T0 vs 345.5 ± 109.8 m at T1, p = 0.1) and 10mWT (10.7 ± 8.4 s at T0 vs 9.35 ± 4.2 s at T1, p = 0.8), without reaching statistical significance (Fig. 1a). Outcome measures related to the impact of the disease on physical and psychological functions, perceived fatigue and perceived walking abilities significantly changed after two-week training period: MSIS-29 (75.06 ± 24.52 at T0 vs 61.6 ± 23.86 at T1, p = 0.001), MFIS (37.6 ± 15.3 at T0 vs 25.9 ± 13.8 at T1, p = 0.04), MSWS-12 (39.86 ± 12.4 at T0 vs 31.66 ± 13.51 at T1, p = 0.03), Fig. 1b. Only the self-reported quality of life investigated with the FAMS presented a trend in improvement after TOCT training (90.6 ± 18.3 at T0 vs 83.06 ± 22.38 at T1, p = 0.3), without reaching statistical significance (Fig. 1b).
Fig 3.

Mechanical (A) and electrical (B) muscle fatigue during sustained isometric contractions before (T0) and after (T1) TOCT. a. knee extension, b. ankle dorsiflexion.
Analysis of muscle mechanical force
As regard mechanical strength sustained during 3-seconds isometric contractions, we revealed a significant increase in force output during knee extension after training intervention (275 ± 155.83 at T0 vs 303.81 ± 138.19 at T1, p = 0.026), Fig. 2a. Concerning ankle dorsiflexion trials, force output remained basically unmodified after training period (118.57 ± 48.38 at T0 vs 121.62 ± 50.78 at T1, p = 0.79), Fig. 2b.
Analysis of mechanical and electrical fatigue
Data showed no significant change in mechanical fatigue after TOCT, as evidenced by force normalized slope both for knee extension (p= 0.55; Fig. 3A.a) and ankle dorsiflexion (p= 0.68; Fig. 3A.b) trials. Likewise, electrical fatigue revealed no significant change after 2-week training period both for knee extension (p= 0.10; Fig. 3B.a) and for ankle dorsiflexion (p= 0.78; Fig. 3B.b), as shown by RMS normalized slope. Moreover, no significant variation was revealed on baseline RMS values during sustained contraction for knee extension after training period (0.039 ± 0.02 mV at T0 vs 0.038 ± 0.02 mV at T1).
Analysis of correlation
As regard the relationship between functional scales, self-reported questionnaires and force output, the analysis of correlation revealed that:
The variations of MSIS-29 scale (ΔMSIS-29) and knee force output (ΔMCV Gin) were significantly (p = 0.042) and negative correlated (r = -0.530) each other, such that the improvement in the perceived disability after treatment corresponded to an increase in knee mechanical force output, Fig. 4.
The analysis of correlation revealed the absence of relationship between the variations of force output (ΔMCV Gin) and all self-reported questionnaires, in particular as regard perceived fatigue (ΔMFIS), defining a lack of interaction between the clinical improvements and subjective fatigue, Fig. 4.
Discussion
Up to now, several randomized controlled trials have been conducted in order to evaluate the efficacy of rehabilitation trainings on force output and MS-related fatigue.11,12 Our study pointed out important training-induced improvements as regard force output, subjective fatigue symptom, health-related quality of life and perceived walking ability. Conversely to what was expected, the rehabilitation program did not induce any relevant changes in performance fatigability. While, correlation analysis revealed a significant relationship between the subjective perception of motor disability and the extent of strength improvement after training intervention. In line with previous findings,17 functional data revealed an improved motor performance after TOCT, especially with respect to dynamic balance and health-related quality of life. Emerging evidences comprehend the improvements both on perceived fatigue and perceived ambulatory function, respectively investigated with MFIS and MSWS-12. As currently evidenced, it was reported a mutual relationship between these two clinical scales,25 therefore it is not surprising that training-induced improvements involved both the subjective measure of fatigue and ambulatory function. To date, literature covers a plenty of studies evidencing how a structured exercise training may be an effective approach to improve fatigue in MS.10 Based on this evidence, we can speculate that our progressive intensive training may have improved subjective fatigue symptom for some contributing factors. The conception of a progressive evolution and intensity of the TOCT, tailored on symptom fluctuations, may have favoured the adherence of patients; while the possibility to personalize the circuit training on the basis of individual competencies and needs may have increased confidence and motivation to exercise.28 This could particularly influence factors, such as fatigue, that have links to mood;3 training-induced gait biomechanics modifications may have reduced the metabolic costs during ambulation,17 and modified the subjective perception of energy expenditure during walking,29 positively influencing patients’ general sense of fatigue and perceived walking ability. 30 The novelty of our data is the fact that a two-week intensive program positively modified knee extensor isometric muscle strength in a group of mildly impaired MS subjects. While, force output during isometric ankle dorsiflexion trials remained basically unmodified after training period. In particular, strength of knee extensors moved its value towards normality parameters, while force output during ankle dorsiflexion persisted lower than normative data.31 The relevance of this finding is represented by the fact that reductions in lower-extremity muscle mechanical function (e.g., muscle strength) appear to have critical implications for lower-limb functional capacity in MS people on all levels of the International Classification of Functioning, Disability and Health model including activity level.2 Nevertheless, the direct relationship between knee muscle strength and walking performance in MS patients are controversial: in a recent review Ramari et al. (2020)2 pointed out that no lower-extremity muscle group is the main driver of lower-limb functional capacity. To date, progressive resistance programs represent the most effective choice for counteracting muscle weakness and peripheral muscular hypotrophy in MS. Anyway, rehabilitation intervention should address other aspects than high-intensity RT, involving elements that target motor control and balance.2 In this light, actual data point out that the 2-week task-oriented exercise training can positively improve peripheral muscle force output beside inducing benefits on functional capacities and gait biomechanics,17 indicating a kind of relationship between physical strength and physical function. As regard motor performance fatigability assessment, the first datum arising is that amplitude parameter, defined by RMS normalized slope, did not present the typical increase observed during sustained contraction in healthy subjects.19 This evidence is consistent with literature: RMS is less in people with MS during 60%–100% MVC contractions, which is a likely reflection of inability to drive units at sufficient firing rates or to perform an adequate motor unit recruitment [4]. Moreover, no significant changes were revealed on mechanical and electrical fatigue both during knee extension and ankle dorsiflexion tasks. Few studies investigated the impact of short rehabilitation training on performance fatigability in MS people13-16 For example, motor fatigability decreased significantly after an acquatic fitness program,13 while a combined 6-month rehabilitation program, including endurance and resistance training, decreased the fatigability of knee extensor muscles in women, but not in men.14 Similarly to our results, Hameau et al. (2018)15 found that a short, intensive, combined 4-week rehabilitation programme, focused on gait, balance, endurance and resistance training, did not modify fatigability in moderate impaired MS subjects. Same findings was revealed by Beretta-Piccoli et al. (2020)16 while testing an exercise intervention based mainly on progressive resistance training for lower limb. Anyway, these studies used different protocols and fatigability was investigated with different tasks, making it difficult to draw unit conclusion. A stimulating result is represented by the fact that, beside the higher exerted force during 3-seconds’ isometric knee extensor task, we did not find a proportional increase in basal RMS values during sustained contraction after TOCT. These data define that the increase in force output after training intervention could be due to an improved motor unit firing rate, rather than an increase in muscle fibers recruitment.19 However, it is noteworthy to specify that others electromyographic parameters should be taken into account to better discuss data and define the motor unit temporal synchronization, such as time-frequency domain analysis. Finally, another stimulating result is related to the analysis of correlations. Indeed, we evidenced a strong relation between the extent of strength increase during isometric knee contraction after training and the clinical improvement assessed with the MSIS-29 scale. Conversely, we did not reveal any interaction between the variation in force output and improvements in the others self-reported questionnaires, in particular perceived fatigue. Then, these findings suggest a strong relationship between the subjective perception of motor disability and strength improvement following the specific training, promoting the idea of a virtuous circle between strength levels, recovery of functional skills and improvement of individual quality of life.32
Fig 4.

Correlation analysis between variations in functional scales, self-reported questionnaires and force output.
The findings of this study must be interpreted in the context of a number of potential limitations. A first limitation is generalizability, as far as our study includes a relatively small sample size (n=15), with an heterogeneity in subjects’ gender, and presents a lack of a control group. Therefore, future perspective will be to assess our approach in a large cohort of patients with a longer follow-up in order to verify whether instrumental and functional changes persist along time and to confirm the generalizability of such an approach. Moreover, we studied muscle force output and performance fatigability of lower limb muscles that are more prone to show signs of deconditioning (knee extensors and ankle dorsiflexors) and represent two of the major muscles responsible for day-to-day mobility. Beside the fact that knee extensors force have been reported to be strongly associated with functional abilities and gait in MS, recent literature on the association of strength to gait also supports the importance of strength in others districts, such as knee flexors and ankle plantarflexors. Therefore, it could be important to extend future evaluations even in such muscle groups. Nevertheless, the analysis of muscle force output, the surface EMG signal and its correlation with functional data and self-reported questionnaires represents an important method to investigate neurophysiological mechanisms underlying motor improvements after training interventions.
In conclusion, the instrumental evaluation of force output evidenced that TOCT positively modifies knee extensor isometric muscle strength in a group of mildly impaired MS subjects. Correlation analysis confirm the strong relationship between the subjective perception of motor disability and the extent of strength improvement following the specific training, promoting the idea of a virtuous circle between strength levels, recovery of functional skills and improvement of individual quality of life.
Acknowledgments
None
List of acronyms
- 10mWT
10 meter Walk Test
- 6MWT
Minutes Walking Test
- ARV
Average Rectified Value
- CV
coefficient of variation
- DGI
Dynamic Gait Index
- EF
electrical fatigue
- ET
endurance training
- ET + RT
combined training
- FAMS
Functional Assessment of Multiple Sclerosis
- MFIS
Modified Fatigue Impact Scale
- MHC
myosin heavy chain
- MS
Multiple Sclerosis
- MSIS-29
Multiple Sclerosis Impact Scale- 29
- MSWS-12
Multiple Sclerosis Walking Scale–12
- MVC
maximum voluntary contraction
- NF
normalized force
- RCTs
randomized controlled trials
- RMS
Root Mean Square
- RT
resistance training
- sEMG
surface electromyography
- SENIAM
surface EMG for non-invasive assessment of muscles
- T0 and T1
evaluations before and after TOCT
- TA
Tibialis Anterior
- TOCT
task-oriented circuit training
- TUG
Timed Up & Go
- VM
Vastus Medialis
- Δ = T1
T0 score difference measured after and before two-weeks training
- ΔDGI
change in DGI,
- ΔMCV Gin
force output variation
- ΔMFIS
change in MFIS
- ΔMSIS-29
change in MSIS-29
- ΔMSWS-12
change in MSWS-12
Funding Statement
Funding None
Contributor Information
Siria Di Martino, Email: siria.dimartino@gmail.com.
Angela Foglia, Email: angela.foglia@gmail.com.
Carmelo Chisari, Email: carmelo.chisari@unipi.it.
References
- 1.Schwid SR, Covington M, Segal BM, Goodman AD. Fatigue in multiple sclerosis: Current understanding and future directions. Journal of Rehabilitation Research and Development 2002;39:211-24. [PubMed] [Google Scholar]
- 2.Ramari C, Hvid LG, David AC, Dalgas U. The importance of lower-extremity muscle strength for lower-limb functional capacity in multiple sclerosis: Systematic review. Ann Phys Rehabil Med 2020;63:123-37. doi: 10.1016/j.rehab.2019. 11.005 [DOI] [PubMed] [Google Scholar]
- 3.Rudroff T, Kindred JH, Ketelhut NB. Fatigue in multiple sclerosis: misconceptions and future research directions. Front Neurol 2016;7:122. doi: 10.3389/fneur.2016.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rice CL, Vollmer TL, Bigland-Richie B. Neuromuscular responses of patients with Multiple Sclerosis. Muscle Nerve 1992;15:1123-32. doi: 10.1002/mus.880151011. [DOI] [PubMed] [Google Scholar]
- 5.Carroll CC, Gallagher PM, Seidle ME, Trappe SW. Skeletal Muscle Characteristics of People With Multiple Sclerosis. Arch Phys Med Rehabil 2005;86:224-9. doi: 10.1016/j.apmr.2004.03.035 [DOI] [PubMed] [Google Scholar]
- 6.Kent-Braun JA, Ng AV, Castro M, et al. Strength, skeletal muscle composition, and enzyme activity in multiple sclerosis. J Appl Physiol 1997;83:1998-2004. doi: 10.1152/jappl.1997.83.6.1998 [DOI] [PubMed] [Google Scholar]
- 7.De Haan A, De Ruiter CJ, Van der Woude LHV, Jongen PJH. Contractile properties and fatigue of quadriceps muscles in Multiple Sclerosis. Muscle Nerve 2000;23:1534–41. doi: 10.1002/1097-4598(200010)23:10<1534::aid-mus9>3.0.co;2-d [DOI] [PubMed] [Google Scholar]
- 8.Braley TJ, Chervin RD. Fatigue in Multiple Sclerosis: Mechanisms, Evaluation, and Treatment. Sleep 2010;33:1061–7. doi: 10.1093/sleep/33.8.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stroud NM, Minahan CL. The impact of regular physical activity on fatigue, depression and quality of life in persons with multiple sclerosis. Health Qual Life Outcomes 2009;7:68. doi: 10.1186/1477-7525-7-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilutti LA, Greenlee TA, Motl RW, et al. Effects of exercise training on fatigue in multiple sclerosis: a meta-analysis. Psychosom Med 2013;75:575-80. doi: 10.1097/PSY.0b013e31829b4525. [DOI] [PubMed] [Google Scholar]
- 11.Andreasen A, Stenager E, Dalgas U. The effect of exercise therapy on fatigue in multiple sclerosis. Mult Scler 2011;17:1041-54. doi: 10.1177/1352458511401120. [DOI] [PubMed] [Google Scholar]
- 12.Asano M, Berg E, Johnson K, et al. A scoping review of rehabilitation interventions that reduce fatigue among adults with multiple sclerosis. Disabil Rehabil 2015;37:729–38. 10.3109/09638 288.2014.944996. [DOI] [PubMed] [Google Scholar]
- 13.Gehlsen GM, Grigsby SA, Winant DM. Effects of an aquatic fitness program on the 24 muscular strength and endurance of patients with multiple sclerosis Phys Ther 1984;64:653–57. doi: 10.1093/ ptj/64.5.653 [DOI] [PubMed] [Google Scholar]
- 14.Surakka J, Romberg A, Ruutiainen J, et al. Effects of aerobic and strength exercise on motor fatigue in men and women with multiple sclerosis: a randomized controlled trial. Clin Rehabil 2004;18:737-46. doi: 10.1212/01.wnl.0000145761.38400.65 [DOI] [PubMed] [Google Scholar]
- 15.Hameau S, Bensmail D, Roche N, Zory R. Adaptations of fatigue and fatigability after a 12 short intensive, combined rehabilitation program in patients with multiple sclerosis. J Rehabil Med 2018;50:59-66. oi: 10.2340/16501977-2277. [DOI] [PubMed] [Google Scholar]
- 16.Beretta-Piccoli M, Cescon C, Barbero M, et al. Upper and lower limb performance fatigability in people with multiple sclerosis investigated through surface electromyography: a pilot study. Physiol Meas 2020;41:1-27. doi: 10.1088/1361-6579/ab6f54. [DOI] [PubMed] [Google Scholar]
- 17.Tramonti C, Di Martino S, Chisari C. An intensive task-oriented circuit training positively impacts gait biomechanics in MS patients. 2020;46(3):321-31. doi: 10.3233/NRE-192997. [DOI] [PubMed] [Google Scholar]
- 18.Scott SM. Surface EMG characteristics of people with multiple sclerosis during static contractions of the knee extensors. Clin Physiol Funct Imaging 2011;31:11-7. doi: 10.1111/j.1475-097X.2010.00972.x [DOI] [PubMed] [Google Scholar]
- 19.Merletti R, Lo Conte LR, Orizio SC. Indices of Muscle Fatigue. J Electromyogr Kinesiol 1991;1: 20-33. doi: 10.1016/1050-6411(91)90023-X [DOI] [PubMed] [Google Scholar]
- 20.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annuals of Neurology 2011;69:292–302. doi: 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurtzke JF. Rating neurological impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444-52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 22.Bennett SE, Bromley LE, Fisher NM, et al. Validity and Reliability of Four Clinical Gait Measures in Patients with Multiple Sclerosis. Int J MS Care 2017;19:247-52. doi: 10.7224/1537-2073.2015-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riazi A, Hobart JC, Lamping DL, et al. Evidence-based measurement in multiple sclerosis: the psychometric properties of the physical and psychological dimensions of three quality of life rating scales. Mult Scler 2003;9:411-9. doi: 10.1191/1352458503ms929oa. [DOI] [PubMed] [Google Scholar]
- 24.Learmonth YC, Dlugonski D, Pilutti LA, et al. Psychometric properties of the Fatigue Severity Scale and the Modified Fatigue Impact Scale. J Neurol Sci 2013;331(1-2):102-7. doi: 10.1016/j.jns.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Hobart JC. Measuring the impact of MS on walking ability: the 12-Item MS Walking Scale (MSWS-12). Neurology 2003;60:31–6. doi: 10.1212/wnl. 60.1.31 [DOI] [PubMed] [Google Scholar]
- 26.Tramonti C, Rossi B, Chisari C. Extensive Functional Evaluations to Monitor Aerobic Training in Becker Muscular Dystrophy: A Case Report. Eur J Transl Myol 2016;26:5873. doi: 10.4081/ejtm.2016.5873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 2000;10:361-74. doi: 10. 1016/s1050-6411(00)00027-4 [DOI] [PubMed] [Google Scholar]
- 28.Moss-Morris R, Harrison AM, Safari R, et al. Which behavioural and exercise interventions targeting fatigue show the most promise in multiple sclerosis? A systematic review with narrative synthesis and meta-analysis. Behav Res Ther 2019; 28:103464. doi: 10.1016/j.brat.2019.103464 [DOI] [PubMed] [Google Scholar]
- 29.Tantucci C, Massucci M, Piperno R, et al. Energy cost of exercise in multiple sclerosis patients with a low degree of disability. Mult Scler 1996;2:161-67. oi: 10.1177/135245859600200307. [DOI] [PubMed] [Google Scholar]
- 30.Guicciardi M, Carta M, Pau M, Cocco E. The Relationships between Physical Activity, Self-Efficacy, and Quality of Life in People with Multiple Sclerosis. Behav Sci (Basel) 2019;9:121. doi: 10.3390/bs9120121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoll T, Huber E, Seifert B, et al. Maximal Isometric Muscle Strength: Normative Values and Gender-Specific Relation to Age. Clinical Rheumatology 2000;19:105-13. doi: 10.1007/s100670050026. [DOI] [PubMed] [Google Scholar]
- 32.Haraldstad K, Rohde G, Stea TH, et al. Changes in health-related quality of life in elderly men after 12 weeks of strength training. Eur Rev Aging Phys Act 2017;14:8. doi: 10.1186/s11556-017-0177-3 [DOI] [PMC free article] [PubMed] [Google Scholar]


