Abstract
Background
There is no consensus regarding the management and postoperative follow-up of non-functioning pituitary adenomas (NFAs) in the setting of recurrent or residual disease. Subsequent treatment options include continued follow-up, re-resection or radiotherapy. To address this gap and better understand current practice patterns, we surveyed neurosurgeons and radiation oncologists in Canada.
Methods
Neurosurgeons and radiation oncologists (ROs) across Canada were invited to complete a standardized online questionnaire. Summary statistics were computed, and Fisher's Exact tests were performed to assess significance. Qualitative analyses were performed through open and axial coding.
Results
Thirty-three participants completed the questionnaires, with neurosurgeons representing a majority of respondents (n = 20 vs n = 13). When treating giant (>3 cm) tumors, 90.9% of neurosurgeons in practice for less than 10 years reported using an endoscopic approach, as compared to only 66.7% of neurosurgeons in practice for 10 years of more. Additionally, neurosurgeons who were newer to practice had a greater tendency to advocate for stereotactic radiosurgery (SRS) or re-resection (54.5% and 36.4%, respectively), as compared to older surgeons who showed a higher propensity (22.2%) to advocate for observation. The presence of cavernous sinus extension appeared to encourage ROs to offer radiotherapy sooner (61.4%), as compared to 40% of neurosurgeons.
Conclusions
Our results identified both variations and commonalities in practice amongst Canadian neurosurgeons. Approaches deviated in the setting of residual tumor based on years of practice. This work provides a critical foundation for future studies aiming to define evidence-based best practices in the management of NFAs.
Keywords: Canada-wide survey, Practice patterns, Radiation oncologist, Recurrent non-functioning pituitary adenoma, Residual non-functioning pituitary adenoma, Skull-base neurosurgeon
Highlights
-
•
No consensus in treatment of residual/recurrent non-functioning pituitary adenomas
-
•
Younger physicians prefer radiotherapy compared to senior physicians.
-
•
Skull base neurosurgeons advocate more aggressively for re-resection.
-
•
There is a need for standardization of treatment guidelines.
1. Introduction
Non-functioning pituitary adenomas (NFAs1) include all pituitary tumors that are not hormonally active, and account for approximately 15% to upwards of 50% of all pituitary adenomas [[1], [2], [3], [4]]. Lacking any hypersecretory characteristics, NFAs often escape detection until patients begin presenting with symptoms of mass effect.
Neurosurgical intervention, either endoscopic or microscopic, remains the gold standard of treatment for symptomatic NFAs, with a majority of cases being performed via an endoscopic transsphenoidal approach due to its slightly lower complication rate in comparison to a microscopic approach [[5], [6], [7], [8], [9], [10]]. Alternatively, conservative approaches to treatment may also be considered for patients with incidentally discovered NFAs, depending on tumor characteristics such as size and extent of tumor invasion [5]. Despite advances in neurosurgical technique, due to the invasiveness, possibility of lateral and suprasellar extension, and size of NFAs, partial resection is common, leaving behind tumor remnants that may lead to recurrence [11,12]. Recurrence after surgery, either due to growth from residual tumor post partial resection, or new growth has been found to be as high as 12–47% [[11], [12], [13], [14], [15], [16]]. In an attempt to directly determine the rate of pituitary tumor proliferation, and better estimate prognosis, the monoclonal antibody Ki-67 is often used as a tool to aid in the postoperative decision making process [17,18]. A wide body of evidence exists showing that higher Ki-67 levels are associated with greater risk of recurrence and shorter disease free survival, with 2004 WHO classifications outlining a Ki-67 of 3% or higher as an indicator of aggressive tumor behaviour [19,20].
Importantly, even after repeat surgery for residual disease, partial resection and the risk of further growth remains high [11]. This has made the use of adjuvant radiotherapy (RT) to treat detectable residual tumor an increasingly attractive option, as it considerably reduces risk of NFA recurrence [13,15,16,21]. However, the exact role RT plays in the adjuvant setting remains unclear. Therefore, the primary objective of this study was to explore practice patterns across Canada in the treatment of residual and recurrent NFAs, with the aim of better understanding the current practice landscape in order to facilitate the optimization of NFA management moving forward.
2. Methods
2.1. Study design and population
Neurosurgeons and ROs across Canada were identified from hospital department staff directories and university faculty directories. The identified surgeons and ROs were then contacted by email and invited to complete a 25-item, online questionnaire. The questionnaire was offered in both English and French. Participation in the study was voluntary and completion of any portion of the survey implied consent to participate. There were no incentives provided for completion of the survey. Responses to all questions from participants were anonymized and tabulated. This study meets the exclusion criteria of the Canadian Tri-Council Policy statement for research that necessitates a review by an institutional research ethics board, since there was no involvement of patients as subjects in this study.
2.2. Questionnaire and administration
The questionnaire was created based on a review of current literature, with consultation from skull-base neurosurgeons. The format of the questionnaire included a mixture of multiple choice and short answer questions, exploring topics ranging from total years in practice and number of current.
patients being treated with NFAs, to treatment preferences in the setting of recurrence/residual tumor; six cases were also presented, two with MRI images, asking for first choice of treatment given certain pathology reports, imaging results, and symptomatology. The questionnaire consisted of 25 questions in total, and was structured using built in survey logic, whereby prior responses influenced which subsequent questions would be asked to each individual respondent. These logic steps ensured that the questionnaire was tailored to the specialty and practice patterns of each respondent. Fig. 1 illustrates a flow diagram of the questions. Prior to distribution, the questionnaire was revised by two staff neurosurgeons and one neurosurgery resident for validity and ease of comprehension and was amended accordingly. Surveys were distributed using the Qualtrics web-based platform (Qualtrics, Provo, UT), including an initial email invitation with subsequent reminders at 4 and 8 weeks after the initial invitation. Twelve weeks after the final invitation, the questionnaire was closed. Upon completion of the questionnaire, answers and aggregate data was exported from Qualtrics to Microsoft Excel (Microsoft Corporation, Redmond, WA) for analysis. Participants were subdivided by specialty, pituitary neoplasm subspecialisation, years in practice, volume of pituitary patients, and neurosurgical approach to resection (neurosurgeons only); subsequently, Fisher's Exact tests were employed, due to small sample size, to analyze frequency tables of relevant survey responses for statistically significant differences between subgroups.
Fig. 1.
Flow diagram depicting questions presented to survey participants.
3. Results
A total of 81 neurosurgeons and ROs were included in the study cohort and invited to participate in the study, with 33 (40.7%) responses collected from November 12, 2018 to January 28, 2019. Forty-one neurosurgeons who perform pituitary surgeries were contacted, from which 20 responses were collected (48.8%), representing 61.6% of total responses. Forty ROs specialising in treating CNS tumors were contacted, from which 13 responses were collected (32.5%), representing 39.4% of total responses. Thirty-one surveys (93.9%) were completed in English, and two (6.1%) were completed in French. Ten of the respondents (30%; five neurosurgeons and five ROs) were from Western Canada (British Columbia, Alberta, Saskatchewan, Manitoba); nineteen of the respondents (58%; fourteen neurosurgeons and five ROs) were from Ontario and Quebec; and the remaining four respondents (12%; one neurosurgeon and three ROs) were from Atlantic Canada (Nova Scotia, Newfoundland). This distribution reflects our attempt to receive a wide variety of questionnaire responses across many sites in different provinces. Respondents' years of practice varied widely: less than 5 years 18.2% (neurosurgery: n = 3, 15%; RO: n = 3, 23.1%), 5–10 years 33.3% (neurosurgery: n = 8, 40%; RO: n = 3, 23.1%), 11–15 years 9.1% (neurosurgery: n = 2, 10%; RO: n = 1, 7.7%), 16–20 years 6.1% (neurosurgery: n = 2, 10%; RO: n = 0, 0%), 21–25 years 9.1% (neurosurgery: n = 1, 5%; RO: n = 2, 15.4%), 26 years and greater 24.2% (neurosurgery: n = 4, 20%; RO: n = 4, 30.8%).
Half of the neurosurgeons surveyed cited following over 100 patients with a pituitary adenoma at present, while 40% were following between 1 and 50 patients, and 10% were following between 51 and 100 patients. All ROs surveyed were currently following fewer than 75 patients (pre- and post-op), with a majority (84.7%) following 50 or less. Patients were followed at various stages of their treatment including pre- and postoperative care. Seventeen (51.5%) respondents were confirmed to have completed relevant fellowship level training (neurosurgery: n = 11, 55%; RO: n = 6, 46.2%). A subset of relevant survey responses is summarized in Table 1.
Table 1.
Select responses of neurosurgeons (n = 20) and radiation oncologists (n = 13) for management of NFAs.
| Neurosurgeons (%) | Radiation Oncologists (%) | |
|---|---|---|
| Practice has a neuro-oncology focus | 80% | 92.3% |
| Practice has a pituitary neoplasm focus | 60% | 46.2% |
| Gross total resection is always surgical aim when operating on NFA | 80% | – |
| Performs NFA resection via endoscopic transsphenoidal approach | 75% | – |
| Postop MRI < 72 h | 30% | 38.5% |
| Your centre routinely performs Ki-67 on pituitary adenomas | 85% | 53.8% |
| Your centre routinely performs MIB1 on pituitary adenomas | 35% | 15.4% |
| Ki-67 status influences short term management of recurrent NFA | 15% | 15.4% |
3.1. Surgical management
An endoscopic transsphenoidal approach, with the goal of gross total resection (GTR), was preferred amongst neurosurgeons, with no significant difference in preference (endoscopic vs. microscopic) amongst respondents when considering years in practice or patient volume. Indications for a craniotomy to resect an NFA were uniformly rare across all respondents and consisted exclusively of extensive suprasellar and lateral extension of the tumor, or prior failure of a transsphenoidal approach. In one instance a craniotomy was performed due to patient preference.
3.2. Postoperative management
3.2.1. Follow-up imaging
Of those ordering an immediate postoperative MRI (within 72 h), subsequent additional imaging was also obtained at 1 month (n = 0), 2 months (n = 1), 3 months (n = 5), 4–6 months (n = 2), and 6 months (n = 2). The remaining surgeons and ROs, who do not routinely order an immediate postoperative MRI, subsequently performed follow-up MRI at 1 month (n = 1), 2 months (n = 4), 3 months (n = 9), and 4–6 months (n = 2).
3.2.2. Residual and recurrent disease
Sixty percent (n = 12) of neurosurgeon respondents have never had to return to the operating room during the same admission for issues of large residual tumor; 35% (n = 7) have had to repeat surgery during the same admission 5 or fewer times in their career, and 5% (n = 1) have had to repeat surgery during the same admission more than 5 times.
When asked if the presence of cavernous sinus extension (Knosp Grade 2 or more) in the postoperative patient would encourage them to offer RT sooner; 40% neurosurgeons responded yes (n = 8), compared to 61.5% of ROs (n = 8), however this trend was not significant. Given the presence of postoperative cavernous sinus extension, 37.5% of surgeons and ROs would offer radiosurgery within 3 months of the initial operation; 25% of surgeons and 37.5% of ROs would offer it between 3 and 6 months, and 37.5% of surgeons and 25% of ROs would offer it after 6 months, respectively. When taking into consideration years of practice, patient volume, and approach to pituitary resection, the distribution of responses did not significantly differ.
Radiation oncologists were also asked about the use of additional radiosurgical modalities in the setting of recurrent adenomas, besides fractioned RT. Sixty-nine percent (n = 9) of ROs indicated that they use a LINAC to provide external beam radiation to patients with NFAs, 23.1% (n = 3) use Gamma Knife SRS, and 15.4% (n = 2) use Cyberknife SRS. Gamma Knife SRS appears to be exclusively performed by ROs with greater than 10 years of practice. Once more, no differences were found between groups when considering years in practice or patient volume. In lieu of SRS for intrasellar recurrent NFAs with cavernous sinus extension (5 mm distance from optic apparatus), respondents were asked if they would advocate for proton therapy if it were an option in Canada; three ROs (23.1%) and one surgeon (5%), indicated they would prefer proton therapy over SRS in this situation.
3.3. Select case examples
Case 1
A 40-year-old patient with a growing residual tumor in the sella (> 1 cm away from optic apparatus, with no cavernous involvement), with obvious growth over serial scans (over 1 year). Treatment options advocated for included “Re-resection” (n = 11, 33.3%), “Stereotactic radiosurgery” (n = 16, 48.5%), “Follow with serial imaging” (n = 5, 15.2%), and “Other: may do repeat imaging sooner” (n = 1, 3.0%). Table 2, Table 3, Table 4 subdivide physicians' responses by years in practice, volume of pituitary patients in their practice, and further subspecialisation, respectively. Of note, 60% of neurosurgeons who indicated they preferred a microscopic approach to macroadenoma (<3 cm) resections endorsed re-resection in this case, as compared to only 26.7% of those preferring an endoscopic approach. Upon grouping and subsequently comparing physicians by these parameters, as well as by specialty, Fisher's Exact tests revealed no significant differences in the distributions of answers.
Case 2
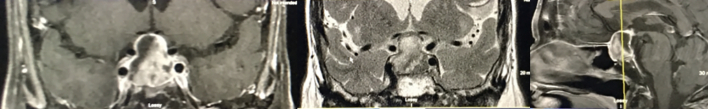
A 40-year-old male with bitemporal hemianopsia, occurring over months; the postoperative MRI showed GTR, and pathology was consistent with an NFA, with Ki-67 of 1–2%. Serial MRIs over 1 year showed slow growth of recurrence at the floor of the sella (Fig. 2). Treatment options advocated for included “Observe/follow with serial imaging” (n = 16, 48.5%), “Offer radiotherapy” (n = 14, 42.4%), and “Operate now” (n = 3, 9.1%).
Table 2.
Case 1: Growing residual tumor in the sella with obvious growth over serial scans; comparing results of younger physicians (neurosurgeons: n = 11; ROs: n = 6; ≤10 years of practice) and senior physicians (neurosurgeons: n = 9; ROs: n = 7; >10 years of practice).
| Re-resection, n (%) | SRS, n (%) | Watchful Waiting, n (%) | ||
|---|---|---|---|---|
| Neurosurgeons | Younger | 4 (36.4) | 6 (54.5) | 1 (9.1) |
| Senior | 3 (33.3) | 3 (33.3) | 3 (33.3) | |
| ROs | Younger | 1 (16.7) | 4 (66.7) | 1 (16.7) |
| Senior | 3 (42.9) | 3 (42.9) | 1 (14.3) | |
| Total | Younger | 5 (29.4) | 10 (58.8) | 2 (11.8) |
| Senior | 6 (37.5) | 6 (37.5) | 4 (25) | |
Table 3.
Case 1: Growing residual tumor in the sella with obvious growth over serial scans; comparing results of physicians with smaller (neurosurgeons: n = 8; ROs: n = 7; ≤50 pituitary adenoma patients) and larger pituitary patient volumes (neurosurgeons: n = 12; ROs: n = 6; >50 pituitary adenoma patients for neurosurgeons, >25 patients for ROs). Pt, patient.
| Re-resection, n (%) | SRS, n (%) | Watchful Waiting, n (%) | ||
|---|---|---|---|---|
| Neurosurgeons | Small Pt Volume | 2 (25) | 4 (50) | 2 (25) |
| Large Pt Volume | 5 (41.7) | 5 (41.7) | 2 (16.7) | |
| ROs | Small Pt Volume | 2 (28.6) | 4 (57.1) | 1 (14.3) |
| Large Pt Volume | 2 (33.3) | 3 (50) | 1 (16.7) | |
| Total | Small Pt Volume | 4 (26.7) | 8 (53.3) | 3 (20) |
| Large Pt Volume | 7 (38.9) | 8 (44.4) | 3 (16.7) | |
Table 4.
Case 1: Growing residual tumor in the sella with obvious growth over serial scans; comparing results between neurosurgeons and ROs who have a practice with pituitary neoplasm focus (neurosurgeons: n = 12; ROs: n = 6) and those who do not (neurosurgeons: n = 8; ROs: n = 7).
| Does your practice have a pituitary neoplasm focus? | Re-resection, n (%) | SRS, n (%) | Watchful Waiting, n (%) | |
|---|---|---|---|---|
| Neurosurgeons | Yes | 5 (41.7) | 4 (33.3) | 3 (25) |
| No | 2 (25) | 5 (62.5) | 1 (12.5) | |
| ROs | Yes | 2 (33.3) | 2 (33.3) | 2 (33.3) |
| No | 2 (28.6) | 5 (71.4) | 0 (0) | |
| Total | Yes | 7 (38.9) | 6 (33.3) | 5 (27.8) |
| No | 4 (26.7) | 10 (66.7) | 1 (6.7) | |
Fig. 2.
A 40-year-old male with bitemporal hemianopsia; postoperative MRI shows gross total resection; serial MRIs over 1 year show slow growth of recurrence in floor of the sella.
Case 3 was nearly identical to Case 2; however, the Ki-67 index was now >3%. Treatment options advocated for included “Observe/follow with serial imaging” (n = 9, 27.3%), “Offer radiotherapy” (n = 22, 66.7%), and “Operate now” (n = 2, 6.1%). Responses to Case 2 and Case 3 are outlined in Table 5, Table 6, Table 7, Table 8, which subdivide physicians' responses by years in practice, volume of pituitary patients in their practice, preference of surgical approach (neurosurgeons only), and further subspecialisation, respectively. Upon grouping and subsequently comparing physicians by these parameters, Fisher's Exact tests revealed no significant differences in the distributions of answers.
Table 5.
Two additional cases (Fig. 2) were presented with multiple options of management; comparing results of younger physicians (neurosurgeons: n = 11; ROs: n = 6; ≤10 years of practice) and senior physicians (neurosurgeons: n = 9; ROs: n = 7; >10 years of practice).
|
Case 2 |
Case 3 |
||||||
|---|---|---|---|---|---|---|---|
| Operate Now, n (%) | RT, n (%) | Watchful Waiting, n (%) |
Operate Now, n (%) | RT, n (%) | Watchful Waiting, n (%) |
||
| Neurosurgeons | Younger | 0 (0) | 5 (45.5) | 6 (54.5) | 0 (0) | 9 (81.8) | 2 (18.2) |
| Senior | 1 (11.1) | 2 (22.2) | 6 (66.7) | 0 (0) | 6 (66.7) | 3 (33.3) | |
| ROs | Younger | 0 (0) | 4 (66.7) | 2 (33.3) | 0 (0) | 4 (66.7) | 2 (33.3) |
| Senior | 2 (28.6) | 3 (42.9) | 2 (28.6) | 2 (28.6) | 3 (42.6) | 2 (28.6) | |
| Total | Younger | 0 (0) | 9 (52.9) | 8 (47.1) | 0 (0) | 13 (76.5) | 4 (23.5) |
| Senior | 3 (18.8) | 5 (31.3) | 8 (50) | 2 (12.5) | 9 (56.3) | 5 (31.3) | |
Table 6.
Two additional cases (Fig. 2) were presented with multiple options of management; comparing results of physicians with smaller (neurosurgeons: n = 8; ROs: n = 7; ≤50 pituitary adenoma patients for neurosurgeons, ≤25 for ROs) and larger pituitary patient volumes (neurosurgeons: n = 12; ROs: n = 6; >50 pituitary adenoma patients for neurosurgeons, >25 patients for ROs). Pt, patient; Vol, volume.
|
Case 2 |
Case 3 |
||||||
|---|---|---|---|---|---|---|---|
| Operate Now, n (%) | RT, n (%) | Watchful Waiting, n (%) | Operate Now, n (%) | RT, n (%) | Watchful Waiting, n (%) | ||
| Neurosurgeons | Small Pt Vol | 0 (0) | 3 (37.5) | 5 (62.5) | 0 (0) | 6 (75) | 2 (25) |
| Large Pt Vol | 1 (8.3) | 4 (33.3) | 7 (58.3) | 0 (0) | 9 (75) | 3 (25) | |
| ROs | Small Pt Vol | 1 (14.3) | 4 (57.1) | 2 (28.6) | 1 (14.3) | 4 (57.1) | 2 (28.6) |
| Large Pt Vol | 1 (16.7) | 3 (50) | 2 (33.3) | 1 (16.7) | 3 (50) | 2 (33.3) | |
| Total | Small Pt Vol | 1 (6.7) | 7 (46.7) | 7 (46.7) | 1 (6.7) | 10 (66.7) | 4 (26.7) |
| Large Pt Vol | 2 (11.1) | 7 (38.9) | 9 (50) | 1 (5.6) | 12 (66.7) | 5 (27.8) | |
Table 7.
Two additional cases (Fig. 2) were presented with multiple options of management; comparing results of neurosurgeons who perform their microadenoma resection via endoscopic approach (n = 15) and microscopic approach (n = 5).
|
Case 2 |
Case 3 |
|||||
|---|---|---|---|---|---|---|
| Operate Now, n (%) | RT, n (%) | Watchful Waiting, n (%) | Operate Now, n (%) | RT, n (%) | Watchful Waiting, n (%) | |
| Endoscopic | 1 (6.7) | 6 (40) | 8 (53.3) | 0 (0) | 11 (73.3) | 4 (26.7) |
| Microscopic | 0 (0) | 1 (20) | 4 (80) | 0 (0) | 4 (80) | 1 (20) |
Table 8.
Two additional cases (Fig. 2) were presented with multiple options of management; comparing results between neurosurgeons and ROs who have a practice with pituitary neoplasm focus (neurosurgeons: n = 12; ROs: n = 6) and those who do not (neurosurgeons: n = 8; ROs: n = 7).
| Does your practice have a pituitary neoplasm focus? |
Case 2 |
Case 3 |
|||||
|---|---|---|---|---|---|---|---|
| Operate Now, n (%) | RT, n (%) | Watchful Waiting, n (%) | Operate Now, n (%) | RT, n (%) | Watchful Waiting, n (%) | ||
| Neurosurgeons | Yes | 1 (8.3) | 3 (25) | 8 (66.7) | 0 (0) | 7 (58.3) | 5 (41.7) |
| No | 0 (0) | 4 (50%) | 4 (50%) | 0 (0) | 8 (100) | 0 (0) | |
| ROs | Yes | 1 (16.7) | 3 (50) | 2 (33.3) | 1 (16.7) | 3 (50) | 2 (33.3) |
| No | 1 (14.3) | 4 (57.1) | 2 (28.6) | 1 (14.3) | 4 (57.1) | 2 (28.6) | |
| Total | Yes | 2 (11.1) | 6 (33.3) | 10 (55.6) | 1 (5.6) | 10 (55.6) | 7 (38.9) |
| No | 1 (6.7) | 8 (53.3) | 6 (40) | 1 (6.7) | 12 (80) | 2 (13.3) | |
3.4. Qualitative analysis
The qualitative component of the survey included questions pertaining to the management of NFAs that the respondents would like to be explored and addressed. Responses were varied widely. Emergent themes included: 1) use of SRS prophylactically in the case of residual tumor versus observation, 2) predicting early recurrence/using better growth surrogates, 3) reliable intraoperative imaging to assess the degree of resection, 4) when exactly to intervene and what intervention is most appropriate.
4. Discussion
4.1. Landscape of postoperative management of NFAs
NFAs are common tumors and account for approximately a third to a half of all pituitary adenomas [22]. They typically present later than functional tumors, and with symptoms of mass effect on the optic apparatus, normal pituitary gland tissue or cranial nerves in the vicinity of the cavernous sinus. Current Congress of Neurological Surgeons (CNS) guidelines recommend transsphenoidal resection, most commonly via an endoscopic approach, as first-line treatment [23]. However, NFAs are difficult to definitively cure. Incomplete resection and recurrence are not uncommon, often due to the presence of lateral or suprasellar extension of the tumor, and large initial size of many NFAs upon diagnosis, making complete tumor resection difficult [24,25]. Therefore, radiosurgical modalities have been employed, in line with CNS guidelines, to complement the treatment options in the setting of recurrent or residual disease in order to lower the risk of further progression. In the setting of little to no residual tumor on postoperative imaging, a watchful waiting approach is often recommended, with patients being followed with serial imaging. Repeat resection may be appropriate in the circumstances of larger, symptomatic residual or recurrent NFAs [22]. With these recommendations in mind, the best management strategy for recurrent or residual NFAs still remains undefined. There is a general lack of consensus on treatment details, ranging from optimal timing of radiotherapy and which radiation modality to employ, to the impact of proliferative tumor markers (e.g. Ki-67) and the timing of postoperative follow-up imaging.
This study aimed to investigate and consolidate the current Canadian landscape of postoperative management of NFAs by surveying neurosurgeons and radiation oncologists who treat pituitary tumors. This thorough analysis is the first to systematically address the variability in clinical practice in the setting of NFAs and develops an essential baseline for further works to spring from.
4.2. Current surgical management practices
When asked how they perform their pituitary macroadenoma (<3 cm) resection, 75% of neurosurgeons indicated they preferred an endoscopic transsphenoidal approach for the resection of NFAs. Respondents who selected the endoscopic approach represented a diverse group of surgeons coming from all regions of Canada, with varying years of experience and volumes of pituitary patients seen within their practices. While the current CNS guidelines for the treatment of NFAs do not provide sufficient evidence to support one approach over the other, over the past few decades there has been a transition towards the endoscopic approach for both initial and repeat re-resections [26,27]. This may be largely due to factors such as better view of the surgical field, shorter hospital stay, reduced perioperative complications, and less patient discomfort associated with the endoscopic approach [22,27]. With these trends in mind, the preference for endoscopy amongst the neurosurgeons represented in our cohort is perhaps not unexpected.
Giant NFAs pose a particularly difficult challenge when considering neurosurgical intervention, due to their large size and frequently accompanying lateral and suprasellar extensions. While there are many definitions, a commonly used criterion in diagnosing a giant NFA is whether the tumor's largest diameter is ≥4 cm [28]. Transsphenoidal surgery remains the first line treatment for giant NFAs; however, complication rates are higher and GTR rates are much lower [28]. Once again, the majority of neurosurgeons in our cohort preferred an endoscopic approach when treating giant NFAs (80%). Of note, all four of the respondents who indicated they preferred the microscopic approach have pituitary patient volumes exceeding 100 patients, and three of the four have been in practice for ≥15 years. This finding may simply reflect the fact that the endoscopic approach is a relatively newer surgical modality in comparison to the microscopic approach, and as such, more senior surgeons are more likely to employ the microscopic technique. It is reasonable to expect that surgeons with practice patterns that have repeatedly proven successful in the past, or that have been maintained over several years, may not change their preferred surgical approach to giant NFAs.
When asked about the aim of resection (<3 cm, no cavernous extensions), 80% (n = 16) of neurosurgeons indicated that GTR was always the surgical aim. Of those who indicated that GTR was not always the surgical aim, a majority were younger neurosurgeons with larger pituitary patient volumes (in practice for 5–10 years; ≥100 pituitary patients). This finding could simply reflect skepticism in younger neurosurgeons treating larger volumes of patients whose pituitary tumors are not always amenable to GTR, especially considering relevant literature reveals subtotal resection rates as high as approximately 30% [29]. Alternatively, more senior neurosurgeons may be more experienced and thus more confident in their ability to consistently perform GTRs. While the CNS guidelines do not specify whether GTR should always be the surgical aim of NFA resections, they do highlight that GTR leads to improved tumor control and improved symptom control [30,31]. Our survey cohort's responses largely fall in line with this recommendation.
4.3. Current postoperative management practices
4.3.1. Follow-up imaging
Presence of residual or recurrent disease must be assessed radiographically. Moreover, postoperative SRS or RT depend on precise neuroanatomical information, and thus follow-up imaging is vital to quality patient care. Relevant MRI studies highlight that delayed regression of sellar contents postoperatively is common, and that detection of residual tumor is greatly improved at 3–4 months post-operation [[32], [33], [34], [35]]. That being said, with more advanced and higher resolution MRI techniques, such as spoiled gradient recalled echo (SPGR) sequencing, immediate imaging within a week of surgery may compare with the 3–4 month standard in its ability to detect residual tumor [32,36]. Particulary in the setting of macroadenomas, accurate and immediate postoperative imaging may play an important role in determining the earliest optimal treatment available. The CNS guidelines recommend serial imaging in two scenarios: 1) absence of residual tumor, or 2) small intrasellar residual [23]. However, they do not outline whether an immediate postoperative MRI should be performed, or how soon after surgery the subsequent imaging study should be ordered. When these questions were posed to the survey cohort, there was an absence of consensus. This parallels the lack of consensus in the literature, with a routine immediate postoperative MRI being ordered by a third of total survey respondents [36]. Furthermore, respondents did not reach consensus on the timing of subsequent MRI studies in the postoperative period, with answers ranging from 1 month to over 6 months. The absence of consensus surrounding postoperative imaging identifies marked variability in postoperative care across neurosurgical centres.
4.3.2. Radiation therapy, Re-resection, and watchful waiting
Radiation therapy is widely considered the most effective adjuvant treatment option for preventing growth of recurrent or residual disease [14]. However, there are conflicting results in the literature as to whether prophylactic radiation therapy administered postoperatively is appropriate, with several studies promoting a wait-and-see approach for a majority of patients [24,30]. When presented a scenario of a patient with residual cavernous sinus extension, 61.5% of ROs would advise for earlier postoperative RT, as opposed to only 40% of neurosurgeons, highlighting a preference for more immediate intervention amongst ROs. This could reflect the baseline diversity of respondents' preferences regarding when to offer or refer patients for RT. This possible explanation is supported by the variation in preferences of when to offer RT in the extent of residual cavernous sinus extension to begin with – as answers were almost evenly split between “within 3 months,” “between 3-6 months,” and “after 6 months.” Once more, these findings indicate a lack of general agreement.
When asked which radiosurgical modality they would advocate for in the setting of recurrent disease (besides fractioned RT), ROs preferred LINAC, followed by Gamma Knife SRS, and Cyberknife SRS being advocated for to a lesser extent. While all of these modalities have proven effective in controlling tumor growth in the context of recurrent disease, LINAC and Gamma Knife SRS remain the most commonly used [23,37]. The survey results in the present study reflect the preference of LINAC and Gamma Knife SRS as revealed in the literature. Of note, geographical discrepancy in the proximity to centres that offer more comprehensive, multidisciplinary approaches to pituitary disease management, including the use of SRS, exist in certain parts of Canada. This may have influenced respondents' answers with regard to preference of not only different radiosurgical treatment modalities, but all treatment approaches in general.
When presented with scenarios of residual tumor in the sella with no cavernous involvement, survey respondents overwhelmingly advocated for a watchful waiting approach, independent of the tumor's histological markers of proliferation (Ki-67). Differences amongst respondents did emerge, however, when presented with scenarios detailing a growing residual tumor in the sella without any cavernous involvement or proximity to the optic chiasm. All three treatment options, re-resection, SRS, and watchful waiting, were advocated for by a subset of respondents, clearly outlining a lack of consensus in suggested treatment. When analyzing response rates by years of practice, focus of practice, and volume of NFA patients seen in practice, some interesting findings came to light. There was a slight preference for SRS amongst all physicians with fewer years of practice and amongst those who do not have a practice with a pituitary neoplasm focus. Moreover, surgeons who have a practice with a pituitary neoplasm focus preferred re-resection. There are several points to discuss here. To begin with, the apparent preference of SRS in younger physicians may be explained by a gradual trend towards becoming more conservative in treatment approaches as more experience is accumulated – a slight shift in preference from SRS and re-resection to watchful waiting over time. Furthermore, the preference of physicians without a pituitary neoplasm focus to advocate for SRS could be illustrative of the fact that they are not as comfortable performing/referring for re-resection in this setting due to a lack of expertise. Meanwhile, those surgeons who do have a pituitary neoplasm focus of practice understandably may be more confident in their ability to perform a re-resection.
Treatment preferences again diverged when respondents were presented with a patient with bilateral hemianopsia with confirmed slow growth of recurrence on the floor of the sella (initial postoperative MRI showing GTR) and Ki-67 of 1–2%. Watchful waiting and RT were strongly preferred over-resection, but, of note, of those who did advocate for re-resection, all were more senior physicians. This could perhaps indicate that more senior physicians with extensive experience are better able to determine which patients are good candidates for repeat surgeries and are then more comfortable suggesting aggressive treatments in those situations. Neurosurgeons in general preferred watchful waiting, whereas ROs preferred RT. This could be explained by simple bias amongst the different specialists. Afterall, watchful waiting approaches are regularly employed for many common intracranial tumors that neurosurgeons would be treating on a daily basis [38]. In the same patient scenario, but with a Ki-67 of >3%, there was a shift amongst surgeons (greatest amongst younger surgeons) from watchful waiting towards RT as the preferred treatment option. No such shift appeared amongst ROs, who preferred RT irrespective of tumor histology. This finding could indicate that neurosurgeons in particular take the Ki-67 index into consideration for clinical decision-making. The 2004 WHO guidelines had included Ki-67 > 3% as a criterion for atypical adenoma – an adenoma with more invasive behaviour [39]. The latest guidelines from WHO, however, have now removed the specific Ki-67% in the diagnostic criteria [40]. Despite the recent changes in WHO classification, this survey shows that it still plays a role in decision making in the management of NFA's.
5. Limitations
This online survey collected a diverse set of responses from both Neurosurgeons and ROs. The cohort ranged in expertise, from neuro-oncology focused to pituitary neoplasm focused, varied in years of practice and patient volume, and represented regions from all across Canada. This format allowed for a focused investigation into the topic of practice patterns in the treatment of recurrent and residual NFAs, a subject lacking clear consensus and worthy of exploration. Despite our best efforts to ensure a thorough and rigorous study, there are of course some limitations and shortcomings. The inclusion of more detailed pre- and post-operative imaging complementing the case questions' descriptions could have benefited respondents and influenced their decision-making. The sample size and response rate may also be a source of bias, leaving the study underpowered and consequently influencing the generalizability of the trends observed and our ability to draw over-arching conclusions. In the future, methods to increase response rates, in addition to formal email invitations, should be explored and employed to complement email's convenience and accessibility. Moreover, while registering a diverse range of responses was a primary goal of this survey, Radiation Oncologists and Neurosurgeons bring with them a different training background and, understandably, potentially different management preferences. Additionally, our data was not amenable to deducing intra-institutional results or trends amongst the responding physicians. These limitations must be taken into account when interpreting and comparing the two groups' responses. Furthermore, because all cohort members were Canadian physicians treating patients in the context of the Canadian healthcare system, it would be irresponsible to over-extend our findings to other jurisdictions without thoughtful consideration. Another shortcoming is that some of the treatment modalities, such as SRS, as well as more integrated multidisciplinary care involving close collaboration between neurosurgeons and radiation oncologists, may not be offered across all regions and healthcare centres in Canada; therefore, the variations in healthcare infrastructure and services between regions may impact responses as treatment choices may largely depend on resource availability.
6. Conclusion
It is evident from our survey results that there are both commonalities and variation in practice patterns with regard to the treatment of residual and recurrent NFAs. There is a lack of consensus amongst neurosurgeons and ROs across Canada concerning the timeline of scheduling postoperative follow-up imagining and offering RT in the setting of residual or recurrent disease. Furthermore, while, RT was preferred in the setting of growing residual disease, and both RT and watchful waiting were preferred in the setting of growth of recurrence post GTR, slight differences emerged when comparing between years of practice, pituitary neoplasm focus of practice, and pituitary patient volume. In particular, younger physicians preferred RT over re-resection and watchful waiting in settings of growing residual disease, while more senior physicians were more evenly split amongst treatment options. Neurosurgeons with a pituitary neoplasm focus, also appeared to be more aggressive in advocating for re-resection in this setting. Qualitative analysis of free text responses did not yield any unexpected themes. Taken together, this systematic survey demonstrates a need for standardization of management in some domains of postoperative care for patients with pituitary adenoma, while emphasizing individualized treatment options. An understanding of the current landscape of care is essential for furthering initiatives to endeavour to establish consensus evidence-based practices. Moving forward, future studies should consider not only targeted analyses aimed at better understanding intra-institutional and regional practice patterns, but also larger retrospective and prospective analyses of pituitary patient cohorts being treated in Canada and internationally, at select centres of expertise in Europe and North America. Expanding these findings to include the responses of international colleagues will be essential to improving relevance and generalizability.
Disclosures
The authors have no conflicts of interest to declare. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations of interest
None.
Footnotes
Abbreviations List: GTR, gross total resection; NFA(s), non-functioning pituitary adenoma(s); RO(s), radiation oncologist(s); RT, radiotherapy; SRS, stereotactic radiosurgery
Contributor Information
Graham Kasper, Email: graham.kasper@mail.utoronto.ca.
Nardin Samuel, Email: nardin.samuel@mail.utoronto.ca.
Ryan Alkins, Email: rda1@queensu.ca.
Osaama H. Khan, Email: osaama.h.khan@gmail.com.
References
- 1.Al-Dahmani K., Mohammad S., Imran F. Sellar masses: an epidemiological study. Can. J. Neurol. Sci. 2016;43(2):291–297. doi: 10.1017/cjn.2015.301. [DOI] [PubMed] [Google Scholar]
- 2.Daly A.F., Rixhon M., Adam C., Dempegioti A., Tichomirowa M.A., Beckers A. High prevalence of pituitary adenomas: a cross-sectional study in the province of Liege, Belgium. J. Clin. Endocrinol. Metabol. 2006;91(12):4769–4775. doi: 10.1210/jc.2006-1668. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez A., Karavitaki N., Wass J.A.H. Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK) Clin. Endocrinol. 2010;72(3):377–382. doi: 10.1111/j.1365-2265.2009.03667.x. [DOI] [PubMed] [Google Scholar]
- 4.Ntali G., Wass J.A. Epidemiology, clinical presentation and diagnosis of non-functioning pituitary adenomas. Pituitary. 2018;21(2):111–118. doi: 10.1111/j.1365-2265.2009.03667.x. [DOI] [PubMed] [Google Scholar]
- 5.Chanson P., Lecoq A.-L., Raverot G. Physiopathology, diagnosis, and treatment of nonfunctioning pituitary adenomas. In: Casanueva F.F., Ghigo E., editors. Hypothalamic-Pituitary Diseases. Springer International Publishing; Cham: 2017. pp. 1–37. [DOI] [Google Scholar]
- 6.Castinetti F., Dufour H., Gaillard S. 2015. Non-Functioning Pituitary Adenoma: When and How to Operate? What Pathologic Criteria for Typing? [DOI] [PubMed] [Google Scholar]
- 7.Ferrante E., Ferraroni M., Castrignanò T. Non-functioning pituitary adenoma database: a useful resource to improve the clinical management of pituitary tumors. Eur. J. Endocrinol. 2006;155(6):823–829. doi: 10.1530/eje.1.02298. [DOI] [PubMed] [Google Scholar]
- 8.Chanson P., Raverot G., Castinetti F., Cortet-Rudelli C., Galland F., Salenave S. 2015. Management of Clinically Non-Functioning Pituitary Adenoma. [DOI] [PubMed] [Google Scholar]
- 9.Ammirati M., Wei L., Ciric I. Short-term outcome of endoscopic versus microscopic pituitary adenoma surgery: a systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2013;84(8):843–849. doi: 10.1136/jnnp-2012-303194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bastos R.V.S., Silva C.M.D.M., Tagliarini J.V. Endoscopic versus microscopic transsphenoidal surgery in the treatment of pituitary tumors: systematic review and meta-analysis of randomized and non-randomized controlled trials. Arch. Endocrinol. Metabol. 2016;60(5):411–419. doi: 10.1590/2359-3997000000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortet-Rudelli C., Bonneville J.-F., Borson-Chazot F. 2015. Post-Surgical Management of Non-Functioning Pituitary Adenoma. [DOI] [PubMed] [Google Scholar]
- 12.Brochier S., Galland F., Kujas M. Factors predicting relapse of nonfunctioning pituitary macroadenomas after neurosurgery: a study of 142 patients. Eur. J. Endocrinol. 2010;163(2):193–200. doi: 10.1530/EJE-10-0255. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y., De Wang C., Su Z.P. Natural history of postoperative nonfunctioning pituitary adenomas: a systematic review and meta-analysis. Neuroendocrinology. 2012;96(4):333–342. doi: 10.1159/000339823. [DOI] [PubMed] [Google Scholar]
- 14.Losa M., Mortini P., Barzaghi R. Early results of surgery in patients with nonfunctioning pituitary adenoma and analysis of the risk of tumor recurrence. J. Neurosurg. 2008;108(3):525–532. doi: 10.3171/JNS/2008/108/3/0525. [DOI] [PubMed] [Google Scholar]
- 15.van den Bergh A.C.M., Van den Berg G., Schoorl M.A. Immediate postoperative radiotherapy in residual nonfunctioning pituitary adenoma: beneficial effect on local control without additional negative impact on pituitary function and life expectancy. Int. J. Radiat. Oncol. Biol. Phys. 2007;67(3):863–869. doi: 10.1016/j.ijrobp.2006.09.049. [DOI] [PubMed] [Google Scholar]
- 16.Park P., Chandler W.F., Barkan A.L. The role of radiation therapy after surgical resection of nonfunctional pituitary macroadenomas. Neurosurgery. 2004;55(1):100–107. doi: 10.1227/01.NEU.0000126885.71242.D7. [DOI] [PubMed] [Google Scholar]
- 17.Knosp E., Kitz K., Perneczky A. Proliferation activity in pituitary adenomas: measurement by monoclonal antibody Ki-67. Neurosurgery. 1989;25(6):927–930. doi: 10.1227/00006123-198912000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Landolt A.M., Shibata T., Kleihues P. Growth rate of human pituitary adenomas. J. Neurosurg. 1987;67(6):803–806. doi: 10.3171/jns.1987.67.6.0803. [DOI] [PubMed] [Google Scholar]
- 19.Grimm F., Maurus R., Beschorner R. Ki-67 labeling index and expression of p53 are non-predictive for invasiveness and tumor size in functional and nonfunctional pituitary adenomas. Acta Neurochir. 2019;161(6):1149–1156. doi: 10.1007/s00701-019-03879-4. [DOI] [PubMed] [Google Scholar]
- 20.Righi A., Agati P., Sisto A. A classification tree approach for pituitary adenomas. Hum. Pathol. 2012;43(10):1627–1637. doi: 10.1016/j.humpath.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Murad M.H., Fernández-Balsells M.M., Barwise A. Outcomes of surgical treatment for nonfunctioning pituitary adenomas: a systematic review and meta-analysis. Clin. Endocrinol. 2010;73(6):777–791. doi: 10.1111/j.1365-2265.2010.03875.x. [DOI] [PubMed] [Google Scholar]
- 22.Drummond J.B., Ribeiro-Oliveira A., Jr., Soares B.S. Endotext [Internet] MDText. com, Inc; 2018. Non-functioning pituitary adenomas. [Google Scholar]
- 23.Sheehan J., Lee C.-C., Bodach M., Tumialan L., Oyesiku N., Patil C., Litvack Z., Zada G., Aghi M. 2016. Management of Patients with Residual or Recurrent Nonfunctioning Pituitary Adenomas. [DOI] [PubMed] [Google Scholar]
- 24.Greenman Y., Ouaknine G., Veshchev I., Reider-Groswasser I.I., Segev Y., Stern N. Postoperative surveillance of clinically nonfunctioning pituitary macroadenomas: markers of tumour quiescence and regrowth. Clin. Endocrinol. 2003;58(6):763–769. doi: 10.1046/j.1365-2265.2003.01784.x. [DOI] [PubMed] [Google Scholar]
- 25.Boelaert K., Gittoes N.J. Radiotherapy for non-functioning pituitary adenomas. Eur. J. Endocrinol. 2001;144(6):569–575. doi: 10.1530/eje.0.1440569. [DOI] [PubMed] [Google Scholar]
- 26.Jankowski R., Auque J., Simon C., Marchal J.C., Hepner H., Wayoff M. How I do it: head and neck and plastic surgery: endoscopic pituitary tumor surgery. Laryngoscope. 1992;102(2):198–202. doi: 10.1288/00005537-199202000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Fries G., Perneczky A. Endoscope-assisted brain surgery: part 2—analysis of 380 procedures. Neurosurgery. 1998;42(2):226–231. doi: 10.1097/00006123-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Iglesias P., Berrocal V.R., Díez J.J. Giant pituitary adenoma: histological types, clinical features and therapeutic approaches. Endocrine. 2018;61(3):407–421. doi: 10.1007/s12020-018-1645-x. [DOI] [PubMed] [Google Scholar]
- 29.Dallapiazza R.F., Grober Y., Starke R.M., Laws E.R., Jr., Jane J.A., Jr. Long-term results of endonasal endoscopic transsphenoidal resection of nonfunctioning pituitary macroadenomas. Neurosurgery. 2014;76(1):42–53. doi: 10.1227/NEU.0000000000000563. [DOI] [PubMed] [Google Scholar]
- 30.Dekkers O.M., Pereira A.M., Roelfsema F. Observation alone after transsphenoidal surgery for nonfunctioning pituitary macroadenoma. J. Clin. Endocrinol. Metabol. 2006;91(5):1796–1801. doi: 10.1210/jc.2005-2552. [DOI] [PubMed] [Google Scholar]
- 31.Mortini P., Losa M., Barzaghi R., Boari N., Giovanelli M. Results of transsphenoidal surgery in a large series of patients with pituitary adenoma. Neurosurgery. 2005;56(6):1222–1233. doi: 10.1227/01.NEU.0000159647.64275.9D. [DOI] [PubMed] [Google Scholar]
- 32.Patel K.S., Dhawan S., Wang R., Carter B.S., Chen J.Y., Chen C.C. Post-operative imaging assessment of non-functioning pituitary adenomas. Acta Neurochir. 2018;160(5):1029–1039. doi: 10.1007/s00701-018-3491-2. [DOI] [PubMed] [Google Scholar]
- 33.Dina T.S., Feaster S.H., Laws E.R., Davis D.O. MR of the pituitary gland postsurgery: serial MR studies following transsphenoidal resection. Am. J. Neuroradiol. 1993;14(3):763–769. [PMC free article] [PubMed] [Google Scholar]
- 34.Kremer P., Forsting M., Ranaei G. Magnetic resonance imaging after transsphenoidal surgery of clinically non-functional pituitary macroadenomas and its impact on detecting residual adenoma. Acta Neurochir. 2002;144(5):433–443. doi: 10.1007/s007010200064. [DOI] [PubMed] [Google Scholar]
- 35.Rajaraman V., Schulder M. Postoperative MRI appearance after transsphenoidal pituitary tumor resection. Surg. Neurol. 1999;52(6):592–599. doi: 10.1016/S0090-3019(99)00157-3. [DOI] [PubMed] [Google Scholar]
- 36.López E.T., Molina M.V., Gregori J.C. Assessment of the extent of pituitary macroadenomas resection in immediate postoperative MRI. Radiología (English Edition). 2018;60(1):64–72. doi: 10.1016/j.rxeng.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Loeffler J.S., Shih H.A. Radiation therapy in the management of pituitary adenomas. J. Clin. Endocrinol. Metabol. 2011;96(7):1992–2003. doi: 10.1210/jc.2011-0251. [DOI] [PubMed] [Google Scholar]
- 38.Cruickshank G. Tumours of the brain. Surgery (Oxford). 2004;22(3):69–72. doi: 10.1383/surg.22.3.69.33531. [DOI] [Google Scholar]
- 39.KK Lloyd R.V., Young W.F., Jr., Farrell W.E., Asa S.L., Trouillas J. Pituitary tumors: Introduction. In: LR DeLellis R.A., Heitz P.U., Eng C., editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Endocrine Organs. 3 ed. IARC Press; Lyon: 2004. [Google Scholar]
- 40.Lloyd R.V., Osamura R.Y., Kloppel G., Rosai J. 4 ed. 2017. WHO Classification of Tumours of Endocrine Organs. Lyon. [Google Scholar]