Abstract
Background
Simvastatin, a drug for lowering serum cholesterol, has been shown to enhance bone regeneration, but few studies have qualitatively and quantitatively tested its effect when used topically in different animal models. This study aims to investigate topical administration of simvastatin as a bone regeneration inducer by testing its effect on bone formation in both long tubular bone and flat bone defect, and the mechanism involved.
Methods
Two animal models were used for testing the effect of simvastatin on entochondrostosis and intramembranous ossification respectively. Simvastatin of different dosages combined with poly lactic acid were implanted in extreme radial defects of 12 adult male New Zealand rabbits. Bone formation was monitored using x-ray and CT-scan and measured using x-ray scales, pixel values and spiral CT-scan for 16 weeks before being subject to histological and immunohistochemistry examination. The result was compared with that of autograft and blank control groups. Simvastatin with thrombin and fibrin sealant were implanted in calvarial defects of three Rhesus monkeys and monitored for 18 weeks. Bone formation was compared between the simvastatin and the blank control group using spiral CT-scan and histological examination.
Results
Both visual and quantitative measurements by x-ray and spiral CT-scan indicated significant bone formation in radial defects in all simvastatin groups and the autograft group whereas no bone formation was found in control groups. There was no significant difference in bone formation quantity between 100 mg simvastatin and autograft. Histological and immunohistochemistry examination indicated entochondrostosis in association with positive expression of BMP-2 and HIF-1 alpha. Spiral CT-scan and histological examination of calvarial defects of monkeys showed intramembranous ossification after simvastatin implantation. No change was found in the control group.
Conclusions
Topical administration of simvastatin induces entochondrostosis and intramembranous ossification by enhancing expression of BMP-2 and HIF-1 alpha. The effect of simvastatin on bone regeneration is comparable to autograft.
The translational potential of this article
Topical administration of simvastatin can repair bone defect in both long tubular bones and flat bones of rabbits and monkeys as effectively as autograft. Given that it is cheap, safe and already in clinical use, simvastatin might be considered as a bone regeneration inducer with great potential.
Keywords: Simvastatin, Bone regeneration, Bone substitute, Entochondrostosis, Intramembranous ossification
Introduction
Bone defect repairmen, as an important yet challenging clinical problem, has long been a topic of tremendous interest in the field of orthopedics. Although significant progress has been made with noticeable success, the issue remains largely unresolved. The repairmen involves a complex bone regeneration process requiring reconstruction of bone structure with adequate function. Conventional treatments including bone graft and various forms of synthetic bone substitutes are flawed either for the significant side effects or for being financially unsound. The latest approach is to utilize tissue-engineering technology whereby osteogenic stem cells and/or bone growth factors are amplified or loaded in extracellular matrices in vitro before they are implanted in vivo to repair bone defect. The stem cells and the growth factors act to induce bone formation and the matrices serves as a scaffold that gradually degrades along with bone ingrowth. This should endow the new bone with both adequate structure and function. Nonetheless, the use of stem cells is still in an experimental stage due to the lack of reliable technique in amplifying and guided differentiation [1]. There has been clinical success in the use of bone growth factors in combination with natural polymers scaffold as bone healing materials. But the rising safety concerns and the high cost significantly limits their clinical application [2].
In earlier study of osteoporosis, it was noticed that simvastatin, a drug for lowering serum cholesterol, had an effect to enhance bone formation by increasing expression of the bone morphogenetic protein-2 (BMP-2) gene in bone cells [3]. More recent clinical studies also associate simvastatin with increased bone density and reduced risk of osteoporotic fracture [4,5]. Because it is safe, cheap and clinically available, simvastatin has been drawing increasing attention as a promising new bone regeneration inducer. However, the exact mechanism and effectiveness of simvastatin on bone formation as well as the optimal dose remains unclear. This study is prompted to investigate these issues in search for a better bone regeneration inducer.
Materials and methods
Ethical statement
All experiments performed in this study were approved by the Peking University Third Hospital Animal Experiment Ethics Committee, Permit No. 2009–0006 (2). Animal care complied with the guideline of animal experiments of the Peking University Third Hospital on the care and use of laboratory animals.
Materials
There were two sets of bone defect animal models tested in this study. The first model involved 12 adult male New Zealand rabbits weighed 2.3–2.8 kg. The second model involved three Rhesus monkeys, aged 5–6 years and weighed 7.5–9.5 kg.
General procedures
In the first model, a 22 mm long bone defect was surgically inflicted on the radius of both forearms of each of 12 New Zealand rabbits (Fig. 1A and B) that were randomly divided into four groups. Note that, each time the periosteum of ulna was also removed along with the radius in the making of the defect. In three of the four groups, the radial bone defect was filled with compounds made up of simvastatin (Merck & Co Inc, USA) of three different dosages and poly lactic acid (PLA, molecular weight 4 × 106, Shandong Medical Instrument Research Center, China) scaffold on one side (the implant side) and PLA scaffold alone on the other (the control side). For the implant side, the compound was made under room temperature by mixing simvastatin (50 mg, 100 mg and 200 mg respectively) with 250 ml PLA and 2 ml acetone solution (Sinopharm Chemical Reagent Beijing Co. Ltd, China), which was subsequently freeze-dried and molded to form a 4 mm thick and 24 mm long cylindrical paste (Fig. 1C). It was sterilized by 60Co radiation each time before implantation. For the control side, the compound was made the same way except that no simvastatin was added to the paste. For the remaining group, removed radial bone was minced and re-implanted to the defect as autogenous bone graft on the implant side while no intervention was applied on the control side. Detailed information concerning each group was given in Table 1. Repairing progress of the radial bone defects was monitored radiologically at 8 and 16 weeks after surgery and histologically as well as immunohistochemically when all rabbits were eventually sacrificed at the 16th week. In the second model, two full-thickness round defects of 15 mm diameter were made on both sides of the sagittal suture of each of three Rhesus monkeys (Fig. 1D–F). A compound of 50 mg simvastatin, 0.4 ml thrombin and 0.4 ml fibrin sealant (FS) was implanted on the left hand side (the implant side) while the same compound without simvastatin was implanted on the right hand side (the control side). CT scans of the involved calvarias were taken at 2, 4, 6, 12 and 18 weeks post-operation before all subjects were sacrificed for histological examination except for one that was sacrificed 4 weeks into the experiment and subject to histological examination.
Fig. 1.
The New Zealand rabbit radial defect and the Rhesus monkey calvarial defect. A-C. The New Zealand rabbit model. D-F. The Rhesus monkey model. A. Surgically inflicted radial bone defect (without implantation). B. Radial bone defect with simvastatin and PLA implantation. C. A Solid block made of simvastatin and PLA. D. Surgically inflicted bone defects on either side of the sagittal suture (without implantation). E. Each defect was made 15 mm in diameter. F. A 4 mm thick pancake shape paste made of simvastatin/thrombin/FS and thrombin/FS in place of the defect.
Table 1.
Groups with different interventions in rabbits radial defect model and Rhesus monkey calvarial defect model.
| Animal model | Groups | Implant side (left) | Control side (right) |
|---|---|---|---|
| New Zealand rabbit model | SIM50mg | simvastatin 50 mg + PLA 250 mg+2 ml acetone | PLA 250 mg+2 ml acetone |
| SIM100mg | simvastatin 100 mg + PLA 250 mg+2 ml acetone | PLA 250 mg+2 ml acetone | |
| SIM200mg | simvastatin 200 mg + PLA 250 mg+2 ml acetone | PLA 250 mg+2 ml acetone | |
| Autograft | autogenous bone | void | |
| Rhesus monkey model | SIM50mg | simvastatin 50 mg + thrombin 0.4 ml + fibrin sealant | 0.4 ml thrombin+0.4 ml fibrin sealant |
Measurements
The rabbit model
Radiological measurements
Bone ingrowth in each radial bone defect was monitored at 8 and 16 weeks post-operation using a digital x-ray machine. For each bone defect, the x-ray pixel values of three identical spots chosen at fixed co-ordinates were measured and scaled using Taria’s x-ray scoring method to quantify bone healing in each circumstance [6]. Spiral CT scans (120 kV, 256 mA, thickness 2.0 mm, GE, USA) were taken at 16 weeks post-operation of both forearms of each sacrificed rabbit to reconstruct a 3D image of the intact radial bones using surface shade display (SSD) method. The area of opaque region (representing new bone formation) against the whole area of bone defect was calculated using computer software (GE AW4.2 software) and compared as the ratio of new bone formation in each experimental group. The same method had been described by Nakayama et al. [7]. One rabbit was randomly selected from the implant groups and examined using high-resolution plain film (Hitachi, Japan) and Micro CT scan (Skyscan, Belgium) to inspect structural changes on the surface and in the bone marrow cavity of the bone defects.
Histological inspection
The section of bone defect in each radium was severed to obtain 5 μm-thick slices and processed with hematoxylin-eosin (HE) and toluidine blue staining before being inspected under optical microscopy.
Immunohistochemistry examination
The same slices were immersed with BMP-2 and Hypoxia-inducible factors (HIF)-1alpha antibodies (Santa Cruz Biotechnology Inc, USA and Abcam plc, USA) and dyed to detect expression of BMP-2 and HIF-1alpha using Polymer HRP Detection System for goat primary antibody and Mouse SP Kit (Santa Cruz Biotechnology Inc, USA).
The monkey model
Radiological measurements
Calvarias of two of the three monkeys were scanned using Spiral CT at 2, 4, 6, 12 and 18 weeks post-operation to reconstruct a 3D image of the entire calvarial bone using surface shade display (SSD) method. The remaining monkey was sacrificed at 4 weeks for histological inspection.
Histological inspection
Bone defect sections obtained from the sacrificed monkey were severed to obtain 5 μm-thick slices and processed with hematoxylin-eosin (HE) and toluidine blue staining before being inspected under optical microscopy.
Statistical methods
All statistical tests were carried out using computer software (SPSS 13.0). Results obtained from the implant sides and the control sides were compared within each group using Student t-test and between different groups using One-way analysis and LSD test. Difference was deemed significant when p value was less than 0.05.
Results
The rabbit model
Radiological measurements
X-ray image
Radiographic images demonstrated significant bone healing at 8 weeks post-operation and complete bone union at 16 weeks on the implant sides in all groups. Fig. 2 & Table 2 show callus formation in the defects changed from blurry into continuous and fracture ends changed from blurry to union. By contrast, neither identifiable callus formation nor signs of union at the fracture ends were found on all the control sides for the same period.
Fig. 2.
Radiographs of radial defects at 8 and 16 weeks after simvastatin implantation. 1a-1h. Radiographs at 8 weeks. 1a. SIM50mg implant side. 1 b. SIM50mg control side. 1c. SIM100mg implant side. 1 d. SIM100mg control side. 1e. SIM200mg implant side. 1f. SIM200mg control side. 1 g. Autograft implant side. 1 h. Autograft control side. 2a-2h. Radiographs at 16 weeks. 2a. SIM50mg implant side. 2 b. SIM50mg control side. 2c. SIM100mg implant side. 2 d. SIM100mg control side. 2e. SIM200mg implant side. 2f. SIM200mg control side. 2 g. Autograft implant side. 2 h. Autograft control side.
Table 2.
Radiographic images of rabbit radial defect model.
| Groups | 8 weeks |
16 weeks |
||||||
|---|---|---|---|---|---|---|---|---|
| callus formation |
fracture ends |
callus formation |
fracture ends |
|||||
| IS∗ | CS∗ | IS∗ | CS∗ | IS∗ | CS∗ | IS∗ | CS∗ | |
| SIM50mg | hazy | none or few | blurry | clear | continuous | none or few | union | Sclerotic |
| SIM100mg | even | none or few | blurry | clear | continuous | none or few | union | Sclerotic |
| SIM200mg | continuous | none or few | continuous | clear | continuous | none or few | union | sclerotic |
| Autograft | abundant | none or few | blurry | clear | continuous | none or few | union | sclerotic |
X-ray scores
For all experimental groups, the x-ray scores were significantly (p < 0.05) greater on the implant sides than the control sides at both 8 and 16 weeks of the experiment. At 8 weeks, the x-ray scores on the implant sides were significantly higher in the SIM200mg and the autograft group than that in the SIM50mg and the SIM100mg group, although there was no significant difference between the former and latter two groups respectively. At 16 weeks, SIM100mg and autograft group were associated with significantly (p < 0.05) greater x-ray scores than the rest two groups, without a significant difference between themselves (Table 3).
Table 3.
Comparison of x-ray scores and pixel values measured from the bone defects at 8 and 16 weeks post-op among all experimental groups.
| Items | Groups | 8 weeks post-op |
16weeks post-op |
||
|---|---|---|---|---|---|
| IS∗ | CS∗ | IS∗ | CS∗ | ||
| x-ray scores | SIM50mg | 7.17 ± 0.75∗△▲ | 5.67 ± 0.82 | 11.83 ± 1.47∗#▲ | 8.67 ± 1.03 |
| SIM100mg | 8.00 ± 0.63∗△▲ | 6.33 ± 1.03 | 17.00 ± 0.89∗△ | 8.50 ± 1.05 | |
| SIM200mg | 9.67 ± 1.03∗# | 4.83 ± 0.98 | 9.67 ± 1.21∗#▲ | 5.67 ± 1.37 | |
| autograft | 9.50 ± 1.05∗# | 5.17 ± 1.17 | 18.17 ± 1.47∗△ | 5.00 ± 0.89 | |
| Pixel values | SIM50mg | 1.42 ± 0.04∗ | 0.83 ± 0.04 | 3.44 ± 0.09∗ | 2.41 ± 0.03 |
| SIM100mg | 1.82 ± 0.12∗ | 1.37 ± 0.06 | 3.68 ± 0.05∗ | 2.59 ± 0.08 | |
| SIM200mg | 1.47 ± 0.93∗ | 1.09 ± 0.05 | 3.49 ± 0.02∗ | 2.48 ± 0.08 | |
| Autograft | 1.43 ± 0.13∗ | 1.10 ± 0.11 | 3.45 ± 0.03∗ | 2.42 ± 0.07 | |
∗ IS=Implant Side; CS=Control Side ∗p < 0.05 as compared with CS; #p < 0.05 as compared with SIM100mg group; △p < 0.05as compared with SIM200mg group; ▲p < 0.05 as compared with autograft group
X-ray pixel values
In all groups, implant sides were associated with significantly greater x-ray pixel values than control sides at both 8 and 16 weeks post-operation. There was no significant difference among implant groups and control groups respectively (Table 3).
Spiral CT scans
Bone union or significant reduction in bone defect was seen on the implant sides whereas non-union and insignificant bone ingrowth was seen on the control sides of all SIM groups and the autograft group (Fig. 3A–D). The ratio of new bone formation was significantly higher on the implant side than the control side in all groups with the SIM 100 mg and autograft group being associated with significantly higher rates than the rest (Fig. 4).
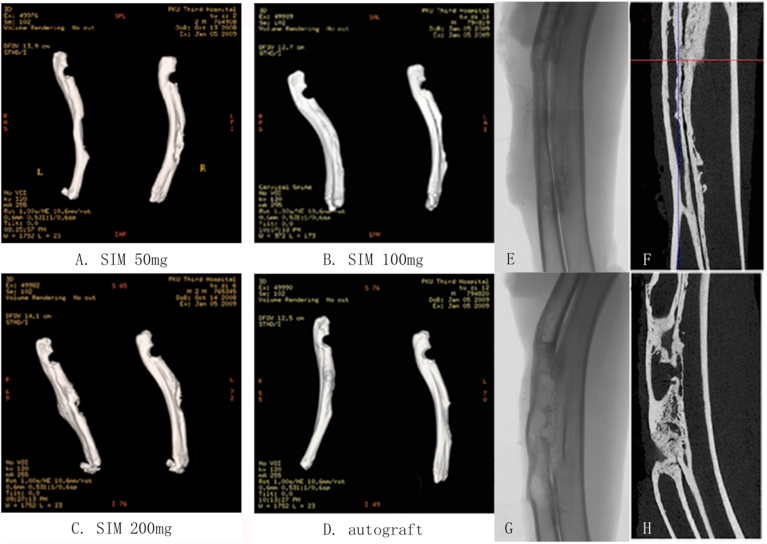
Fig. 3.
Spiral CT scans of radial defects on the implant and the control sides in all experimental groups at 16 weeks after simvastatin implantation. (A-D). Spiral CT scans (Right: Implant side; Left: Control side). E&F. High-resolution plain film (E) and Micro CT scan (F) of the defect on the implant side in the SIM100mg group. G&H. High-resolution plain film (G) and Micro CT scan (H) of the defect on the control side in the SIM100mg group.
Fig. 4.
Rates of new bone formation in radial defects measured from spiral CT scans at 16 weeks after simvastatin implantation. Note that there were 6 radial bone defects from 3 different rabbits tested in each group in the plot. ∗significant difference between Implant and Control side (p < 0.05); #significant difference between SIM 100 mg, autograft group and SIM 50 mg, SIM 200 mg group (p < 0.05). 120 kV 256 mA thin-bed layer thickness 0.625 mm.
High-resolution plain film and micro CT scans
Both forearms of one rabbit in the SIM 100 mg group (Fig. 3B) were scanned. The images of the implant side showed the defect was replaced by continuous new bone formation with union at both fracture ends, complete cortical bone formation on the outside and rechanellization of bone marrow cavity on the inside (Fig. 3E&F). On the control side, neither continuous bone formation nor rechanellization of bone marrow was seen (Fig. G&H).
Histological inspection
HE staining
Radial bone defects were replaced by new bone formation with complete cortical bone and closed medullary cavities on the implant sides in all groups whereas no bone formation but hyperplasia was found on the control sides (Fig. 5A).
Fig. 5.
HE and Toluidine blue staining images of radial defects under optical microscope at 16 weeks after simvastatin implantation. A. HE staining ( × 10) of the implant side (left column) and the control side (right column). U: ulna; R: radius. B. Toluidine blue staining ( × 100) of the implant side (left) and the control side (right).
Toluidine blue staining
Visual inspection under microscopy showed transformation of mesenchymal cells to chondrocytes and eventually to bone tissues in most area of the defect on the implant side (SIM 100 mg). There were a few residual chondrocytes in sight (Fig. 5B). On the control side, large portion of deep blue staining area indicates lack of transformation from chondrocytes to bone tissues.
Immunohistochemistry examination
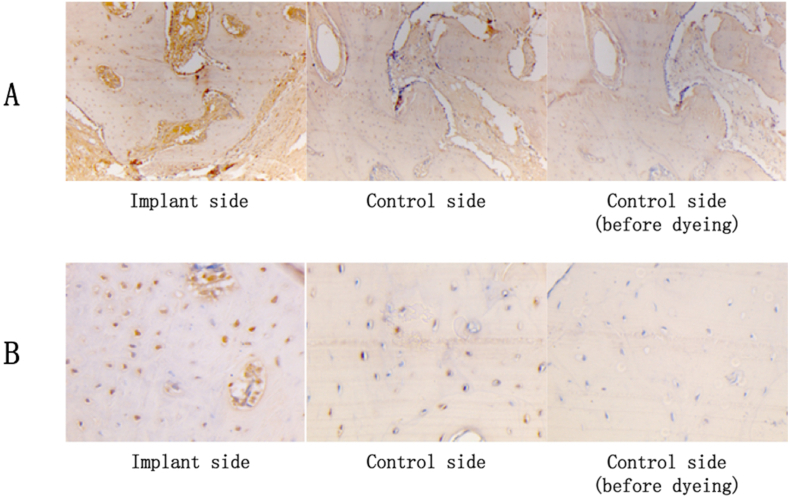
Expression of BMP-2 (Fig. 6A) and HIF-1 alpha (Fig. B) was positive under optical microscope on the implant side at 16 weeks after implantation.
Fig. 6.
Expression of BMP-2 in cytoplasm and HIF-1 alpha in cell nucleus under optical microscope at 16 weeks after simvastatin implantation. A. Expression of BMP-2 in cytoplasm ( × 100). B. Expression of HIF-1 alpha in cell nucleus ( × 400).
The monkey model
Spiral CT scans
Traces of high-density bone formation started to emerge in calvarial defects of all three monkeys since 6 weeks into the study and gradually developed on the implant side until the end of the experiment (18 weeks) when the defect was significantly reduced. By contrast, no change was visible on the control side all through the experiment (Fig. 7).
Fig. 7.
Spiral CT scans of calvarial defects on the implant and the control sides at 6 and 18 weeks after simvastatin implantation. A-C. 6 weeks post-op. A. Coronal plane showing the implant side (left) and the control side (right). B&C. 3D-reconstruction images showing the implant side (left) and the control side (right). D-F. 18 weeks post-op. D. Coronal plane showing the implant side (left) and the control side (right). E&F. 3D-reconstruction image showing the implant side (left) and the control side (right).
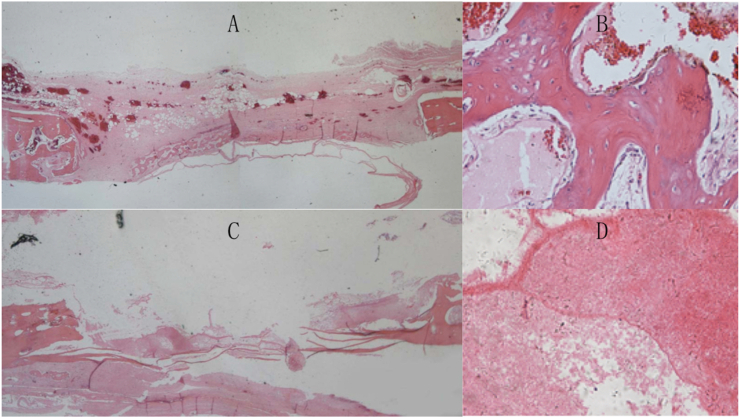
HE staining
HE staining of the calvarial bone defect at 4 weeks after simvastatin implantation showed islands of newly formed woven bone spreading in a matrix of mesenchymal cells in absence of chondrocytes on the implant side (Fig. 8A and B). On the control side, however, there was no bone formation but inflammatory and fibrous tissue (Fig. 8C and D).
Fig. 8.
HE staining images of calvarial defects under optical microscope at 4 weeks after simvastatin implantation. A-B. The implant side. A. Woven bone formation can be seen spreading across the defect ( × 10). B. Magnified image showing woven bone formation ( × 200). C-D. The control side. No bone formation but inflammatory tissue can be seen ( × 10, × 200).
Discussion
This study is designed to explore effects of simvastatin on different mechanisms of bone development. Rabbit radial defect and monkey calvarial defect model were used in this study to investigate the effect of simvastatin on both entochondrostosis of long tubular bones and intramembranous ossification of flat bones respectively. Radial defect of rabbits is considered the standard model in study of bone formation where a 20 mm long bone defect is deemed the upper limit for self-repairmen [8]. We used 22 mm long bone defects in excess of the maximal limit for self-repairmen. The periosteum of the ulna was also removed to eliminate interference from bone formation originated from the ulna. Design of the self-controlled study also helps to minimize the potential interference from the ulna. Monkeys are considered the most genetically close to human.
This study compared the result of the experiment and the control group on two symmetrical sides of the same subject, which eliminated influence of individual difference. In the rabbit radial model, X-ray images showed union of bone defect in all simvastatin-implanted groups in comparison with absence of bone formation in all control groups. Micro CT scans showed newly formed bone with complete cortex and rechanellization of bone marrow cavity. These suggested that simvastatin acted to induce natural bone healing while being released and biodegraded in the bone defect. X-ray scores, X-ray pixel values and spiral CT scans measurements are widely accepted standard methods for quantitative assessment of osteogenesis, which confirmed the visual finding by showing a significantly higher density and ratio of new bone formation on the implant sides than that on the control sides [9]. These quantitative radiological findings were further verified histologically by HE and Toluidine blue staining. Toluidine blue is known to have an affinity for proteoglycans in the chondrocytes [10]. Changes in the staining demonstrated transformation of mesenchymal cells to chondrocytes and to bone tissues. Residual chondrocytes were also evidences of entochondrostosis.
The function of simvastatin to enhance bone formation and reduce bone loss was initially demonstrated after gastric administration and subcutaneous tissue injection in rats [11,12]. Seto et al. and Lee et al. later reported significant repairmen of bone defects in the alveolar bone and the mandibles of rats after topical application of simvastatin [13,14]. More recent studies succeed to test the effect of local administration of simvastatin on bone regeneration using bigger animal models. Chalisserry et al. created 24 5 × 8 mm bone defects on the lateral aspect of the femoral condyle of 12 adult New Zealand white rabbits to compare the result of bone formation induced by Nano-Hydroxyapatite (nHA) particles alone and nHA with simvastatin. The condyles were retrieved after 8 weeks and analyzed using micro CT and histology. Their results showed significantly higher bone mineral density and bone volume in the simvastatin loaded nHA group compared with the nHA alone group [15]. Zhang et al. reported the same result when compared the effect of simvastatin loaded Polylactic Acid-Polyglycolic Acid Copolymer (PLGA-CPC) with that of PLGA-CPC alone on rabbit femoral condyle defects [16]. These are concordant with our results even though we used maximum size radial bone defects that represent the most extreme model of entochondrostosis in long tubular bones. Positive effect of simvastatin on bone formation in calvarias has been reported using different carriers in rats and rabbits [[17], [18], [19]]. But there is a lack of similar study using nonhuman primates. Yasuhiko et al. implanted a basic fibroblast growth factor (bFGF) loaded biodegradable hydrogel to skull defects (6 mm in diameter) in a monkey [20]. The defects were replaced by new bone formation in 21 weeks after implantation. We used monkeys calvarial model because monkeys were genetically closer to human than any other animals and the calvarial bone was the site to choose for investigation of intramembranous ossification in flat bones. In our study, Spiral CT scans showed significantly reduced bone defects at 18 weeks after implantation on the implant side and no change on the control side. This was verified by HE staining, which showed widespread newly formed woven bone in a matrix of mesenchymal cells on the implant side. The absence of chondrocytes indicates origin of bone formation from transformation of mesenchymal cells, i.e. intramembranous ossification.
Our results of immunohistochemistry examination indicated positive expression of BMP-2 and HIF-1 alpha after simvastatin implantation. HIFs are oxygen-dependent transcriptional activators that play crucial roles in angiogenesis, erythropoiesis, energy metabolism, and cell fate decisions. HIFs and their target gene, vascular endothelial growth factor (VEGF), are believed to promote bone regeneration by stimulating new blood vessels formation in the bone [[21], [22], [23]]. BMP-2 is a known growth factor that induces bone regeneration. Numerous studies suggest that simvastatin can promote osteoblast viability and differentiation by enhancing expression of BMP-2 [3,18,24]. The potential mechanism is that simvastatin inhibits the secretion of mevalonic acid, which per se is an inhibitor of BMP-2, by blocking the action of the HMG-CoA reductase [25]. Moreover, inhibition of mevalonic acid also impedes activation of osteoclast [26].
Simvastatin presents a reduced risk in comparison with growth factors or gene therapy, but undesired high local congregation may still pose a risk of inflammatory response [27]. Therefore, it requires a carrier that can slow the release process allowing simvastatin to function for an extended period while limiting the inflammatory process. PLA is known as a biocompatible and biodegradable polymer with a three dimensional porous structure that facilitates bone ingrowth [28]. Previous studies of PLA and simvastatin compound proved that release of simvastatin could be controlled by the slow degradation of PLA due to the strong chemical interaction between the two [[29], [30], [31]]. Moreover, mechanical property of the scaffold was not influenced during the release process.
Although simvastatin has been investigated as a bone regeneration inducer in multiple forms of administration, there has no general consent on the optimal dose to date. The dosage used in this study was determined based on relevant reviews in the literature [32]. In this study, x-ray scores of the SIM100mg and autograft were significant higher than the rest groups whereas no difference was found between each other. The same result was confirmed by spiral CT scans in calculation of the ratio of new bone formation. Both quantitatively verified the effect of simvastatin. Our results of significant association between higher concentration of simvastatin and higher X-ray scores can be explained as previous studies indicated a positive correlation between the dose of simvastatin and expression of BMP-2 [3,33]. However, the finding that 100 mg simvastatin group had significantly higher X-ray scores and new bone formation rate than 200 mg simvastatin group at 16 weeks after implantation seemed to indicate an optimal dose for topical administration. In fact, we found in preparation of SIM200mg implant that its porosity was significantly lower than that of SIM50mg and SIM100mg implant. Low porosity could impede bone growth by limiting ingrowth of blood vessels and mesenchymal cells. Other authors suggested that time also had significantly influences on the effect of simvastatin [32].
The weakness of this study is that it is an animal experiment. But it is the first time that simvastatin is tested in non-human primate. In addition, the limited number of samples involved in this study limits the statistical power of the result and makes it erring on the side of caution. The encouraging results of ours favor further clinical tests for topical administration of simvastatin as a bone regeneration inducer.
Conclusions
Topical administration of simvastatin can promote bone regeneration in both forms of entochondrostosis and intramembranous ossification with a capacity comparable to autograft. It also acts in different species of mammals, including non-human primates. Its effect on entochondrostosis is associated with increased expression of BMP-2 and HIF-1 alpha. Therefore, simvastatin is a promising candidate for the best bone regeneration inducer.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant Number 81672133, 81874010) and the National High Technology Research and Development Program of China (863 Program, grant number 2015AA020304).
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2020.11.009.
Contributor Information
Lei Dang, Email: mph595@126.com.
Chunli Song, Email: schl@bjmu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Walmsley G.G., Ransom R.C., Zielins E.R., Leavitt T., Flacco J.S., Hu M.S. Stem cells in bone regeneration. Stem cell reviews and reports. 2016;12(5):524–529. doi: 10.1007/s12015-016-9665-5. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dumic-Cule I., Peric M., Kucko L., Grgurevic L., Pecina M., Vukicevic S. Bone morphogenetic proteins in fracture repair. Int Orthop. 2018;42(11):2619–2626. doi: 10.1007/s00264-018-4153-y. [eng] [DOI] [PubMed] [Google Scholar]
- 3.Mundy G., Garrett R., Harris S., Chan J., Chen D., Rossini G. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286(5446):1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 4.An T., Hao J., Sun S., Li R., Yang M., Cheng G. Efficacy of statins for osteoporosis: a systematic review and meta-analysis. Osteoporos Int : a J. Establish. Res. Cooper. Betn. Eur. Found. Osteopor. Natl. Osteopor. Found. USA. 2017;28(1):47–57. doi: 10.1007/s00198-016-3844-8. [eng] [DOI] [PubMed] [Google Scholar]
- 5.Chiadika S.M., Shobayo F.O., Naqvi S.H., Saraykar S.S., Ambrose C.G., Rianon N.J. Lower femoral neck bone mineral density (BMD) in elderly women not on statins. Women Health. 2019;59(8):845–853. doi: 10.1080/03630242.2019.1567646. [eng] [DOI] [PubMed] [Google Scholar]
- 6.Taira H., Moreno J., Ripalda P., Forriol F. Radiological and histological analysis of cortical allografts: an experimental study in sheep femora. Arch Orthop Trauma Surg. 2004;124(5):320–325. doi: 10.1007/s00402-004-0653-x. [DOI] [PubMed] [Google Scholar]
- 7.Nakayama Y., Li Q., Katsuragawa S., Ikeda R., Hiai Y., Awai K. Automated hepatic volumetry for living related liver transplantation at multisection CT. Radiology. 2006;240(3):743–748. doi: 10.1148/radiol.2403050850. [eng] [DOI] [PubMed] [Google Scholar]
- 8.Saito A., Suzuki Y., Kitamura M., Ogata S., Yoshihara Y., Masuda S. Repair of 20-mm long rabbit radial bone defects using BMP-derived peptide combined with an alpha-tricalcium phosphate scaffold. J Biomed Mater Res. 2006;77(4):700–706. doi: 10.1002/jbm.a.30662. [eng] [DOI] [PubMed] [Google Scholar]
- 9.Hazra S., Song H.R., Biswal S., Lee S.H., Lee S.H., Jang K.M. Quantitative assessment of mineralization in distraction osteogenesis. Skeletal Radiol. 2008;37(9):843–847. doi: 10.1007/s00256-008-0495-7. [DOI] [PubMed] [Google Scholar]
- 10.Vidal B.C., Mello M.L.S. Toluidine blue staining for cell and tissue biology applications. Acta Histochem. 2019;121(2):101–112. doi: 10.1016/j.acthis.2018.11.005. [eng] [DOI] [PubMed] [Google Scholar]
- 11.Serin-Kilicoglu S., Erdemli E. New addition to the statin’s effect. J Trauma. 2007;63(1):187–191. doi: 10.1097/01.TA.0000240974.07999.bc. [DOI] [PubMed] [Google Scholar]
- 12.Oxlund H., Dalstra M., Andreassen T.T. Statin given perorally to adult rats increases cancellous bone mass and compressive strength. Calcif Tissue Int. 2001;69(5):299–304. doi: 10.1007/s00223-001-2027-5. [DOI] [PubMed] [Google Scholar]
- 13.Seto H., Ohba H., Tokunaga K., Hama H., Horibe M., Nagata T. Topical administration of simvastatin recovers alveolar bone loss in rats. J Periodontal Res. 2008;43(3):261–267. doi: 10.1111/j.1600-0765.2007.01024.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee Y., Schmid M.J., Marx D.B., Beatty M.W., Cullen D.M., Collins M.E. The effect of local simvastatin delivery strategies on mandibular bone formation in vivo. Biomaterials. 2008;29(12):1940–1949. doi: 10.1016/j.biomaterials.2007.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalisserry E.P., Nam S.Y., Anil S. Simvastatin loaded Nano hydroxyapatite in bone regeneration: a study in the rabbit femoral condyle. Curr Drug Deliv. 2019;16(6):530–537. doi: 10.2174/1567201816666190610164303. [eng] [DOI] [PubMed] [Google Scholar]
- 16.Zhang H.X., Xiao G.Y., Wang X., Dong Z.G., Ma Z.Y., Li L. Biocompatibility and osteogenesis of calcium phosphate composite scaffolds containing simvastatin-loaded PLGA microspheres for bone tissue engineering. J Biomed Mater Res. 2015;103(10):3250–3258. doi: 10.1002/jbm.a.35463. [eng] [DOI] [PubMed] [Google Scholar]
- 17.Venkatesan N., Liyanage A.D.T., Castro-Nunez J., Asafo-Adjei T., Cunningham L.L., Dziubla T.D. Biodegradable polymerized simvastatin stimulates bone formation. Acta Biomater. 2019;93:192–199. doi: 10.1016/j.actbio.2019.04.059. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J., Wang H., Shi J., Wang Y., Lai K., Yang X. Combination of simvastatin, calcium silicate/gypsum, and gelatin and bone regeneration in rabbit calvarial defects. Sci Rep. 2016;6:23422. doi: 10.1038/srep23422. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C.Z., Wang Y.H., Lin C.W., Lee T.C., Fu Y.C., Ho M.L. Combination of a bioceramic scaffold and simvastatin nanoparticles as a synthetic alternative to autologous bone grafting. Int J Mol Sci. 2018;19(12) doi: 10.3390/ijms19124099. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabata Y., Yamada K., Hong L., Miyamoto S., Hashimoto N., Ikada Y. Skull bone regeneration in primates in response to basic fibroblast growth factor. J Neurosurg. 1999;91(5):851–856. doi: 10.3171/jns.1999.91.5.0851. [eng] [DOI] [PubMed] [Google Scholar]
- 21.Fan L., Li J., Yu Z., Dang X., Wang K. The hypoxia-inducible factor pathway, prolyl hydroxylase domain protein inhibitors, and their roles in bone repair and regeneration. BioMed Res Int. 2014;2014:239356. doi: 10.1155/2014/239356. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weimin P., Zheng C., Shuaijun J., Dan L., Jianchang Y., Yue H. Synergistic enhancement of bone regeneration by LMP-1 and HIF-1alpha delivered by adipose derived stem cells. Biotechnol Lett. 2016;38(3):377–384. doi: 10.1007/s10529-015-1988-9. [eng] [DOI] [PubMed] [Google Scholar]
- 23.Jiang X., Zhang Y., Fan X., Deng X., Zhu Y., Li F. The effects of hypoxia-inducible factor (HIF)-1alpha protein on bone regeneration during distraction osteogenesis: an animal study. Int J Oral Maxillofac Surg. 2016;45(2):267–272. doi: 10.1016/j.ijom.2015.09.021. [eng] [DOI] [PubMed] [Google Scholar]
- 24.Chen P.Y., Sun J.S., Tsuang Y.H., Chen M.H., Weng P.W., Lin F.H. Simvastatin promotes osteoblast viability and differentiation via Ras/Smad/Erk/BMP-2 signaling pathway. Nutr Res (NY) 2010;30(3):191–199. doi: 10.1016/j.nutres.2010.03.004. [eng] [DOI] [PubMed] [Google Scholar]
- 25.Montagnani A., Gonnelli S., Cepollaro C., Pacini S., Campagna M.S., Franci M.B. Effect of simvastatin treatment on bone mineral density and bone turnover in hypercholesterolemic postmenopausal women: a 1-year longitudinal study. Bone. 2003;32(4):427–433. doi: 10.1016/s8756-3282(03)00034-6. [eng] [DOI] [PubMed] [Google Scholar]
- 26.Luckman S.P., Hughes D.E., Coxon F.P., Graham R., Russell G., Rogers M.J. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res : Off. J. Am. Soc. Bone and Miner. Res. 1998;13(4):581–589. doi: 10.1359/jbmr.1998.13.4.581. [eng] [DOI] [PubMed] [Google Scholar]
- 27.Encarnacao I.C., Xavier C.C., Bobinski F., dos Santos A.R., Correa M., de Freitas S.F. Analysis of bone repair and inflammatory process caused by simvastatin combined with PLGA+HA+betaTCP scaffold. Implant Dent. 2016;25(1):140–148. doi: 10.1097/ID.0000000000000359. [eng] [DOI] [PubMed] [Google Scholar]
- 28.Kotsar A., Nieminen R., Isotalo T., Mikkonen J., Uurto I., Kellomaki M. Biocompatibility of new drug-eluting biodegradable urethral stent materials. Urology. 2010;75(1):229–234. doi: 10.1016/j.urology.2009.03.016. [eng] [DOI] [PubMed] [Google Scholar]
- 29.Hu Z., Liu Y., Yuan W., Wu F., Su J., Jin T. Effect of bases with different solubility on the release behavior of risperidone loaded PLGA microspheres. Colloids Surf B Biointerfaces. 2011;86(1):206–211. doi: 10.1016/j.colsurfb.2011.03.043. [eng] [DOI] [PubMed] [Google Scholar]
- 30.Montazerolghaem M., Rasmusson A., Melhus H., Engqvist H., Karlsson Ott M. Simvastatin-doped pre-mixed calcium phosphate cement inhibits osteoclast differentiation and resorption. J Mater Sci Mater Med. 2016;27(5):83. doi: 10.1007/s10856-016-5692-7. [eng] [DOI] [PubMed] [Google Scholar]
- 31.Encarnacao I.C., Sordi M.B., Aragones A., Muller C.M.O., Moreira A.C., Fernandes C.P. Release of simvastatin from scaffolds of poly(lactic-co-glycolic) acid and biphasic ceramic designed for bone tissue regeneration. J Biomed Mater Res B Appl Biomater. 2019;107(6):2152–2164. doi: 10.1002/jbm.b.34311. [eng] [DOI] [PubMed] [Google Scholar]
- 32.Sendyk D.I., Deboni M.C., Pannuti C.M., Naclerio-Homem M.G., Wennerberg A. The influence of statins on osseointegration: a systematic review of animal model studies. J Oral Rehabil. 2016;43(11):873–882. doi: 10.1111/joor.12438. [eng] [DOI] [PubMed] [Google Scholar]
- 33.Mankani M.H., Kuznetsov S.A., Shannon B., Nalla R.K., Ritchie R.O., Qin Y. Canine cranial reconstruction using autologous bone marrow stromal cells. Am J Pathol. 2006;168(2):542–550. doi: 10.2353/ajpath.2006.050407. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.