Abstract
Introduction:
Polycystic ovary syndrome (PCOS) is a common endocrine disorder that affects women of reproductive age. Metabolic consequences associated with PCOS include, but are not limited to, insulin resistance (IR), type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD). This narrative review aims to provide a comprehensive overview of the potential therapeutic roles of the incretin-based therapies in the management of PCOS.
Methods:
We performed a systematic search of databases including PubMed, MEDLINE and EMBASE up to 1 October 2020. We developed a search string of medical subject headings (MeSH) including the terms PCOS, incretin mimetics, glucagon-like peptide-1 (GLP-1), glucagon-like peptide-1 receptor antagonists (GLP-1 RAs), liraglutide, exenatide, semaglutide, dipeptidyl peptidase-4 (DPP-4) inhibitors, combined with IR, testosterone and sex hormone-binding globulin (SHBG).
Results:
We identified 854 relevant articles and, after the initial screening, eight interventional animal studies, one observational animal study, 14 interventional human studies, two case–control studies and one systematic review were included. These studies showed the potential significant roles of GLP-1 RAs and DPP-4 inhibitors in the management of PCOS, with significant improvements in the metabolic parameters, including substantial weight reduction and improved insulin sensitivity. These agents also improved the hormonal parameters through decreased free androgen and increased SHBG. Moreover, they improved menstrual regularity, increased fertility with enhanced ovulation and pregnancy in obese women with PCOS.
Conclusion:
GLP-1 RAs and DPP-4 inhibitors have a promising therapeutic role in PCOS; however, larger clinical trials are needed to establish the role of incretin-based therapies in the management of PCOS.
Keywords: dipeptidyl peptidase-4 inhibitors, DPP-4, exenatide, GLP-1 RAs, glucagon-like peptide-1, infertility, insulin resistance, liraglutide, obesity, PCOS, polycystic ovary syndrome, sitagliptin, vildagliptin
Introduction
Polycystic ovary syndrome (PCOS) is an endocrine disorder that affects approximately 10–20% of women of reproductive age.1 There are various diagnostic criteria for PCOS including the Rotterdam 2003 criteria, Androgen Excess Society criteria and the National Institutes of Health (NIH) criteria 1990; however, the international evidence-based guidelines for the assessment and management of PCOS have advocated the use of the Rotterdam 2003 diagnostic criteria.2–4 According to the Rotterdam criteria, to diagnose a woman with PCOS, she must have at least two out of the following three clinical and biochemical features: clinical and biochemical androgen excess, anovulation/oligo-ovulation, and polycystic ovarian morphology verified by ultrasound.2 PCOS is also associated with a wide range of comorbidities including infertility, metabolic syndrome, increased risk of type 2 diabetes (T2DM), obesity, eating disorders, insulin resistance (IR), dyslipidaemia, mood swings and hepatic steatosis. Furthermore, women with PCOS are also at increased risk of miscarriages, premature birth, gestational diabetes, and high blood pressure.5–7
Management strategies for PCOS include lifestyle modifications such as diet and physical activity that are the first-line approach to treatment; however, they are reported to be minimally effective in reducing weight or treating PCOS-related symptoms.8 Pharmacological options are also available; however, they are not explicitly approved for PCOS treatment as they have been primarily used to treat other conditions such as T2DM. The recent development of multiple new therapeutic agents for the management of T2DM has broadened the options for patient-specific therapies in PCOS. An example of these are the incretin-based therapeutic agents.
A significant increase in the plasma insulin level has been observed after oral glucose administration compared to intravenous glucose infusion; this phenomenon is known as the ‘incretin effect’ and contributes to 80% of the total insulin secretion after oral glucose ingestion.9,10 Incretins are the gut-secreted hormones including glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide 1 (GLP-1) that are both secreted in response to meal ingestion, and they enhance glucose-stimulated insulin secretion.11 Incretin hormones also maintain glucose homeostasis by reducing hepatic glucagon release, slowing gastric emptying and suppressing appetite, thereby helping to control body weight and improve glycaemic control.10 However, most of the studies in PCOS have found impaired incretin secretion and activity in overweight/obese individuals, and relatively small studies have reported reduced, normal, or increased GLP-1 levels in patients with PCOS.12,13 Increased GIP and lower GLP-1 concentrations have been reported after an oral glucose tolerance test (OGTT) in women with PCOS.14 A reduction of GLP-1 was also reported in individuals with impaired glucose tolerance (IGT) and impaired fasting glucose (IFG), early markers for prediabetes and the progression to T2DM.15 Initial incretin-based therapy was suggested to reverse the risk of prediabetes by preserving β-cell function in patients with IFG and IGT.16,17
Endogenous GLP-1 has a relatively short half-life of 1–2 min and it is quickly degraded by the proteolytic enzyme dipeptidyl peptidase-4 (DPP-4) more rapidly than GIP, which has a half-life of 5 min.18 The DPP-4 inhibitors are a class of oral anti-diabetes medications that improve glycaemic control by increasing endogenous physiological levels of both GLP-1 and GIP.19 Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) mimic the action of native GLP-1, achieving pharmacological levels, and they are resistant to DPP-4 degradation. They have been shown to improve glycaemic parameters significantly and some agents, such as semaglutide,20 show remarkable weight reduction in overweight and obese patients with or without diabetes.21 Studies in both animal models and clinical settings have demonstrated that both DPP-4 inhibitors and GLP-1 RAs are effective therapeutic agents in the management of PCOS and the prevention of its metabolic consequences.22 The current review summarises the recent evidence on the incretin-based therapies, including GLP-1 RAs, and DPP-4 inhibitors in the management of PCOS, both in animal models and in human studies. This study was conducted following guidance from the international evidence-based guidelines for the assessment and management of PCOS-2018.4
The expression of GLP-1 receptors in the hypothalamic–pituitary–gonadal system
GLP-1 mRNAs are densely expressed on the cerebral cortex, hippocampus, thalamus, and hypothalamus.23 GLP-1 has the potential to regulate gonadotropin-releasing hormone (GnRH) release from the hypothalamic neurons and it has been shown to modulate nitric oxide and the endocannabinoid pathways that regulate the GABAergic current in the postsynaptic GnRH neurons.24 There is also increased expression of the GLP-1 receptors in the area overlapping the arcuate nucleus of the hypothalamus that are occupied by proopiomelanocortin (POMC) neurons. Increased activity in POMC neurons reduces appetite and its inhibition causes obesity.25 The data showed that GLP-1 has the potential to increase the electrical activity in the hypothalamic POMC neurons by upregulating the protein kinase A (PKA) and increasing the L-type calcium channel (Ca²+), which accounts for the action of GLP-1 in suppressing appetite.25 In an experimental study, activation of GLP-1 RAs in the lateral hypothalamus of male rats reduced food reinforcement, food intake and the ingestive behaviour.26
Others have shown that GLP-1 RAs, including liraglutide, stimulate brown adipose tissue thermogenesis by activating AMPK in the ventromedial nucleus of the hypothalamus, which led to weight reduction independent of food intake.27 In humans, changes in energy expenditure do not seem to contribute significantly to the weight-lowering effect of these drugs. In comparison to the hypothalamus, lower GLP-1 mRNA is expressed in the pituitary gland, where GLP-1 increases the release of luteinising hormone (LH) via its effect on releasing GnRH.28 Acute intracerebral injection of GLP-1 promoted an immediate increase in the preovulatory LH, which provoked a significant rise in the level of estrogen and progesterone, and the number of mature follicles.29 GLP-1 RA is also expressed in ovaries and the effects of GLP-1 RA have been observed in both preclinical and clinical studies.30 Treatment of obese women with PCOS with liraglutide resulted in a significant reduction of androstenedione, free testosterone, and an increased level of sex hormone-binding globulin (SHBG).31 GLP-1 also significantly suppressed the level of progesterone with no effect on estrogen synthesis.30
The potential mechanisms by which GLP-1 RAs and DPP-4 inhibitors improve the metabolic parameters in PCOS
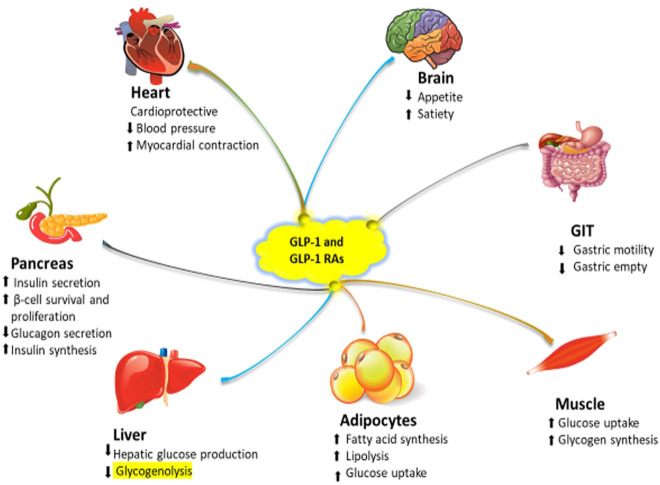
In addition to its glycaemic effect, there is considerable evidence that GLP-1 improves insulin sensitivity in peripheral tissues. An increase in GLP-1 concentration achieved by administering GLP-1 RAs or DPP-4 inhibitors can enhance insulin sensitivity and glucose uptake both in animal and human muscle as well as in adipose tissue32 (Figure 1). However, the primary role for GLP-1 therapy in achieving these outcomes is through weight reduction and the central anorectic effects. However, not all reported studies found an improvement in insulin sensitivity in obese women with PCOS. It has also been proposed that GLP-1 facilitates glucose disposal in an insulin-independent fashion; however, this could be attributed to the overall reduction of glucagon secretion and changing the insulin/glucagon ratio.33 There is evidence suggesting that GLP-1 possesses anti-inflammatory properties. In obese individuals, inflammation of the adipose tissue is the main driver for IR, and treatment with GLP-1 analogues suppresses the inflammatory response by reducing macrophage secretion of inflammatory cytokines including interleukin-1 β (IL1β), interleukin-6 (IL6) and tumour necrosis factor-β (TNF-β).34 Therefore, by reducing the inflammatory response, GLP-1 facilitates insulin sensitivity.
Figure 1.
The mechanism by which glucagon-like peptide-1 (GLP-1) and GLP-1 receptor agonists (RAs) enhance insulin sensitivity and weight loss.
GLP-1 reduces the stress in the endoplasmic reticulum (ER) and improves IR in adipose tissues by modulating the protein kinase R-like endoplasmic reticulum (PERK) pathway by targeting activating transcription factor 4 (ATF4) and C/EBP homologous protein (CHOP) expression.35 Furthermore, it increases the inhibitory effect of insulin on glucose, decreases very low density lipoprotein (VLDL) triglyceride release and facilitates glucose disposal.36 GLP-1 has a significant impact on eating behaviour, intestinal motility, appetite, and gastric emptying (Figure 1). It also has a direct effect on the feeding centre in the hypothalamus where there are GLP-1 receptors in the hypothalamic nuclei.37 GLP-1 decreases both gastric emptying as well as intestinal motility directly by reducing gastric smooth muscle activity, thereby delaying glucose absorption and inhibiting postprandial glucose excursions.38 In addition, GLP-1 has a significant effect in suppressing appetite and inducing satiety, thereby decreasing food intake and facilitating weight loss in both humans and animals.37
The GLP-1 receptors are also expressed in β-cells of the pancreas where GLP-1 exerts multiple actions. GLP-1 stimulates insulin release via various molecular pathways including the production of cyclic adenosine monophosphate (cAMP), activation of voltage-dependent Ca2+ channels, and Ca2+ influx with increased intracellular Ca2+, which stimulates insulin-containing secreting granules and facilitates insulin release into the bloodstream.10,39 In addition to its insulinotropic effects, GLP-1 expands pancreatic β-cell mass by promoting β-cell growth, differentiation and proliferation by activating the epidermal growth factor receptors which, in turn, promote phosphatidylinositol-3 kinase (PI3-K) to synthesise DNA.10,40 GLP-1 utilises its β-cell proliferative effect by down-regulating PI3-K, protein kinase B (PKB/Akt), extracellular signal-related kinase (ERK), p38, protein kinase and mitogen-activated protein kinase (MAPK).41,42 It has also been reported that GLP-1 enhances β-cell survival by reducing apoptosis caused by various cytotoxic stimuli.10 Currently, GLP-1-based therapies are used more often in the management of patients with T2DM and for the treatment of obesity.
Methods
We performed a systematic search of databases including PubMed, MEDLINE and EMBASE up to 1 October 2020. We developed a search string of medical subject headings (MeSH) including the terms polycystic ovary syndrome, PCOS, incretin-based therapy, incretin hormones, incretin mimetics, glucagon-like-peptide 1, GLP-1, GLP-1 receptor agonists, GLP-1 RAs, liraglutide, exenatide, semaglutide, dipeptidyl peptidase 4 inhibitors, DPP-4 inhibitors, gliptins, sitagliptin, alogliptin, saxagliptin, linagliptin, vildagliptin combined with insulin resistance, androgen, testosterone, sex hormone-binding globulin (SHBG), follicular stimulating hormone, FSH, luteinising hormone, LH, androstenedione, free androgen, menstrual irregularity, menstrual cycle, fertility, infertility, subfertility, gonads, ovaries, expression of GLP-1, hypothalamus, pituitary, pregnancy rate and ovulation. The search was limited to English language only with no limit to the year of publication. An initial screening was conducted using titles and abstracts. For eligible studies, the full texts were retrieved, and one reviewer extracted data in consultation with the co-authors.
Results
The review included eight interventional animal studies, one observational animal study, 14 interventional human studies, two case–control human studies and one systematic review. Due to the high level of heterogeneity among the included studies, it was not possible to conduct a meta-analysis; therefore, the results were narratively presented (Figure 2).
Figure 2.
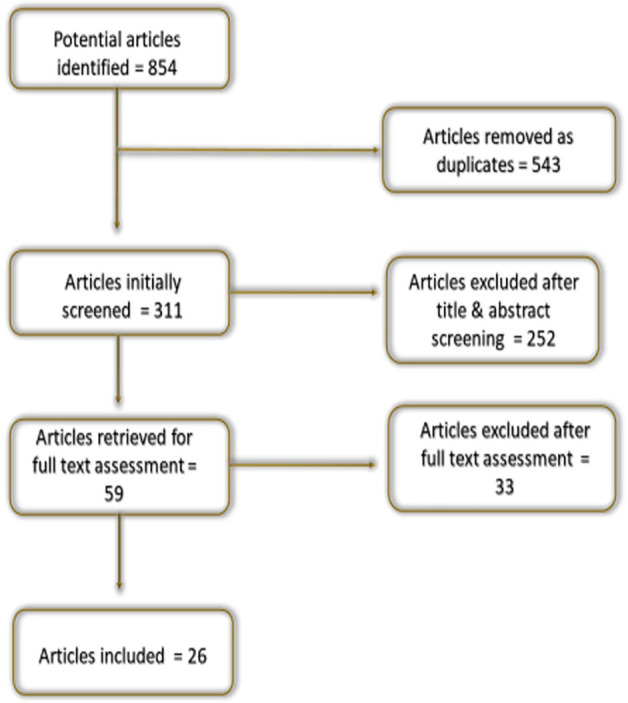
Flowchart for the method of study selection.
Evidence for the therapeutic potentials of GLP-1 RA in PCOS
Exenatide
Studies in animal models
Exenatide is one of the newest therapeutic agents for the treatment of T2DM, and its use in PCOS has increased recently. In a rodent study, 50 female rats (25 days old) were randomly allocated to a PCOS-induced group (n = 37) or a control group (n = 13). The rats in the PCOS group were injected with dehydroepiandrosterone (DHEA) at a dose of 6 mg/100 g/day and 0.2 ml of soybean oil to induce PCOS; meanwhile, 0.2 ml of soybean oil only was administered to the control group. The PCOS-induced rats were then divided into three groups; the PCOS-exenatide group were injected with 10 µg/kg/day of exenatide, the PCOS metformin group were given metformin 300 mg/kg/day, and a PCOS normal saline group was injected with 0.2 ml of saline together with the control group for 4 weeks. In the exenatide-treated group, the number and the size of endometrial glands were reduced due to the increase in the expression of AMP-activated protein kinase α (AMPKα) and the deacetylates enzyme (SIRT1).43 Moreover, the high expression of AMPKα and SIRT1 improved the endocrine and reproductive profiles in PCOS-induced rats treated with exenatide; for instance, there was a significant weight loss (from 222.64 ± 16.57 g to 218.63 ± 13.18 g) in the exenatide group versus 238.30 ± 12.26 g in the metformin group.44 The homeostatic model assessment of insulin resistance (HOMA-IR) was also lower in the exenatide group (from 8.26 ± 2.50 to 7.71 ± 1.23) versus 12.66 ± 1.44 in the metformin group. There was also a significant reduction in the level of serum androgen (0.09 ± 0.03 ng/ml versus 0.53 ± 0.41 ng/ml) in the exenatide and metformin groups, respectively.44 The androgen reduction effect of GLP-1 RA occurred despite the ongoing daily injection of DHEA.
In a randomised trial study, 45 female rats (3 weeks old) were randomly allocated to a DHEA-induced group and a control group.45 The DHEA group was further divided into three groups: metformin treatment group (265 mg/kg); exenatide treatment group (10 µg/kg) and a saline group (1 ml) in addition to the control group for a total duration of 4 weeks. The results showed that there was a comparable effect in weight reduction between metformin and exenatide, and both exenatide and metformin significantly reduced the levels of testosterone, LH and the LH/FSH ratio and increased the level of SHBG.
Clinical studies
Sixty overweight/obese women diagnosed with PCOS were randomly allocated to receive either metformin (1 g BID), exenatide (10 µg BID) or a combined dose of metformin + exenatide for 24 weeks.46 The combination therapy was superior in regulating the menstrual cycle, improved the ovulation rate, insulin sensitivity, and reduced androgen levels, weight and abdominal adiposity. A 24-week open-label randomised trial of 176 overweight/obese women with PCOS randomly allocated to receive either metformin 1 g BID (n = 88) or exenatide 10 µg BID (n = 88) for the initial period of 12 weeks was performed, following which all women continued to receive metformin alone for a second period of 12 weeks. After the initial 12-week period, the exenatide group showed significant weight loss (4.29 ± 1.29 kg) compared to 2.28 ± 0.55 kg with metformin. HOMA-IR was also improved significantly with exenatide therapy (1.30 ± 0.58) compared to metformin (0.59 ± 0.12). The total fat mass was reduced considerably with exenatide (4.67 ± 0.09%) compared to metformin (1.11 ± 0.32%). The ratio of menstrual frequency was also increased with exenatide (0.62 ± 0.12) compared to the metformin group (0.37 ± 0.01). In the second period of 12 weeks, there was a higher rate of spontaneous pregnancy in the previously treated exenatide patients (43.60%) than in metformin (18.70%)47 (Table 1).
Table 1.
Main clinical studies of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) in PCOS.
| References | Study population | Study arms | Duration | Primary outcome | Weight loss (kg) | Metabolic changes |
|---|---|---|---|---|---|---|
| Liraglutide | ||||||
| Rasmussen and Lindenberg48 | Overweight and obese women BMI 35 kg/m², weight 98.9 kg pre-treated with metformin and lifestyle for 6 months. | Liraglutide add on 1.2 mg,1.8 mg (n = 84) | 27.8 weeks | Weight loss | 9.0 kg (95% CI 7.8–10.1, p < 0.0001) | BMI reduced to 3.2 kg/m2 (95% CI 2.8–3.6, p < 0.0001) |
| Jensterle et al.31 | Obese women with PCOS diagnosed by the Rotterdam criteria. Aged 30.7 ± 7.9 years, BMI 38.6 ± 6.0 kg/m2, mean ± SD. | (1) LIRA 1.2 mg QD (n = 14), (2) MET 1000 mg BID (n = 13), (3) ROF 500 mg QD (n = 14) |
12 weeks | Change in body weight | (1) –3.1 ± 3.5 (2) –0.2 ± 1.83 (3) –2.1 ± 2.0 |
BMI reduced by 1.1 ± 1.26 kg/m in LIRA arm, 0.8 ± 0.99 kg/m2 in ROF arm and 0.1 ± 0.67 kg/m2 in MET arm. LIRA reduced VAT (p = 0.015), LIRA was superior to MET in reducing waist circumference and improved glucose homeostasis after OGTT. ROF reduced testosterone (p = 0.05) and increased menstrual frequencies (p = 0.009). |
| Frossing et al.49 | Obese women with PCOS, BMI > 25, diagnosed with the Rotterdam criteria. Pre-treated with contraceptives (6 weeks) and insulin sensitisers (3 months). | (1) LIRA 1.8 mg QD (n = 48) (2) Placebo (n = 24) |
26 weeks | Liver fat contents VAT Prevalence of NAFLD |
(1) –5.2 ± 0.7 (2) +0.2 ± 0.9 |
LIRA reduced liver fat content by 44%, VAT by 18% and the prevalence of NAFLD by two-thirds, SHBG increased by 19%, Free testosterone reduced by 19%. |
| Jensterle et al.50 | Obese women with PCOS, BMI (BMI 38.7 ± 0.1 kg/m2) pre-treated with MET 1000 mg BID. | LIRA 1.2 mg QD SC | 12 weeks | Adiposity parameters eating behaviour | 3.8 ± 0.1 | Significant reductions in VAT, waist circumference, eating behaviour (p < 0.001). |
| Jensterle et al.51 | Obese women with PCOS BMI (38.3 ± 5.4 kg/m2) diagnosed by ASRM-ESHRE Rotterdam criteria. | (1) COMBO (MET 1000 mg BID, LIRA 1.2 mg QD, n = 15). (2) LIRA 3 mg QD (n = 15) |
12 weeks | Changes in anthropo metric measurements of obesity. | (1) −3.6 ± 2.5 (2) −6.3 ± 3.7 |
Greater reduction in BMI and waist circumference in LIRA arm. Both reduced post-OGTT glucose levels. |
| Kahal et al.52 | Young and obese women with PCOS and control | (1) LIRA 1.8 mg QD (2) control |
24 weeks | Body weight, depression and QOL | (1) –3.0 ± 4.2 (2) –3.8 ± 3.4 |
Improvement of physical health (82.6 ± 11.2), psychological health (62.4 ± 16.5 v 57.5 ± 16.4). |
| Kahal et al.53 | Women with PCOS and control | (1) LIRA 1.8 mg QD (2) control |
24 weeks | Markers of liver fibrosis (PIIINP) AST | (1) –3.0 ± 4.2 (2) –3.8 ± 3.4 |
PIIINP (4.4 ± 0.8 versus 3.5 ± 0.84) AST (22.4 ± 5.24 versus 18.8 ± 3.36). |
| Nylander et al.54 | Overweight women with PCOS | (1) LIRA 1.8 mg QD (n = 48) (2) placebo (n = 24) |
26 weeks | Markers of ovarian dysfunction (bleeding ratio, AMH, androgen) | –5.2 kg | Bleeding ratio (0.28 (95% CI 0.20–0.36, p < 0.001), SHBG increased (7.4 nmol/L), testosterone reduced by (0.005 nmol/L), ovarian volume reduced by (0.005 nmol/L) versus placebo. |
| Salamun et al.55 | Infertile obese women with PCOS | (1) LIRA 1.2 mg + MET 1 g BID (2) MET 1 g BID |
12 weeks | Pregnancy rate | (1) –7.5 ± 3.9 (2) –7.0 ± 6.0 |
(1) pregnancy rate 85.7% (2) pregnancy rate 28.6% |
| Exenatide | ||||||
| Liu et al.47 | Overweight and obese women with PCOS diagnosed by the Rotterdam criteria. | (1) EXE 10 µg BID (n = 88) (2) MET 1000 mg BID (n = 88). |
24 weeks | Body weight, fat mass, IR, glycaemic control, hormonal levels. | (1) –4.29 ± 1.29 (2) –2.28 ± 0.55 |
(1) fat mass (–4.67 ± 0.09%), HOMA-IR (1.30 ± 0.58) and increased the menstrual frequencies (0.62 ± 0.12) (2) fat mass (–1.11 ± 0.32%), HOMA-IR (0.59 ± 0.12) and increased the menstrual frequencies (0.37 ± 0.01). |
| Dawson et al.56 | Obese/anovulatory women With PCOS diagnosed by Rotterdam criteria. | EXE 5 µg BID for 4 weeks then EXE 10 µg BID for 12 weeks (n = 30). | 16 weeks | Change in weight | 111.8 ± 4.8–108.6 ± 4.6 | No effects on LDL-C and HDL-C. No effects on glucose, insulin and HOMA-IR. |
| Elkind-Hirsch et al.46 | Overweight/oligo-ovulatory women with PCOS BMI > 27 | (1) MET 1000 mg BID (n = 14) (2) EXE 10 µg BID (n = 14) (3) COMB (MET 1000 mg BID& EXE 10 µg BID, n = 14) |
24 weeks | Menstrual frequency | (1) 113.4 ± 7–111.8 ± 6 (2) 110.5 ± 6–107.3 ± 6 (3) 112 ± 8–106.4 ± 6 |
The COMB was superior to MET or EXE monotherapy in improving menstrual the regularity, ovulation rate, the free androgen levels and insulin sensitivity. |
AMH, anti-Mullerian hormone; BMI, body mass index; COMB, combination; EXE, exenatide; GLP-1, glucagon peptide-1; HOMA-IR, homeostatic model analysis of insulin resistance; IR, insulin resistance; LIRA, liraglutide; MET, metformin; NAFLD, non-alcoholic fatty liver disease; OGTT, oral glucose tolerance test; PCOS, polycystic ovary syndrome; VAT, visceral adiposity.
Recently, exenatide was also shown to improve the parameters of lipid profiles and amino acid metabolism in women with PCOS.57 A 4-month clinical study was conducted in a population of 30 overweight/obese women with PCOS randomly assigned to receive exenatide (5 µg BID) for 4 weeks followed by exenatide (10 µg BID) for 12 weeks. Treatment with exenatide showed a significant reduction in body weight (from 111.8 ± 4.8 to 108.6 ± 4.6 kg), reduction in cardiovascular risk markers including p-selectin (from 101.1 ± 8.2 to 87.4 ± 6.6 ng/ml) as well as e-selectin (from 38.5 ± 3.3 to 33.6 ± 2.6 ng/ml), and an improvement in the inflammatory marker C-reactive protein (CRP)56 (Table 1).
Zinc-α-2-glycoprotein (ZAG) is a protein that stimulates and enhances lipolysis and drives a reduction in body fat mass. In a recent 12-week randomised clinical trial, 82 overweight/obese women with PCOS were assigned to receive exenatide (10 µg BID) or metformin (1 g BID). After 12 weeks, there were no significant differences observed within the two groups (p > 0.05); however, the level of the circulating ZAG was increased in both treatment groups (p < 0.01)58 (Table 1).
Liraglutide
Studies in animal models
Liraglutide is a long-acting GLP-1 analogue with 97% similarity to human GLP-1. Compared to endogenous GLP-1, liraglutide possesses an additional 16 carbon chain that delays its absorption and slows its degradation by DPP-4, thereby prolonging its half-life to over 13 h.59 The clinical effectiveness of liraglutide in the management of T2DM has led to its approval by the European Medicines Agency (EMA) in 2009 and by the US Food and Drug Administration (FDA) in 2010.60 There has been increased interest in considering liraglutide as a prospective therapeutic option for the management of PCOS.61 In addition to its glycaemic reducing effect, liraglutide also has significant effects on weight reduction, lowering blood pressure and improving the lipid profile. A study of 50 C57BL6 female mice aged 3 weeks was performed, randomly assigning them into two groups: the DHEA group (n = 40) and a vehicle group (n = 10). The first group was injected with DHEA (6 mg/100 g/day) for 20 successive days while the vehicle group received sesame oil at a dose of 0.1 ml/100 g. At the age of 32 days, the DHEA mice received a liraglutide injection at a dose of 0.2 mg/kg BID for 21 days, and the control group received saline injections daily. After 6 weeks, liraglutide induced granulosa cell proliferation and promoted their viability in DHEA-induced PCOS mice by modifying the forkhead box protein O1 (FOXO1) phosphorylation site.62 In another study of 20 Parkes strain mice, PCOS was induced using DHEA at a dose of 6 mg/100 g of body weight per day. The PCOS-induced mice then received liraglutide, either 100 or 200 µg/day twice a day for 14 days. Liraglutide was shown to enhance adiponectin and IL6 synthesis and to reduce serum levels of triglyceride, glucose and testosterone. It also improved ovarian function and elevated the level of adiponectin by increasing the expression of Akt and PI3K.63
Clinical studies
In an observational trial in 84 overweight/obese women with PCOS, the women received liraglutide at an initial dose of 0.6 mg subcutaneously once a day and then this was increased to 1.8 mg a day for 4 weeks. There was a significant reduction in weight of up to 9.0 kg, and the body mass index (BMI) was also reduced by 3.2 kg/m² in the liraglutide treatment period of 27.8 weeks48 (Table 1).
A 12-week randomised open-label trial compared treatment with metformin 1 g QD, liraglutide 1.2 mg QD and roflumilast 500 mcg in 45 overweight/obese women with PCOS. The results showed that liraglutide was superior in reducing weight (3.1 ± 3.5 kg) compared to roflumilast (2.1 ± 2.0) and metformin (0.2 ± 1.8 kg) compared to baseline.31
A double-blind, randomised placebo controlled trial of 72 overweight women with PCOS who were treated with liraglutide 1.8 mg/day or placebo for 26 weeks was performed. Treatment with liraglutide reduced body weight significantly by over 5%, liver fat by 44%, visceral adipose tissue by 18% and free testosterone level by 19%.49 Moreover, an even smaller dose of liraglutide of 1.2 mg/day for a shorter period of 12 weeks was also associated with significant weight reduction and improved eating behaviour of women with PCOS50 (Table 1).
Another study of 30 obese women diagnosed with PCOS evaluating the effectiveness of a smaller dose of liraglutide (1.2 mg) combined with metformin versus high dose liraglutide (3 mg) alone was performed. Treatment with high dose liraglutide alone showed superiority to low dose liraglutide combined with metformin. It significantly reduced both BMI and waist circumference (–2.2 ± 1.3 kg/m² and 4.2 ± 3.4 cm) compared to −1.3 ± 1.9 kg/m², –2.2 ± 6.2 cm, respectively in low dose liraglutide combined with metformin51 (Table 1).
In a case–control trial of 19 women diagnosed with PCOS and 17 matched controls, 6 months of liraglutide therapy significantly reduced body weight from baseline (3.0 ± 4.2 kg and 3.8 ± 3.4 kg), respectively. An improvement in the procollagen type 3 amino-terminal peptide (PIIINP), which is a parameter of liver cirrhosis, was seen in the PCOS group that was reduced significantly from 4.4 ± 0.8 to 3.7 ± 0.9 ug/ml, in contrast to a non-significant decrease in the control group (3.5 ± 0.8–3.2 ± 0.7 ug/ml).52
A recent case–control study evaluated the effects of 6 months management with liraglutide 1.8 mg/day on body weight, depression scale and quality of life (QOL) in 19 young and obese women with PCOS compared to 17 age-matched controls. Liraglutide treatment resulted in significant weight reduction in the PCOS group (3.0 ± 4.2 kg) compared to 3.8 ± 3.4 kg in the control group. There was also a significant improvement in the social health, physical and psychological components of the World Health Organization QOL questionnaire (WHOQOL-BREF).53
In a double-blind, randomised trial of 72 women with PCOS randomly assigned in a 2:1 ratio to receive either liraglutide 1.8 mg once a day or placebo for 26 weeks, women treated with liraglutide had a significant improvement in their menstrual bleeding, weight reduction of 5.2 kg, increased levels of SHBG and a reduction in levels of free androgen compared to placebo54 (Table 1).
In a prospective open-label randomised trial of 28 infertile obese women with PCOS treated with metformin (1 g/day) alone or in combination with low dose liraglutide (1.2 mg/day) for 12 weeks, patients treated with metformin lost an average of 7 kg of weight compared to 7.5 kg in the combination group. There was also an increase in the pregnancy rate in the combination group compared to the metformin group (85.7% versus 28.6%), respectively. Therefore, intervention with low dose liraglutide in combination with metformin is superior to metformin alone in increasing the pregnancy rate.55
The results of a systematic review of seven Randomised Clinical Trials (RCTs), including a total of 178 women with PCOS indicated that, following liraglutide treatment, BMI was reduced significantly (–1.65 kg), although no changes in waist circumference, IR or SHBG were observed. However, serum testosterone dropped by 0.29 nmol/L in 88 patients.64
Semaglutide
Semaglutide is a genetically engineered GLP-1 analogue with a long half-life of 168–184 hours and is used in the management of T2DM either alone or combined with other anti-diabetes therapies. Recently, oral semaglutide has been approved for the treatment of T2DM.65 Most of the trials have been performed in patients with T2DM in which treatment with semaglutide has shown significant improvements in glycaemic parameters, considerable weight reduction and decreasing cardiometabolic risk factors. Thus, semaglutide might potentially be the next therapeutic agent in the management of PCOS; however, robust clinical trials are needed.
Evidence for the therapeutic potentials of DPP-4 inhibitors in PCOS
Sitagliptin
Studies in animal models
Sitagliptin was the first DPP-4 inhibitor to be introduced in clinical practice and the most studied class of DPP-4 inhibitors. It inhibits the action of DPP-4, the enzyme that inactivates incretin hormones, allowing endogenous GLP-1 to facilitate insulinotropic glucose-dependent postprandial insulin release.
A study of spontaneously hypertensive obese strain (SHROB) rats with IR treated with the sulfonylurea glyburide (1 mg/kg/day) or sitagliptin (30 mg/kg/day) for 6 weeks was performed and compared with lean rats with hypertension. Sitagliptin enhanced insulin secretion, normalised excess glucagon secretion and lowered plasma glucose.66
Sitagliptin was also administered in a dietary-induced obese mouse model using C57Bl/6J mice given a fat-rich diet and treated for 12 weeks. Sitagliptin treatment reduced body weight in those rats fed a high fat diet, reduced the inflammation in adipose tissues and pancreatic islet cells, and reduced fasting blood glucose as well as insulin.67 Moreover, in streptozotocin-induced diabetic mice, long term sitagliptin for 2–3 months showed a significant rise in the number of insulin-positive β-cells in the pancreatic islets leading to the normalisation of the mass of the β-cell and an increased β-to-α-cell ratio.68
An experimental study of 6-week-old Sprague Dawley (SD) rats injected with insulin and human chorionic gonadotropin (HCG) to establish a PCOS model was performed. Thereafter, the rats were treated with a combination of dimethylbiguanide (DMBG; 300 mg/kg QD) and sitagliptin (10 mg/kg QD) for 12 days. As a result, co-treatment with sitagliptin and DMBG significantly decreased the levels of LH and oestradiol and attenuated IR via upregulating the expression of H19.69 Another study of 30 rats (21 days old) randomly assigned into a PCOS group (given DHEA to induce PCOS) and a control group was performed. The PCOS group was given sitagliptin (63 mg/100 g) while the control group were given 2 ml of distilled water. At 28 days, the treatment group showed a significant reduction in the levels of blood glucose and androgen levels and delayed the progression of ovarian fibrosis. This was suggested to be related to the reduction of factors associated with the tumour growth factor-β1 (TGF-β1) and smad 2/3 signalling pathways.70 The TGF-β1 signalling pathway has also been implicated in adipocyte pathology in women with PCOS. TGF-β signalling is crucial for adipocyte differentiation and is implicated in the development of the visceral fat accumulation in PCOS.71,72
Clinical studies
A double-blind crossover study of 18 obese women with PCOS receiving sitagliptin 100 mg or placebo for 30 days was undertaken. At 1 month, treatment with sitagliptin increased GLP-1 levels and reduced the peak glucose response to OGTT (–17.2 mg/dL), significantly reduced the visceral fat (from 1141.9 ± 700.7 to 1055.1 ± 710.1 g) and increased growth hormone half-life (from 13.9 ± 3.6 to 17.0 ± 6.8 min).73 It also reduced the level of cholesterol (from 168.8 mg/dL ± 26.3 to 162.5 ± 22.2 mg/dL) and improved IR74 (Table 2).
Table 2.
Main clinical studies of dipeptidyl peptidase-4 inhibitors (DPP-4 i) in PCOS.
| References | Study population | Study arms | Duration | Primary outcome | Weight loss (kg) | Metabolic changes |
|---|---|---|---|---|---|---|
| Devin et al.73 | Women with PCOS | (1) Sitagliptin 100 mg QD (n = 18). (2) Placebo QD (n = 18). |
1 month | Increases GH levels Improves glucose levels and vascular function |
– | Decreased peak glucose [−17.2 mg/dL (95% CI −27.7 to −6.6); p < 0.01]. Decreased VAT (1141.9 ± 700.7–1055.1 ± 710.1 g; p = 0.02). Increase GH-half-life (13.9 ± 3.6–17.0 ± 6.8 min, n = 16; p = 0.04). |
| Ferjan et al.75 | Obese women with PCOS and metformin intolerant BMI (36.9 ± 5.5 kg/m2). |
(1) Sitagliptin 100 mg QD (n = 30). (2) Lifestyle intervention (placebo, n = 30). |
12 weeks | Glycaemic control (OGTT, HOMA-B) | – | (1) Sitagliptin BMI (+37 ± 6.2–37.8 ± 5.9kg/m²). (2) Placebo BMI (+36.8 ± 4.9–38 ± 5kg/m²) |
| Ferjan et al.76 | Obese women with PCOS pre-treated with LIRA 3 mg BMI (36.3 ± 5.2 kg/m2). |
(1) COMBO (sitagliptin 100 mg QD and MET 1000 mg BID, n = 15) (2) MET 1000 mg BID (n = 12) |
12 weeks | Anthropometric measures | (1) +0.9 ± 2.5 (2) +4.7 ± 2.7 |
(1) BMI (+0.3 ± 0.8 kg/m²), regain body weight (0.8% ± 2.6%). (2) BMI (+1.7 ± 0.9 kg/m²), regain body weight (4.5% ± 2.5%). |
| Jensterle et al.77 | Obese women with PCOS and persistent IR pre-treated with MET 1000 mg BID | (1) ALO 25 mg QD (n = 15) (2) ALO 25 mg QD and PIO 30 mg QD (n = 15) |
12 weeks | β-cell function and IR | – | (1) MET + ALO reduced HOMA-IR (1.6 ± 2.3, p = 0.039) increased IS (31.4 ± 97.5 ml·min–1·m–2, p = 0.007). (2) MET + ALO + PIO reduced HOMA-IR (2.9 ± 3.3, p = 0.001), IS (39.0 ± 58.1 ml·min–1·m–2 (p = 0.039) |
| El-Halwagy et al.78 | Women with PCOS diagnose by the Rotterdam criteria | (1) PIO 30 mg QD (n = 35) (2) Vildagliptin 50 mg QD (n = 35) (3) MET 500 mg TDS (n = 35) |
6 months | Menstrual regularity, changes in BMI, F–G hirsutism score | – | (1) BMI (27.5 ± 5.2–27.0 ± 4.5, p < 0.016), FG scores (12.0 ± 7.4–10.74 ± 6.12), DHEA (263.7 ± 117–221.0 ± 82.2). (2) BMI (28.0 ± 5.1–27.3 ± 4.5, p < 0.001), F–G scores (11.8 ± 7.9–12.4 ± 7.1), DHEA (271.9 ± 126.5–353.1 ± 362.5). (3) BMI (25.8 ± 5.7–25.3 ± 5.0, p < 0.010), F–G scores (12.3 ± 7.4–10.8 ± 5.5), DHEA (280.7 ± 132.2–243.3 ± 103.5). |
ALO, alogliptin; BMI, body mass index; GH, growth hormone; HOMA-B, homeostatic model analysis for β-cell; HOMA-IR, homeostatic model analysis of insulin resistance; IR, insulin resistance; IS, insulin sensitivity; LIRA, liraglutide; MET, metformin; OGTT, oral glucose tolerance test; PCOS, polycystic ovary syndrome; PIO, pioglitazone; VAT, visceral adiposity.
In a 12-week randomised open-label trial of 30 obese women with PCOS intolerant to metformin, the women were randomly allocated to receive sitagliptin 100 mg/day or lifestyle intervention alone. Treatment with sitagliptin increased β-cell function and prevented the conversion from IGT to T2DM in metformin-intolerant obese subjects with PCOS75 (Table 2).
A 12-week randomised open-label trial of 24 obese women with PCOS (BMI 36.3 ± 5.2 kg/m2) who had previously received liraglutide 3.0 mg for obesity management, were randomly allocated to a combination of either metformin 1 g BID and sitagliptin 100 mg/day or metformin 1 g BID alone. The results showed women treated with metformin regained 4.5 ± 2.5% (4.7 ± 2.7 kg, p = 0.002) of their weight compared to 0.8 ± 0.6% (0.9 ± 2.5 kg, p = 0.147) in the combination group. Furthermore, BMI was increased significantly with the metformin treatment (1.7 ± 0.9 kg/m2) compared to 0.3 ± 0.8 kg/m2 in the combination treatment. Therefore, treatment with sitagliptin as an add on to metformin inhibits weight regain in obese women with PCOS treated with liraglutide76 (Table 2). However, most studies to date in both diabetes and PCOS show that the DPP inhibitors are essentially weight neutral with little weight loss seen.79
Other DPP-4 inhibitors in PCOS
Alogliptin is a class of DPP-4 inhibitors approved for managing T2DM either as monotherapy or in combination with other anti-diabetes medications.80
In a 12-week randomised controlled study, 30 obese women with PCOS aged 34.4 ± 6.5 years and BMI 39.0 ± 4.9 kg/m2 were assigned to receive either alogliptin 25 mg QD or a combination of alogliptin 25 mg QD and pioglitazone 30 mg QD in addition to continuing metformin 1 g BID. Treatment with alogliptin–metformin alone or alogliptin–pioglitazone–metformin significantly reduced IR (HOMA-IR), and improved insulin sensitivity and the androgen index77 (Table 2).
In a 16-week randomised single-blinded study, 38 prediabetic women with PCOS were randomly assigned to a combination of saxagliptin 5 mg/day and metformin 2000 mg/day or saxagliptin 5 mg or metformin 2000 mg as monotherapy. Treatment with saxagliptin + metformin was superior to monotherapy in terms of normalising glucose tolerance, the insulin sensitivity index, waist/height ratio and the free androgen index.81 Furthermore, saxagliptin + metformin was also effective in reducing weight, improving the parameters of lipid profiles, and inhibiting the inflammatory response in women with PCOS newly diagnosed with T2DM.82
A double-blind, randomised clinical trial of 105 women with PCOS randomly allocated the women into three groups to receive pioglitazone 30 mg/QD (group 1), metformin 500 mg/three times per day (group 2, control group) or vildagliptin 50 mg/once a day (group 3) for 6 months was performed. In group 1, patients receiving pioglitazone showed a significant reduction of BMI (p < 0.016), Ferriman–Gallwey score (F–G scores) (p < 0.003), DHEA (p < 0.001) and improvement of menstrual irregularities (p < 0.035). A similar result was found with metformin in which BMI was reduced significantly (p < 0.010), F–G score improved (p < 0.002), free androgen level reduced (p < 0.034) and menstrual irregularity improved (p < 0.004). However, the vildagliptin group demonstrated significant reductions in BMI (p < 0.001) and F–G score (p < 0.046), but with no effect on the free androgen levels, menstrual irregularity and the F–G score78 (Table 2).
Limitations
This current review has its limitations. Firstly, the number of clinical trials of treatment with GLP-1 RAs and DPP-4 inhibitors in patients with PCOS are limited. Secondly, it is not clear whether the preconception intervention with GLP-1 RAs and DPP-4 inhibitors could potentially improve fertility in an obese population with PCOS.
Conclusion
In recent years, there has been intense research aimed at understanding the pathophysiology of PCOS and its metabolic consequences. The health burden resulting from PCOS has driven the need for novel therapeutic strategies to prevent health-related complications. The beneficial efficacy of incretin-based therapies in the management of T2DM, together with their glucose-dependent mechanism of action, indicates their potential benefits in the management of PCOS and its related metabolic consequences. GLP-1 RAs have shown significant efficacy in weight reduction in overweight and obese patients with PCOS as well as improving the parameter of IR, together with potential improvement in β-cell mass and function in preclinical studies.
On the other hand, DPP-4 inhibitors have also shown beneficial effects on both β-cell function and mass and free androgen levels. However, the clinical data are not conclusive although some experimental data are encouraging for sitagliptin.
Despite the available evidence for GLP-1 RAs and DPP-4 inhibitors, more clinical trials are required before they can be recommended routinely in this young female population to promote sustainable weight reduction and to improve the parameters of IR in PCOS. More studies with liraglutide 3 mg are needed to determine the precise indications of liraglutide and to evaluate safety issues.
Footnotes
Author contributions: Mohammed Altigani Abdalla: Conceptualisation; methodology, writing-original draft; writing-review and editing.
Harshal Deshmukh: Conceptualisation; methodology; writing-review and editing.
Stephen Atkin: Conceptualisation; methodology; supervision; writing-review and editing.
Thozhukat Sathyapalan: Conceptualisation; methodology; supervision; writing-review and editing.
Conflict of interest: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Data availability: Data are available from the corresponding author upon request.
ORCID iD: Mohammed Altigani Abdalla  https://orcid.org/0000-0002-6016-3157
https://orcid.org/0000-0002-6016-3157
Contributor Information
Mohammed Altigani Abdalla, Department of Academic Diabetes, Endocrinology and Metabolism, Hull York Medical School, University of Hull, Hull, UK.
Harshal Deshmukh, Department of Academic Diabetes, Endocrinology and Metabolism, Hull York Medical School, University of Hull, Hull, UK.
Stephen Atkin, School of Postgraduate Studies and Research, RCSI Medical University of Bahrain, Kingdom of Bahrain.
Thozhukat Sathyapalan, Department of Academic Diabetes, Endocrinology and Metabolism, Hull York Medical School, University of Hull, Hull, UK.
References
- 1. March WA, Moore VM, Willson KJ, et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 2010; 25: 544–551. [DOI] [PubMed] [Google Scholar]
- 2. Azziz R. Controversy in clinical endocrinology: diagnosis of polycystic ovarian syndrome: the Rotterdam criteria are premature. J Clin Endocrinol Metab 2006; 91: 781–785. [DOI] [PubMed] [Google Scholar]
- 3. Azziz R, Carmina E, Dewailly D, et al. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab 2006; 91: 4237–4245. [DOI] [PubMed] [Google Scholar]
- 4. Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril 2018; 110: 364–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goodarzi MO, Dumesic DA, Chazenbalk G, et al. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol 2011; 7: 219–231. [DOI] [PubMed] [Google Scholar]
- 6. Apridonidze T, Essah PA, Iuorno MJ, et al. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2005; 90: 1929–1935. [DOI] [PubMed] [Google Scholar]
- 7. Boyle JA, Teede HJ. PCOS: refining diagnostic features in PCOS to optimize health outcomes. Nat Rev Endocrinol 2016; 12: 630–631. [DOI] [PubMed] [Google Scholar]
- 8. Moran LJ, Hutchison SK, Norman RJ, et al. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev 2011; CD007506. [DOI] [PubMed] [Google Scholar]
- 9. Creutzfeldt W. The incretin concept today. Diabetologia 1979; 16: 75–85. [DOI] [PubMed] [Google Scholar]
- 10. Papaetis GS. Incretin-based therapies in prediabetes: current evidence and future perspectives. World J Diabetes 2014; 5: 817–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tzotzas T, Karras SN, Katsiki N. Glucagon-Like Peptide-1 (GLP-1) receptor agonists in the treatment of obese women with polycystic ovary syndrome. Curr Vasc Pharmacol 2017; 15: 218–229. [DOI] [PubMed] [Google Scholar]
- 12. Madsbad S. The role of glucagon-like peptide-1 impairment in obesity and potential therapeutic implications. Diabetes Obes Metab 2014; 16: 9–21. [DOI] [PubMed] [Google Scholar]
- 13. Ferjan S, Jensterle M, Oblak T, et al. An impaired glucagon-like peptide-1 response is associated with prediabetes in polycystic ovary syndrome with obesity. J Int Med Res 2019; 47: 4691–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vrbikova J, Hill M, Bendlova B, et al. Incretin levels in polycystic ovary syndrome. Eur J Endocrinol 2008; 159: 121–127. [DOI] [PubMed] [Google Scholar]
- 15. Zhang F, Tang X, Cao H, et al. Impaired secretion of total glucagon-like peptide-1 in people with impaired fasting glucose combined impaired glucose tolerance. Int J Med Sci 2012; 9: 574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garber AJ. Incretin effects on beta-cell function, replication, and mass: the human perspective. Diabetes Care 2011; 34 (Suppl. 2): S258–S263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Byrne MM, Gliem K, Wank U, et al. Glucagon-like peptide 1 improves the ability of the beta-cell to sense and respond to glucose in subjects with impaired glucose tolerance. Diabetes 1998; 47: 1259–1265. [DOI] [PubMed] [Google Scholar]
- 18. Yabe D, Seino Y, Seino Y. Incretin concept revised: the origin of the insulinotropic function of glucagon-like peptide-1 – the gut, the islets or both? J Diabetes Investig 2018; 9: 21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crepaldi G, Carruba M, Comaschi M, et al. Dipeptidyl peptidase 4 (DPP-4) inhibitors and their role in type 2 diabetes management. J Endocrinol Invest 2007; 30: 610–614. [DOI] [PubMed] [Google Scholar]
- 20. Hjerpsted JB, Flint A, Brooks A, et al. Semaglutide improves postprandial glucose and lipid metabolism, and delays first-hour gastric emptying in subjects with obesity. Diabetes Obes Metab 2018; 20: 610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Triplitt C, Solis-Herrera C. GLP-1 receptor agonists: practical considerations for clinical practice. Diabetes Educ 2015; 41: 32S–46S. [DOI] [PubMed] [Google Scholar]
- 22. Abdalla MA, Deshmukh H, Atkin S, et al. A review of therapeutic options for managing the metabolic aspects of polycystic ovary syndrome. Ther Adv Endocrinol Metab 2020; 11: 2042018820938305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alvarez E, Martinez MD, Roncero I, et al. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J Neurochem 2005; 92: 798–806. [DOI] [PubMed] [Google Scholar]
- 24. Farkas I, Vastagh C, Farkas E, et al. Glucagon-like peptide-1 excites firing and increases GABAergic miniature Postsynaptic Currents (mPSCs) in Gonadotropin-Releasing Hormone (GnRH) neurons of the male mice via activation of Nitric Oxide (NO) and suppression of endocannabinoid signaling pathways. Front Cell Neurosci 2016; 10: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma X, Bruning J, Ashcroft FM. Glucagon-like peptide 1 stimulates hypothalamic proopiomelanocortin neurons. J Neurosci 2007; 27: 7125–7129. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26. Lopez-Ferreras L, Richard JE, Noble EE, et al. Lateral hypothalamic GLP-1 receptors are critical for the control of food reinforcement, ingestive behavior and body weight. Mol Psychiatry 2018; 23: 1157–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beiroa D, Imbernon M, Gallego R, et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 2014; 63: 3346–3358. [DOI] [PubMed] [Google Scholar]
- 28. Heppner KM, Baquero AF, Bennett CM, et al. GLP-1R signaling directly activates arcuate nucleus kisspeptin action in brain slices but does not rescue luteinizing hormone inhibition in ovariectomized mice during negative energy balance. eNeuro 2017; 4: ENEURO.0198-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Outeirino-Iglesias V, Romani-Perez M, Gonzalez-Matias LC, et al. GLP-1 increases preovulatory LH source and the number of mature follicles, as well as synchronizing the onset of puberty in female rats. Endocrinology 2015; 156: 4226–4237. [DOI] [PubMed] [Google Scholar]
- 30. Nishiyama Y, Hasegawa T, Fujita S, et al. Incretins modulate progesterone biosynthesis by regulating bone morphogenetic protein activity in rat granulosa cells. J Steroid Biochem Mol Biol 2018; 178: 82–88. [DOI] [PubMed] [Google Scholar]
- 31. Jensterle M, Salamun V, Kocjan T, et al. Short term monotherapy with GLP-1 receptor agonist liraglutide or PDE 4 inhibitor roflumilast is superior to metformin in weight loss in obese PCOS women: a pilot randomized study. J Ovarian Res 2015; 8: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McClenaghan NH. Physiological regulation of the pancreatic {beta}-cell: functional insights for understanding and therapy of diabetes. Exp Physiol 2007; 92: 481–496. [DOI] [PubMed] [Google Scholar]
- 33. Drucker DJ. Minireview: the glucagon-like peptides. Endocrinology 2001; 142: 521–527. [DOI] [PubMed] [Google Scholar]
- 34. Guo C, Huang T, Chen A, et al. Glucagon-like peptide 1 improves insulin resistance in vitro through anti-inflammation of macrophages. Braz J Med Biol Res 2016; 49: e5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jiang Y, Wang Z, Ma B, et al. GLP-1 improves adipocyte insulin sensitivity following induction of endoplasmic reticulum stress. Front Pharmacol 2018; 9: 1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parlevliet ET, de Leeuw van Weenen JE, Romijn JA, et al. GLP-1 treatment reduces endogenous insulin resistance via activation of central GLP-1 receptors in mice fed a high-fat diet. Am J Physiol Endocrinol Metab 2010; 299: E318–E324. [DOI] [PubMed] [Google Scholar]
- 37. MacDonald PE, El-Kholy W, Riedel MJ, et al. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes 2002; 51 (Suppl. 3): S434–S442. [DOI] [PubMed] [Google Scholar]
- 38. Smits MM, Tonneijck L, Muskiet MH, et al. Gastrointestinal actions of glucagon-like peptide-1-based therapies: glycaemic control beyond the pancreas. Diabetes Obes Metab 2016; 18: 224–235. [DOI] [PubMed] [Google Scholar]
- 39. Meloni AR, DeYoung MB, Lowe C, et al. GLP-1 receptor activated insulin secretion from pancreatic β-cells: mechanism and glucose dependence. Diabetes Obes Metab 2013; 15: 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Buteau J, Roduit R, Susini S, et al. Glucagon-like peptide-1 promotes DNA synthesis, activates phosphatidylinositol 3-kinase and increases transcription factor pancreatic and duodenal homeobox gene 1 (PDX-1) DNA binding activity in beta (INS-1)-cells. Diabetologia 1999; 42: 856–864. [DOI] [PubMed] [Google Scholar]
- 41. Buteau J, Foisy S, Rhodes CJ, et al. Protein kinase Czeta activation mediates glucagon-like peptide-1-induced pancreatic beta-cell proliferation. Diabetes 2001; 50: 2237–2243. [DOI] [PubMed] [Google Scholar]
- 42. Buteau J, Foisy S, Joly E, et al. Glucagon-like peptide 1 induces pancreatic beta-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes 2003; 52: 124–132. [DOI] [PubMed] [Google Scholar]
- 43. He Junxian, Cai Lisi, Li Jing, Li Yunhui, Tao Xin, Zhang Yu. Exenatide Improves Endometrial Glands in PCOS Rats through AMPKα-SIRT1. 2020. 10.21203/rs.3.rs-28388/v1. [Google Scholar]
- 44. Tao X, Cai L, Chen L, et al. Effects of metformin and exenatide on insulin resistance and AMPKα-SIRT1 molecular pathway in PCOS rats. J Ovarian Res 2019; 12: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sun L, Ji C, Jin L, et al. Effects of exenatide on metabolic changes, sexual hormones, inflammatory cytokines, adipokines, and weight change in a DHEA-treated rat model. Reprod Sci 2016; 23: 1242–1249. [DOI] [PubMed] [Google Scholar]
- 46. Elkind-Hirsch K, Marrioneaux O, Bhushan M, et al. Comparison of single and combined treatment with exenatide and metformin on menstrual cyclicity in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab 2008; 93: 2670–2678. [DOI] [PubMed] [Google Scholar]
- 47. Liu X, Zhang Y, Zheng S-Y, et al. Efficacy of exenatide on weight loss, metabolic parameters and pregnancy in overweight/obese polycystic ovary syndrome. Clin Endocrinol (Oxf) 2017; 87: 767–774. [DOI] [PubMed] [Google Scholar]
- 48. Rasmussen CB, Lindenberg S. The effect of liraglutide on weight loss in women with polycystic ovary syndrome: an observational study. Front Endocrinol (Lausanne) 2014; 5: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Frossing S, Nylander M, Chabanova E, et al. Effect of liraglutide on ectopic fat in polycystic ovary syndrome: a randomized clinical trial. Diabetes Obes Metab 2018; 20: 215–218. [DOI] [PubMed] [Google Scholar]
- 50. Jensterle M, Kocjan T, Kravos NA, et al. Short-term intervention with liraglutide improved eating behavior in obese women with polycystic ovary syndrome. Endocr Res 2015; 40: 133–138. [DOI] [PubMed] [Google Scholar]
- 51. Jensterle M, Kravos NA, Goricar K, et al. Short-term effectiveness of low dose liraglutide in combination with metformin versus high dose liraglutide alone in treatment of obese PCOS: randomized trial. BMC Endocr Disord 2017; 17: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kahal H, Abouda G, Rigby AS, et al. Glucagon-like peptide-1 analogue, liraglutide, improves liver fibrosis markers in obese women with polycystic ovary syndrome and nonalcoholic fatty liver disease. Clin Endocrinol (Oxf) 2014; 81: 523–528. [DOI] [PubMed] [Google Scholar]
- 53. Kahal H, Kilpatrick E, Rigby A, et al. The effects of treatment with liraglutide on quality of life and depression in young obese women with PCOS and controls. Gynecol Endocrinol 2019; 35: 142–145. [DOI] [PubMed] [Google Scholar]
- 54. Nylander M, Frossing S, Clausen HV, et al. Effects of liraglutide on ovarian dysfunction in polycystic ovary syndrome: a randomized clinical trial. Reprod Biomed Online 2017; 35: 121–127. [DOI] [PubMed] [Google Scholar]
- 55. Salamun V, Jensterle M, Janez A, et al. Liraglutide increases IVF pregnancy rates in obese PCOS women with poor response to first-line reproductive treatments: a pilot randomized study. Eur J Endocrinol 2018; 179: 1–11. [DOI] [PubMed] [Google Scholar]
- 56. Dawson AJ, Sathyapalan T, Vince R, et al. The effect of exenatide on cardiovascular risk markers in women with polycystic ovary syndrome. Front Endocrinol (Lausanne) 2019; 10: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tang L, Yuan L, Yang G, et al. Changes in whole metabolites after exenatide treatment in overweight/obese polycystic ovary syndrome patients. Clin Endocrinol (Oxf) 2019; 91: 508–516. [DOI] [PubMed] [Google Scholar]
- 58. Zheng S, Liu E, Zhang Y, et al. Circulating zinc-alpha2-glycoprotein is reduced in women with polycystic ovary syndrome, but can be increased by exenatide or metformin treatment. Endocr J 2019; 66: 555–562. [DOI] [PubMed] [Google Scholar]
- 59. Sekar R, Singh K, Arokiaraj AW, et al. Pharmacological actions of glucagon-like peptide-1, gastric inhibitory polypeptide, and glucagon. Int Rev Cell Mol Biol 2016; 326: 279–341. [DOI] [PubMed] [Google Scholar]
- 60. Ladenheim EE. Liraglutide and obesity: a review of the data so far. Drug Des Devel Ther 2015; 9: 1867–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Papaetis GS, Filippou PK, Constantinidou KG, et al. Liraglutide: new perspectives for the treatment of polycystic ovary syndrome. Clin Drug Investig 2020; 40: 695–713. [DOI] [PubMed] [Google Scholar]
- 62. Sun Z, Li P, Wang X, et al. GLP-1/GLP-1R signaling regulates ovarian PCOS-associated granulosa cells proliferation and antiapoptosis by modification of forkhead box protein O1 phosphorylation sites. Int J Endocrinol 2020; 2020: 1484321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Singh A, Fernandes JRD, Chhabra G, et al. Liraglutide modulates adipokine expression during adipogenesis, ameliorating obesity, and polycystic ovary syndrome in mice. Endocrine 2019; 64: 349–366. [DOI] [PubMed] [Google Scholar]
- 64. Niafar M, Pourafkari L, Porhomayon J, et al. A systematic review of GLP-1 agonists on the metabolic syndrome in women with polycystic ovaries. Arch Gynecol Obstet 2016; 293: 509–515. [DOI] [PubMed] [Google Scholar]
- 65. Hughes S, Neumiller JJ. Oral semaglutide. Clin Diabetes 2020; 38: 109–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen B, Moore A, Escobedo LV, et al. Sitagliptin lowers glucagon and improves glucose tolerance in prediabetic obese SHROB rats. Exp Biol Med (Maywood) 2011; 236: 309–314. [DOI] [PubMed] [Google Scholar]
- 67. Dobrian AD, Ma Q, Lindsay JW, et al. Dipeptidyl peptidase IV inhibitor sitagliptin reduces local inflammation in adipose tissue and in pancreatic islets of obese mice. Am J Physiol Endocrinol Metab 2011; 300: E410–E421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mu J, Woods J, Zhou YP, et al. Chronic inhibition of dipeptidyl peptidase-4 with a sitagliptin analog preserves pancreatic beta-cell mass and function in a rodent model of type 2 diabetes. Diabetes 2006; 55: 1695–1704. [DOI] [PubMed] [Google Scholar]
- 69. Wang Q, Shang J, Zhang Y, et al. Metformin and sitagliptin combination therapy ameliorates polycystic ovary syndrome with insulin resistance through upregulation of lncRNA H19. Cell Cycle 2019; 18: 2538–2549.31405334 [Google Scholar]
- 70. Wang F, Zhang ZF, He YR, et al. Effects of dipeptidyl peptidase-4 inhibitors on transforming growth factor-beta1 signal transduction pathways in the ovarian fibrosis of polycystic ovary syndrome rats. J Obstet Gynaecol Res 2019; 45: 600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dumesic DA, Phan JD, Leung KL, et al. Adipose insulin resistance in normal-weight women with polycystic ovary syndrome. J Clin Endocrinol Metab 2019; 104: 2171–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Xu N, Kwon S, Abbott DH, et al. Epigenetic mechanism underlying the development of polycystic ovary syndrome (PCOS)-like phenotypes in prenatally androgenized rhesus monkeys. PLoS One 2011; 6: e27286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Devin JK, Nian H, Celedonio JE, et al. Sitagliptin decreases visceral fat and blood glucose in women with polycystic ovarian syndrome. J Clin Endocrinol Metab 2020; 105: dgz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Devin J, Nian H, Wright P, et al. MON-474 Dipeptidyl Peptidase-4 (DPP4) inhibition decreases visceral fat and improves glucose metabolism in overweight women with polycystic ovarian syndrome. J Endocr Soc 2019; 3 (Suppl. 1): MON-474. [Google Scholar]
- 75. Ferjan S, Janez A, Jensterle M. Dpp4 inhibitor sitagliptin as a potential treatment option in metformin-intolerant obese women with polycystic ovary syndrome: a pilot randomized study. Endocr Pract 2018; 24: 69–77. [DOI] [PubMed] [Google Scholar]
- 76. Ferjan S, Janez A, Jensterle M. Dipeptidyl peptidase-4 inhibitor sitagliptin prevented weight regain in obese women with polycystic ovary syndrome previously treated with liraglutide: a pilot randomized study. Metab Syndr Relat Disord 2017; 15: 515–520. [DOI] [PubMed] [Google Scholar]
- 77. Jensterle M, Goricar K, Janez A. Add on DPP-4 inhibitor alogliptin alone or in combination with pioglitazone improved beta-cell function and insulin sensitivity in metformin treated PCOS. Endocr Res 2017; 42: 261–268. [DOI] [PubMed] [Google Scholar]
- 78. El-Halwagy AS, Al-Gergawy AA, Eleslam ES, et al. Clinical and biochemical changes in polycystic ovarian syndrome patients in response to 3 different oral hypoglycemic drugs: a double blind randomized controlled study. Open J Obstet Gynecol 2016; 7: 117–128. [Google Scholar]
- 79. Foley JE, Jordan J. Weight neutrality with the DPP-4 inhibitor, vildagliptin: mechanistic basis and clinical experience. Vasc Health Risk Manag 2010; 6: 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dineen L, Law C, Scher R, et al. Alogliptin (nesina) for adults with type-2 diabetes. P T 2014; 39: 186–202. [PMC free article] [PubMed] [Google Scholar]
- 81. Elkind-Hirsch KE, Paterson MS, Seidemann EL, et al. Short-term therapy with combination dipeptidyl peptidase-4 inhibitor saxagliptin/metformin extended release (XR) is superior to saxagliptin or metformin XR monotherapy in prediabetic women with polycystic ovary syndrome: a single-blind, randomized, pilot study. Fertil Steril 2017; 107: 253–260e251. [DOI] [PubMed] [Google Scholar]
- 82. Tao T, Wu P, Wang Y, et al. Comparison of glycemic control and beta-cell function in new onset T2DM patients with PCOS of metformin and saxagliptin monotherapy or combination treatment. BMC Endocr Disord 2018; 18: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]