Abstract
Background:
Superior capsular reconstruction (SCR) is an alternative surgical option for young active patients with irreparable rotator cuff tears without arthritis. Although cadaveric studies have shown superior stability of the humerus, it remains unclear whether the humerus migrates superiorly after SCR in vivo.
Purpose:
To analyze the change in glenohumeral translation in patients before and after SCR.
Study Design:
Case series; Level of evidence, 4.
Methods:
A total of 22 patients who underwent SCR by a single surgeon for irreparable rotator cuff tears were included. Among them, 14 patients had intact grafts, and 8 patients were diagnosed with retears on 1-year postoperative magnetic resonance imaging (MRI) scans. Fluoroscopy was performed in all patients preoperatively and at 3-month, 6-month, and 1-year follow-up. Moreover, 3-dimensional bone models from computed tomography, MRI, and fluoroscopic scans during scapular plane abduction of the shoulder joints were analyzed.
Results:
In the intact graft group, 3-dimensional dynamic shoulder kinematics at 6 months (P = .026) and 1 year (P = .032) postoperatively showed statistically significant decreases in humeral head superior translation compared with preoperatively. The ≥6-mm acromiohumeral distance (AHD) subgroup had a larger decrease in humeral head superior translation from preoperatively than did the <6-mm AHD subgroup (6 months: P = .026; 1 year: P = .032). The retear group had significantly greater humeral head superior translation at 1 year postoperatively than did the ≥6-mm and <6-mm AHD subgroups (post hoc test: P < .001; ≥6-mm AHD vs retear group: P = .001; <6-mm AHD vs retear group: P = .012).
Conclusion:
SCR with intact grafts resulted in decreased humeral head superior translation after 6 months. Patients with retears showed no improvement in humeral head superior translation. Patients with a preoperative AHD <6 mm had less improvement in humeral head superior translation than did those with a preoperative AHD ≥6 mm. Early surgical treatment before an excessive decrease in preoperative AHD could be helpful for postoperative humeral head translation recovery.
Keywords: irreparable rotator cuff tear, superior capsular reconstruction, acromiohumeral distance, 3-dimensional dynamic shoulder kinematics
Massive irreparable rotator cuff tears are difficult to treat because of the high rate of complications and poor clinical outcomes.9 The shoulder capsule is an important static stabilizer of the glenohumeral joint, and superior capsular defects increase glenohumeral translation in all directions, particularly with superior translation at 5° and 30° of abduction.14 Massive irreparable rotator cuff tears disrupt the superior capsule, given its large, shared footprint with the supraspinatus and infraspinatus tendons of the greater tuberosity of the humerus.29 Attempts to resolve superior translation to physiologic conditions through reconstruction of the superior capsule have been demonstrated in a biomechanical cadaveric model.24 After Mihata et al22 reported the clinical outcomes of 23 patients who underwent superior capsular reconstruction (SCR) using a fascia lata autograft, some authors reported favorable clinical outcomes of SCR.1,6,12,16,17,21 In biomechanical studies, Mihata et al23,25 also showed that SCR decreased superior migration of the humerus. Although cadaveric studies have demonstrated superior stability of the humerus, it remains unclear whether the humerus migrates superiorly after SCR in vivo.
The purpose of this study was to analyze which dynamic changes occurred in shoulders after SCR according to the state of the grafted capsule and difference in preoperative acromiohumeral distance (AHD). We hypothesized that the course of adaptation of the grafted capsule would depend on the postoperative state (intact vs torn) and that the preoperative state would also have an effect. Therefore, in the present study, we analyzed the change in glenohumeral translation in patients before and after SCR using 3-dimensional (3D)/2-dimensional (2D) model image registration techniques.
Methods
Patients
This study was a retrospective case series of 45 patients who underwent SCR, and it included prospectively collected data. This study was approved by an institutional review board, and all patients agreed to participate and signed the institutional review board–approved consent form before the study. Between March 2015 and July 2017, eligible patients underwent SCR performed by a single surgeon (S.-J.L.). The inclusion criteria were as follows: (1) a large to massive full-thickness rotator cuff tear detected on preoperative magnetic resonance imaging (MRI) scans and confirmed arthroscopically, (2) failure of nonoperative treatment (medication and strengthening of the rotator cuff, deltoid, and scapular stabilizer), (3) age <70 years, (4) a rotator cuff tear considered to be irreparable despite interval slides, (5) SCR performed to enable the patient to participate in an occupation and/or hobby, and (6) preoperative and postoperative fluoroscopy. The exclusion criteria included (1) a history of fractures or surgery on the affected shoulder and (2) severe arthritic changes in the glenohumeral joint on radiographs (Hamada stage 4b or 5). Ultimately, 22 patients were included. All patients underwent fluoroscopy preoperatively and at 3 months, 6 months, and 1 year postoperatively. All patients also underwent MRI at 1 year postoperatively, and a retear was confirmed in 8 patients. Patients with an intact graft (14 patients) were divided into 2 subgroups based on the AHD measured preoperatively: <6-mm AHD subgroup (n = 8) and ≥6-mm AHD subgroup (n = 6). An AHD of <6 mm suggested a long-standing total full-thickness infraspinatus tendon tear according to Goutallier et al10 (Figure 1).
Figure 1.

Flowchart of the study. AHD, acromiohumeral distance; CT, computed tomography; f/u, follow-up; MRI, magnetic resonance imaging.
Surgical Technique
All patients received an interscalene nerve block before anesthesia and subsequently underwent general anesthesia. A bilateral examination of the shoulders under anesthesia was performed. Surgery was performed with the patients in the beach-chair position. Generally, 6 portal sites (posterior viewing portal, anterior working portal, lateral working portal, posterolateral viewing portal, Neviaser portal, and anterosuperior portal for anteromedial anchor insertion) were used, and the accessory portal was added as needed. For lesions identified within the glenohumeral joint, immediate treatment was performed intra-articularly. In the presence of subscapularis tendon tears, a single-row technique was employed to repair the lesser tuberosity footprint using a 5.5-mm Bio-Corkscrew suture anchor (Arthrex) in the intra-articular space, and in the presence of biceps lesions, mini-open subpectoral tenodesis was performed after SCR. Subsequently, we accessed the subacromial space and confirmed the presence of the rotator cuff tear and its reparability as well as the features of the rotator cuff tear. For subacromial spurs, subacromial decompression was performed on the lesions that were identified by size and location preoperatively. Subacromial bursectomy was also performed, and the anteroposterior (AP) and mediolateral (ML) dimensions of the rotator cuff tear were measured. The AP size of the superior capsular defect and the distance (ML) from the glenoid superior pole to the humeral greater tuberosity footprint were measured at 30° of abduction of the shoulder.
Regarding the autograft, the fascia lata tendon was harvested from the ipsilateral thigh to create a graft corresponding to the tear size: that is, AP diameter × (ML diameter + 1.5 cm). A length corresponding to 2 to 3 times the required size (ML diameter + 1.5 cm) was measured; subsequently, the fascia lata was harvested. Autograft thickness was measured after folding the graft 2 to 3 times. The difference in thickness of the graft occurred between different harvest sites, that is, the midthigh and proximal thigh. We attempted to use autografts in all patients; however, in patients with a history of surgery at the thigh level or patients who did not want an autograft, allografts were used. With the allograft (MegaDerm; L&C BIO), similar to that with the autograft, a length corresponding to 2 to 3 times the tear size (ML diameter + 1.5 cm) was obtained. Allograft thickness was also measured after folding the graft 2 to 3 times.
Capsular reconstruction was performed at 30° of abduction of the shoulder. There were 2 anchors used for the glenoid superior pole (anterior: 3.0-mm GRYPHON suture anchor [Mitek]; posterior: 2.4-mm SutureTak suture anchor [Arthrex]) and for the humeral greater tuberosity footprint (two 5.0-mm Corkscrew suture anchors; Arthrex). The sutures were taken from the glenoid anchor, pretied to the prepared graft, and subsequently inserted in the joint. After the graft was deployed well in the joint, medial fixation was performed using a matrix suture technique on the glenoid. On the humeral side, lateral fixation was performed via a single-row technique using 6 knot ties. When posterior remnant tissue, such as the infraspinatus tendon, was present, a capsule-to-tendon suture was used. In the absence of posterior remnant tissue, surgery was completed without suturing.
AHD Measurement
The AHD was measured using picture archiving and communication system software (Infinitt) on a high-resolution LCD monitor using conventional true AP shoulder radiographs taken with the patients standing and the arm held in neutral rotation. The AHD was defined as the shortest distance between the dense cortical bone at the inferior aspect of the acromion and the subchondral cortex at the superior aspect of the humeral head.28 This assessment of the AHD is known to be a reliable and reproducible method of measurement.11
MRI Protocol
Patients were instructed to do gentle stretching exercises with their shoulders for 15 minutes, after which time MRI scans were obtained using a 3-T MRI unit (Achieva 3.0T TX; Philips) and an 8-element phased-array shoulder coil. All scans were obtained with the patient in the supine position with the arm by the side and the hand on the lateral aspect of the thigh. Conventional 2D MRI was performed first and was followed by 3D isotropic T1-weighted fast spin echo (FSE) imaging. Conventional 2D MRI consisted of axial, oblique coronal, and oblique sagittal fat-suppressed T1-weighted FSE sequences and oblique coronal and oblique sagittal T2-weighted FSE sequences. We performed the 3D isotropic fat-suppressed T1-weighted FSE (VISTA; Philips) sequence with 0.5-mm thickness in the oblique coronal plane, and the source data were reformatted into axial and oblique sagittal planes with 1-mm thickness. Immediately after MRI, postprocessing was performed at an imaging workstation by a technologist who was not involved in the study; the time required for image reformation was approximately 1 minute. An evaluation of the MRI scans was performed by a consultant musculoskeletal radiologist with 16 years of experience in the diagnostic investigation of MRI of the shoulder.
Image Acquisition and 3D Modeling
We asked patients to position their shoulders toward a monoplane radiographic system. All images were obtained at 30 Hz while patients performed 3 trials preoperatively. Patients performed arm elevation in the scapular plane, starting from the arm at the side, to maximum elevation while keeping their elbow fully extended and the arm externally rotated (thumbs-up position) at a rate of approximately 2 seconds per cycle. One cycle was defined as full arm elevation, followed by a return to the initial position. For postoperative measurements, patients were asked to elevate their arm as much as possible. When performing fluoroscopy before and after surgery, we first obtained fluoroscopic images in the XY plane (coronal plane or AP view of the shoulder radiograph) and then in the ZX plane (axial plane or axial view of the shoulder radiograph). To allow natural arm motion, we did not constrain the patients or strictly control the speed of motion. The patients practiced the activity until they felt comfortable, and then 3 cycles of shoulder elevation were recorded. To obtain more precise data and provide a suitable environment for patients, approximately 30- to 40-second breaks were given between cycles. Among the 3 timed trials, we selected the second cycle.
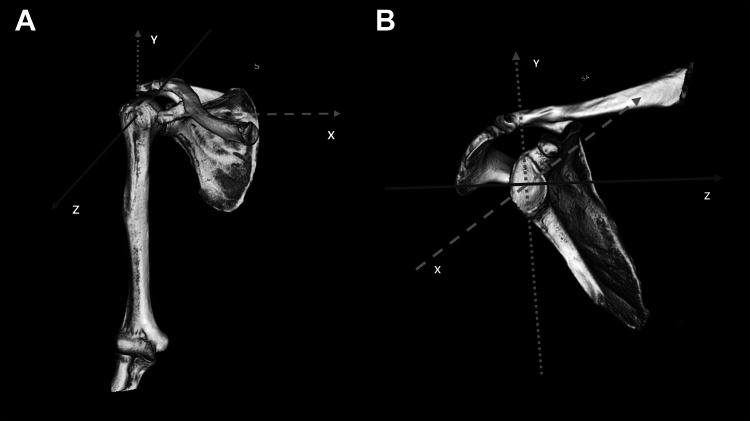
Preoperative computed tomography (CT) scans of the shoulder were acquired with a 1-mm slice pitch (image matrix: 512 × 512; pixel size: 0.62 × 0.62 mm). The CT images were segmented, and 3D surface models of the proximal humerus and scapula were created.30 Anatomic coordinate systems were embedded in each model according to reported conventions.15,28 The humeral origin was placed at the centroid of the humeral head. The y-axis was parallel to the humeral shaft, and the z-axis was defined as a line through the intertubercular groove from the origin. The scapular origin was defined as the midpoint of the line connecting the most superior and inferior bony edges of the glenoid, and the y- and z-axes were pointed superiorly and anteriorly, respectively20 (Figure 2).
Figure 2.
Three-dimensional models of the humeral head and scapula. (A) Humeral axis. The y-axis was parallel to the humeral shaft at the humeral origin, the z-axis was defined as a line through the intertubercular groove from the origin, and the x-axis was the line perpendicular to the YZ plane at the humeral origin. (B) Scapular axis. The z-axis was a line that met the glenoid plane and the perpendicular plane of the line connecting the most superior and inferior bony edges of the glenoid at the glenoid origin, the y-axis was the line at which the glenoid plane met the plane perpendicular to the z-axis at the glenoid origin, and the x-axis was the line perpendicular to the ZY plane at the glenoid origin. S, superior; SA, superior-anterior.
The clavicular origin was defined as the midpoint of the line connecting the most anterior and posterior bony edges of the distal clavicle in the acromioclavicular joint (Figure 3).
Figure 3.
Three-dimensional model of the clavicle. The x-axis was a line formed by the plane of the lateral curve to the joint of the lateral distal clavicle and a plane that passed through the clavicular origin of the plane perpendicular to the line connecting the most anterior and posterior bony edges of the distal clavicle, the z-axis was the line formed by the plane of the lateral curve to the joint of the lateral distal clavicle and the plane perpendicular to the x-axis and passing through the clavicular origin, and the y-axis was the line perpendicular to the XZ plane at the clavicular origin.
Image Registration and Data Processing
The 3D position and orientation of the humerus and scapula were determined via model image registration techniques using the open-source software JointTrack (http://www.sourceforge.net/projects/jointtrack) (Figure 4).2,19
Figure 4.

Three-dimensional position and orientation of the humerus and clavicle. Three-dimensional/2-dimensional model image registration techniques were used to determine the 3-dimensional position and orientation of the shoulder bones.
The bone model was projected onto the distortion-corrected fluoroscopic image, and its 3D pose was iteratively adjusted to match its silhouette with the silhouette of the fluoroscopic image.26 Model image registration measurements were performed by a senior operator (S.-J.L.), who used a series of fluoroscopic images from the second cycle of the activity. The kinematics of the humerus relative to the radiographic coordinate system and the scapula were determined using Cardan angles (Z-X-Y order).3,18 Elevation of the humerus was defined as rotation about the z-axis, and internal/external rotation of the humerus was defined as rotation about its y-axis. Superior/inferior translation was defined as the motion of the humeral origin relative to the scapular origin along the y-axis of the scapula. Kinematic data were individually plotted as a function of the humeral elevation angle, and polynomial regression lines were used to calculate interpolating values at each 15° increment of humeral elevation. R 2 values of the polynomial regression lines ranged from 0.90 to 0.99 for rotational data and 0.47 to 0.96 for translational data.
Clinical Assessment
For all patients, preoperative range of motion (ROM) values and pain visual analog scale (VAS) scores were obtained by the surgeon. There were 3 movements to measure active ROM: forward flexion in the scapular plane, external rotation with the arm at the side, and internal rotation at the back. Internal rotation was estimated by determining how far the patient could reach with the thumb along the spine. For statistical analysis, the spinal segment was converted into numbers: segments from T1 to T12 were designated as 1 to 12; segments from L1 to L5, as 13 to 17; and the sacrum, as 18.
Statistical Analysis
All statistical analyses were performed using SPSS Version 22.0 (IBM Corp). The Student t test was used to analyze continuous variables, and the Mann-Whitney U test was used to analyze data with nonnormal distributions. Categorical variables were analyzed by using the chi-square test or Fisher exact test. Preoperative shoulder motion measurements were analyzed using repeated-measures analysis of variance (ANOVA). One-way ANOVA was used to perform comparisons of subgroups. If 1-way ANOVA indicated a significant difference, the Bonferroni method and post hoc test were used. Correlation analysis was performed to investigate correlations between 2 variables. All tests were analyzed using a 95% confidence level. The level of significance was set at .05.
Results
Descriptive Data
A total of 22 patients were included in the final analysis (16 men and 6 women). The overall mean age was 60.12 ± 4.29 years, the mean body mass index was 24.59 ± 2.42, and the mean duration of preoperative symptoms was 24.01 ± 16.12 months. The pain VAS score at the first hospital visit was 5.69 ± 1.22, and the ROM values were 102.25° ± 36.98° for forward flexion, 42.24° ± 15.21° for external rotation, and 12.97° ± 4.13° for internal rotation at the back. Overall, 10 patients had only a supraspinatus tendon tear, 2 patients had supraspinatus and infraspinatus tendon tears, 4 patients had supraspinatus and subscapularis tendon tears, and 6 patients had tears of all 3 tendons. The mean ML size of the superior capsular defect was 44.89 ± 6.29 mm. Additionally, 17 patients received an autograft, and 5 received an allograft. The descriptive data stratified by patients with an intact graft and those with a retear are shown in Table 1.
Table 1.
Descriptive Dataa
| Intact: ≥6-mm AHD (n = 6) | Intact: <6-mm AHD (n = 8) | Retear (n = 8) | P Valueb | |
|---|---|---|---|---|
| Sex, n | .240 | |||
| Male | 4 | 5 | 7 | |
| Female | 2 | 3 | 1 | |
| Age, y | 59.13 ± 4.57 | 59.79 ± 4.01 | 60.51 ± 4.24 | .965 |
| Body mass index | 24.56 ± 2.18 | 24.46 ± 2.23 | 24.78 ± 2.57 | .984 |
| Duration of preoperative symptoms, mo | 23.42 ± 15.89 | 25.33 ± 16.01 | 23.78 ± 15.71 | .890 |
| VAS pain score | 5.77 ± 1.23 | 5.38 ± 1.09 | 5.78 ± 1.33 | .926 |
| ROM | ||||
| Forward flexion, deg | 105.02 ± 38.89 | 102.45 ± 33.53 | 101.75 ± 37.12 | .886 |
| External rotation, deg | 41.43 ± 15.01 | 42.88 ± 15.89 | 41.67 ± 15.19 | .912 |
| Internal rotation at back, degc | 12.98 ± 4.03 | 13.29 ± 4.14 | 12.44 ± 4.09 | .898 |
| Tear pattern, n | .791 | |||
| Supraspinatus tendon | 4 | 3 | 3 | |
| Supraspinatus and infraspinatus tendons | 0 | 1 | 1 | |
| Supraspinatus and subscapularis tendons | 1 | 2 | 1 | |
| Supraspinatus, infraspinatus, and subscapularis tendons | 1 | 2 | 3 | |
| Mediolateral size of superior capsular defect, mm | 44.12 ± 6.24 | 44.55 ± 6.09 | 45.87 ± 6.12 | .843 |
| Graft type, n | .848 | |||
| Autograft | 5 | 6 | 6 | |
| Allograft | 1 | 2 | 2 |
aValues are presented as mean ± SD unless otherwise indicated. AHD, acromiohumeral distance; ROM, range of motion; VAS, visual analog scale.
bP values are shown for differences between intact and retear groups.
cInternal rotation was estimated by determining how far the patient could reach with his or her thumb along one’s spine. For statistical analysis, the spinal segment was converted into numbers: segments from T1 to T12 were designated as 1 to 12, segments from L1 to L5 as 13 to 17, and the sacrum as 18.
3D Dynamic Shoulder Kinematics
Mean preoperative AHD values were 5.47 ± 1.60 and 3.36 ± 2.10 mm in patients with an intact graft and those with a retear, respectively (P = .015). At 1 year postoperatively, the mean AHD was significantly higher in patients with an intact graft (6.17 ± 2.06 mm) than in those with a retear (3.25 ± 1.37 mm) (P = .002). No difference in preoperative and postoperative AHD values was detected between patients with an intact graft and those with a retear (P = .227 and P = .874, respectively). Preoperative superior translation of the humeral head was significantly different between patients with an intact graft (2.62 ± 2.72 mm) and those with a retear (6.45 ± 4.41 mm) (defined as a positive value in the superior direction and a negative value in the inferior direction; P = .038). Superior translation of the humeral head in patients with an intact graft and those with a retear were 1.99 ± 4.20 and 8.02 ± 1.01 mm at 3 months postoperatively (P = .001), 0.24 ± 3.68 and 7.16 ± 3.98 mm at 6 months postoperatively (P = .020), and 0.59 ± 3.57 and 7.46 ± 3.57 mm at 1 year postoperatively (P < .001), respectively. Among patients with an intact graft, there was no significant difference in preoperative and 3-month postoperative superior and inferior translation of the humeral head (P = .637). However, superior and inferior translation of the humeral head at 6 months and 1 year postoperatively were statistically significantly different from that preoperatively (P = .026 and P = .032, respectively). By contrast, among patients with a retear, there was no significant difference in preoperative and postoperative superior and inferior translation of the humeral head at all time points (P = .357, P = .747, and P = .495, respectively) (Figure 5).
Figure 5.
Dynamic humeral head kinematics over time. *P < .05 between groups. # P < .05 from preoperatively in the intact graft subgroups. postop, postoperatively; preop, preoperative.
Essentially, 2 subgroups of patients with an intact graft were classified based on the preoperative AHD (6 mm), and 3D dynamic shoulder kinematics were compared between these subgroups and patients with a retear (Table 2).
Table 2.
Superior Translation of Humeral Head (in mm)a
| Intact: ≥6-mm AHD (n = 6) | Intact: <6-mm AHD (n = 8) | P Valueb | Retear (n = 8) | P Valuec | P Valued | |
|---|---|---|---|---|---|---|
| Preoperative | 1.43 ± 2.50 | 3.51 ± 2.68 | .166 | 6.45 ± 4.41 | .038 | .039 (≥6-mm AHD vs retear) |
| 3 mo | 1.53 ± 5.01 | 2.32 ± 3.81 | .743 | 8.02 ± 1.01 | .001 | .010 (≥6-mm AHD vs retear) .019 (<6-mm AHD vs retear) |
| 6 mo | –2.19 ± 2.29 | 2.06 ± 3.55 | .026 | 7.16 ± 3.98 | .020 | .007 (≥6-mm AHD vs retear) |
| 1 y | –1.69 ± 3.38 | 2.30 ± 2.78 | .032 | 7.46 ± 3.57 | <.001 | .001 (≥6-mm AHD vs retear) .012 (<6-mm AHD vs retear) |
aValues are presented as mean ± SD. AHD, acromiohumeral distance.
bComparison of 2 intact groups.
cComparison of all 3 groups.
dPost hoc test using Bonferroni method.
Superior and inferior translation of the humeral head were significantly different between the ≥6-mm and <6-mm AHD subgroups at 6 months (–2.19 ± 2.29 and 2.06 ± 3.55 mm, respectively; P = .026) and 1 year (–1.69 ± 3.38 and 2.30 ± 2.78 mm, respectively; P = .032) postoperatively. Scapular abduction was significantly different between the ≥6-mm and <6-mm AHD subgroups at 1 year postoperatively (30.37° ± 9.15° and 14.47° ± 5.97°, respectively; P = .002). In addition, a comparison of the 3 groups including patients with a retear showed that superior and inferior translation of the humeral head were significantly different at all time points (Figure 6).
Figure 6.

Dynamic humeral head kinematics over time. The comparison between the 3 groups of superior and inferior translation of the humeral head showed significant differences at all times. AHD, acromiohumeral distance; postop, postoperatively; preop, preoperative.
Correlation analysis for each variable among patients with an intact graft showed a significant correlation between preoperative AHD and 1-year postoperative superior and inferior translation of the humeral head (correlation coefficient = –0.734; P = .003).
Discussion
In this single-surgeon case series, we investigated 12-month dynamic humeral kinematics of intact grafted capsules after SCR. Per our hypothesis, intact grafted capsules resulted in less superior translation of the humeral head than did grafted capsules with retears over time after surgery. Additionally, we observed larger decreases in superior translation of the humeral head when the preoperative AHD was ≥6 mm.
SCR restores superior translation of the humerus and tension of the remnant rotator cuff (subscapularis and infraspinatus tendons).24 Because patients with irreparable rotator cuff tears often have deteriorating stabilization of the humeral head resulting from supraspinatus and infraspinatus muscle dysfunction, the humeral head could migrate superiorly and become fixed to the surrounding soft tissue (ie, muscles including the deltoid, triceps, and latissimus dorsi; joint capsules; and ligaments). In the present study, there was no statistical difference between preoperative and postoperative AHD in patients with an intact graft and patients with retear. Although SCR is known to biomechanically reduce superior translation of the humeral head,14,24 altered glenohumeral kinematics cannot be restored immediately; thus, a longer follow-up may be necessary. We aimed to investigate the changes in kinematics over time after SCR in patients with irreparable rotator cuff tears, and to the best of our knowledge, this is the first study to analyze in vivo kinematics after SCR.
The thin membrane structure under the rotator cuff has been considered the superior capsule of the glenohumeral joint. In the cadaveric study by Mihata et al,23 SCR without side-to-side suturing did not decrease glenohumeral superior translation, although subacromial peak contact pressure decreased. However, adding side-to-side suturing completely restored superior stability to the intact level. This restoration of stability results in a stable fulcrum and may allow the deltoid and remaining rotator cuff to function more effectively. However, in the cadaveric study of Hu et al,13 the values of superior translation in the superior capsular defect model were not larger than those of the supraspinatus defect model. Therefore, they concluded that the supraspinatus rather than the superior capsule plays a primary role in preventing superior translation of the humeral head. All of these experimental studies used cadaveric specimens; there are no similar studies on living patients.
AHD has been used by researchers as a simple measurable value to infer the rotator cuff function. Chung et al5 reported that a reduced postoperative AHD is predictive of a poor functional outcome after massive rotator cuff repair. According to Nové-Josserand et al,27 a reduced AHD indicates a full-thickness infraspinatus tendon tear with or without other associated rotator cuff tendon lesions. We divided and analyzed patients based on an AHD <6 or ≥6 mm.10 A preoperative AHD was correlated with postoperative superior and inferior translation of the humeral head. While our study showed a benefit to performing SCR in patients with an AHD ≥6 mm, we are unable to answer the question of whether it is worthwhile to perform SCR in patients with an AHD <6 mm. In our study, even if the grafted capsule was intact, there was no significant difference in superior migration of the humeral head at 3 months postoperatively; however, the ≥6-mm AHD subgroup had significantly decreased superior migration of the humeral head at 6 and 12 months after surgery compared with the <6-mm AHD subgroup. We thought that this result was obtained because the strength of the infraspinatus and subscapularis tendons were improved in the ≥6-mm AHD subgroup. In the case of the infraspinatus tendon, the integrity of posterior remnant tissue has been reported to have a significant relationship with retears in a previous study investigating factors related to a retear after SCR.17 These results can only be obtained in an in vivo follow-up study and not in a cadaveric study, and we consider this to be an advantage of our study.
Many studies have quantified shoulder and glenohumeral joint kinematics. Most earlier studies used conventional single-plane radiographs4,7,8 that do not show the 3D motion of the shoulder and do not provide dynamic kinematics. The possibility of errors may also be increased by the imaging method, beam projection angle, patient or cassette position, and other factors. Therefore, we measured shoulder kinematics of patients before and after surgery using CT-derived 3D bone models that were matched with the silhouette of the bones on fluoroscopic images using 3D/2D model image registration techniques devised by Matsuki et al.20
This study has some limitations; therefore, the findings should be interpreted with caution. First, the small number of patients limited our analysis, and there may have been some nonsignificant findings that were a result of type II errors. Nevertheless, we were able to obtain statistically significant results and draw conclusions. Second, the patients’ postoperative pain may have caused shoulder motion limitations. Therefore, there is a possibility that a difference could be observed if patients’ motion was not limited secondary to pain, especially at 3 months postoperatively. To solve this problem, all patients underwent the same operative technique (surgical procedure and similar graft thickness) performed by 1 surgeon, and we tried to minimize the difference by prescribing the same protocol and rehabilitation therapy. Third, our findings resulted from combining data for SCR and heterogeneous graft materials (dermal graft and autogenous fascia lata graft). The different mechanical properties between the 2 grafts could have caused bias in the biomechanical evaluation.
Conclusion
SCR with intact grafts resulted in decreased humeral head superior translation after 6 months. Patients with retears showed no improvement in humeral head superior translation. Patients with a preoperative AHD <6 mm had less improvement in humeral head superior translation than did those with a preoperative AHD ≥6 mm. Early surgical treatment before an excessive decrease in preoperative AHD could be helpful for postoperative humeral head translation recovery.
Footnotes
Final revision submitted June 25, 2020; accepted July 7, 2020.
The authors declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Pusan National University Yangsan Hospital (study No. 05-2019-035).
References
- 1. Altintas B, Scheidt M, Kremser V, et al. Superior capsule reconstruction for irreparable massive rotator cuff tears: does it make sense? A systematic review of early clinical evidence. Am J Sports Med. 2020;48(13):3365–3375. [DOI] [PubMed] [Google Scholar]
- 2. Banks SA, Hodge WA. Accurate measurement of three-dimensional knee replacement kinematics using single-plane fluoroscopy. IEEE Trans Biomed Eng. 1996;43:638–649. [DOI] [PubMed] [Google Scholar]
- 3. Braman JP, Engel SC, LaPrade RF, Ludewig PM. In vivo assessment of scapulohumeral rhythm during unconstrained overhead reaching in asymptomatic subjects. J Shoulder Elbow Surg. 2009;18:960–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen S, Simonian PT, Wickiewicz TL, Otis JC, Warren RF. Radiographic evaluation of glenohumeral kinematics: a muscle fatigue model. J Shoulder Elbow Surg. 1999;8:49–52. [DOI] [PubMed] [Google Scholar]
- 5. Chung SW, Kim JY, Kim MH, Kim SH, Oh JH. Arthroscopic repair of massive rotator cuff tears. Am J Sports Med. 2013;41:1674–1683. [DOI] [PubMed] [Google Scholar]
- 6. Denard PJ, Brady PC, Adams CR, Tokish JM, Burkhart SS. Preliminary results of arthroscopic superior capsule reconstruction with dermal allograft. Arthroscopy. 2018;34:93–99. [DOI] [PubMed] [Google Scholar]
- 7. Deutsch A, Altchek DW, Schwartz E, Otis JC, Warren RF. Radiologic measurement of superior displacement of the humeral head in the impingement syndrome. J Shoulder Elbow Surg. 1996;5:186–193. [DOI] [PubMed] [Google Scholar]
- 8. Freedman L, Munro R. Abduction of the arm in the scapular plane: scapular and glenohumeral movements. A roentgenographic study. J Bone Joint Surg Am. 1966;48:1503–1510. [PubMed] [Google Scholar]
- 9. Gerber C, Wirth SH, Farshad M. Treatment options for massive rotator cuff tears. J Shoulder Elbow Surg. 2011;20:S20–S29. [DOI] [PubMed] [Google Scholar]
- 10. Goutallier D, Le Guilloux P, Postel J, Radier C, Bernageau J, Zilber S. Acromiohumeral distance less than six millimeter: its meaning in full-thickness rotator cuff tear. Orthop Traumatol Surg Res. 2011;97:246–251. [DOI] [PubMed] [Google Scholar]
- 11. Gruber G, Bernhardt GA, Clar H, Zacherl M, Glehr M, Wurnig C. Measurement of the acromiohumeral interval on standardized anteroposterior radiographs: a prospective study of observer variability. J Shoulder Elbow Surg. 2010;19:10–13. [DOI] [PubMed] [Google Scholar]
- 12. Hirahara AM, Andersen WJ, Panero AJ. Superior capsular reconstruction: clinical outcomes after minimum 2-year follow-up. Am J Orthop (Belle Mead NJ). 2017;46:266–278. [PubMed] [Google Scholar]
- 13. Hu Q, Ding Z, Zhang H, He Y. The superior glenohumeral joint capsule alone does not prevent superior translation of the humeral head: an in vitro biomechanical study. Arthroscopy. 2018;34:2962–2970. [DOI] [PubMed] [Google Scholar]
- 14. Ishihara Y, Mihata T, Tamboli M, et al. Role of the superior shoulder capsule in passive stability of the glenohumeral joint. J Shoulder Elbow Surg. 2014;23:642–648. [DOI] [PubMed] [Google Scholar]
- 15. Kon Y, Nishinaka N, Gamada K, Tsutsui H, Banks SA. The influence of handheld weight on the scapulohumeral rhythm. J Shoulder Elbow Surg. 2008;17:943–946. [DOI] [PubMed] [Google Scholar]
- 16. Lacheta L, Horan MP, Schairer WW, et al. Clinical and imaging outcomes after arthroscopic superior capsule reconstruction with human dermal allograft for irreparable posterosuperior rotator cuff tears: a minimum 2-year follow-up. Arthroscopy. 2020;36:1011–1019. [DOI] [PubMed] [Google Scholar]
- 17. Lee SJ, Min YK. Can inadequate acromiohumeral distance improvement and poor posterior remnant tissue be the predictive factors of re-tear? Preliminary outcomes of arthroscopic superior capsular reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018;26:2205–2213. [DOI] [PubMed] [Google Scholar]
- 18. Ludewig PM, Cook TM. Alterations in shoulder kinematics and associated muscle activity in people with symptoms of shoulder impingement. Phys Ther. 2000;80:276–291. [PubMed] [Google Scholar]
- 19. Mahfouz MR, Hoff WA, Komistek RD, Dennis DA. A robust method for registration of three-dimensional knee implant models to two-dimensional fluoroscopy images. IEEE Trans Med Imaging. 2003;22:1561–1574. [DOI] [PubMed] [Google Scholar]
- 20. Matsuki K, Matsuki KO, Yamaguchi S, et al. Dynamic in vivo glenohumeral kinematics during scapular plane abduction in healthy shoulders. J Orthop Sports Phys Ther. 2012;42:96–104. [DOI] [PubMed] [Google Scholar]
- 21. Mihata T, Lee TQ, Hasegawa A, et al. Five-year follow-up of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. J Bone Joint Surg Am. 2019;101:1921–1930. [DOI] [PubMed] [Google Scholar]
- 22. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29:459–470. [DOI] [PubMed] [Google Scholar]
- 23. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44:1423–1430. [DOI] [PubMed] [Google Scholar]
- 24. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40:2248–2255. [DOI] [PubMed] [Google Scholar]
- 25. Mihata T, Watanabe C, Fukunishi K, Ohue M, Tsujimura T, Kinoshita M. Arthroscopic superior capsular reconstruction restores shoulder stability and function in patients with irreparable rotator cuff tears: a prospective study (SS-15). Arthroscopy. 2011;27:e36–e37. [Google Scholar]
- 26. Nishinaka N, Tsutsui H, Mihara K, et al. Determination of in vivo glenohumeral translation using fluoroscopy and shape-matching techniques. J Shoulder Elbow Surg. 2008;17:319–322. [DOI] [PubMed] [Google Scholar]
- 27. Nové-Josserand L, Edwards TB, O’Connor DP, Walch G. The acromiohumeral and coracohumeral intervals are abnormal in rotator cuff tears with muscular fatty degeneration. Clin Orthop Relat Res. 2005;433:90–96. [DOI] [PubMed] [Google Scholar]
- 28. Saupe N, Pfirrmann CWA, Schmid MR, Jost B, Werner CML, Zanetti M. Association between rotator cuff abnormalities and reduced acromiohumeral distance. Am J Roentgenol. 2006;187:376–382. [DOI] [PubMed] [Google Scholar]
- 29. Sethi P, Franco WG. The role of superior capsule reconstruction in rotator cuff tears. Orthop Clin North Am. 2018;49:93–101. [DOI] [PubMed] [Google Scholar]
- 30. Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. [DOI] [PubMed] [Google Scholar]