Abstract
Background
Inflammatory bowel disease, including ulcerative colitis and Crohn’s disease, is characterized by chronic inflammation that could be a risk factor for extraintestinal cancer. The aim of this study is to evaluate whether inflammatory bowel disease is related to the risk of lower urinary tract tumors.
Methods
A systematical research was performed on various medical databases including PubMed, the Cochrane Library, Embase and Web of Science from inception to April 2020. Data were independently extracted by two reviewers. The Newcastle-Ottawa Scale and the Oxford Centre for Evidence-Based Medicine criteria were used to assess the quality of included articles. The analysis was completed by STATA version 14.2.
Results
Six hundred and twelve of records were initially identified and 16 studies were included in the final analysis. In general, inflammatory bowel disease patients were not at increased risk of prostate cancer, bladder cancer and male genital cancer. In the subgroup analysis, Crohn’s disease patients seemed to have borderline increased risk of prostate cancer [standardized incidence ratio: 1.05; 95% confidence interval (CI): 0.90–1.21; I2=15.1%] and bladder cancer (standardized incidence ratio: 1.19; 95% CI: 0.94–1.44; I2=0.0%), and ulcerative colitis patients seemed to have borderline increased risk of prostate cancer (standardized incidence ratio: 1.13; 95% CI: 0.93–1.33; I2=73.5%).
Conclusions
Inflammatory bowel disease did not significantly increase the risk of prostate cancer, bladder cancer and male genital cancer. Crohn’s disease patients seemed to have a higher risk of prostate cancer and bladder cancer, and ulcerative colitis patients seemed to have a higher risk of prostate cancer. ulcerative colitis patients in East Asian countries have significantly increased prostate cancer risk.
Keywords: Inflammatory bowel disease (IBD), Crohn’s disease, prostate cancer (PCa), bladder cancer (BCa), male genital cancer (MGCa)
Introduction
Inflammatory bowel disease (IBD), comprising ulcerative colitis (UC) and Crohn’s disease (CD), is characterized by chronic gastrointestinal tract inflammation. In the past decades, a significantly increased incidence of IBD has been observed worldwide, especially in eastern countries (1). IBD has been a great health burden to the society for its high morbidity and tight association with gastrointestinal malignancy (2-4), as well as numerous extraintestinal malignancies (5,6).
Though mechanism underlying IBD and oncogenesis has not been clearly elucidated, it was reported that inflammation not only serves as host`s response to malignant tumor but also elicit carcinogenesis (7,8). Moreover, immunosuppressant medications, which are widely used for IBD treatment, are believed to be responsible for elevated risk of cancers like non-Hodgkin lymphoma, acute myeloid leukemia, non-melanoma skin cancers and urinary tract cancers (9-12). From these knowledges, a concept about the interplay between IBD and malignancy could be established, which depicts the inflammation induced immune dysregulation and immunosuppressant medications use as key factors in IBD related malignancies. However, this indirect evidence-based hypothesis may not apply to all kinds of malignant tumors, especially extraintestinal tumors. Direct evidence from studies evaluating the association between certain malignancy and IBD could be of great value in prognosis predicting and providing guidance for cancer surveillance. To our knowledge, no meta-analysis has been performed to evaluating the risk of bladder cancer (BCa) or male genital cancer (MGCa) in IBD patients. A recent study reported an increased risk of prostate cancer (PCa) in IBD patients (13). With the aim of evaluating the risk of lower urinary tract cancers including PCa, BCa and MGCa in IBD patients, we performed this meta-analysis. We presented the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/tau-20-1020).
Methods
Search strategy
We conducted this meta-analysis according to the guideline of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (14). Electronic databases including PubMed, the Cochrane Library, Embase and Web of Science was initially searched from inception to April 2020 with no language limitation. We used the following Medical Subject Heading (MeSH) terms and related synonyms: “Inflammatory Bowel Diseases”, “Urinary Bladder Neoplasms”, “Prostatic Neoplasms”, and “Genital Neoplasms, Male”. The complete search strategy was described in the Supplementary file. We scrutinized all potentially eligible studies, regardless of the primary outcomes or language. Besides, references from related articles were also retrieved manually to ensure a comprehensive search.
Study selection
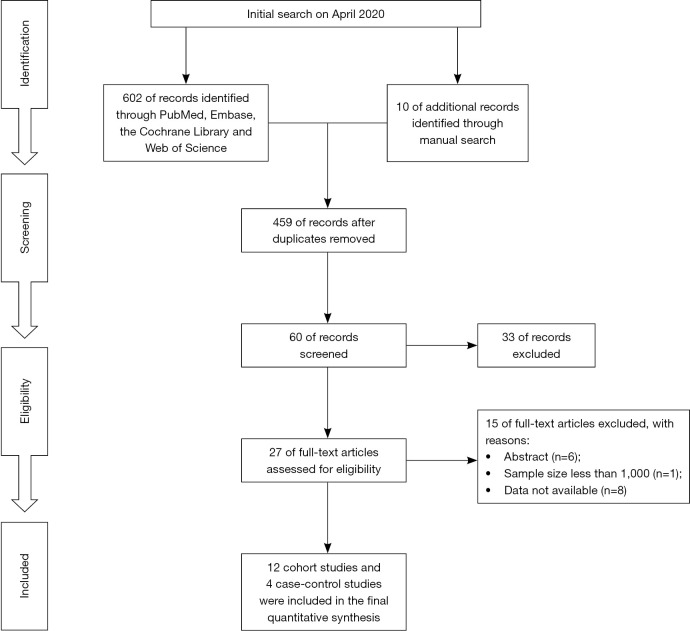
Studies were regarded as eligible if they satisfied the following inclusion criteria: (I) patients developed with BCa, PCa, or MGCa after being diagnosed with IBD, irrespective of UC or CD; (II) standardized incidence ratio (SIR) or relative risk (RR) with 95% confidence intervals (CIs) were used to evaluate the association between IBD and risk of lower urinary tract tumors (BCa, PCa or MGCa); (III) population-based cohort studies or case-control studies; (IV) the follow-up of included studies should be more than 1 year. If the study population overlapped, we used the most recently published or most informative literature. Exclusion criteria were as follows: (I) any study which did not meet the inclusion criteria; (II) meeting abstracts, review or meta-analysis; (III) data not available. Figure 1 sketches the PRISMA flow diagram of this meta-analysis.
Figure 1.
The PRISMA flowchart showing the study selection process of this meta-analysis.
Data extraction and quality assessment
Two independent authors screened the search results based on title, abstract, and, finally, full text. Disagreements were resolved by discussion or a third party. We prospectively developed a homogeneous form to extract data. Data were independently extracted by two reviewers. The following data were extracted: the first author’s name, year of publication, country, study design, period, sample size, cancer types, outcomes (SIRs or RRs).
Two authors independently evaluated the methodological bias of included studies through the Newcastle-Ottawa Scale (NOS) (15). The NOS used a “star system” to assess the quality of study from three perspectives: the selection of the studies, the comparability of studies, and the assessment of outcome. Whenever seven or more of nine stars were received, the study was considered as high quality. Moreover, two reviewers independently rated the level of evidence of the included articles using the Oxford Centre for Evidence-Based Medicine criteria (16); This scale graded studies from strongest (level 1) to weakest (level 5) strength of evidence on the basis of study design and data quality.
Statistical analysis
Data were expressed as SIR or RR with 95% CIs. The Q and I2 statistics were calculated to estimate the heterogeneity among studies. If P>0.1 or I2≤50%, we used the fixed-effect models. Otherwise, a random-effect model was used. Besides, we reported the pooled analysis according to the cancer types and IBD subtype. Funnel plots were used to assess the possibility of publication bias, and the asymmetry of funnel plots was evaluated by Egger’s test and Begg’s test; We considered significant publication bias as a p value less than 0.1. Furthermore, we conducted sensitivity analyses to evaluate the robustness of the pooled results. Statistical significance was set at P<0.05. This meta-analysis was completed by STATA version 14.2 (StataCorp LP, College Station, TX, USA).
Results
Search results
Six hundred and twelve of records were initially identified through PubMed, Embase, the Cochrane Library and Web of Science from inception to April 2020, resulting 12 cohort studies (12,17-27) and 4 case-control (28-31) studies included in the final analysis based on title, abstract, and full text (Figure 1). A total of 511,259 participants were included in this population-based study. In the cohort studies, 10 of 12 studies with 174,094 patients reported available data for PCa, 9 of 12 studies with 136,502 patients were available for BCa and 6 of 12 studies were available for MGCa. In the case-control studies, a total of 4 studies containing 335,167 participants reported related outcomes of PCa, and 2 of 4 studies with 285,534 participants were available for BCa. Table 1 details the main characteristics of included studies in this study.
Table 1. the main characteristics of included studies in this meta-analysis.
| Study | Country | Design | Period | Population | Cancer type | Outcomes | NOS | LoE |
|---|---|---|---|---|---|---|---|---|
| Hemminki et al. 2009 | Sweden | Cohort | 1964–2004 | 21,788 CD | BCa, PCa and MGCa | SIR | 9 | 2b |
| Jung et al. 2017 | Korea | Cohort | 2011–2014 | 5,825 UC and 3,918 CD | BCa and PCa | SIR | 8 | 2b |
| Jussila et al. 2013 | Finland | Cohort | 2000–2010 | 16,649 UC and 5,315 CD | BCa and PCa | SIR | 7 | 2b |
| Kappelman et al. 2014 | Denmark | Cohort | 1978–2010 | 13,756 CD and 35,152 UC | BCa and PCa | SIR | 9 | 2b |
| Loo et al. 2019 | Canada | Cohort | 1998–2015 | 20,644 CD, 14,000 UC and 1,341 unspecified | PCa | SIR | 9 | 2b |
| Ekbom et al. 1991 | Sweden | Cohort | 1965–1983 | 1,655 CD and 3,121 UC | BCa, PCa and MGCa | SIR | 7 | 2b |
| Hemminki et al. 2008 | Sweden | Cohort | 1964–2004 | 27,606 UC | BCa, PCa and MGCa | SIR | 9 | 2b |
| Jess et al. 2013 | Denmark | Cohort | 1978–2010 | 1,515 UC and 810 CD | MGCa and PCa | SIR | 8 | 2b |
| So et al. 2017 | China | Cohort | 1990–2016 | 1,603 UC and 1,018 CD | PCa | SIR | 7 | 2b |
| Karlén et al. 1999 | Sweden | Cohort | 1955–1989 | 1,547 UC | BCa, PCa and MGCa | SIR | 8 | 2b |
| Bourrier et al. 2016 | France | Cohort | 2004–2005 | 11,759 CD and 7,727 UC | BCa | SIR | 9 | 2b |
| Persson et al. 1994 | Sweden | Cohort | 1955–1989 | 1,251 CD | BCa and MGCa | SIR | 7 | 2b |
| Mosher et al. 2018 | USA | Case-control | 1996–2015 | 2,080 IBD and 271,898 IBD-free | BCa and PCa | RR | 7 | 3b |
| Wilson et al. 2016 | UK | Case-control | 1995–2012 | 19,647 IBD and 19,647 IBD-free | PCa | HR | 8 | 3b |
| Burns et al. 2019 | USA | Case-control | 1996–2017 | 1,033 IBD and 9,306 IBD-free | PCa | HR | 9 | 3b |
| Bernstein et al. 2001 | Canada | Case-control | 1984–1997 | 6,027 IBD and 5,529 IBD-free | BCa | IRR | 9 | 3b |
CD, Crohn’s disease; UC, ulcerative colitis; IBD, inflammatory bowel disease; BCa, bladder cancer; PCa, prostate cancer; MGCa, male genital cancer; SIR, standardized incidence ratio; RR, relative risk; HR, hazard ratio; IRR, incidence rate ratio; NOS, Newcastle-Ottawa Scale; LoE, level of evidence.
Meta-analysis results
PCa
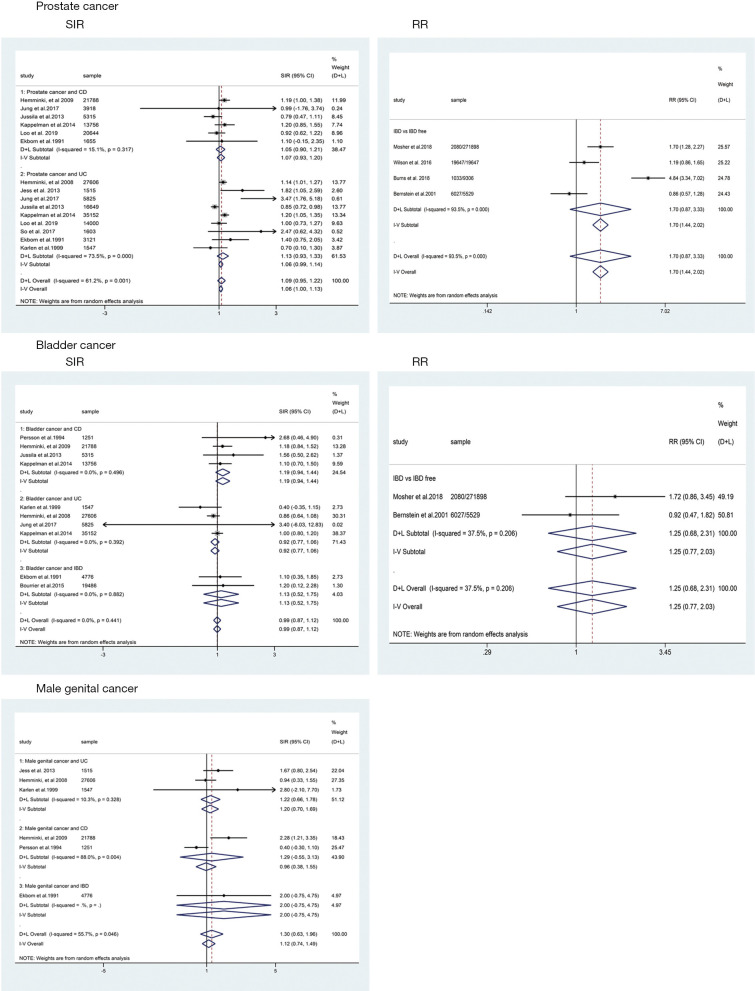
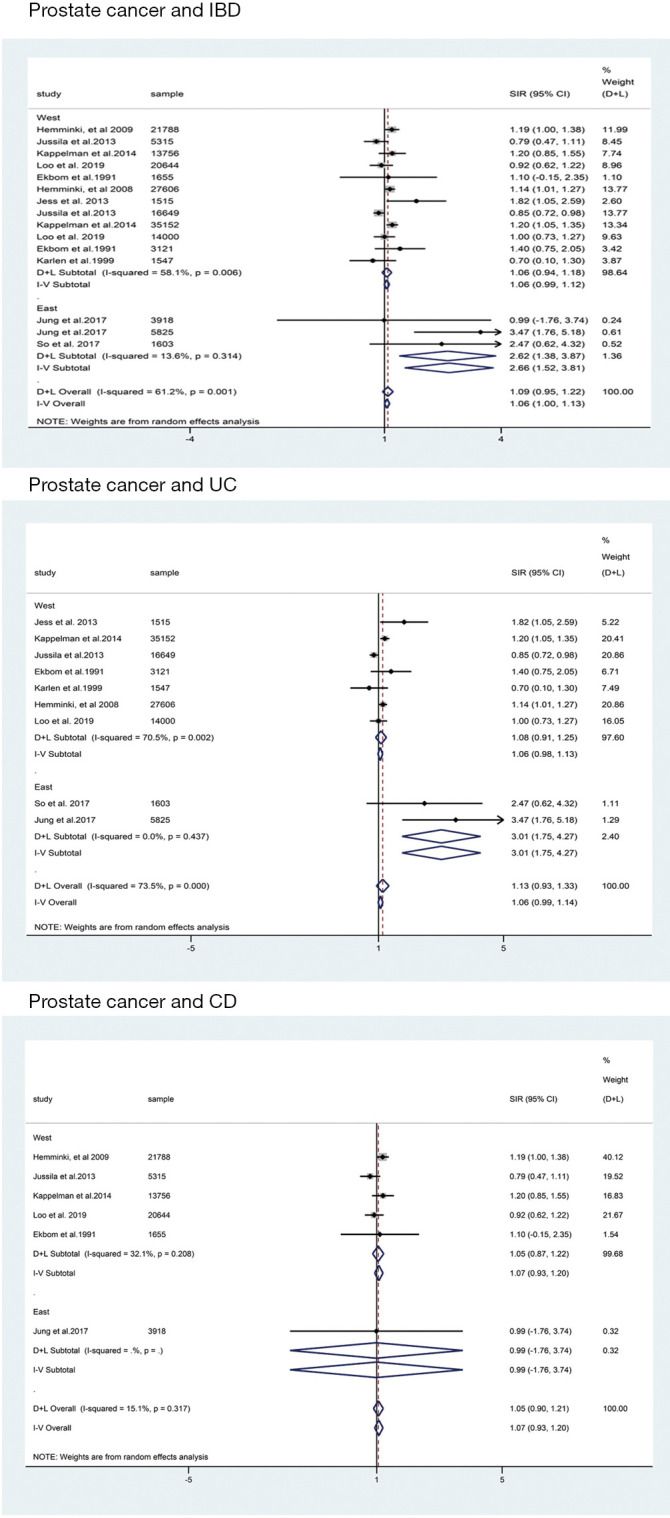
For cohort studies, the risk of PCa in patients with IBD were of borderline significant increase (SIR: 1.09; 95% CI: 0.95–1.22; I2=61.2%; Figure 2) when compared with the background population, as was the risk of subgroup of UC (SIR: 1.13; 95% CI: 0.93–1.33; I2=73.5%; Figure 2) and CD (SIR: 1.05; 95% CI: 0.90–1.21; I2=15.1%; Figure 2). For case-control studies, the results indicated that there was no significant difference between IBD group and IBD-free group regarding the risk of PCa (RR: 1.70; 95% CI: 0.87–3.33; Figure 2) with great between-study heterogeneity (I2=93.5%; P=0.000; Figure 2). Subgroup analysis revealed that IBD patients in Eastern countries have significantly increased PCa risk (SIR: 2.62; 95% CI: 1.38–3.87; I2=13.6%; Figure 3), especially for UC patients (SIR: 3.01; 95% CI: 1.75–4.27; I2=0.0%; Figure 3).
Figure 2.
The pooled results of this meta-analysis. IBD, inflammatory bowel disease; UC, ulcerative colitis; CD, Crohn’s disease; SIR, standardized incidence ratio; RR, relative risk; CI, confidence interval; D+L, DerSimonian-Laird; I-V, inverse-variance.
Figure 3.
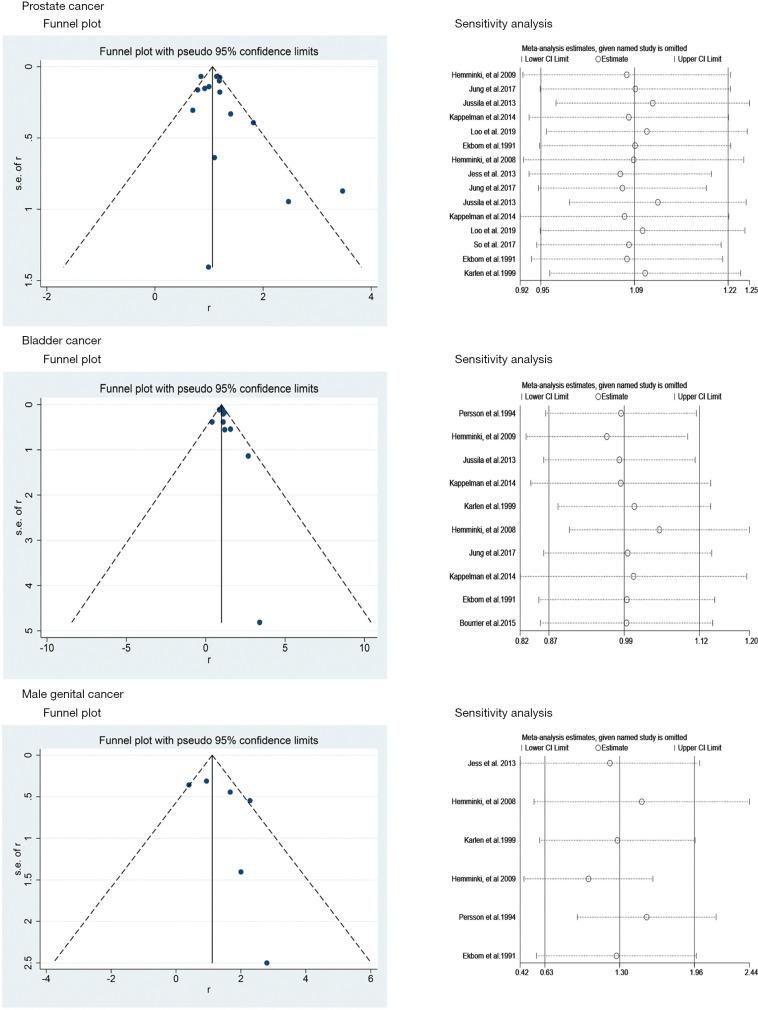
The funnel plots and sensitivity analyses of outcomes in this meta-analysis. IBD, inflammatory bowel disease; UC, ulcerative colitis; CD, Crohn’s disease; SIR, standardized incidence ratio; CI, confidence interval; D+L, DerSimonian-Laird; I-V, inverse-variance.
BCa
For cohort studies, no significantly decreased risk of BCa was observed in patients with IBD (SIR: 0.99; 95% CI: 0.87–1.12; I2=0.0%; Figure 2) and UC subgroup (SIR: 0.92; 95% CI: 0.77–1.06; I2=0.0%; Figure 2), whereas a trend toward an increased risk of BCa was detected in patients with CD subgroup (SIR: 1.19; 95% CI: 0.94–1.44; I2=0.0%; Figure 2). Only two case-control studies provided data on BCa in UC and CD combined, revealing that IBD patients seemed to have a higher risk of BCa than IBD-free patients (RR: 1.25; 95% CI: 0.68–2.31; I2=37.5%; Figure 2).
MGCa
Data from six cohort studies (17,18,21,22,24,26) showed that IBD or IBD subtype (UC and CD) did not significantly increase the risk of MGCa. The pooled SIRs were 1.30, 1.20 and 1.29 for IBD, UC and CD, respectively (Figure 2).
Publication bias and sensitivity analysis
No significant asymmetry was observed in the present study in terms of PCa and BCa through the corresponding funnel plots (Figure 4). The P values of Egger’s test for PCa, BCa and MGCa were 0.234, 0.194 and 0.276, respectively. Furthermore, sensitivity analysis revealed that there was no significant change of the pooled results for all outcomes observed through excluding each study from the meta-analysis sequentially (Figure 4).
Figure 4.
The subgroup analysis of prostate cancer according to geographical region. CI, confidence interval.
Discussion
In this study, we found there was no significant difference between IBD patients and background population regarding the risk of PCa, BCa or MGCa. However, a trend toward higher risk of PCa was suggested in patients with IBD, CD, and UC. For BCa, CD patients seemed to have a higher risk. As was previously reported, lower incidence rate of PCa was observed in East Asian countries when compared with that in other regions (32), we performed a subgroup analysis to evaluate the risk of IBD related PCa in East Asian countries. Different from the general results above, a significantly increased risk of PCa in East Asian IBD patients was detected (SIR: 2.62; 95% CI: 1.38–3.87; I2=13.6%; Figure 3), especially for UC patients (SIR: 3.01; 95% CI: 1.75–4.27; I2=0.0%; Figure 3).
Great improvement has been made in understanding the interaction between inflammation and carcinogenesis. Current knowledge about the mechanism underlying inflammation related carcinogenesis mainly depicts inflammation induced epigenetic alterations and DNA damage as the key factors in carcinogenesis (33). IBD related malignancy has been the hot point for researchers in the past decades and agreement has been achieved on the increased risk of IBD related colorectal cancer (34). As discussed above, it is widely accepted that the chronic local inflammation in bowel is involved in the carcinogenesis of colorectal cancer (35). For extraintestinal cancers, previous epidemiological studies revealed a tight association between IBD and increased risk of numerous cancers like hematologic cancer, lung cancer and non-Hodgkin lymphoma (26,36). However, studies about the association between IBD and lower urinary tract cancers including BCa and MGCa are limited and no conclusive results are available. A previous meta-analysis evaluating the risk of extraintestinal cancers in IBD patients reported an increased risk of BCa (6). However, only 2 eligible studies were included in their pool analysis for risk of BCa, which could weaken the strength of their conclusion. For the risk of PCa in IBD patients, controversies still remain. Some studies reported that there was an association between increased risk of PCa and IBD (23,26,31). In contrast, in a previous meta-analysis of population-based studies, they found there was no increased incidence of PCa among IBD patients (6).
Though mechanism underlying increased risk of PCa among IBD patients in East Asian countries remains elusive, the findings of our study could still be of value in providing guidance for PCa surveillance in IBD patients, especially for those in East Asian countries. Meanwhile, as the risk of BCa was not statistically different between IBD patients and background population, we detected from the results a trend toward higher risk in IBD group. We believed that this might be due to the lack of data from Eastern countries in the included studies. These findings could be inconsistent with previous meta-analysis (6) and more qualitative studies are needed to yield a conclusive result in the future. Besides, although chronic inflammation was thought to be an important risk factor for extraintestinal cancer in IBD patients, mechanism for IBD related colorectal cancer and extraintestinal cancer might be different. Instead, immunosuppression medications that are widely used for IBD treatment have been demonstrated to be related to increased risk of numerous malignancies including non-Hodgkin lymphoma, acute myeloid leukemia and myelodysplastic syndromes, non-melanoma skin cancers and urinary tract cancers (9-12). Concerned the large range of immunosuppression agents currently available for IBD treatment and insufficient data from studies evaluating their effect on carcinogenesis in IBD patients, we were unable to perform a subgroup analysis to evaluate the effect of immunosuppression agents on the risk of cancer in IBD. Future studies conducted on this topic could be of great value in providing guidance in immunosuppression agent option for IBD treatment.
Conclusions
IBD did not significantly increase the risk of PCa, BCa and MGCa. CD patients seemed to have a higher risk of PCa and BCa, and UC patients seemed to have a higher risk of PCa. However, IBD patients, especially for UC patients, in Eastern countries have significantly increased PCa risk.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/tau-20-1020
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-1020). The authors have no conflicts of interest to declare.
References
- 1.Chi KR. Epidemiology: Rising in the East. Nature 2016;540:S100-2. 10.1038/540S100a [DOI] [PubMed] [Google Scholar]
- 2.Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn’s disease. Aliment Pharmacol Ther 2006;23:1097-104. 10.1111/j.1365-2036.2006.02854.x [DOI] [PubMed] [Google Scholar]
- 3.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001;48:526-35. 10.1136/gut.48.4.526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jess T, Simonsen J, Jørgensen KT, et al. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology 2012;143: 375-81.e1; quiz e13-4. 10.1053/j.gastro.2012.04.016 [DOI] [PubMed] [Google Scholar]
- 5.Yadav S, Singh S, Harmsen WS, et al. Effect of Medications on Risk of Cancer in Patients with Inflammatory Bowel Diseases: A Population-Based Cohort Study from Olmsted County, Minnesota. Mayo Clin Proc 2015;90:738-46. 10.1016/j.mayocp.2015.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedersen N, Duricova D, Elkjaer M, et al. Risk of extra-intestinal cancer in inflammatory bowel disease: meta-analysis of population-based cohort studies. Am J Gastroenterol 2010;105:1480-7. 10.1038/ajg.2009.760 [DOI] [PubMed] [Google Scholar]
- 7.Trikha M, Corringham R, Klein B, et al. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin Cancer Res 2003;9:4653-65. [PMC free article] [PubMed] [Google Scholar]
- 8.Gakis G. The role of inflammation in bladder cancer. Adv Exp Med Biol 2014;816:183-96. 10.1007/978-3-0348-0837-8_8 [DOI] [PubMed] [Google Scholar]
- 9.Long MD, Herfarth HH, Pipkin CA, et al. Increased risk for non-melanoma skin cancer in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2010;8:268-74. 10.1016/j.cgh.2009.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez A, Mounier M, Bouvier AM, et al. Increased risk of acute myeloid leukemias and myelodysplastic syndromes in patients who received thiopurine treatment for inflammatory bowel disease. Clin Gastroenterol Hepatol 2014;12:1324-9. 10.1016/j.cgh.2014.02.026 [DOI] [PubMed] [Google Scholar]
- 11.Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet 2009;374:1617-25. 10.1016/S0140-6736(09)61302-7 [DOI] [PubMed] [Google Scholar]
- 12.Bourrier A, Carrat F, Colombel JF, et al. Excess risk of urinary tract cancers in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Aliment Pharmacol Ther 2016;43:252-61. 10.1111/apt.13466 [DOI] [PubMed] [Google Scholar]
- 13.Ge Y, Shi Q, Yao W, et al. The association between inflammatory bowel disease and prostate cancer risk: a meta-analysis. Prostate Cancer Prostatic Dis 2020;23:53-8. 10.1038/s41391-019-0177-7 [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Ottawa Hospital Research Institute. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 16.Centre for Evidence-Based Medicine. Oxford Centre for evidence-based medicine: levels of evidence, 2009. Available online: http://www.cebm.net/oxford-centreevidence-based-medicine-levels-evidence-march-2009/
- 17.Persson PG, Karlén P, Bernell O, et al. Crohn's disease and cancer: a population-based cohort study. Gastroenterology 1994;107:1675-9. 10.1016/0016-5085(94)90807-9 [DOI] [PubMed] [Google Scholar]
- 18.Hemminki K, Li X, Sundquist J, et al. Cancer risks in Crohn disease patients. Ann Oncol 2009;20:574-80. 10.1093/annonc/mdn595 [DOI] [PubMed] [Google Scholar]
- 19.Jussila A, Virta LJ, Pukkala E, et al. Malignancies in patients with inflammatory bowel disease: a nationwide register study in Finland. Scand J Gastroenterol 2013;48:1405-13. 10.3109/00365521.2013.846402 [DOI] [PubMed] [Google Scholar]
- 20.Kappelman MD, Farkas DK, Long MD, et al. Risk of cancer in patients with inflammatory bowel diseases: a nationwide population-based cohort study with 30 years of follow-up evaluation. Clin Gastroenterol Hepatol 2014;12:265-73.e1. 10.1016/j.cgh.2013.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlén P, Löfberg R, Broström O, et al. Increased risk of cancer in ulcerative colitis: a population-based cohort study. Am J Gastroenterol 1999;94:1047-52. 10.1111/j.1572-0241.1999.01012.x [DOI] [PubMed] [Google Scholar]
- 22.Hemminki K, Li X, Sundquist J, et al. Cancer risks in ulcerative colitis patients. Int J Cancer 2008;123:1417-21. 10.1002/ijc.23666 [DOI] [PubMed] [Google Scholar]
- 23.Jung YS, Han M, Park S, et al. Cancer Risk in the Early Stages of Inflammatory Bowel Disease in Korean Patients: A Nationwide Population-based Study. J Crohns Colitis 2017;11:954-62. 10.1093/ecco-jcc/jjx040 [DOI] [PubMed] [Google Scholar]
- 24.Ekbom A, Helmick C, Zack M, et al. Extracolonic malignancies in inflammatory bowel disease. Cancer 1991;67:2015-9. [DOI] [PubMed] [Google Scholar]
- 25.Loo SY, Vutcovici M, Bitton A, et al. Risk of Malignant Cancers in Inflammatory Bowel Disease. J Crohns Colitis 2019;13:1302-10. 10.1093/ecco-jcc/jjz058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jess T, Horváth-Puhó E, Fallingborg J, et al. Cancer risk in inflammatory bowel disease according to patient phenotype and treatment: a Danish population-based cohort study. Am J Gastroenterol 2013;108:1869-76. 10.1038/ajg.2013.249 [DOI] [PubMed] [Google Scholar]
- 27.So J, Tang W, Leung WK, et al. Cancer Risk in 2621 Chinese Patients with Inflammatory Bowel Disease: A Population-based Cohort Study. Inflamm Bowel Dis 2017;23:2061-8. 10.1097/MIB.0000000000001240 [DOI] [PubMed] [Google Scholar]
- 28.Mosher CA, Brown GR, Weideman RA, et al. Incidence of Colorectal Cancer and Extracolonic Cancers in Veteran Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis 2018;24:617-23. 10.1093/ibd/izx046 [DOI] [PubMed] [Google Scholar]
- 29.Bernstein CN, Blanchard JF, Kliewer E, et al. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer 2001;91:854-62. [DOI] [PubMed] [Google Scholar]
- 30.Wilson JC, Furlano RI, Jick SS, et al. A population-based study examining the risk of malignancy in patients diagnosed with inflammatory bowel disease. J Gastroenterol 2016;51:1050-62. 10.1007/s00535-016-1199-8 [DOI] [PubMed] [Google Scholar]
- 31.Burns JA, Weiner AB, Catalona WJ, et al. Inflammatory Bowel Disease and the Risk of Prostate Cancer. Eur Urol 2019;75:846-52. 10.1016/j.eururo.2018.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu J, Goodman M, Jemal A, et al. Prostate Cancer Prognostic Factors Among Asian Patients Born in the US Compared to Those Born Abroad. J Immigr Minor Health 2015;17:625-31. 10.1007/s10903-014-0023-x [DOI] [PubMed] [Google Scholar]
- 33.Murata M. Inflammation and cancer. Environ Health Prev Med 2018;23:50. 10.1186/s12199-018-0740-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herrinton LJ, Liu L, Levin TR, et al. Incidence and mortality of colorectal adenocarcinoma in persons with inflammatory bowel disease from 1998 to 2010. Gastroenterology 2012;143:382-9. 10.1053/j.gastro.2012.04.054 [DOI] [PubMed] [Google Scholar]
- 35.Dulai PS, Sandborn WJ, Gupta S. Colorectal Cancer and Dysplasia in Inflammatory Bowel Disease: A Review of Disease Epidemiology, Pathophysiology, and Management. Cancer Prev Res (Phila) 2016;9:887-94. 10.1158/1940-6207.CAPR-16-0124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Heuvel TRA, Wintjens DSJ, Jeuring SFG, et al. Inflammatory bowel disease, cancer and medication: Cancer risk in the Dutch population-based IBDSL cohort. Int J Cancer 2016;139:1270-80. 10.1002/ijc.30183 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as