Abstract
Background
Expression of Long non-coding RNA (LncRNA) small nucleolar RNA host gene 9 (SNHG9) is observed in some cancer types, while its role in prostate cancer (PCa) is unclear. We aimed to demonstrate the relationship between SNHG9 and PCa based on The Cancer Genome Atlas (TCGA) database.
Methods
Kruskal-Wallis test, Wilcoxon signed-rank test, and logistic regression were used to evaluate relationships between clinical-pathologic features and SNHG9 expression. Receiver operating characteristic (ROC) curves were used to describe binary classifier value of SNHG9 using area under curve (AUC) score. Kaplan-Meier method and Cox regression analysis were used to evaluate factors contributing to prognosis. Gene set enrichment analysis (GSEA) and immune infiltration analysis were performed to identify the significantly involved functions of SNHG9.
Results
Increased SNHG9 expression in PCa was associated with N stage (P<0.001), Gleason score (P=0.002), primary therapy outcome (P=0.001), residual tumor (P<0.001) and prostate specific antigen (PSA) (P=0.007). ROC curve suggested the significant diagnostic and prognostic ability of SNHG9 (AUC =0.815). High SNHG9 expression predicted a poorer progression-free survival (PFS) (P=0.002), and SNHG9 expression (HR: 1.776; 95% CI: 1.067–2.955; P=0.027) was independently correlated with PFS in PCa patients. GSEA and immune infiltration analysis showed that SNHG9 expression was correlated with regulating the function of ribosome and some types of immune infiltrating cells.
Conclusions
SNHG9 expression was significantly correlated with poor survival and immune infiltrations in PCa, and it may be a promising prognostic biomarker in PCa.
Keywords: Small nucleolar RNA host gene 9 (SNHG9), biomarker, prostate cancer (PCa), prognosis
Introduction
Prostate cancer (PCa) is currently ranked as the second cancer affecting the health of men worldwide (1). For most PCa patients, although the growth of the tumor begins to significantly slow down or even stagnate after undergoing physical or chemical castration treatment, castration resistance and metastasis or recurrence will still inevitably occur, resulting in high mortality of patients (2-5). Although prostate specific antigen (PSA) has been put into clinical use as a biomarker for the diagnosis and prognosis of PCa, it still has some limitations, such as differences in various races or ethnic groups and low sensitivity. Thus, it is important to find new biomarkers for diagnosing the occurrence, development and recurrence of PCa.
Small nucleolar RNA host gene (SNHG) family is a group of lncRNAs which acts as novel oncogenes in various cancers (6-10). A recent study demonstrated that SNHG1 can be regulated by miRNAs and promote the immune escape of breast cancer (11,12). SNHG6 can promote the progression of colon and rectal adenocarcinoma and tumorigenesis of glioma via modulating the expression levels of miR-101-3p (13,14). Moreover, SNHG8 is a potential biomarker and therapeutic target for hepatocellular carcinoma and non-small cell lung cancer (15,16). Although a few studies have shown that SNHG9 can play a key role in cancer development (17-20), no literature has explored the correlation between SNHG9 and PCa. Therefore, the present study aimed to clarify the expression of SNHG9 in PCa tissues, and its potential therapeutic and prognostic values.
In this research, we used the PCa RNA-seq data in The Cancer Genome Atlas (TCGA) database to compare the difference of SNHG9 expression between tumor tissues and normal samples, and investigate the correlation between SNHG9 expression levels and clinical pathological features of PCa. Next, we evaluated the prognostic value of SNHG9 in PCa. In addition, gene-set enrichment analysis (GSEA) was performed on the high and low expression groups of SNHG9 to reveal its possible functions. Finally, by analyzing the correlation between SNHG9 expression and immune infiltration, we comprehensively explored and discussed the potential mechanism by which SNHG9 modulates the occurrence and development of PCa. We present the following article in accordance with the MDAR reporting checklist (available at: http://dx.doi.org/10.21037/tau-20-1134).
Methods
RNA-sequencing data and bioinformatics analysis
We used TCGA database (https://genome-cancer.ucsc.edu/) to collect RNA-seq data and clinical information from 495 cases of PCa projects, including 52 cases with matched adjacent tissues. The downloaded data format was level 3 HTSeq-fragments per kilobase per million (FPKM) and then was converted into transcripts per million (TPM) format for subsequent analysis. We also download TPM format RNA-seq data in TCGA and Genotype-Tissue Expression (GTEx) database that uniformly processed by Toil process from UCSC Xena (https://xenabrowser.net/datapages/) (21). All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013).
Gene set enrichment analysis (GSEA)
The GSEA and MSigDB v6.2 (Molecular Signatures Database) were downloaded from the GSEA website (http://software.broadinstitute.org/gsea/index.jsp). The R package clusterProfiler (version 3.6.0) was used to perform GSEA between high- and low-SNHG9 groups (22,23). According to the default statistical method, the process was repeated 1,000 times for each analysis and selected c5.all.v7.0.symbols.gmt (Gene ontology) in MSigDB Collections as the reference gene collection, false discovery rate (FDR) q-value <0.25 and adjusted P adjust <0.05 are considered to be significantly enriched.
Immune infiltration analysis by ssGSEA
The immune infiltration analysis of PCa was performed by single sample GSEA (ssGSEA) method from R package GSVA (version 3.6) (http://www.bioconductor.org/packages/release/bioc/html/GSVA.html), and we quantified the infiltration levels of 24 immune cell types from gene expression profile in the literature (24). In order to discover the correlation between SNHG9 and the infiltration levels of 24 immune cells, P values were determined by the Spearman and Wilcoxon rank sum test.
Statistical analysis
All statistical analyses were performed using R (v.3.6.3). The relationship between clinical pathologic features and SNHG9 was analyzed using the Wilcoxon rank sum test, Chi-square test, Fisher exact test, and logistic regression. TCGA patient survival rates were calculated using the Kaplan-Meier method. Univariate and multivariate analysis were performed to estimate the association between clinical and genetic clinical characteristics and progression-free survival (PFS) using Cox proportional hazard models. P values less than 0.05 were considered statistically significant.
Results
SNHG9 expression is correlated with poor clinicopathological features of PCa
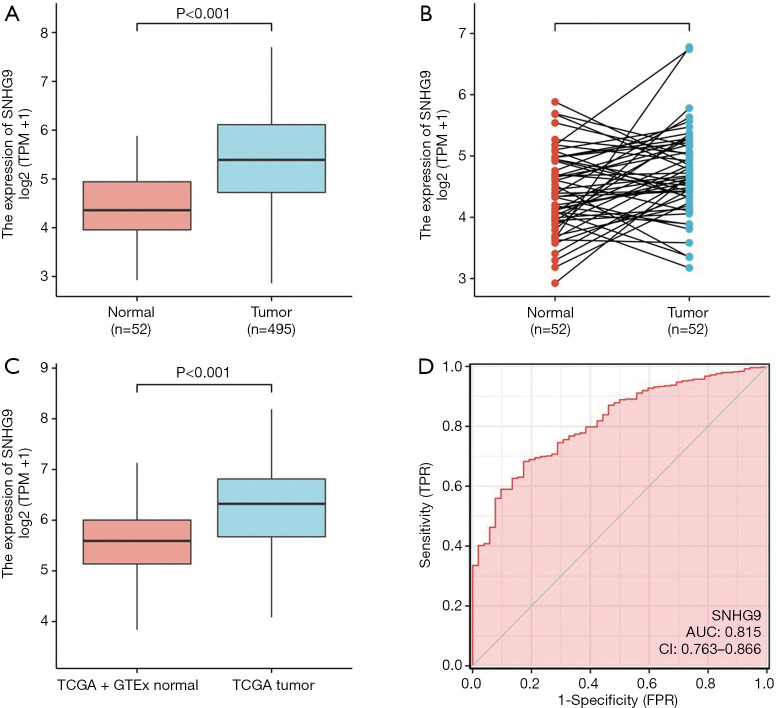
In order to identify the difference of SNHG9 expression between PCa and normal tissues, we analyzed the expression level of SNHG9 in 495 PCa tissues and 52 adjacent prostate tissues, and found that SNHG9 was highly expressed in PCa tissues (P<0.001, Figure 1A). Meanwhile, we also analyzed the expression of SNHG9 in 52 PCa tissues and their matched adjacent tissues. The results indicated that PCa tissues highly expressed SNHG9 (P=0.031, Figure 1B). Moreover, the expression of SNHG9 in normal samples of GTEx combined TCGA database and PCa samples of TCGA database was compared. We also found that SNHG9 was significantly overexpressed in PCa samples (P<0.001, Figure 1C). To determine differences of SNHG9 expression in tumors and normal tissues, the SNHG9 transcription levels in different multiple cancer types and normal tissues were analyzed using the TCGA and GTEx database. This analysis revealed that the SNHG9 expression was higher in multiple types of cancers compared to the normal tissues (Figure S1). In addition, we used the receiver operating characteristic (ROC) curve to analyze the effectiveness of SNHG9 expression level to distinguish PCa tissues from non-tumor tissues. The area under curve (AUC) of SNHG9 was 0.815, suggesting that SNHG9 could be served as an ideal biomarker to distinguish PCa from non-tumor tissue (Figure 1D).
Figure 1.
SNHG9 expression and clinicopathological features of PCa. (A) Wilcoxon rank sum test was used to analyze the difference expression of SNHG9 in PCa tissues and adjacent prostate tissues. (B) Wilcoxon signed rank sum test was used to detect the difference expression of SNHG9 in PCa tissues and adjacent prostate tissues. (C) Wilcoxon rank sum test was used to analyze the difference expression of SNHG9 in normal prostate tissues of GTEx combined with TCGA and PCa tissues of TCGA. (D) ROC curve showed the efficiency of SNHG9 expression level to distinguishing PCa tissue from non-tumor tissue. X-axis represents false positive rate, and Y-axis represents true positive rate.
The characteristics of patients were shown in Table 1, in which 495 primary PCa with both clinical and gene expression data were collected from TCGA database. According to the mean value of relative SNHG9 expression, the patients with PCa were divided into high (n=248) and low (n=247) expression groups. The association between the expression level of SNHG9 and the clinicopathological characteristics of PCa patients was evaluated. Chi-square test or Fisher’s exact test revealed that SNHG9 expression was associated with N stage (P<0.001), Gleason score (P=0.002), primary therapy outcome (P=0.001) and residual tumor (P<0.001). Using Student’s t-test or Wilcoxon signed rank sum test, we found that SNHG9 expression was associated with PSA level (P=0.007).
Table 1. Correlation between SNHG9 expression and clinicopathological characteristics in prostate cancer.
| Characters | Level | Low expression of SNHG9 | High expression of SNHG9 | P | Test |
|---|---|---|---|---|---|
| n | 248 | 247 | |||
| T stage, n (%) | T2 | 105 (43.4) | 82 (33.3) | 0.064 | Exact |
| T3 | 133 (55.0) | 158 (64.2) | |||
| T4 | 4 (1.7) | 6 (2.4) | |||
| N stage, n (%) | N0 | 185 (88.9) | 159 (74.3) | <0.001 | |
| N1 | 23 (11.1) | 55 (25.7) | |||
| M stage, n (%) | M0 | 222 (99.6) | 231 (99.1) | 1.000 | Exact |
| M1 | 1 (0.4) | 2 (0.9) | |||
| Gleason score, n (%) | 10 | 3 (1.2) | 1 (0.4) | 0.002 | Exact |
| 6 | 20 (8.1) | 25 (10.1) | |||
| 7 | 144 (58.1) | 102 (41.3) | |||
| 8 | 27 (10.9) | 36 (14.6) | |||
| 9 | 54 (21.8) | 83 (33.6) | |||
| Primary therapy outcome, n (%) | CR | 188 (85.1) | 149 (70.0) | 0.001 | |
| PD | 13 (5.9) | 15 (7.0) | |||
| PR | 12 (5.4) | 28 (13.1) | |||
| SD | 8 (3.6) | 21 (9.9) | |||
| Residual tumor, n (%) | R0 | 173 (74.6) | 141 (60.5) | <0.001 | Exact |
| R1 | 55 (23.7) | 91 (39.1) | |||
| R2 | 4 (1.7) | 1 (0.4) | |||
| Race, n (%) | Asian | 7 (2.9) | 5 (2.1) | 0.211 | |
| Black or African American | 34 (14.0) | 22 (9.2) | |||
| White | 201 (83.1) | 211 (88.7) | |||
| Zone of origin, n (%) | Central zone | 3 (3.2) | 1 (0.6) | 0.363 | Exact |
| Overlapping/multiple zones | 44 (46.8) | 82 (45.6) | |||
| Peripheral zone | 44 (46.8) | 92 (51.1) | |||
| Transition zone | 3 (3.2) | 5 (2.8) | |||
| TP53 status, n (%) | Mut | 21 (8.5) | 35 (14.2) | 0.065 | |
| WT | 225 (91.5) | 211 (85.8) | |||
| Age [median (IQR)] | 61.00 [56.00, 66.00] | 62.00 [56.00, 66.00] | 0.443 | Nonnorm | |
| PSA (ng/mL) [median (IQR)] | 0.10 [0.03, 0.11] | 0.10 [0.01, 0.12] | 0.007 | Nonnorm |
CR, complete response; PD, progressive disease; SD, stable disease; PR, partial response; PSA, prostate specific antigen.
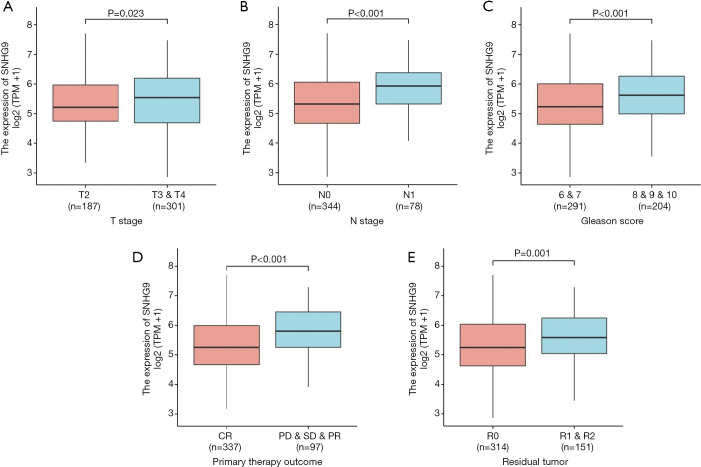
Logistic regression method was also used to show the relationship between the clinicopathological characteristics of PCa and expression level of SNHG9. The results suggested that SNHG9 was significantly related to T stage (P=0.023), N stage (P<0.001), Gleason score (P<0.001), primary therapy outcome (P<0.001) and residual tumor (P=0.001) (Table 2, Figure 2).
Table 2. SNHG9 expression associated with clinicopathologic characteristics (logistic regression).
| Characteristics | Odds ratio in SNHG9 expression | Odds ratio (OR) | P value |
|---|---|---|---|
| T stage (T3&T4 vs. T2) | 488 | 1.53 (1.06–2.22) | 0.023 |
| N stage (N1 vs. N0) | 422 | 2.78 (1.66–4.81) | <0.001 |
| M stage (M1 vs. M0) | 456 | 1.92 (0.18–41.53) | 0.595 |
| Gleason score (8&9&10 vs. 6&7) | 495 | 1.84 (1.29–2.66) | <0.001 |
| Primary therapy outcome (PD&SD&PR vs. CR) | 434 | 2.45 (1.54–3.96) | <0.001 |
| Residual tumor (R1&R2 vs. R0) | 465 | 1.91 (1.29–2.85) | 0.001 |
| PSA(ng/mL) (≥4 vs. <4) | 438 | 0.92 (0.42–2.02) | 0.843 |
| TP53 status (Mut vs. WT) | 492 | 1.78 (1.01–3.20) | 0.049 |
CR, complete response; PD, progressive disease; SD, stable disease; PR, partial response; PSA, prostate specific antigen.
Figure 2.
Association between SNHG9 expression and clinicopathologic characteristics in PCa. (A) Wilcoxon rank sum test was used to compare the relationship between the expression of SNHG9 and T stage of PCa patients in TCGA database. (B) Wilcoxon rank sum test was used to compare the relationship between the expression of SNHG9 and N stage of PCa patients in TCGA database. (C) Wilcoxon rank sum test was used to compare the relationship between the expression of SNHG9 and Gleason score of PCa patients in TCGA database. (D) Wilcoxon rank sum test was used to compare the relationship between the expression of SNHG9 and residual tumor of PCa patients in TCGA database. (E) Wilcoxon rank sum test was used to compare the relationship between the expression of SNHG9 and primary therapy outcome of PCa patients in TCGA database. CR, complete response; PD, progressive disease; SD, stable disease; PR, partial response.
SNHG9 expression is correlated with poor prognosis of patients with PCa
The association between SNHG9 expression and PFS of patients with PCa was evaluated by Kaplan-Meier analysis, which indicated that expression of SNHG9 is positively correlated with poor PFS of PCa patients (P=0.002, Figure 3A). In addition, to expand our observation to a pan-cancer level, expression of SNHG9 and patient survival was further analyzed in multiple cancer types other than PCa. As shown in Figure S2, significant association between SNHG9 expression and poor PFS was also observed in patients diagnosis of bladder carcinoma (BLCA), Cervical Squamous Cell Carcinoma (CESC), Kidney Renal Clear Cell Carcinoma (KIRC), Low Grade Glioma (LGG), Pancreatic adenocarcinoma (PAAD) and Uveal Melanoma (UVM).
Figure 3.
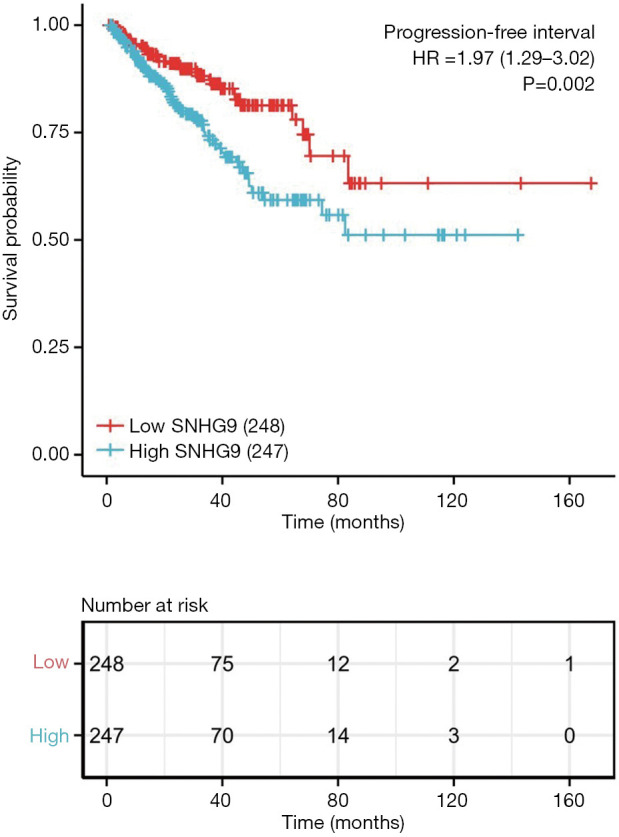
SNHG9 expression and prognosis in patients with PCa. Kaplan-Meier curve was drawn using the R package survminer to evaluate the prognostic value of SNHG9 in PFS of PCa patients. SNHG9 expression value was divided into high and low expression group according to median value.
Cox univariate and multivariate analysis of prognostic factors in PCa
Table 3 showed the results of Cox univariate and multivariate analysis of PFS in PCa patients. In the Cox univariate regression model, the variables with P<0.01 were T stage (P<0.001), N stage (P=0.013), Gleason score (P<0.001), primary therapy outcome (P<0.001), residual tumor (P<0.001), PSA (P<0.001), TP53 status (P=0.004) and SNHG9 (P=0.002). Multivariate analysis further revealed that Gleason score (P<0.001), primary therapy outcome (P<0.001) and SNHG9 (P<0.001) were independent prognostic factors in PFS of PCa patients.
Table 3. Associations with progression-free survival and clinicopathological characteristics in TCGA patients using Cox regression.
| Characteristics | Total(N) | HR (95% CI), univariate analysis | P value, univariate analysis | HR (95% CI), multivariate analysis | P value, multivariate analysis |
|---|---|---|---|---|---|
| T stage (T3 & T4 vs. T2) | 488 | 3.716 (2.100–6.575) | <0.001 | 1.471 (0.692–3.124) | 0.316 |
| N stage (N1 vs. N0) | 422 | 1.854 (1.137–3.026) | 0.013 | 0.726 (0.411–1.285) | 0.272 |
| M stage (M1 vs. M0) | 456 | 3.648 (0.505–26.354) | 0.200 | ||
| Gleason score (8 & 9 & 10 vs. 6 & 7) | 495 | 4.603 (2.909–7.284) | <0.001 | 3.120 (1.677–5.805) | <0.001 |
| Primary therapy outcome (PD & SD & PR vs. CR) | 434 | 6.793 (4.430–10.416) | <0.001 | 3.657 (2.085–6.414) | <0.001 |
| Residual tumor (R1 & R2 vs. R0) | 465 | 2.320 (1.533–3.510) | <0.001 | 1.028 (0.601–1.757) | 0.921 |
| PSA (ng/mL) (≥4 vs. <4) | 438 | 4.246 (2.119–8.510) | <0.001 | 1.437 (0.630–3.278) | 0.389 |
| Age (>60 vs. ≤60) | 495 | 1.274 (0.843–1.923) | 0.250 | ||
| Race (White vs. Asian & Black or African American) | 480 | 1.309 (0.726–2.360) | 0.371 | ||
| Zone of origin (overlapping/multiple zones vs. peripheral zone) | 262 | 1.293 (0.794–2.108) | 0.302 | ||
| TP53 status (Mut vs. WT) | 492 | 2.086 (1.258–3.461) | 0.004 | 0.956 (0.553-1.655) | 0.874 |
| SNHG9 (high vs. low) | 495 | 1.974 (1.290–3.020) | 0.002 | 1.776 (1.067-2.955) | 0.027 |
CR, complete response; PD, progressive disease; SD, stable disease; PR, partial response; PSA, prostate specific antigen.
SNHG9-related signaling pathways based on GSEA
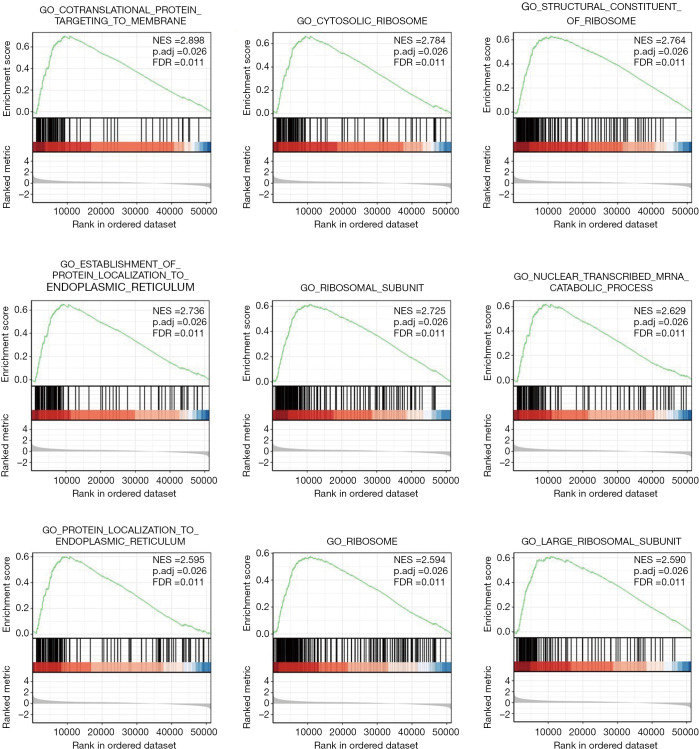
GSEA was used to identify SNHG9-related signaling pathways. GSEA revealed significant differences (Padj <0.05, FDR <0.25) in enrichment of MSigDB Collection (c5.all.v7.0.symbols.gmt). There were 3,461 data sets which showed significantly differential enrichment in SNHG9 high expression phenotype, and we selected the top 9 data sets with high value of normalized enrichment score (NES), with most of which were related to the function of ribosome (Table 4, Figure 4).
Table 4. GO terms enriched in high- and low-SNHG9 groups by using GSEA.
| Gene set name | NES | P.adjust | FDR |
|---|---|---|---|
| GO_COTRANSLATIONAL_PROTEIN_TARGETING_TO_MEMBRANE | 2.898 | 0.026 | 0.011 |
| GO_CYTOSOLIC_RIBOSOME | 2.784 | 0.026 | 0.011 |
| GO_STRUCTURAL_CONSTITUENT_OF_RIBOSOME | 2.764 | 0.026 | 0.011 |
| GO_ESTABLISHMENT_OF_PROTEIN_LOCALIZATION_TO_ENDOPLASMIC_RETICULUM | 2.736 | 0.026 | 0.011 |
| GO_RIBOSOMAL_SUBUNIT | 2.725 | 0.026 | 0.011 |
| GO_NUCLEAR_TRANSCRIBED_MRNA_CATABOLIC_PROCESS_NONSENSE_MEDIATED_DECAY | 2.629 | 0.026 | 0.011 |
| GO_PROTEIN_LOCALIZATION_TO_ENDOPLASMIC_RETICULUM | 2.595 | 0.026 | 0.011 |
| GO_RIBOSOME | 2.594 | 0.026 | 0.011 |
| GO_LARGE_RIBOSOMAL_SUBUNIT | 2.59 | 0.026 | 0.011 |
NES, normalized enrichment score; FDR, false discovery rate.
Figure 4.
Enrichment plot from the GSEA. The data set was on the left significantly enriched in red area (SNHG9 high expression group). NES, normalized NS; Padj, adjust P value; FDR, false discovery rate.
The correlation between SNHG9 expression and immune infiltration
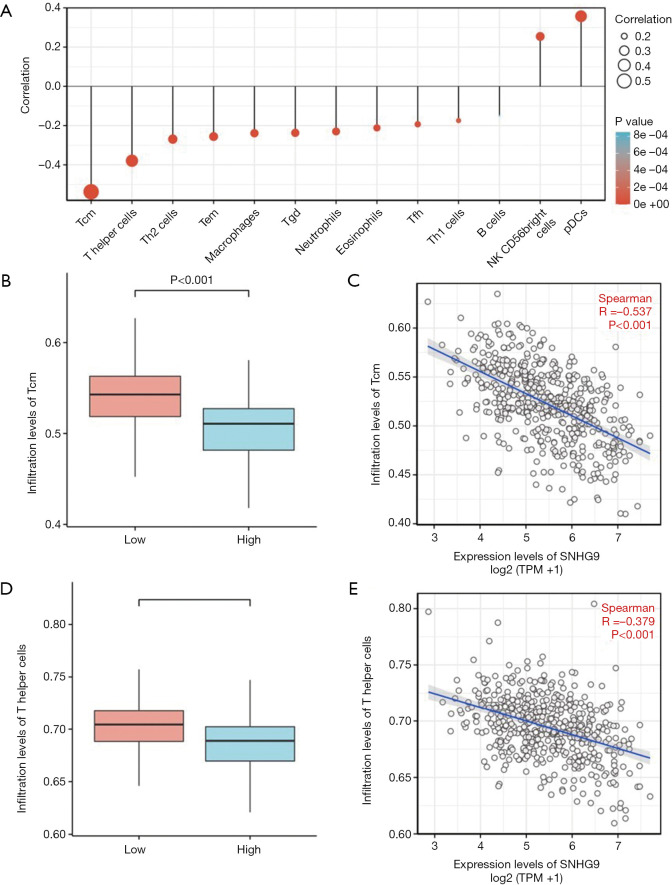
We further analyzed the correlation between expression of SNHG9 and immune infiltration by ssGSEA with Spearman r. The results showed that SNHG9 expression was negatively correlated with infiltration levels of T central memory (Tcm) cells and T helper cells (P<0.001, Figure 5), and positively correlated with that of plasmacytoid dendritic cells (pDCs) and natural killer (NK) CD56 bright cells (P<0.001, Figure S3).
Figure 5.
The expression level of SNHG9 was related to the immune infiltration in the tumor microenvironment. (A) The forest plot shows the correlation between SNHG9 expression level and 24 immune cells. The size of dots indicates the absolute value of Spearman r. The Wilcoxon rank sum test was used to analyze the difference of Tcm cells infiltration level between SNHG9 high and low expression groups (B), and the correlation between SNHG9 expression and Tcm cells was detected by Spearman correlation method (C). The Wilcoxon rank sum test was used to analyze the difference of T helper cells infiltration level between SNHG9 high and low expression groups (D), and the correlation between SNHG9 expression and T helper cells was detected by Spearman correlation method (E). Tcm, T central memory; Tem, T effector memory; Tgd, T gamma delta; Tfh, T follicular helper; NK, natural killer; pDCs, plasmacytoid dendritic cells.
Discussion
In this study, the expression of LncRNA SNHG9 in PCa and its correlation with PCa diagnosis and prognosis were explored. Dysregulation of SNHG9 has been reported in several cancer types, which plays an essential role during cancer initiation and progression. For instance, a recent study demonstrated that SNHG9 expression was significantly altered in the tissues and serum of pancreatic cancer patients, and its expression was significantly correlated with the TNM stages. In addition, silencing of SNHG9 promoted pancreatic cell proliferation (20). In addition, SNHG9 has been reported to promote aerobic glycolysis and glioblastoma cell proliferation, and this effect might be exerted via a miR-199a-5p/Wnt2-dependent pathway (19). Knockdown of SNHG9 in lung cancer cells reduced the cell growth, proliferation and invasion both in vitro and in vivo (18). Another study revealed that SNHG9 directly binds to TRADD, activates the NF-κB signaling, thus suppresses inflammation and apoptosis, eventually fulfills its protective effect on endothelial function (17). In contrast, down-regulation of SNHG9 was observed in non-small cell lung cancer. The authors further revealed that SNHG9 could downregulate miR-21 by increasing the methylation level of this oncogenic miRNA, and thus suppress cancer cell proliferation (25). All these studies suggest that SNHG9 may play different roles in various cancer types. The present study demonstrated the elevated level of SNHG9 in PCa tissues, which is associated with poor patient outcomes. Similarly, high expression of SNHG9 was also inversely associated with overall survival (OS) of lung cancer patients (18). In addition to PCa, ROC analysis and survival analysis showed that SNHG9 could act as a prognostic indictor of pancreatic cancer as well (20).
A highlight of this work is to predict the potential mechanisms by which SNHG9 regulates the development of PCa. Through GSEA, SNHG9 was found to be involved in targeting to membrane, locating to endoplasmic reticulum and affecting the ribosome function, indicating that SNHG9 may play a role in the maintenance of cell metabolism and protein synthesis. Another important aspect of this study is to investigate the relationship between SNHG9 expression and diverse immune infiltration levels in PCa. Our results revealed a moderate relationship between SNHG9 expression and infiltration level of Tcm, T helper cells, pDCs and NK CD56bright cells in PCa. These correlations could be indicative of a potential mechanism by which SNHG9 inhibits the function of Tcm and T helper cells, subsequently promotes the function of pDCs and NK CD56 bright cells, and thus exerts its inhibitory effect on PCa.
To our best of knowledge, it is the first work to explore the relationship between SNHG9 and PCa, despite the existence of some limitations. First, the current study was performed primarily based on bioinformatic analyses, which could be further strengthened by experimental research. Second, the number of healthy subjects used as controls is largely different from that of cancer patients. Last but not most, retrospective studies still have their own limits, especially nonuniform intervening measures, and lacking of some information. As a result, follow-up studies are needed to further validate our findings.
Conclusions
Collectively, we observed increased SNHG9 in PCa, which was also related to poor PFS. Moreover, SNHG9 might participate in the development of PCa via affecting the function of ribosome and immune infiltrating cells. The current study partially unveiled the roles of SNHG9 in PCa and provided a potential biomarker for the diagnosis and prognosis of PCa.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013).
Footnotes
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/tau-20-1134
Peer Review File: Available at http://dx.doi.org/10.21037/tau-20-1134
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-1134). The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411-22. 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- 3.Clyne M. Prostate cancer: androgen deprivation causes EMT in the prostate. Nat Rev Urol 2011;9:4. 10.1038/nrurol.2011.208 [DOI] [PubMed] [Google Scholar]
- 4.Kassi E, Moutsatsou P. Glucocorticoid receptor signaling and prostate cancer. Cancer Lett 2011;302:1-10. 10.1016/j.canlet.2010.10.020 [DOI] [PubMed] [Google Scholar]
- 5.Attard G, Parker C, Eeles RA, et al. Prostate cancer. Lancet 2016;387:70-82. 10.1016/S0140-6736(14)61947-4 [DOI] [PubMed] [Google Scholar]
- 6.Zimta AA, Tigu AB, Braicu C, et al. An Emerging Class of Long Non-coding RNA With Oncogenic Role Arises From the snoRNA Host Genes. Front Oncol 2020;10:389. 10.3389/fonc.2020.00389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Gao J, Kan A, et al. SNHG and UCA1 as prognostic molecular biomarkers in hepatocellular carcinoma: recent research and meta-analysis. Minerva Med 2017;108:568-74. [DOI] [PubMed] [Google Scholar]
- 8.Shuwen H, Xi Y, Quan Q, et al. Can small nucleolar RNA be a novel molecular target for hepatocellular carcinoma? Gene 2020;733:144384. 10.1016/j.gene.2020.144384 [DOI] [PubMed] [Google Scholar]
- 9.Yang H, Jiang Z, Wang S, et al. Long non-coding small nucleolar RNA host genes in digestive cancers. Cancer Med 2019;8:7693-704. 10.1002/cam4.2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Q, Yang H, Cheng P, et al. Bioinformatic analysis of the prognostic value of the lncRNAs encoding snoRNAs in hepatocellular carcinoma. Biofactors 2019;45:244-52. 10.1002/biof.1478 [DOI] [PubMed] [Google Scholar]
- 11.Xiong X, Feng Y, Li L, et al. Long non-coding RNA SNHG1 promotes breast cancer progression by regulation of LMO4. Oncol Rep 2020;43:1503-15. 10.3892/or.2020.7530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pei X, Wang X, Li H. LncRNA SNHG1 regulates the differentiation of Treg cells and affects the immune escape of breast cancer via regulating miR-448/IDO. Int J Biol Macromol 2018;118:24-30. 10.1016/j.ijbiomac.2018.06.033 [DOI] [PubMed] [Google Scholar]
- 13.Shao Q, Xu J, Deng R, et al. SNHG 6 promotes the progression of Colon and Rectal adenocarcinoma via miR-101-3p and Wnt/β-catenin signaling pathway. BMC Gastroenterol 2019;19:163. 10.1186/s12876-019-1080-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng Q, Yang BY, Liu B, et al. Long non-coding RNA SNHG6 promotes glioma tumorigenesis by sponging miR-101-3p. Int J Biol Markers 2018;33:148-55. 10.1177/1724600817747524 [DOI] [PubMed] [Google Scholar]
- 15.Dong J, Teng F, Guo W, et al. lncRNA SNHG8 Promotes the Tumorigenesis and Metastasis by Sponging miR-149-5p and Predicts Tumor Recurrence in Hepatocellular Carcinoma. Cell Physiol Biochem 2018;51:2262-74. 10.1159/000495871 [DOI] [PubMed] [Google Scholar]
- 16.Chen C, Zhang Z, Li J, et al. SNHG8 is identified as a key regulator in non-small-cell lung cancer progression sponging to miR-542-3p by targeting CCND1/CDK6. Onco Targets Ther 2018;11:6081-90. 10.2147/OTT.S170482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song Y, Li H, Ren X, et al. SNHG9, delivered by adipocyte-derived exosomes, alleviates inflammation and apoptosis of endothelial cells through suppressing TRADD expression. Eur J Pharmacol 2020;872:172977. 10.1016/j.ejphar.2020.172977 [DOI] [PubMed] [Google Scholar]
- 18.Lin Y, Holden V, Dhilipkannah P, et al. A Non-Coding RNA Landscape of Bronchial Epitheliums of Lung Cancer Patients. Biomedicines 2020;8:88. 10.3390/biomedicines8040088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H, Qin D, Jiang Z, et al. SNHG9/miR-199a-5p/Wnt2 Axis Regulates Cell Growth and Aerobic Glycolysis in Glioblastoma. J Neuropathol Exp Neurol 2019;78:939-48. 10.1093/jnen/nlz078 [DOI] [PubMed] [Google Scholar]
- 20.Zhang B, Li C, Sun Z. Long non-coding RNA LINC00346, LINC00578, LINC00673, LINC00671, LINC00261, and SNHG9 are novel prognostic markers for pancreatic cancer. Am J Transl Res 2018;10:2648-58. [PMC free article] [PubMed] [Google Scholar]
- 21.Vivian J, Rao AA, Nothaft FA, et al. Toil enables reproducible, open source, big biomedical data analyses. Nat Biotechnol 2017;35:314-6. 10.1038/nbt.3772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu G, Wang LG, Han Y, et al. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 2012;16:284-7. 10.1089/omi.2011.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005;102:15545-50. 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bindea G, Mlecnik B, Tosolini M, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013;39:782-95. 10.1016/j.immuni.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 25.Wang D, Cao X, Han Y, et al. LncRNA SNHG9 is Downregulated in Non-Small Cell Lung Cancer and Suppressed miR-21 Through Methylation to Promote Cell Proliferation. Cancer Manag Res 2020;12:7941-8. 10.2147/CMAR.S253052 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as