Abstract
TMPRSS3 belongs to the large type II transmembrane serine protease (TTSP) family, which plays an important role in the development and progression of tumors. However, the function of TMPRSS3 in nasopharyngeal carcinoma (NPC) remains unclear. The present study aimed to examine the impact of TMPRSS3 on the proliferation, migration, and invasion of NPC cells and their potential mechanisms. Our results demonstrated that the expression of TMPRSS3 was obviously upregulated in human NPC tissues and cell lines. Knockdown of TMPRSS3 expression significantly suppressed the proliferation and tumorigenicity of NPC cells in vitro and in vivo. Furthermore, knockdown of TMPRSS3 inhibited migration and invasion, as well as prevented the EMT process in NPC cells. Finally, knockdown of TMPRSS3 attenuated activation of the PI3K/Akt signaling pathway in NPC cells. Taken together, the present study demonstrates that the knockdown of TMPRSS3 inhibits proliferation, migration, and invasion in human NPC cells through the inactivation of the PI3K/Akt signaling pathway. This study suggests that TMPRSS3 may be a potential therapeutic target for the treatment of NPC.
Key words: Nasopharyngeal carcinoma (NPC), TMPRSS3, Invasion, PI3K/Akt pathway
INTRODUCTION
Nasopharyngeal carcinoma (NPC) is a unique cancer arising from the epithelial lining of the nasopharynx1. NPC occurring with a high incidence rate is mainly in Southeast Asia and Southern China2. Despite advances in the development of radiation against NPC3,4, the survival of patients with NPC remains poor because most NPC patients are diagnosed in the later stages, and the majority of patients present with cervical lymph node metastasis5,6. Thus, elucidation of the molecular mechanisms underlying NPC metastasis and tumorigenesis is critical to the individual treatment of NPC.
TMPRSS3 belongs to the large type II transmembrane serine protease (TTSP) family, which plays an important role in a variety of biological and physiological processes7–9. It encodes for a protease that contains low-density lipoprotein receptor class A (LDLRA) and scavenger receptor cysteine-rich (SRCR) domains10. In addition, TMPRSS3 is found to be overexpressed in several cancers, and high expression levels of TMPRSS3 are associated with metastasis and poor prognosis for malignancies7,11,12. A recent study showed that downregulation of TMPRSS3 significantly suppressed the motility and invasion of ovarian cancer cells13. However, the function of TMPRSS3 in NPC remains unclear. The present study aimed to examine the impact of TMPRSS3 on the proliferation, migration, and invasion of NPC cells and their potential mechanisms. Our results demonstrated that knockdown of TMPRSS3 inhibits proliferation, migration, and invasion in human NPC cells. These results suggest that TMPRSS3 functions as an oncogene in the development of NPC.
MATERIALS AND METHODS
Clinical Specimens
Eight pairs of primary NPC biopsies and matched adjacent normal nasopharyngeal epithelium biopsies were collected from newly diagnosed NPC patients at the Department of ENT, Huai’an First People’s Hospital, Nanjing Medical University (P.R. China) from 2014 to 2015. This study was approved by the ethics committees of Huai’an First People’s Hospital, Nanjing Medical University (P.R. China). The patients were informed regarding sample collection and signed consent forms.
Cell Culture
Human NPC cell lines (HONE1, SUNE-5-8F, and CNE-1) and the immortalized nasopharyngeal epithelial cell line NP69 were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured at 37°C with 5% CO2 in Dulbecco’s modified Eagle’s medium with 10% heat-inactivated fetal bovine serum, 100 U of penicillin, and 100 μg/ml streptomycin.
ShRNA Transfection
The short hairpin RNA against TMPRSS3 (sh-TMPRSS3) and negative control shRNA (sh-NC) were purchased from GenePharma Co. (Shanghai, P.R. China). CNE-1 cells at a density of 1 × 105 cells/well were transfected with sh-TMPRSS3 or sh-NC using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted from NPC tissues and cells using the TRIzol reagent (Invitrogen) and reverse-transcribed into first-strand cDNA using M-MLV reverse transcriptase (Clontech, Palo Alto, CA, USA). qRT-PCR was performed using an ABI Prism 7500 System (Applied Biosystems, Foster City, CA, USA) with the SYBR Green Supermix (Invitrogen). The specific primers for TMPRSS3 were 5′-ATGGTGAGTAAAATGGGTGTGAGGA-3′ (sense) and 5′-CTTGGAAGTAGAAAGGGTGGGTTTG-3′ (antisense), and for β-actin were 5′-AAATCGTGCGTGACATCAAAGA-3′ (sense) and 5’-GGCCATCTCCTGCTCGAA-3’ (antisense). Gene expression was analyzed using the 2−ΔΔCT method.
Western Blot
NPC tissues or cells were homogenized and lysed with RIPA lysis buffer [100 mM NaCl, 50 mM Tris-HCl (pH 7.5), 1% Triton X-100, 1 mM EDTA, 10 mM β-glycerophosphate, 2 mM sodium vanadate, and protease inhibitor]. An equal amount of protein samples was separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis followed by transference to a polyvinylidene difluoride membrane (Millipore, Boston, MA, USA). After blocking with 5% nonfat milk for 1 h at 37°C, the membrane was incubated with primary antibodies against TMPRSS3, E-cadherin, N-cadherin, p-PI3K, PI3K, p-Akt, Akt, and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight. The membrane was then incubated with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology) for 1 h. Finally, the reactive bands were visualized using the ECL chemiluminescence system (Amersham, Piscataway, NJ, USA).
Cell Proliferation Assay
Cell proliferation was measured using the 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Briefly, NPC cells transfected with sh-TMPRSS3 or sh-NC were seeded into 96-well plates at a density of 1 × 105 cells/well. After culturing for 0, 24, 48, 72, and 96 h, 20 μl of MTT solution (5 mg/ml; Sigma-Aldrich, St. Louis, MO, USA) was added to each well for an additional 4 h at 37°C. Dimethyl sulfoxide (DMSO; 150 μl; Sigma-Aldrich) was then added to each well to dissolve MTT formazan crystals. Absorbance at 490 nm was measured using a microplate reader (Bio-Rad, Hercules, CA, USA).
Migration and Invasion Assays
For the migration assay, the transfected CNE-1 cells at a density of 1 × 105 cells per well were added into the upper chamber of Transwell chambers (BD Biosciences, San Jose, CA, USA) containing serum-free medium. For the invasion assay, the transfected CNE-1 cells were seeded into the upper chamber, which was precoated with Matrigel (BD Biosciences). For both assays, 600 μl of DMEM supplemented with 10% FBS was added to the lower chamber. After incubation in a humidified atmosphere of 5% CO2/95% air at 37°C for 24 h at 37°C, noninvasive cells in the upper chamber were removed using a cotton swab, and cells adhering to the lower membrane were fixed in methanol and stained with 0.05% crystal violet for 15 min. The number of invaded cells per four high-power fields was counted under a light microscope (magnification: 100×).
In Vivo Tumorigenicity
Animal experiments were approved by the Institutional Animal Care and Use Committee of Huai’an First People’s Hospital, Nanjing Medical University. Ten female Balb/c nude mice (4–5 weeks old) were obtained from the Animal Breeding Center of Nanjing Medical University and housed under standard conditions of room temperature, humidity, and dark–light cycles in pathogen-free microisolator cages with free access to water and food. For tumor cell implantation, CNE-1 cells (1 × 106 cells in 0.2 ml of PBS) stably transfected with sh-TMPRSS3 or sh-NC were injected subcutaneously into the left flank of female Balb/c nude mice (n = 5 per group). The tumors were measured every 7 days. The tumor volume was calculated according to the following formula: length × width2 × 0.5. All mice were sacrificed 28 days after inoculation, and tumors were isolated and weighed.
Statistical Analysis
Data are presented as the mean ± SD. Statistical analysis was carried out using one-way analysis of variance (ANOVA) followed by Bonferroni test for multiple groups or Student’s t-test between two groups. A value of p < 0.05 was considered statistically significant.
RESULTS
TMPRSS3 Is Overexpressed in Human NPC
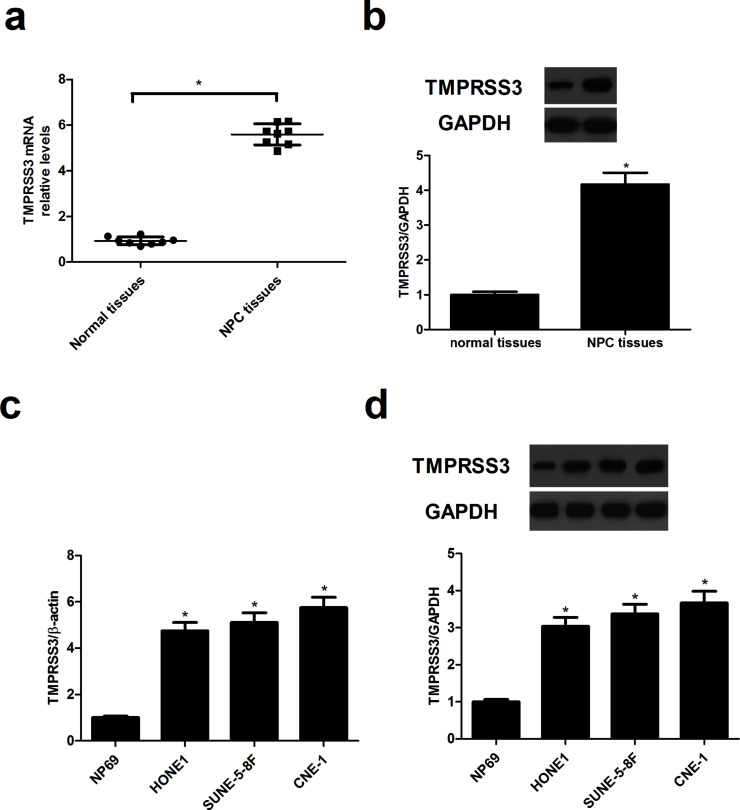
To determine the expression profiles of TMPRSS3 in NPC, we first detected the expression levels of TMPRSS3 in NPC tissues using qRT-PCR and Western blot. The results demonstrated that TMPRSS3 mRNA (Fig. 1a) and protein expression levels (Fig. 1b) were significantly upregulated in NPC tissues when compared with the matched adjacent normal nasopharyngeal epithelium biopsies. Similarly, TMPRSS3 was overexpressed in NPC cell lines (Fig. 1c and d) compared with the NP69 group.
Figure 1.
TMPRSS3 is overexpressed in human nasopharyngeal carcinoma (NPC). (a) The mRNA expression of TMPRSS3 in human NPC tissues was evaluated using quantitative real-time (qRT)-PCR. (b) The protein expression of TMPRSS3 in NPC tissues was detected by Western blot. (c) The mRNA expression of TMPRSS3 in NPC cell lines was measured using qRT-PCR. (d) The protein expression of TMPRSS3 in NPC cell lines was detected by Western blot. *p < 0.05.
Knockdown of TMPRSS3 Inhibits the Proliferation of NPC Cells
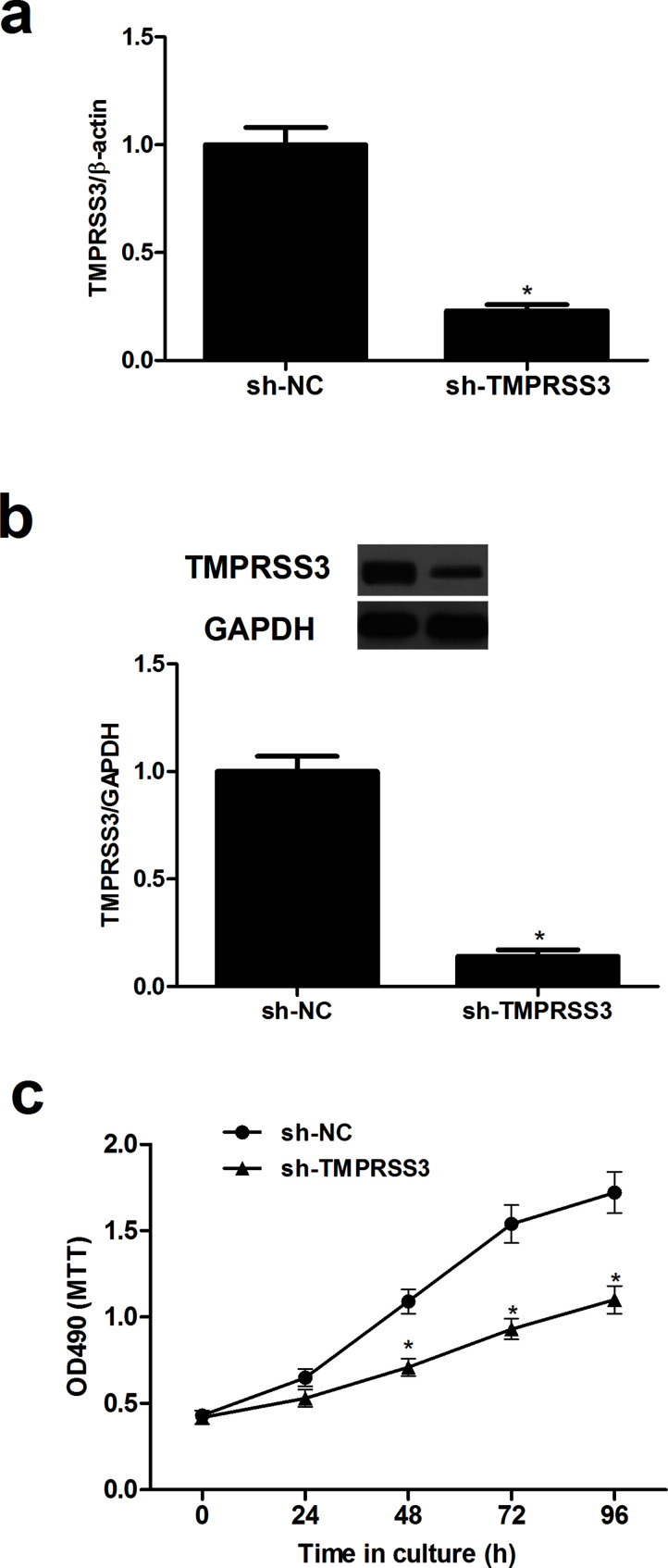
To investigate the effect of TMPRSS3 on NPC cell proliferation, we generated TMPRSS3 knockdown CNE-1 cells by transfection with sh-TMPRSS3. The knockdown efficiency was confirmed by qRT-PCR and Western blot (Fig. 2a and b). We then evaluated the effect of TMPRSS3 knockdown on NPC cell proliferation using the MTT assay. The results showed that knockdown of TMPRSS3 significantly inhibited the proliferation of CNE-1 cells compared with its matched control (Fig. 2c).
Figure 2.
Knockdown of TMPRSS3 inhibits the proliferation of NPC cells. CNE-1 cells were transfected with short hairpin RNA against TMPRSS3 (sh-TMPRSS3) or negative control shRNA (sh-NC) for 48 h. (a) qRT-PCR analysis was used to detect the mRNA expression levels of TMPRSS3. (b) Western blot analysis was used to detect the protein expression levels of TMPRSS3. (c) Cell proliferation was evaluated using the MTT assay. *p < 0.05.
Knockdown of TMPRSS3 Inhibits the Migration and Invasion of NPC Cells
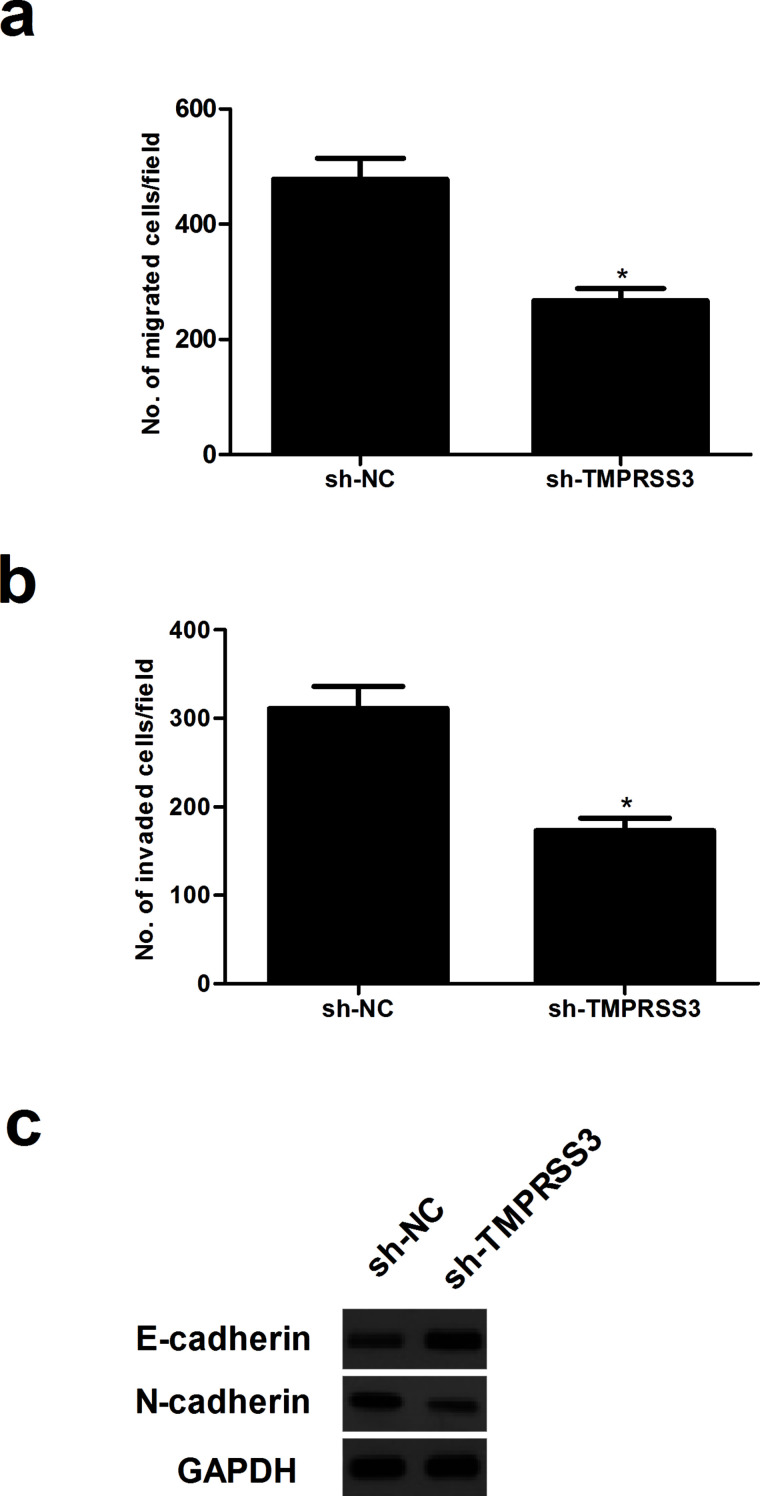
To further examine the effects of TMPRSS3 knockdown on NPC cell migration and invasion, we performed in vitro migration and invasion assays. The number of CNE-1 cells that migrated was obviously reduced after transfection with sh-TMPRSS3 when compared with the sh-NC group (Fig. 3a). In addition, the number of CNE-1 cells passing through the Transwell coated with Matrigel in the TMPRSS3-silenced group was significantly lower than those in the sh-NC group (Fig. 3b). Furthermore, the results of the Western blot analysis indicated that the protein expression levels of E-cadherin were higher while the levels of N-cadherin were lower in sh-TMPRSS3-transfected cells relative to cells transfected with the negative control (Fig. 3c).
Figure 3.
Knockdown of TMPRSS3 inhibits the migration and invasion of NPC cells. (a, b) Transwell invasion and migration assays of CNE-1 cells transfected with sh-TMPRSS3 or sh-NC were performed to investigate the effects of TMPRSS3 on the migration and invasion of CNE-1 cells. (c) Western blot analysis was used to assay the expression level of E-cadherin and N-cadherin in CNE-1 cells after transfection with sh-TMPRSS3 or sh-NC. *p < 0.05.
Knockdown of TMPRSS3 Suppresses NPC Progression In Vivo
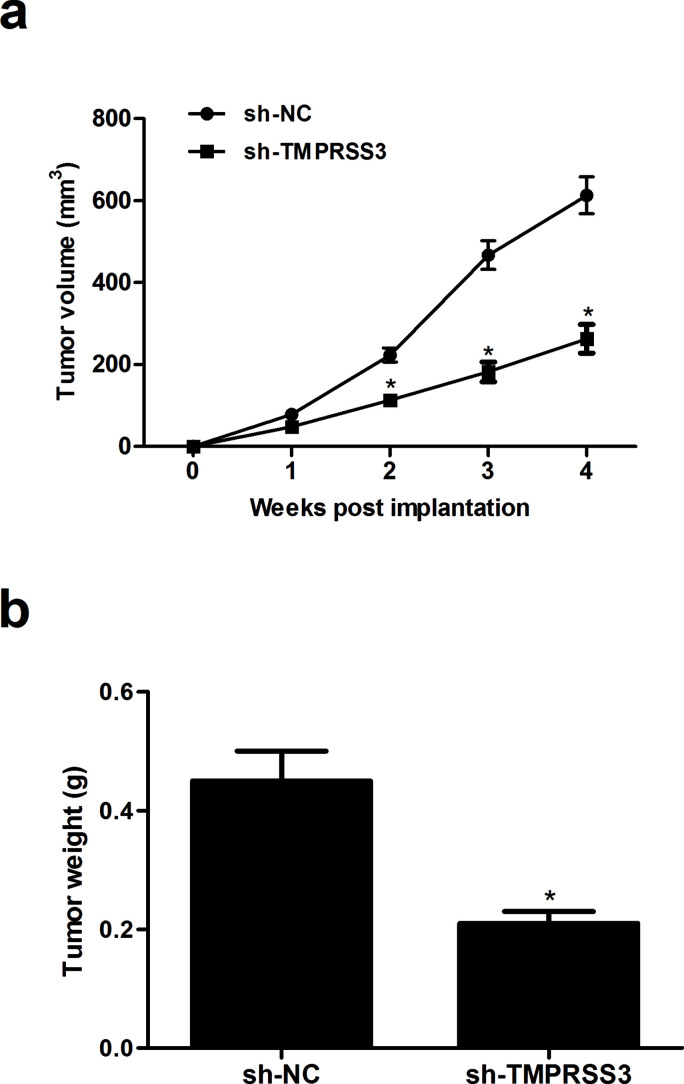
To evaluate the role of TMPRSS3 in NPC progression in vivo, the xenografted tumor in nude mice was employed. The tumor volumes formed by TMPRSS3-silenced cells were significantly suppressed, compared with the sh-NC group (Fig. 4a). In addition, detection of tumor weight at the end of the experiment revealed a significant decrease in this parameter in tumors that arose from CNE-1 cells where TMPRSS3 was depleted (Fig. 4b).
Figure 4.
Knockdown of TMPRSS3 suppresses NPC progression in vivo. CNE-1 cells (1 × 106 cells in 0.2 ml of PBS) stably transfected with sh-TMPRSS3 or sh-NC were injected subcutaneously into the left flank of female Balb/c nude mice. (a) Tumor volume was monitored every week and measured. (b) After 4 weeks, the mice were sacrificed, and the tumors were dissected out and weighed. *p < 0.05 versus sh-NC group.
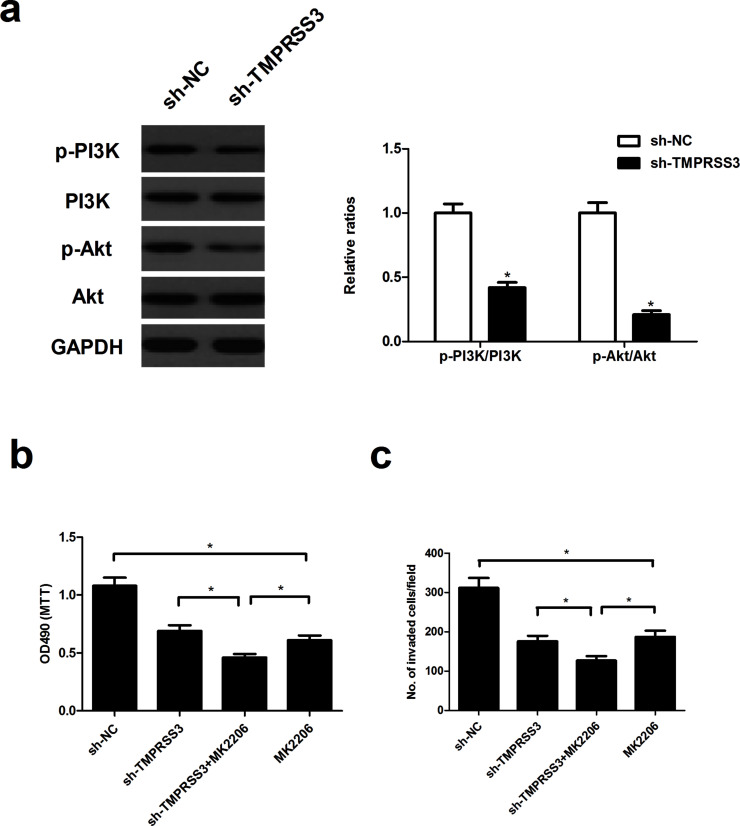
Knockdown of TMPRSS3 Suppresses the Proliferation and Invasion of NPC Cells Through Inactivation of the PI3K/Akt Signaling Pathway
To determine the mechanism by which TMPRSS3 knockdown inhibited NPC cell proliferation and invasion, we examined the effect of TMPRSS3 knockdown on the activation of the PI3K/Akt signaling pathway in CNE-1 cells. Knockdown of TMPRSS3 remarkably reduced the levels of phosphorylated PI3K (p-PI3K) and phosphorylated Akt (p-Akt) in CNE-1 cells, compared with the sh-NC group (Fig. 5a). Additionally, the Akt inhibitor MK2206 significantly enhanced the inhibitory effects of sh-TMPRSS3 on CNE-1 cell proliferation (Fig. 5b) and invasion (Fig. 5c).
Figure 5.
Knockdown of TMPRSS3 suppresses the proliferation and invasion of NPC cells through the inactivation of the PI3K/Akt signaling pathway. (a) CNE-1 cells were transfected with sh-TMPRSS3 or sh-NC for 48 h. (a) The expression of PI3K, phosphorylated PI3K, Akt, and phosphorylated Akt was evaluated by Western blot. (b, c) CNE-1 cells silencing TMPRSS3 were treated with the Akt inhibitor MK2206 (10 μM) for 30 min, and then the MTT and Transwell assays were performed 24 h later. *p < 0.05.
DISCUSSION
In this study, we found a significant increase in the expression of TMPRSS3 in human NPC tissues and cell lines. Knockdown of TMPRSS3 expression significantly suppressed the proliferation and tumorigenicity of NPC cells in vitro and in vivo. Furthermore, knockdown of TMPRSS3 inhibited migration and invasion, as well as prevented the EMT process in NPC cells. Finally, knockdown of TMPRSS3 attenuated activation of the PI3K/Akt signaling pathway in NPC cells.
TMPRSS3 is upregulated in several types of tumors, for instance, ovarian, breast, and pancreatic cancers. Wallrapp et al. reported that TMPRSS3 was overexpressed in a subset of pancreatic cancer tissues, and its expression correlates with the metastatic potential of pancreatic cancer cells11. Similarly, in this study we found that TMPRSS3 mRNA and protein expression levels were significantly upregulated in NPC tissues and cell lines. Given the above results, we then investigated the role of TMPRSS3 in NPC cell proliferation in vitro and NPC progression in vivo and found that knockdown of TMPRSS3 expression significantly suppressed the proliferation of NPC cells. In line with these in vitro data, we found that knockdown of TMPRSS3 markedly attenuated tumor growth in vivo in an allograft murine model. These data suggest that TMPRSS3 may be a novel candidate oncogene in the development and progression of NPC.
Uncontrolled metastasis, which is an important feature of malignant tumors, is the main cause of death of patients with NPC14. EMT is considered to be the key step by which tumor cells gain the higher ability of invasiveness and metastasis15. During EMT, epithelial cells lose their characteristic marker E-cadherin and gain mesenchymal markers including N-cadherin and vimentin16. In this study, we observed that knockdown of TMPRSS3 inhibited migration and invasion, as well as suppressed the EMT progress in CNE-1 cells. These data suggest that TMPRSS3 silencing inhibited the EMT process, consequently affecting NPC cell migration and invasion in vitro.
The PI3K/Akt pathway is one of the most important signaling pathways identified as being implicated in tumorigenesis17–19. It is activated in the progression of NPC20, and hyperactivation of the PI3K/Akt pathway is correlated with tumor development, progression, and a poor prognosis in human solid tumors21. Several studies have shown that aberrant activation of Akt is involved in NPC metastasis and tumorigenesis22–24. Therefore, targeting the PI3K/Akt pathway may represent a promising approach to the treatment of NPC. For example, PI3K/Akt inhibitors, GSK216458, and PKI-587 significantly inhibited cell proliferation and motility in NPC cells, and the combination of ionizing radiation with GSK2126458 or PKI-587 effectively inhibited NPC growth25. In this study, we found that knockdown of TMPRSS3 remarkably reduced the levels of p-PI3K and p-Akt in CNE-1 cells. Additionally, the Akt inhibitor MK2206 significantly enhanced the inhibitory effects of sh-TMPRSS3 on CNE-1 cell proliferation and invasion. These findings suggest a possible mechanism by which sh-TMPRSS3 suppresses NPC cell proliferation and invasion through negatively regulating the PI3K/Akt signaling pathway.
In summary, the present study demonstrates that knockdown of TMPRSS3 inhibits proliferation, migration, and invasion in human NPC cells through the inactivation of the PI3K/Akt signaling pathway. This study suggests that TMPRSS3 may be a potential therapeutic target for the treatment of NPC.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Wei KR, Xu Y, Liu J, Zhang WJ, Liang ZH. Histopathological classification of nasopharyngeal carcinoma. Asian Pac J Cancer Prev. 2011;12:1141–7. [PubMed] [Google Scholar]
- 2. Xu ZX, Lin ZX, Fang JY, Wu KS, Du PL, Zeng Y, Tang WR, Xu XL, Lin K. Mortality characteristic and prediction of nasopharyngeal carcinoma in China from 1991 to 2013. Asian Pac J Cancer Prev. 2015;16:6729–34. [DOI] [PubMed] [Google Scholar]
- 3. Zhang L, Zhao C, Ghimire B, Hong MH, Liu Q, Zhang Y, Guo Y, Huang YJ, Guan ZZ. The role of concurrent chemoradiotherapy in the treatment of locoregionally advanced nasopharyngeal carcinoma among endemic population: A meta-analysis of the phase iii randomized trials. BMC Cancer 2010;10:558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tian YM, Tian YH, Zeng L, Liu S, Guan Y, Lu TX, Han F. Prognostic model for survival of local recurrent nasopharyngeal carcinoma with intensity-modulated radiotherapy. Br J Cancer 2014;110:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wei WI, Sham JST. Nasopharyngeal carcinoma. Lancet 2005;365:2041–54. [DOI] [PubMed] [Google Scholar]
- 6. Zheng W, Zong J, Huang C, Chen J, Wu J, Chen C, Lin S, Pan J. Multimodality treatment may improve the survival rate of patients with metastatic nasopharyngeal carcinoma with good performance status. PLoS One 2016;11:e0146771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Webb SL, Sanders AJ, Mason MD, Jiang WG. Type II transmembrane serine protease (TTSP) deregulation in cancer. Front Biosci. 2011;16:539–52. [DOI] [PubMed] [Google Scholar]
- 8. Béliveau F, Brulé C, Désilets A, Zimmerman B, Laporte SA, Lavoie CL, Leduc R. Essential role of endocytosis of the type II transmembrane serine protease TMPRSS6 in regulating its functionality. J Biol Chem. 2011;286:29035–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tanabe LM, List K. The role of type II transmembrane serine protease mediated signaling in cancer. FEBS J. 2017;284:1421–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guipponi M, Antonarakis SE, Scott HS. TMPRSS3, a type II transmembrane serine protease mutated in non-syndromic autosomal recessive deafness. Front Biosci. 2008;13:1557–67. [DOI] [PubMed] [Google Scholar]
- 11. Wallrapp C, Hähnel S, Müllerpillasch F, Burghardt B, Iwamura T, Ruthenbürger M, Lerch MM, Adler G, Gress TM. A novel transmembrane serine protease (TMPRSS3) overexpressed in pancreatic cancer. Cancer Res. 2000;60:2602–6. [PubMed] [Google Scholar]
- 12. Rui X, Li Y, Jin F, Li F. TMPRSS3 is a novel poor prognostic factor for breast cancer. Int J Clin Exp Pathol. 2015;8:5435–42. [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang D, Qiu S, Wang Q, Zheng J. TMPRSS3 modulates ovarian cancer cell proliferation, invasion and metastasis. Oncol Rep. 2015;35:81–8. [DOI] [PubMed] [Google Scholar]
- 14. Ma J, Liu L, Tang L, Zong J, Lin A, Lu T, Cui N, Cui C, Li L. Retropharyngeal lymph node metastasis in nasopharyngeal carcinoma: Prognostic value and staging categories. Clin Cancer Res. 2007;13:1445–52. [DOI] [PubMed] [Google Scholar]
- 15. Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metast Rev. 2009;28:15–33. [DOI] [PubMed] [Google Scholar]
- 16. Kalluri R. EMT: When epithelial cells decide to become mesenchymal-like cells. J Clin Invest. 2009;119:1417–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cho D, Mier JW, Atkins MB. PI3K/Akt/mTOR pathway: A growth and proliferation pathway. In: Bukowski RM, Figlin RA, Motzer RJ, editors. Renal cell carcinoma. New York (NY): Springer; 2009. p. 267–85. [Google Scholar]
- 18. Morgensztern D, McLeod HL. PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs 2005;16:797–803. [DOI] [PubMed] [Google Scholar]
- 19. Castaneda CA, Cortesfunes H, Gomez HL, Ciruelos EM. The phosphatidyl inositol 3-kinase/AKT signaling pathway in breast cancer. Cancer Metast Rev. 2010;29:751–9. [DOI] [PubMed] [Google Scholar]
- 20. Guo Q, Lu T, Chen Y, Su Y, Zheng Y, Chen Z, Chen C, Lin S, Pan J, Yuan X. Genetic variations in the PI3K-PTEN-AKT-mTOR pathway are associated with distant metastasis in nasopharyngeal carcinoma patients treated with intensity-modulated radiation therapy. Sci Rep. 2016;6:37576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Toulany M, Rodemann HP. Phosphatidylinositol 3-kinase/Akt signaling as a key mediator of tumor cell responsiveness to radiation. Semin Cancer Biol. 2015;35:180–90. [DOI] [PubMed] [Google Scholar]
- 22. Yip WK, Leong VC, Abdullah MA, Yusoff S, Seow HF. Overexpression of phospho-Akt correlates with phosphorylation of EGF receptor, FKHR and BAD in nasopharyngeal carcinoma. Oncol Rep. 2008;19:319–28. [PubMed] [Google Scholar]
- 23. Liu Y, Chen LH, Yuan YW, Li QS, Sun AM, Guan J. Activation of AKT is associated with metastasis of nasopharyngeal carcinoma. Tumor Biol. 2012;33:241–5. [DOI] [PubMed] [Google Scholar]
- 24. Wang W, Wen Q, Xu L, Xie G, Li J, Luo J, Chu S, Shi L, Huang D, Li J, Fan S. Activation of Akt/mTOR pathway is associated with poor prognosis of nasopharyngeal carcinoma. PLoS One 2014;9:e106098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu T, Sun Q, Li Q, Yang H, Zhang Y, Wang R, Lin X, Xiao D, Yuan Y, Chen L, Wang W. Dual PI3K/mTOR inhibitors, GSK2126458 and PKI-587, suppress tumor progression and increase radiosensitivity in nasopharyngeal carcinoma. Mol Cancer Ther. 2015; 4:429–39. [DOI] [PubMed] [Google Scholar]