Abstract
Rap2B, belonging to the Ras superfamily, has been implicated in cancer development and functions as a tumor promoter. However, the role of Rap2B in cervical cancer is unknown. In this study, we investigated the expression pattern and biological functions of Rap2B in cervical cancer. The results showed that Rap2B was overexpressed in cervical cancer tissues and cell lines. Knockdown of Rap2B inhibited the proliferation, migration, and invasion of cervical cancer cells. In addition, our tumorigenesis assay showed that Rap2B knockdown suppressed cervical cancer cell growth and metastasis in vivo. We also found that the ERK1/2 signaling pathway is involved in the inhibitory effect of Rap2B knockdown on cervical cancer development. In conclusion, we suggest that Rap2B is an oncogene and may be a promising therapeutic target for cervical cancer.
Key words: Rap2B, Proliferation, Migration, Invasion, Cervical cancer
INTRODUCTION
Cervical cancer, the third most common malignant tumor type in the world, is one of the main reasons for high mortality in women1,2. This neoplastic disease has multiple risk factors such as smoking, immunosuppression, hormonal contraceptives, and certain infections3,4. With the development of therapeutic means, the 5-year survival rate has increased to 65% for patients in stage I after radical surgery or radiotherapy5. However, for patients in stage IV, the survival rate is only 15%, even after radiotherapy or chemotherapy6. The failure in treatment is mainly due to local recurrence or distant metastasis7–9. Therefore, further exploration of novel biomarkers for early diagnosis and better prognosis is urgently needed. Additionally, it is imperative that we improve our understanding of the molecular mechanisms underlying the progression of cervical cancer.
The Ras superfamily of proteins comprises five members, namely, Rap1A, Rap1B, Rap2A, Rap2B, and Rap2C10–12. All of the members are small GTP-binding proteins and can be divided into two groups; Rap1 and Rap213. These proteins share almost 60% sequence identity with a small difference in the effector region14. In addition, their expression can be observed in all multicellular eukaryotes15. Many studies have reported the essential role of Rap family members in various biological functions such as apoptosis, proliferation, and migration10,16. More importantly, increasing evidence has demonstrated the implication of Rap1 and Rap2 in cancer development. For example, Rap2B was found to function as an oncogene in lung cancer17. However, less is known about its specific role in cervical cancer.
In this study, we explored the biological functions of Rap2B in the progression of cervical cancer. Our data showed that Rap2B was overexpressed in cervical cancer tissues and cell lines. Knockdown of Rap2B inhibited cervical cancer cell proliferation, migration, and invasion in vitro and suppressed cervical cancer cell growth and metastasis in vivo. Furthermore, we found that the ERK1/2 signaling pathway might be responsible for the inhibitory effect of Rap2B knockdown on cervical cancer development.
MATERIALS AND METHODS
Patients and Tissue Samples
Thirty-two cervical cancer patients from the Women’s Hospital, School of Medicine, Zhejiang University (Hangzhou, P.R. China) participated in the study and provided informed consent. These patients received no chemotherapy or radiotherapy before surgery. After collection, cervical cancer tissues and adjacent normal tissues were frozen in liquid nitrogen and then stored at −80°C for further analysis. The study was approved by the ethics committee of Zhejiang University.
Cell Lines and Cell Culture
Human cervical cancer cell lines (HeLa and C33A) and immortalized human cervical mucosa epithelial cell line H8 were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). All cells were cultured at 37°C in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Rockville, MD, USA) supplemented with 10% fetal bovine serum (FBS; Gibco) and 1% penicillin–streptomycin (Gibco) in a humidified incubator containing 5% CO2.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated from tissues or cells with the RNeasy Kit (Qiagen, Hilden, Germany) and used to synthesize cDNA with the PrimeScript RT Reagent Kit (TaKaRa, Osaka, Japan). RT-PCR was performed using a SYBR GREEN PCR Master Mix (Invitrogen, Carlsbad, CA, USA) on a 7900 HT Fast Real-time PCR System (Applied Biosystems, Foster City, CA, USA). The reactions were carried out under the following conditions: 50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15 s, and 60°C for 1 min. The following primers were used: Rap2B, 5′-GAAGCTTATGCGCGAGTACAAAG-3′ (forward) and 5′-GGAATTCCTATTGTATGTTACATG-3′ (reverse); β-actin, 5′-GCGCGGCTACAGCTTCA-3′ (forward) and 5′-CTTAATGTCACGCACGATTTCC-3′ (reverse). The results were normalized to β-actin. Data were collected on an IQ5 instrument (Bio-Rad, Hercules, CA, USA).
Western Blot Analysis
Tissues or cells were lysed in RIPA buffer and put in a vortex instrument for 30 s of shaking every 10 min. After centrifugation for 10 min at 4°C and 10,000 rpm, the supernatant was collected. Protein concentration was measured using a BCA kit (Pierce, Rockford, IL, USA). Proteins were subjected to 12% SDS-PAGE and blotted on PVDF membranes. After blocking in 5% skim milk for 2 h, the membranes were incubated overnight at 4°C with primary antibodies against Rap2B, p-ERK1/2, ERK1/2, and β-actin (Invitrogen). The membranes were then washed and incubated with HRP-conjugated secondary antibodies (Invitrogen) at room temperature for 1 h. Proteins were visualized using enhanced chemiluminescence (Bio-Rad) and quantified using the Quantity One software (Bio-Rad).
Lentivirus Infection
Rap2B-specific shRNA (sh-Rap2B) and control shRNA (sh-NC) in eukaryotic lentiviral vectors/puromycin were purchased from GenePharma Company (Shanghai, P.R. China). Before infection, cells were seeded into six-well plates to reach 80% confluence. Subsequently, the culture medium was replaced with 2 ml of polybrene (6 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and lentiviral particles were added. After overnight incubation, the culture medium was removed, and complete medium was added. Forty-eight hours later, puromycin (6 μg/ml; Santa Cruz Biotechnology) was added. The culture medium was changed every 3 days. Three weeks later, colonies resistant to puromycin were separated and cultured in 96-well plates for further experiments.
MTT Assay
Cell proliferation was detected by the MTT assay. In brief, cells were seeded onto 96-well culture plates at a density of 2 × 103 cells/well. After culturing for different times, 20 ml of MTT (5 mg/ml; Sigma-Aldrich, St. Louis, MO, USA) was added to each well, and cell culturing was continued for 2 h at 37°C. The culture medium was then removed, and 150 ml of DMSO (Sigma-Aldrich) was added. The OD value was measured at a wavelength of 570 nm.
Transwell Assay
Cell migration and invasion were assessed using Transwell chambers. For the cell migration assay, cells (5 × 104) in RPMI-1640 medium were cultured in the upper chamber for 24 h at 37°C, and RPMI-1640 medium supplemented with 10% FBS was added to the lower chamber. Nonmigrating cells were removed with cotton swabs, and migrating cells were stained with 0.1% crystal violet. The number of migrating cells from four random fields was calculated under a microscope. The cell invasion assay was performed according to the same procedure as mentioned above, except that Matrigel-coated polycarbonate membranes were used.
In Vivo Xenograft Tumor Assay
Six-week-old female BALB/c nude mice were purchased from Shanghai Laboratory Animal Center (Shanghai, P.R. China). All mice were handled in accordance with the guidelines of the Animal Care and Use Committee of Zhejiang University. Transfected HeLa cells (5 × 106) were subcutaneously injected into the left flank of mice (n = 6). Tumor volume was measured every week. After 5 weeks, mice were sacrificed, and tumors were weighed. For tumor metastasis assay, 5 × 106 transfected HeLa cells were intravenously injected into mice (n = 6). After 5 weeks, mice were sacrificed, and their lungs were checked. The number of metastatic nodules in the lungs was counted under a microscope.
Statistical Analysis
Data were shown as means ± standard deviation (SD). Student’s t-tests were used for comparison of different groups. Statistical analysis was performed by the SPSS software. Each experiment was carried out in triplicate. A value of p < 0.05 was considered statistically significant.
RESULTS
Rap2B Is Overexpressed in Cervical Cancer Tissues and Cell Lines
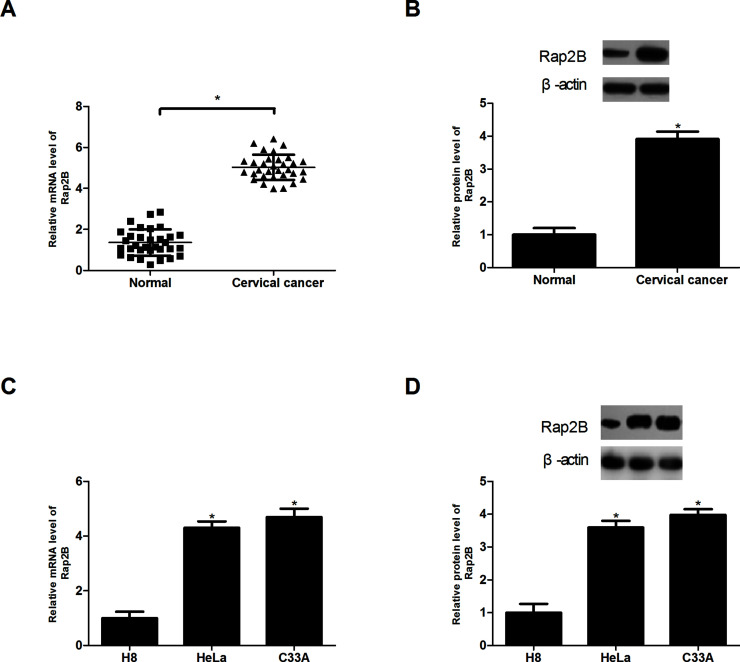
We collected 32 pairs of cervical cancer tissues and adjacent normal tissues to examine the expression of Rap2B by RT-PCR and Western blot. The analysis demonstrated that Rap2B was obviously elevated in cervical cancer tissues at both the mRNA and protein levels, in comparison with normal tissues (Fig. 1A and B). In addition, we investigated the expression of Rap2B in cervical cancer cell lines (HeLa and C33A) and cervical mucosa epithelial cell H8. The results suggested a higher expression level of Rap2B in HeLa and C33A than in H8 (Fig. 1C and D).
Figure 1.
Rap2B is overexpressed in cervical cancer tissue and cell lines. (A, B) Real-time polymerase chain reaction (RT-PCR) and Western blot analysis of Rap2B expression in cervical cancer tissues compared with normal tissues. (n = 32). (C, D) RT-PCR and Western blot analysis of Rap2B expression in cervical cancer cell lines (HeLa and C33A) and cervical mucosa epithelial cell H8. *p < 0.05.
Knockdown of Rap2B Inhibits the Proliferation of Cervical Cancer Cells
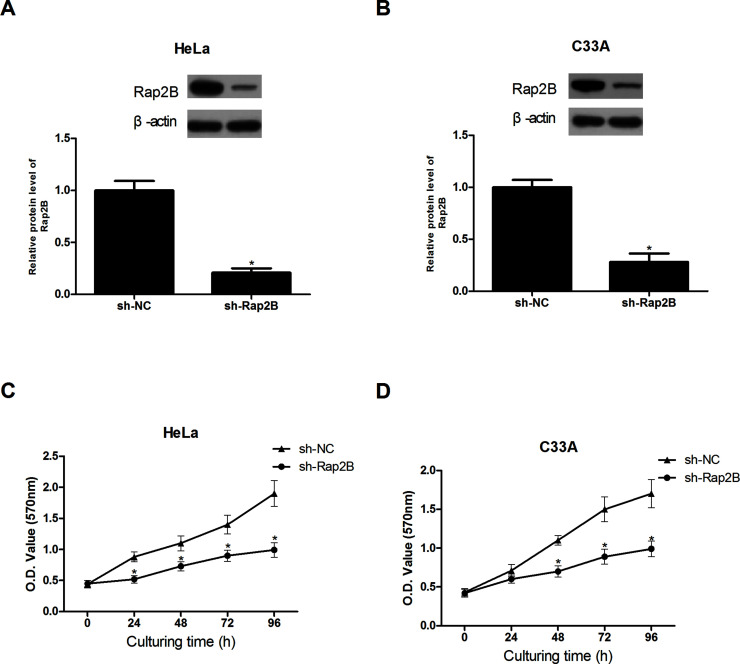
Before investigation of the biological functions of Rap2B in cervical cancer, we reduced the expression of Rap2B in cervical cancer cell lines (HeLa and C33A) via lentivirus infection. The protein expression levels of Rap2B were significantly decreased in HeLa and C33A cells after shRNA transfection, in comparison with H8 cells (Fig. 2A and B).
Figure 2.
Knockdown of Rap2B inhibits the proliferation of cervical cancer cells. (A, B) Rap2B knockdown in HeLa and C33A cells was confirmed by Western blot analysis. (C, D) Knockdown of Rap2B significantly decreased the proliferation of HeLa and C33A cells as determined by the MTT assay, in comparison with the control group. *p < 0.05.
We detected cell proliferation by MTT assay. A decrease in Rap2B markedly inhibited the proliferation of HeLa and C33A cells in comparison with the control cells (Fig. 2C and D).
Knockdown of Rap2B Inhibits the Migration and Invasion of Cervical Cancer Cells
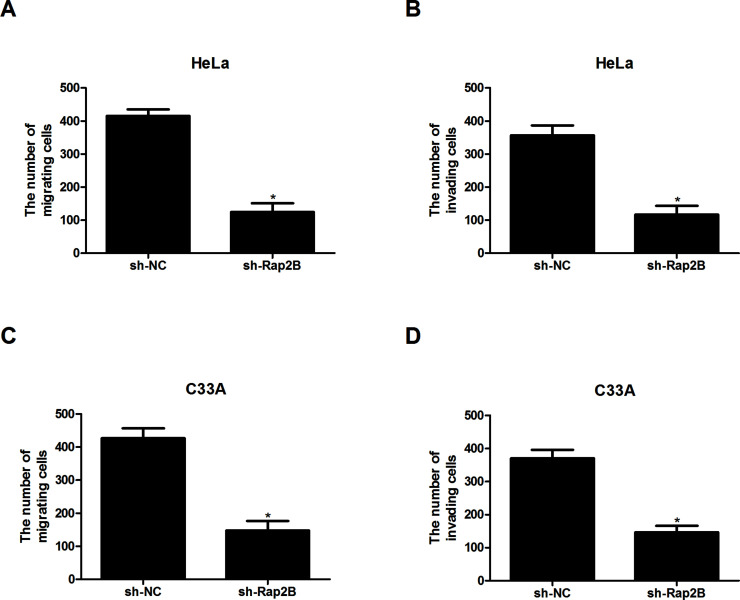
We also tested the effect of Rap2B knockdown on cervical cancer cell migration and invasion. The Transwell assay indicated that Rap2B downregulation greatly reduced the number of migrating and invading HeLa cells, in comparison with the control group (Fig. 3A and B). Similar results were obtained for C33A cells (Fig. 3C and D).
Figure 3.
Knockdown of Rap2B inhibits the migration and invasion of cervical cancer cells. (A, B) The migratory and invasive abilities of HeLa cells were significantly decreased after Rap2B knockdown, in comparison with the control group. (C, D) The migratory and invasive abilities of C33A cells were obviously reduced after Rap2B knockdown, in comparison with the control group. *p < 0.05.
Knockdown of Rap2B Inhibits Cervical Cancer Cell Growth and Metastasis In Vivo
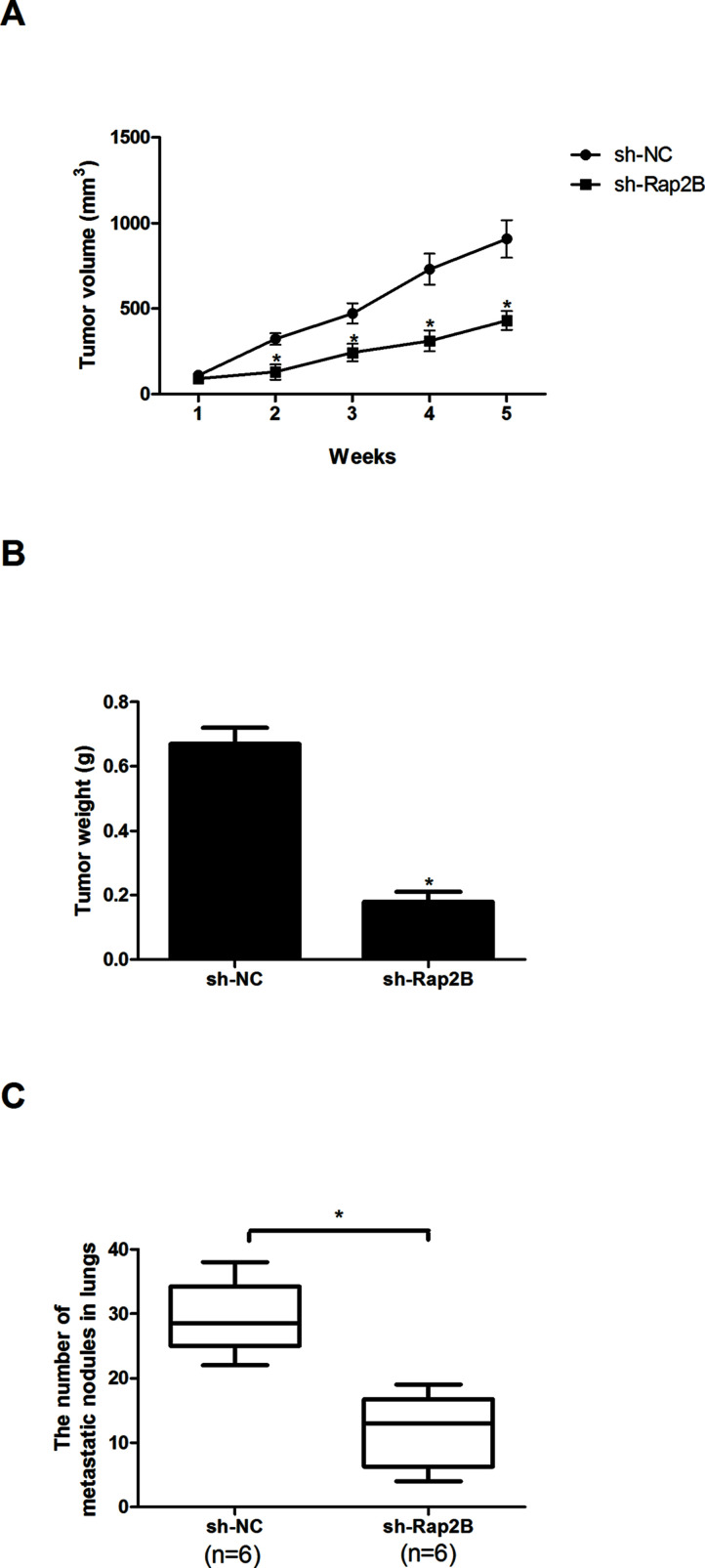
We explored the in vivo tumorigenic effect of Rap2B knockdown via the xenograft tumor assay. For the tumor growth assay, mice were subcutaneously injected with sh-Rap2B- or sh-NC-transfected HeLa cells (n = 6). Tumor volume and mass were significantly decreased by Rap2B knockdown in HeLa cells compared with the control cells (Fig. 4A and B). For the tumor metastasis assay, mice were intravenously injected with sh-Rap2B- or sh-NC-transfected HeLa cells (n = 6). The anatomical results showed a remarkable reduction in the number of metastatic nodules in the lungs after Rap2B knockdown in HeLa cells compared with the control group (Fig. 4C).
Figure 4.
Knockdown of Rap2B inhibits cervical cancer cell growth and metastasis in vivo. (A) Tumor growth curves in mice. (B) Tumor weight measured 5 weeks after subcutaneous injection of HeLa cells. (C) Quantification of metastatic nodules in lungs 5 weeks after intravenous injection of HeLa cells (n = 6). *p < 0.05.
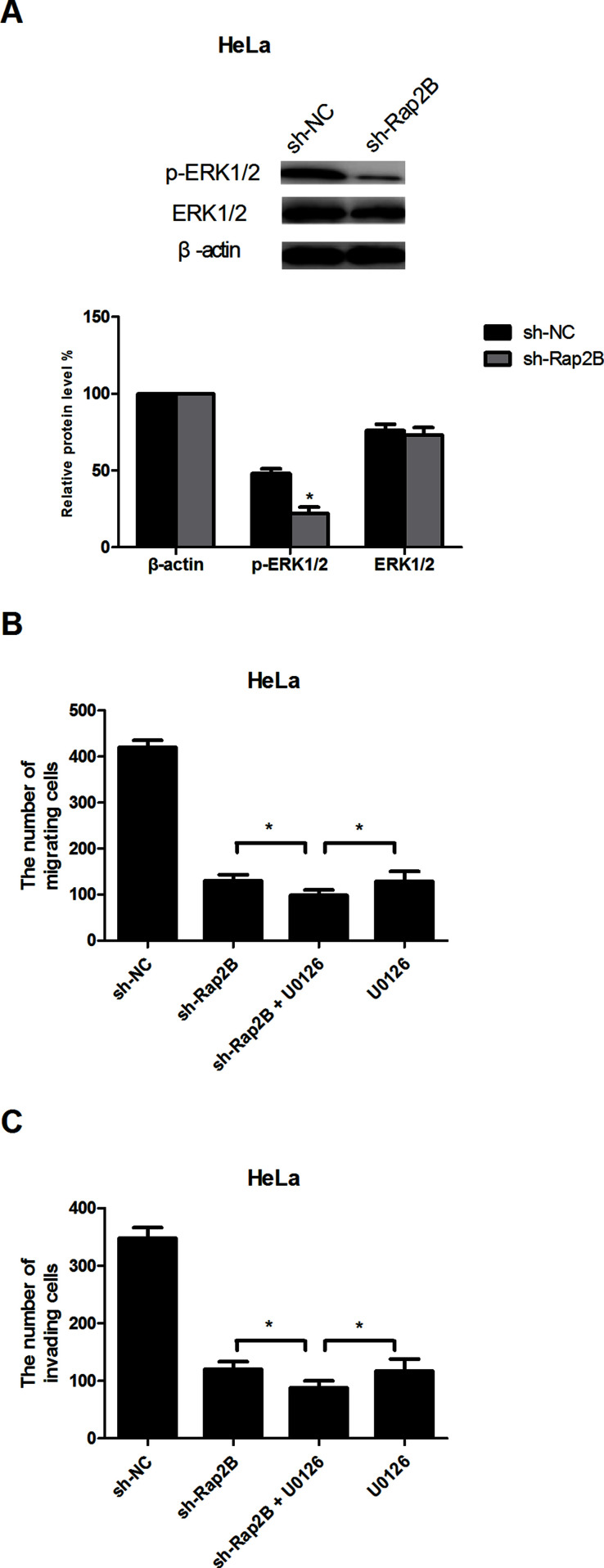
Knockdown of Rap2B Inhibits Cell Migration and Invasion via the ERK1/2 Signaling Pathway
The ERK1/2 signaling pathway is frequently deregulated in a diverse variety of cancers, including cervical cancer18,19. Rap2B has also been reported to be closely related to the ERK1/2 signaling pathway when it functions as a tumor promoter20. Therefore, we hypothesized that Rap2B knockdown inhibited cervical cancer cell migration and invasion via regulating the ERK1/2 signaling pathway. Downregulation of Rap2B drastically decreased the protein expression levels of p-ERK1/2 in HeLa cells, without affecting the total ERK1/2 expression (Fig. 5A). To further prove our assumption, we used the ERK inhibitor U0126 (10 μM) to investigate the involvement of ERK1/2 in the sh-Rap2B-inhibited migration and invasion of cervical cancer cells. As expected, U0126 enhanced the sh-Rap2B-suppressed effect on cervical cancer cell migration and invasion (Fig. 5B and C).
Figure 5.
Knockdown of Rap2B inhibits cell migration and invasion via the ERK1/2 signaling pathway. (A) Western blot analysis of the protein levels of p-ERK1/2 and ERK1/2 in HeLa cells. (B, C) HeLa cells were incubated in the presence or absence of U0126 (10 μM). Thirty minutes later, cell migration and invasion were measured by Transwell assays. *p < 0.05.
DISCUSSION
It is estimated that 275,000 women die from cervical cancer every year, and this number increases year by year. As a widely considered threat to women, cervical cancer remains a global public health problem to solve.
Rap2B, first identified from the cDNA library of human platelets, belongs to the Ras family of small GTP-binding proteins21–23. It is acknowledged that Rap2B is mainly localized at recycling endosomes and cell membranes24,25. In addition, Rap2B has been reported in many studies to have a close relationship with the development of various cancers and thus has received broad attention. For example, Di et al. demonstrated upregulation of Rap2B in breast cancer and its promoting effect on cell proliferation, migration, and invasion20. In renal carcinoma, Rap2B was found to accelerate cell migration and invasion after its overexpression26. Similar to the previous observations, a study by Xie et al. showed that Rap2B significantly enhanced lung cancer cell proliferation and invasion27. All these findings suggest that Rap2B might serve as an oncogene, and this notion has been proven by many researchers in many types of cancers, such as prostate and bladder cancers28,29. In this study, we explored whether Rap2B played a similar role in cervical cancer. We first examined the expression of Rap2B. In line with previous studies, our results showed that Rap2B was overexpressed in cervical cancer tissues and cell lines. We then performed related functional experiments and found that knockdown of Rap2B inhibited cervical cancer cell proliferation, migration, and invasion in vitro. We also carried out xenograft tumor assays to further verify our in vitro results. Consistently, the in vivo results showed that Rap2B knockdown inhibited cervical cancer cell growth and metastasis in mice. Thus, we proved that Rap2B functions as a tumor promoter in the development of cervical cancer.
To our knowledge, the underlying mechanism of Rap2B in cervical cancer migration and invasion has not been well elucidated. Here we made some related investigations. We examined the ERK1/2 signaling pathway. ERK1/2 acts as a multiple molecule target, and increasing evidence has demonstrated its involvement in a diverse variety of cellular functions such as proliferation, apoptosis, and invasion30. Furthermore, deregulation of the ERK1/2 pathway is frequently observed in many cancers18,19. There have been findings in support of the tumor-promoting role of ERK1/2 as well as studies revealing the suppressive effect of ERK1/2 on tumorigenesis31. More importantly, Rap2B has been reported to play a regulatory role in the ERK pathway20. In the present study, we found that a decrease in the expression of Rap2B drastically reduced the protein expression levels of p-ERK1/2 while the total levels of ERK1/2 remained unchanged. Our data also showed that the ERK inhibitor U0126 potentiated the sh-Rap2B-suppressed effect on cervical cancer cell migration and invasion. These results suggest that knockdown of Rap2B inhibited cervical cancer cell migration and invasion, at least in part, via the ERK1/2 signaling pathway.
In summary, our study provides the first in vitro and in vivo evidence that Rap2B knockdown could inhibit the proliferation, migration, and invasion of cervical cancer cells. We also showed that Rap2B downregulation blocked the ERK1/2 signaling pathway by reducing the protein levels of p-ERK1/2, which probably accounted for its inhibitory effect on the biological behaviors of cervical cancer cells. Based on these results, we concluded that Rap2B might be a promising therapeutic target for cervical cancer.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917. [DOI] [PubMed] [Google Scholar]
- 2. Torbé B, Falco M, Torbé A, Ciepiela P, Kurzawa R. Radiotherapy versus radiochemotherapy with cisplatin in treatment of cervical cancer. Med Oncol. 2010;27:1–8. [DOI] [PubMed] [Google Scholar]
- 3. Zhang B, Zhou AF, Zhu CC, Zhang L, Xiang B, Chen Z, Hu RH, Zhang YQ, Qiu L, Zhang YM. Risk factors for cervical cancer in rural areas of Wuhan China: A matched case-control study. Asian Pac J Cancer Prev. 2013;14:7595–600. [DOI] [PubMed] [Google Scholar]
- 4. Bonneau C, Perrin M, Koskas M, Genin AS, Rouzier R. [Epidemiology and risk factors for cancer of the uterus]. La Revue Du Praticien 2014;64:774–9. [PubMed] [Google Scholar]
- 5. Lilic V, Lilic G, Filipovic S, Milosevic J, Tasic M, Stojiljkovic M. Modern treatment of invasive carcinoma of the uterine cervix. J BUON 2009;14:587–92. [PubMed] [Google Scholar]
- 6. Villalba SR, Planell DC, Grau JMC. Current opinion in cervix carcinoma. Clin Transl Oncol. 2011;13:378–84. [DOI] [PubMed] [Google Scholar]
- 7. Delli Carpini J, Karam AK, Montgomery L. Vascular endothelial growth factor and its relationship to the prognosis and treatment of breast, ovarian, and cervical cancer. Angiogenesis 2010;13:43–58. [DOI] [PubMed] [Google Scholar]
- 8. del Campo JM, Prat A, Gil-Moreno A, Pérez J, Parera M. Update on novel therapeutic agents for cervical cancer. Gynecol Oncol. 2008;110:S72–6. [DOI] [PubMed] [Google Scholar]
- 9. Zagouri F, Sergentanis TN, Chrysikos D, Filipits M, Bartsch R. Molecularly targeted therapies in cervical cancer. A systematic review. Gynecol Oncol. 2012;126:291–303. [DOI] [PubMed] [Google Scholar]
- 10. Itoh M, Nelson CM, Myers CA, Bissell MJ. Rap1 integrates tissue polarity, lumen formation, and tumorigenic potential in human breast epithelial cells. Cancer Res. 2007;67:4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin KBL, Tan P, Freeman SA, Lam M, Mcnagny KM, Gold MR. The Rap GTPases regulate the migration, invasiveness and in vivo dissemination of B-cell lymphomas. Oncogene 2009;29:608–15. [DOI] [PubMed] [Google Scholar]
- 12. Park HO, Chant J, Herskowitz I. BUD2 encodes a GTPase-activating protein for Bud1/Rsr1 necessary for proper bud-site selection in yeast. Nature 1993;365:269–74. [DOI] [PubMed] [Google Scholar]
- 13. Torti M, Lapetina EG. Structure and function of Rap proteins in human platelets. Thromb Haemost. 1994;71:533–43. [PubMed] [Google Scholar]
- 14. Reuther GW, Der CJ. The Ras branch of small GTPases: Ras family members don’t fall far from the tree. Curr Opin Cell Biol. 2000;12:157. [DOI] [PubMed] [Google Scholar]
- 15. Gunzburg JD. Ras Family Proteins. In: Der C., editor. RAS family GTPases. Amsterdam, Netherlands: Springer; 2006. p. 295–339. [Google Scholar]
- 16. Kooistra MR, Dubé N, Bos JL. Rap1: A key regulator in cell-cell junction formation. J Cell Sci. 2007;120:17–22. [DOI] [PubMed] [Google Scholar]
- 17. Fu G, Liu Y, Yuan J, Zheng H, Shi T, Lei W, Xiao T, Gao Y, Cheng S. [Identification and functional analysis of a novel candidate oncogene RAP2B in lung cancer.]. Zhongguo Fei Ai Za Zhi 2009;12:273–6. [DOI] [PubMed] [Google Scholar]
- 18. Li J, Zhu J, Cao B, Mao X. The mTOR signaling pathway is an emerging therapeutic target in multiple myeloma. Curr Pharm Des. 2014;20:125–35. [DOI] [PubMed] [Google Scholar]
- 19. Wang J, Su H, Han X, Xu K. Inhibition of fibroblast growth factor receptor signaling impairs metastasis of hepatocellular carcinoma. Tumor Biol. 2014;35:11005–11. [DOI] [PubMed] [Google Scholar]
- 20. Di J, Huang H, Qu D, Tang J, Cao W, Lu Z, Cheng Q, Yang J, Bai J, Zhang Y. Rap2B promotes proliferation, migration, and invasion of human breast cancer through calcium-related ERK1/2 signaling pathway. Sci Rep. 2015;5:12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang X, He Y, Lee KH, Dubois W, Li Z, Wu X, Kovalchuk A, Zhang W, Huang J. Rap2bx, a novel p53 target, regulates p53-mediated pro-survival function. Cell Cycle 2013;12:1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Farrell FX, Ohmstede CA, Reep BR, Lapetina EG. cDNA sequence of a new Ras-related gene (Rap2b) isolated from human platelets with sequence homology to rap2. Nucleic Acids Res. 1990;18:4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ohmstede CA, Farrell FX, Reep BR, Clemetson KJ, Lapetina EG. RAP2B: A RAS-related GTP-binding protein from platelets. Proc Natl Acad Sci USA 1990;87:6527–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sun W, Zhang K, Zhang X, Lei W, Xiao T, Ma J, Guo S, Shao S, Zhang H, Liu Y. Identification of differentially expressed genes in human lung squamous cell carcinoma using suppression subtractive hybridization. Cancer Lett. 2004;212:83. [DOI] [PubMed] [Google Scholar]
- 25. An Q, Pacyna-Gengelbach M, Schlüns K, Deutschmann N, Guo S, Gao Y, Zhang J, Cheng S, Petersen I. Identification of differentially expressed genes in immortalized human bronchial epithelial cell line as a model for in vitro study of lung carcinogenesis. Int J Cancer 2003;103:194–204. [DOI] [PubMed] [Google Scholar]
- 26. Di JH, Qu DB, Lu Z, Li LT, Cheng Q, Xin Y, Zhang LZ, Zhang Y, Zheng JN. Rap2B promotes migration and invasion of human suprarenal epithelioma. Tumor Biol. 2014;35:9387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xie X, Liu H, Wang M, Ding F, Xiao H, Hu F, Hu R, Mei J. miR-342-3p targets RAP2B to suppress proliferation and invasion of non-small cell lung cancer cells. Tumor Biol. 2015;36:5031–8. [DOI] [PubMed] [Google Scholar]
- 28. Di J, Cao H, Tang J, Zheng L, Gao K, Zhu Z, Zheng J. Rap2B promotes cell proliferation, migration and invasion in prostate cancer. Med Oncol. 2016;33:1–10. [DOI] [PubMed] [Google Scholar]
- 29. Zhang M, Zhuang Q, Cui L. MiR-194 inhibits cell proliferation and invasion via repression of RAP2B in bladder cancer. Biomed Pharmacother. 2016;80:268. [DOI] [PubMed] [Google Scholar]
- 30. Roskoski R Jr. ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacol Res. 2012;66:105–43. [DOI] [PubMed] [Google Scholar]
- 31. Deschênes-Simard X, Kottakis F, Meloche S, Ferbeyre G. ERKs in cancer: Friends or foes? Cancer Res. 2014;74:412–9. [DOI] [PubMed] [Google Scholar]