Abstract
Systematic treatment of advanced non-small cell lung cancer (NSCLC) includes targeted treatment with epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs). The development of skin rash and its intensity have been associated with EGFR TKI’s efficacy. The main purpose of this study was to further investigate the potential value of erlotinib-associated rash as a predictor of prognosis and treatment response in a real-world cohort of patients with advanced NSCLC. The medical records of all NSCLC patients treated with erlotinib at the Oncology Unit of GPP, Sotiria Athens General Hospital between January 1, 2014 and August 31, 2016 were retrospectively reviewed. Seventy-nine patient medical records fulfilled the criteria and were included in the study. Development of erlotinib-associated rash was correlated with clinicopathological characteristics of patients, treatment response, and overall survival (OS) using univariate and multivariate Cox regression analysis. The number of patients with rash was greater in the responders group (90% vs. 46.4%, p = 0.015). In univariate analysis, there was a statistically significant association between rash development and time to progression (TTP) [HR: 0.32 (0.17–0.57), p < 0.001]. With multivariate Cox regression analysis, it was found that PS ≥ 2 (HR: 2.01, 95% CI: 1.12–3.60, p = 0.018) and rash (HR: 0.34, 95% CI: 0.18–0.63, p = 0.001) were independently associated with TTP and also that the duration of treatment with erlotinib (HR: 0.58, 95% CI: 0.42–0.80, p = 0.001) and rash (HR: 0.10, 95% CI: 0.20–0.48, p = 0.004) was an independent predictor of survival. Our results suggest that erlotinib-associated rash may represent a clinically valuable biomarker for the prediction of treatment response and OS in patients with advanced NSCLC.
Key words: Erlotinib, Rash, Non-small cell lung cancer (NSCLC), Response
INTRODUCTION
Systematic treatment of advanced non-small cell lung cancer (NSCLC) includes targeted treatment with epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs). Approximately 15% of NSCLC patients have tumors that harbor EGFR-sensitizing mutations (i.e., the exon 19 deletion and the L858R point mutation in exon 21). Erlotinib is a member of the EGFR TKI family. It has been approved since 2004 for the treatment of patients with locally advanced or metastatic NSCLC after progression on at least one prior chemotherapy regimen1 and as first-line treatment in patients with sensitizing EGFR mutations2. Erlotinib is generally well tolerated. The most common side effects are diarrhea and dermatological toxicity, mostly papulopustular rash3. The mechanism of rash development is not yet clearly understood. One theory states that erlotinib-induced rash results from direct EGFR inhibition in the skin4 and another that rash is the result of a systemic immunological reaction5. The inhibition of EGFR signaling pathways causes the arrest of keratinocyte growth and apoptosis, decreased migration, increased cell attachment, and premature differentiation and also stimulates inflammation6–8. EGFR polymorphisms and/or polymorphisms in drug transporters and metabolizing enzymes may be of important significance in the development of rash in patients receiving EGFR TKIs9–11.
Besides sensitizing EGFR mutations and also EGFR gene amplification12, the development of skin rash and its intensity have been associated with EGFR TKI’s efficacy13. This association was first noted in patients with colorectal cancer treated with cetuximab14. Patients who develop rash seem to have a better response to EGFR TKIs, and also the greater the intensity of the cutaneous toxicity the better the response15. Since then, the predictive value of erlotinib-induced rash has been confirmed in a number of trials enrolling patients with various solid tumors, including NSCLC13,16–22. However, real-world data on this issue are sparse.
The aim of this study was the retrospective correlation of rash development in patients receiving erlotinib for NSCLC with clinicopathological characteristics, response to treatment, and prognosis. The abovementioned data from existing literature suggest that the appearance and grade of rash during treatment with erlotinib may be markers of response to treatment and prognosis.
MATERIALS AND METHODS
Patients
Five hundred medical records of patients with NSCLC who were treated at the Oncology Unit GPP, Sotiria Athens General Hospital, between January 1, 2014 and August 31, 2016 were retrospectively reviewed. Inclusion criteria were age > 18 years, histologically or cytologically confirmed NSCLC stage IIIB/IV (IASCL 7th edition), and treatment with erlotinib in the first-, second-, third-, or fourth-line setting. OS was measured from initiation of treatment with erlotinib until death or loss to follow-up. Erlotinib (150 mg) was administered once daily (q1–28) until disease progression or unacceptable toxicity. Patients were excluded if they had received a TKI other than erlotinib or if they had a preexisting dermatological condition. Seventy-nine patient medical records fulfilled the criteria and were included in the study. Of these, 34 patients were tested for EGFR mutation, and 18 had sensitizing mutations. EGFR mutation testing was performed by the kit Cobas® EGFR mutation Test v2 by Roche. Demographics, comorbidities, treatment data, and skin-related toxicities were documented. Response was defined per the Response Evaluation Criteria in Solid Tumors (RECIST 1.1). Grade of toxicity evaluation of the cutaneous lesions was performed according to International EGFR Inhibitors Dermatological Toxicity Forum Grading System.
Statistical Analysis
Quantitative variables are expressed as mean values (SD) or as median values [interquartile range (IQR)]. Qualitative variables are expressed as absolute and relative frequencies. For the comparisons of proportions, Fisher’s exact tests were used. Mann–Whitney test was used to compare duration of treatment between those with and without complete or partial response. Life table analyses were used to calculate cumulative survival rate (standard errors) for specific time intervals. The association of each study variable with time to progression (TTP) and survival was first assessed by univariate Cox regression analysis. Variables that showed significant association with the outcome were included in the multivariate Cox proportional hazard model in a forward–backward stepwise method in order to determine the independent predictors for TTP and survival. The assumption of proportional hazards was evaluated by testing for interaction with a continuous time variable. Kaplan–Meier survival estimates were graphed over the follow-up period. All reported p values are two tailed. Statistical significance was set at p < 0.05, and analyses were conducted using STATA statistical software (version 6.0).
RESULTS
Patient Characteristics
A total of 79 patients with a mean age of 67.6 years (SD = 10.9 years), 56 men and 23 women, were included in the study. Demographics, clinicopathological characteristics, and treatment data of patients are presented in Table 1. Sixty percent of the patients were current or former smokers. Prevailing histology was adenocarcinoma (73.4%), followed by squamous cell carcinoma (20.3%). EGFR mutation testing was performed on 34 of the 79 patients; 18 had sensitizing mutations, and 16 had wild-type EGFR. The majority of patients were stage IV (88.6%) and had an ECOG performance status (PS) ≥2 (53.2%). Twenty-five patients received erlotinib as the first-line, 34 as the second-line, 18 as the third-line, and 2 as the fourth-line treatment.
Table 1.
Demographics and Clinical Characteristics
| n | |
|---|---|
| Age [mean (SD)] | 67.6 (10.9%) |
| Gender | |
| Men | 56 (70.9%) |
| Women | 23 (29.1%) |
| Smoking | |
| No | 19 (24.1%) |
| Yes | 60 (75.9%) |
| Pack/years [mean (SD)] | 75.3 (42.1) |
| Comorbidity | |
| No | 17 (21.5%) |
| Yes | 62 (78.5%) |
| Histological type | |
| NOS | 5 (6.3%) |
| Adenocarcinoma | 58 (73.4%) |
| Squamous cell carcinoma | 16 (20.3%) |
| Differentiation (n = 44) | |
| Low | 24 (54.5%) |
| Moderate | 20 (45.5%) |
| EGFR (n = 34) | |
| Negative | 16 (47.1%) |
| Positive | 18 (52.9%) |
| Performance status | |
| 0–1 | 37 (46.8%) |
| ≥2 | 42 (53.2%) |
| Stage | |
| IIIB | 9 (11.4%) |
| IV | 70 (88.6%) |
| Duration of treatment with erlotinib (months), median (IQR) | 3 (2–8) |
| Treatment line | |
| First | 25 (31.6%) |
| Second | 34 (43.0%) |
| Third | 18 (22.8%) |
| Fourth | 2 (2.5%) |
| Rash | |
| No | 38 (48.1%) |
| Yes | 41 (51.9%) |
| Rash grade | |
| I | 24 (58.5%) |
| II | 14 (34.1%) |
| III | 3 (7.3%) |
| Time to rash (days) [median (IQR)] | 23 (18–44.9) |
Treatment Administration and Toxicity
The median duration of treatment with erlotinib was 3 months (IQR: 2–8); 31.6% of the patients received erlotinib as the first line of chemotherapy, 43% at the second line, 22.8% at the third line, and 2.5% at the fourth line. Papulopustular rash presented in 51.9% of the participants and was, in most cases (58.5%), grade I; 12.7% of the patients developed torso rash, 22.8% facial rash, 8.9% torso and facial rash, 3.8% facial and extremities rash, and 3.8% torso, facial, and extremities rash. The median time to rash development after treatment initiation was 23 days (IQR: 18–44.9).
Response to Treatment
There was 12.6% of the patients who had an objective response, namely, 2.5% complete response (CR) and 10.1% partial response (PR). All patients with CR/PR had adenocarcinoma except for one that had squamous cell carcinoma. Patients that achieved CR/PR were lighter smokers (p = 0.010), had activating EGFR mutations, had a greater duration of treatment with erlotinib (p < 0.001), and mainly received erlotinib as the first-line treatment (70%). The number of patients with rash was greater in the responders group (90% vs. 46.4%, p = 0.015).
Time to Disease Progression
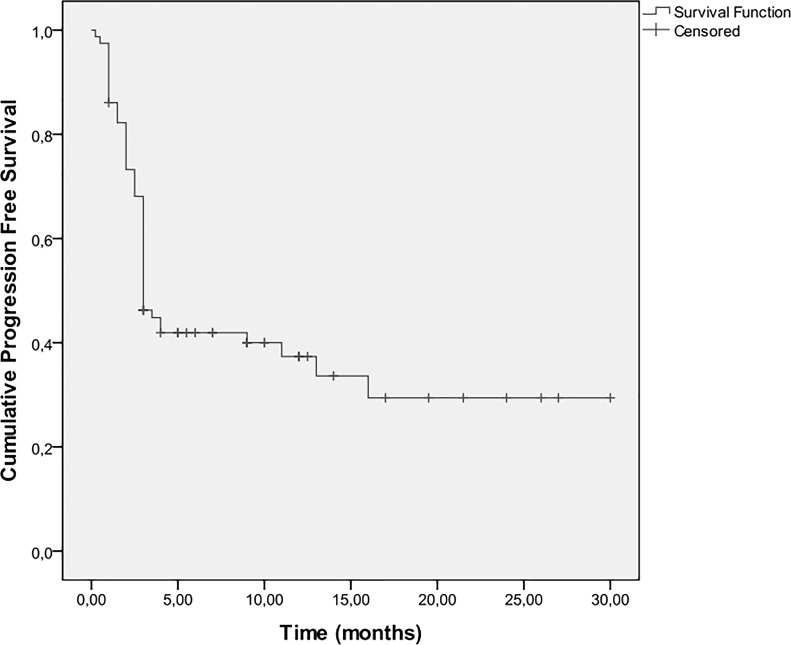
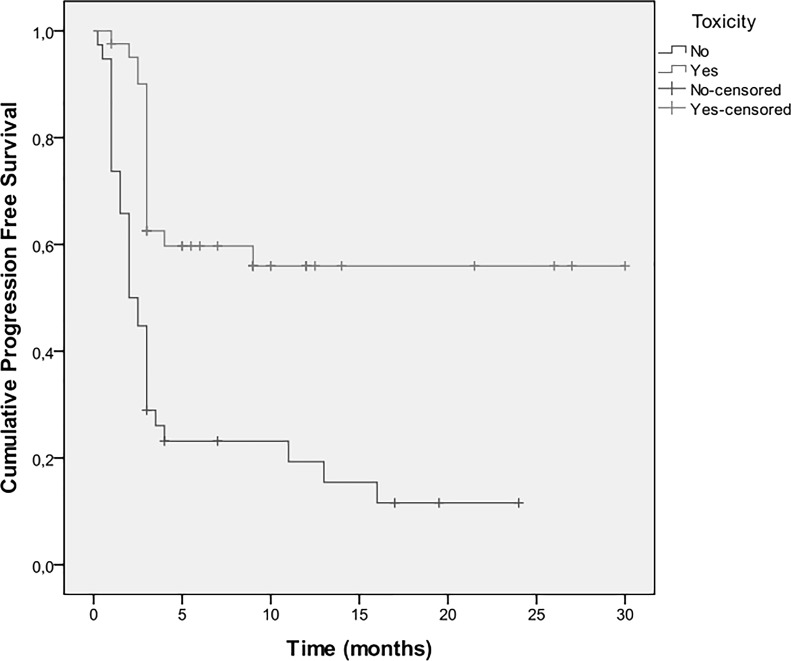
Three-month progression-free survival (PFS) was 68%, 6-month PFS was 40%, and 1-year PFS was 36%. The median TTP was 3 months (IQR: 9–16 months) (Fig. 1). Univariate Cox regression analyses results for disease progression are shown in Table 2. A heavier smoking history and PS ≥ 2 were associated with greater risk for progression. On the other hand, activating EGFR mutations, female sex, increased duration of treatment with erlotinib, and skin rash (Fig. 2) were associated with a lower risk for disease progression. When multiple Cox regression analysis was conducted in a stepwise method, it was found that PS ≥ 2 [hazard ratio (HR): 2.01, 95% CI: 1.12–3.60, p = 0.018] and the presence of rash (HR: 0.34, 95% CI: 0.18–0.63, p = 0.001) were independently associated with TTP.
Figure 1.
Kaplan–Meier estimates for disease progression-free survival.
Table 2.
Univariate Cox Regression Analyses Results for Disease Progression
| Hazard Ratio (HR) (95% CI) | p Value | |
|---|---|---|
| Age | 0.98 (0.96–1.00) | 0.099 |
| Gender | ||
| Men | 1.00* | |
| Women | 0.48 (0.24–0.96) | 0.039 |
| Smoking | ||
| No | 1.00* | |
| Yes | 1.18 (0.60–2.31) | 0.627 |
| Pack/years | 1.01 (1.00–1.02) | 0.023 |
| Comorbidity | ||
| No | 1.00* | |
| Yes | 1.44 (0.67–3.07) | 0.347 |
| Histological type | ||
| NOS | 1.00* | |
| Adenocarcinoma | 0.72 (0.23–2.30) | 0.580 |
| Squamous cell carcinoma | 0.69 (0.24–1.94) | 0.477 |
| Differentiation (n = 44) | ||
| Low | 1.00* | |
| Moderate | 0.70 (0.31–1.61) | 0.402 |
| EGFR (n = 34) | ||
| Negative | 1.00* | |
| Positive | 0.22 (0.08–0.61) | 0.004 |
| Performance status | ||
| 0–1 | 1.00* | |
| ≥2 | 2.09 (1.17–3.74) | 0.013 |
| Stage | ||
| IIIB | 1.00* | |
| IV | 2.51 (0.78–8.09) | 0.124 |
| Duration of treatment with erlotinib (days) | 0.76 (0.67–0.86) | <0.001 |
| Treatment line | ||
| First | 1.00* | |
| Second | 1.96 (0.98–3.94) | 0.059 |
| Third–fourth | 1.50 (0.68–3.29) | 0.314 |
| Rash | ||
| No | 1.00* | |
| Yes | 0.32 (0.17–0.57) | <0.001 |
| Rash grade | ||
| I | 1.00* | |
| II/III | 1.05 (0.4–2.76) | 0.923 |
| Time to rash (days) | 1.00 (0.99–1.01) | 0.583 |
Reference category.
Figure 2.
Kaplan–Meier estimates for disease progression-free survival according to the presence of toxicity.
Overall Survival
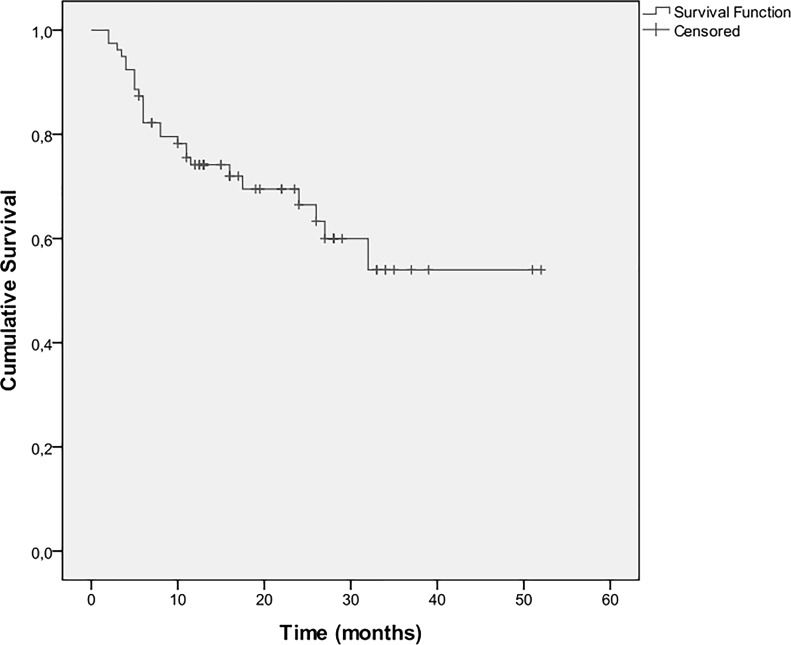
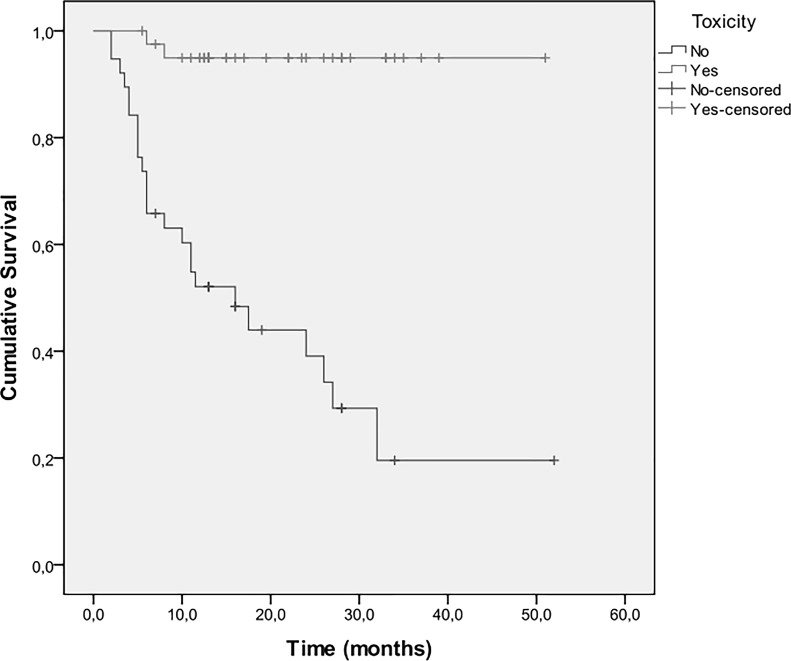
Twenty-six patients died during the follow-up period. OS at 6 months was 87%, at 1 year was 74%, at 2 years was 70%, and at 3 years was 53%. Median overall survival was 13 months (IQR: 10–26 months) (Fig. 3). Univariate analyses for survival (Table 3) showed that an increased smoking history and a PS ≥ 2 were associated with a greater hazard, whereas sensitizing EGFR mutations, increased duration of treatment with erlotinib, and rash (Fig. 4) were associated with a lower hazard. Multiple Cox regression analysis indicated that duration of treatment with erlotinib and rash were independent predictors of survival. Specifically, the hazard decreases as the duration of treatment increases (HR: 0.58, 95% CI: 0.42–0.80, p = 0.001), whereas patients that developed rash had a 90% lower death hazard (HR: 0.10, 95% CI: 0.20–0.48, p = 0.004).
Figure 3.
Kaplan–Meier estimates for survival.
Table 3.
Univariate Cox Regression Analyses Results for Survival
| Hazard Ratio (95% CI)* | p Value | |
|---|---|---|
| Age | 0.98 (0.95–1.01) | 0.258 |
| Gender | ||
| Men | 1.00* | |
| Women | 0.56 (0.21–1.48) | 0.240 |
| Smoking | ||
| No | 1.00* | |
| Yes | 0.83 (0.33–2.08) | 0.696 |
| Pack/years | 1.01 (1.00–1.03) | 0.012 |
| Comorbidity | ||
| No | 1.00* | |
| Yes | 2.36 (0.71–7.89) | 0.162 |
| Histological type | ||
| NOS | 1.00* | |
| Adenocarcinoma | 0.99 (0.11–8.98) | 0.996 |
| Squamous cell carcinoma | 1.46 (0.19–10.91) | 0.715 |
| Differentiation (n = 44) | ||
| Low | 1.00* | |
| Moderate | 0.50 (0.15–1.67) | 0.262 |
| EGFR (n = 34) | ||
| No | 1.00* | |
| Yes | 0.11 (0.01–1.00) | 0.049 |
| Performance status | ||
| 0–1 | 1.00* | |
| ≥2 | 5.97 (2.05–17.35) | 0.001 |
| Stage | ||
| IIIB | 1.00* | |
| IV | 1.90 (0.45–8.07) | 0.382 |
| Duration of treatment with erlotinib (days) | 0.48 (0.33–0.69) | <0.001 |
| Treatment line | ||
| First | 1.00* | |
| Second | 0.98 (0.41–2.37) | 0.967 |
| Third–fourth | 0.54 (0.18–1.65) | 0.279 |
| Rash | ||
| No | 1.00* | |
| Yes | 0.06 (0.01–0.24) | <0.001 |
| Rash grade | ||
| I | 1.00* | |
| II/III | 1.36 (0.09–21.81) | 0.826 |
| Time to rash (days) | 0.97 (0.87–1.09) | 0.639 |
Reference category.
Figure 4.
Kaplan–Meier estimates for survival according to the presence of toxicity.
DISCUSSION
Our study found that the development of rash in patients with NSCLC receiving erlotinib is independently associated with longer TTP and OS. Patients that developed skin toxicity had a TTP of 5 months versus 2.25 months for the patients who did not, and an OS of 15 months versus 12.3 months, respectively. These results concur with the existing literature. A meta-analysis included 24 publications (17 prospective trials and 7 retrospective case series) and found that the presence of skin rash was an independent predictive factor for survival (HR: 0.30, p < 0.00001) and disease progression (HR: 0.50, p < 0.00001). Also, patients who developed National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) grades 2–4 rash were more likely to respond to treatment than patients with no rash (42% vs. 7%)23. A retrospective analysis of patients who took erlotinib in the pivotal phase III trial of erlotinib in locally advanced or metastatic NSCLC, BR.21, also supports this fact. In this subanalysis, 75% of patients that took erlotinib developed a rash at any point in time during treatment, while 17% of the placebo group patients also developed a rash. Ninety-five percent of rash cases in the erlotinib group were reported within 10 weeks of treatment, and the median OS of patients with rash was 37.4 weeks versus 11.1 weeks in patients with no rash (HR: 0.51, 95% CI: 0.38–0.68, p < 0.0001). These correlations increased with rash severity grade: grade 1 versus no rash (HR: 0.41, p < 0.001) and grade ≥2 versus no rash (HR: 0.29, p < 0.001)24. However, the development of a rash does not necessarily mean clinical activity, as was shown in a trial of high-dose erlotinib in patients with NSCLC25.
Interestingly, EGFR mutation status was not found to correlate with rash development, while the rash grade was not associated with treatment response or OS in our patient population. The latter is in discordance with some previous studies that have shown a positive correlation between rash severity and EGFR TKI efficacy13,24,26–29. However, it must be noted that methods of rash grade evaluation vary significantly among different observers and may thus be subject to interpretational bias. EGFR mutation status, although it is the principal determinant of erlotinib’s efficacy, has not been shown to relate to rash development or any other erlotinib-related toxicity30.
The NCI-CTCAE is used in most erlotinib trials to grade cutaneous toxicity. However, there are limitations to their use for EGFR TKIs. In these criteria, body surface area coverage is incorporated in the evaluation of rash grade. Erlotinib-associated rash presents mostly in the face and upper torso, and while it may remain confined to those areas, it can be of significant severity. This is why a simplified system has been proposed to grade EGFR TKI-associated rash by the International EGFR Inhibitors Dermatological Toxicity Forum Grading System. It consists of three categories (mild, moderate, and severe toxicity), which do not include the extent of affected skin but the intensity of the cutaneous reaction and the presence of superinfection31,32.
It has been proposed that the correlation of rash with treatment efficacy may be the reflection of sufficient plasma and, consequently, tumor drug concentrations. Until now, the “dose to achieve rash” approach has not shown favorable results in NSCLC. In a study of gefitinib 250 mg versus 500 mg, no relation was shown between plasma concentrations and rash severity33. Regarding erlotinib, a dose escalation study was conducted where the dose was escalated up from 150 mg by 25 mg until patients presented a grade 2 (CTCAE) rash or the maximum dose of 250 mg of erlotinib was reached. The study showed that while a grade 2 rash was achieved in 59% of patients, the response rate was only 7%34. However, it was shown recently that the assessment of drug-metabolizing activity might be of use in these cases. Indeed, drug-metabolizing activity assessed by the erlotinib/O-desmethyl-erlotinib metabolic ratio has been correlated with the severity of skin rash (i.e., a high metabolic activity lowers the occurrence of skin rash). The erlotinib/O-desmethyl-erlotinib metabolic ratio was also highly associated with PFS and OS in NSCLC and pancreatic adenocarcinoma patients. The individual metabolic activity of erlotinib determined in the serum may be helpful for therapeutic monitoring and individual “dosing to rash” in rash-negative cancer patients35.
A recent trial showed that genetic polymorphisms in drug transporters and metabolizing enzymes are important factors in the variability of efficacy and toxicity of erlotinib between patients and are significantly correlated with the appearance of skin rash10. The study indicates that a single nucleotide polymorphism (SNP) in the CYP27B1 gene is significantly correlated with erlotinib-induced skin rash in NSCLC patients, probably through a mechanism mediated by vitamin D3 and inflammation at the skin level. A study of EGFR polymorphisms in patients with NSCLC that had received gefitinib showed that patients homozygous for the shorter length alleles of the intron 1 dinucleotide CA repeat polymorphism or patients carrying at least one T allele of the −216G/T promoter polymorphism had an improved PFS and OS and also that there was a correlation between the T allele of the −216G/T polymorphism and the development of any grade of treatment-related rash or diarrhea9. A previous study on EGFR polymorphisms also demonstrated that shorter repeat lengths of the intron 1 dinucleotide CA repeat correlate with improved response to gefitinib in head and neck cancer cell lines and with skin toxicity in head and neck cancer patients treated with an EGFR TKI11. The relationships between EGFR mutation, amplification, and genetic polymorphism, as well as transporting and metabolizing enzymes polymorphisms, still require further study. These genetic alterations may provide an explanation for the association of skin rash with EGFR TKI efficacy.
Erlotinib skin-related toxicity is seen in over 50% of patients36, as was also the case in our study. Rash usually presents in the face and upper torso37,38 and is in most cases mild to moderate. Erlotinib is a generally well-tolerated drug, but rash is often the cause for dose reduction or discontinuation39,40. Approximately 10%–12% of patients will discontinue treatment due to this cutaneous toxicity or will require a dose reduction41,42, leading to a less effective treatment. Rash usually presents 1–2 weeks after the start of treatment and may improve or resolve spontaneously while erlotinib is continued and seems to be dose related43. The spontaneous improvement that has been seen in some patients, and also the fact that it can be managed with immunosuppressants (i.e., corticosteroids), is the basis of the immunological theory of erlotinib-associated rash development31.
Dermatological toxicity can have a huge effect on the patients’ quality of life and may interfere with adherence to erlotinib. Patients may experience skin sensitivity, causalgia, and even pain44. Skin rash changes one’s image of oneself and so impacts the patients’ physical, emotional, and social well-being. This leads to frustration and depression and a consequent withdrawal from social activities. Symptom reframing, for example, stating that the presence of rash means that the treatment is efficient, may help patients deal with their altered image and physical discomfort44,32. As has been mentioned above, approximately 10%–12% of patients will experience moderate to severe rash, resulting in dose reduction or even treatment discontinuation41,42. This highlights the need for the effective management, and a proactive approach is very important in minimizing or alleviating cutaneous toxicity45.
While rash development is a strong indicator of EGFR TKI efficacy, the absence of rash is not synonymous to treatment failure. Albeit small, a percentage of patients that do not develop rash have a clinical benefit from erlotinib treatment29. The limitations of our study are its retrospective design, potential bias introduced during evaluation, grading of rash from different observers, the heterogeneity of patient population with regard to treatment history, and small sample size; nonetheless, the results contribute to existing literature to highlight the prognostic significance of rash development in patients receiving treatment with EGFR TKIs. The mechanism behind this intriguing observation is still ambiguous and remains to be clarified in future studies.
Our study shows that patients with NSCLC that develop rash while on erlotinib have a better response to this agent (90% vs. 46.4%, p = 0.015). In univariate and multivariate analysis, there is a statistically significant association between rash development and TTP [HR: 0.32 (0.17–0.57), p < 0.001] and (HR: 0.34, 95% CI: 0.18–0.63, p = 0.001), respectively]. In multivariate analysis, PS ≥ 2 also correlates with TTP (HR: 2.01, 95% CI: 1.12–3.60, p = 0.018). Duration of treatment with erlotinib (HR: 0.58, 95% CI: 0.42–0.80, p = 0.001) and rash (HR: 0.10, 95% CI: 0.20–0.48, p = 0.004) are independent predictors of survival. These results suggest that erlotinib-associated rash may represent a clinically valuable biomarker for the prediction of treatment response and OS in patients with advanced NSCLC. There is a need for larger, prospective real-world studies in order to validate these results.
REFERENCES
- 1. Johnson JR, Cohen M, Sridhara R, Chen Y-F, Williams GM, Duan J, Gobburu J, Booth B, Benson K, Leighton J, Hsieh LS, Chidambaram N, Zimmerman P, Pazdur R. Approval summary for erlotinib for treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen. Clin Cancer Res. 2005;11(18):6414–21. [DOI] [PubMed] [Google Scholar]
- 2. Khozin S, Blumenthal GM, Jiang X, He K, Boyd K, Murgo A, Justice R, Keegan P, Pazdur R. U.S. Food and Drug Administration approval summary: Erlotinib for the first-line treatment of metastatic non-small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 (L858R) substitution mutations. Oncologist 2014;19(7):774–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burotto M, Manasanch EE, Wilkerson J, Fojo T. Gefitinib and erlotinib in metastatic non-small cell lung cancer: A meta-analysis of toxicity and efficacy of randomized clinical trials. Oncologist 2015;20(4):400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer 2006;6(10):803–12. [DOI] [PubMed] [Google Scholar]
- 5. Peréz-Soler R, Saltz L. Cutaneous adverse effects with HER1/EGFR-targeted agents: Is there a silver lining? J Clin Oncol. 2005;23(22):5235–46. [DOI] [PubMed] [Google Scholar]
- 6. Peus D, Hamacher L, Pittelkow MR. EGF-receptor tyrosine kinase inhibition induces keratinocyte growth arrest and terminal differentiation. J Invest Dermatol. 1997;109(6):751–6. [DOI] [PubMed] [Google Scholar]
- 7. Woodworth CD, Michael E, Marker D, Allen S, Smith L, Nees M. Inhibition of the epidermal growth factor receptor increases expression of genes that stimulate inflammation, apoptosis, and cell attachment. Mol Cancer Ther. 2005;4(4):650–8. [DOI] [PubMed] [Google Scholar]
- 8. Lacouture ME, Lai SE. The PRIDE (Papulopustules and/or paronychia, Regulatory abnormalities of hair growth, Itching, and Dryness due to Epidermal growth factor receptor inhibitors) syndrome. Br J Dermatol. 2006;155(4):852–4. [DOI] [PubMed] [Google Scholar]
- 9. Liu G, Gurubhagavatula S, Zhou W, Wang Z, Yeap BY, Asomaning K, Su L, Heist R, Lynch TJ, Christiani DC. Epidermal growth factor receptor polymorphisms and clinical outcomes in non-small-cell lung cancer patients treated with gefitinib. Pharmacogenomics J. 2008;8(2):129–38. [DOI] [PubMed] [Google Scholar]
- 10. Arbitrio M, Di Martino MT, Barbieri V, Agapito G, Guzzi PH, Botta C, Iuliano E, Scionti F, Altomare E, Codispoti S, Conforti S, Cannataro M, Tassone P, Tagliaferri P. Identification of polymorphic variants associated with erlotinib-related skin toxicity in advanced non-small cell lung cancer patients by DMET microarray analysis. Cancer Chemother Pharmacol. 2016;77(1):205–9. [DOI] [PubMed] [Google Scholar]
- 11. Amador ML, Oppenheimer D, Perea S, Maitra A, Cusatis G, Cusati G, Iacobuzio-Donahue C, Baker SD, Ashfaq R, Takimoto C, Forastiere A, Hidalgo M. An epidermal growth factor receptor intron 1 polymorphism mediates response to epidermal growth factor receptor inhibitors. Cancer Res. 2004;64(24):9139–43. [DOI] [PubMed] [Google Scholar]
- 12. Bethune G, Bethune D, Ridgway N, Xu Z. Epidermal growth factor receptor (EGFR) in lung cancer: An overview and update. J Thorac Dis. 2010;2(1):48–51. [PMC free article] [PubMed] [Google Scholar]
- 13. Pérez-Soler R, Chachoua A, Hammond LA, Rowinsky EK, Huberman M, Karp D, Rigas J, Clark GM, Santabárbara P, Bonomi P. Determinants of tumor response and survival with erlotinib in patients with non-small-cell lung cancer. J Clin Oncol. 2004;22(16):3238–47. [DOI] [PubMed] [Google Scholar]
- 14. Saltz LB, Meropol NJ, Loehrer PJ, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22(7):1201–8. [DOI] [PubMed] [Google Scholar]
- 15. Cutsem EV, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Laethem JLV, Maurel J, Richardson G, Wolf M, Amado RG. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25(13):1658–64. [DOI] [PubMed] [Google Scholar]
- 16. Kris MG, Natale RB, Herbst RS, Lynch TJ, Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H, Sandler A, Albain KS, Cella D, Wolf MK, Averbuch SD, Ochs JJ, Kay AC. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: A randomized trial. JAMA. 2003;290(16):2149–58. [DOI] [PubMed] [Google Scholar]
- 17. Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard J-Y, Nishiwaki Y, Vansteenkiste J, Kudoh S, Rischin D, Eek R, Horai T, Noda K, Takata I, Smit E, Averbuch S, Macleod A, Feyereislova A, Dong RP, Baselga J. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 2003;21(12):2237–46. [DOI] [PubMed] [Google Scholar]
- 18. Hanna N, Lilenbaum R, Ansari R, Lynch T, Govindan R, Jänne PA, Bonomi P. Phase II trial of cetuximab in patients with previously treated non-small-cell lung cancer. J Clin Oncol. 2006;24(33):5253–8. [DOI] [PubMed] [Google Scholar]
- 19. Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, Kris MG, Tran HT, Klein P, Li X, Ramies D, Johnson DH, Miller VA. TRIBUTE: A phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23(25):5892–9. [DOI] [PubMed] [Google Scholar]
- 20. Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, Rosa FD, Milanowski J, Karnicka-Mlodkowski H, Pesek M, Serwatowski P, Ramlau R, Janaskova T, Vansteenkiste J, Strausz J, Manikhas GM, Pawel JV. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: The Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25(12):1545–52. [DOI] [PubMed] [Google Scholar]
- 21. Giaccone G, Herbst RS, Manegold C, Scagliotti G, Rosell R, Miller V, Natale RB, Schiller JH, Von Pawel J, Pluzanska A, Gatzemeier U, Grous J, Ochs JS, Averbuch SD, Wolf MK, Rennie P, Fandi A, Johnson DH. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: A phase III trial—INTACT 1. J Clin Oncol. 2004;22(5):777–84. [DOI] [PubMed] [Google Scholar]
- 22. Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C, Scagliotti G, Rosell R, Oliff I, Reeves JA, Wolf MK, Krebs AD, Averbuch SD, Ochs JS, Grous J, Fandi A, Johnson DH. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: A phase III trial—INTACT 2. J Clin Oncol. 2004;22(5):785–94. [DOI] [PubMed] [Google Scholar]
- 23. Petrelli F, Borgonovo K, Cabiddu M, Lonati V, Barni S. Relationship between skin rash and outcome in non-small-cell lung cancer patients treated with anti-EGFR tyrosine kinase inhibitors: A literature-based meta-analysis of 24 trials. Lung Cancer 2012;78(1):8–15. [DOI] [PubMed] [Google Scholar]
- 24. Pérez-Soler R, Leon L, Wojtowicz-Praga S. Cutaneous toxicity secondary to erlotinib therapy in patients with non-small cell lung cancer in the NCIC CTG BR.21 study: Time course and correlation with survival. J Clin Oncol. 2012;30(Suppl; abstr 7573). [Google Scholar]
- 25. Milton DT, Azzoli CG, Heelan RT, Venkatraman E, Gomez JE, Kris MG, Krug LM, Pao W, Rizvi NA, Dunne M, Miller VA. A phase I/II study of weekly high-dose erlotinib in previously treated patients with nonsmall cell lung cancer. Cancer 2006;107(5):1034–41. [DOI] [PubMed] [Google Scholar]
- 26. Tsao MS, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S, Squire J, Lorimer I, Zhang T, Liu N, Daneshmand M, Marrano P, da Cunha Santos G, Lagarde A, Richardson F, Seymour L, Whitehead M, Ding K, Pater J, Shepherd FA. Erlotinib in lung cancer—Molecular and clinical predictors of outcome. N Engl J Med. 2005;353(2):133–44. [DOI] [PubMed] [Google Scholar]
- 27. Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, Insa A, Massuti B, Gonzalez-Larriba JL, Paz-Arez L, Bover I, Garcia-Campelo R, Moreno MA, Catot S, Rolfo C, Reguart N, Palmero R, Sanchez JM, Bastus R, Mayo C, Bertran-Alamillo J, Molina MA, Sanchez JJ, Taron M. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958–67. [DOI] [PubMed] [Google Scholar]
- 28. Clark GM, Pérez-Soler R, Siu L, Gordon A, Santabárbara P. Rash severity is predictive of increased survival with erlotinib hydrochloride. Proc Am Soc Clin Oncol. 2003;abstr 786:196. [Google Scholar]
- 29. Wacker B, Nagrani T, Weinberg J, Witt K, Clark G, Cagnoni PJ. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res. 2007;13(13):3913–21. [DOI] [PubMed] [Google Scholar]
- 30. Charpidou A, Blatza D, Anagnostou V, Anagnostou E, Syrigos KN. Review. EGFR mutations in non-small cell lung cancer—Clinical implications. In Vivo 2008;22(4):529–36. [PubMed] [Google Scholar]
- 31. Kiyohara Y, Yamazaki N, Kishi A. Erlotinib-related skin toxicities: Treatment strategies in patients with metastatic non-small cell lung cancer. J Am Acad Dermatol. 2013;69(3):463–72. [DOI] [PubMed] [Google Scholar]
- 32. Lynch TJ, Kim ES, Eaby B, Garey J, West DP, Lacouture ME. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: An evolving paradigm in clinical management. Oncologist 2007;12(5):610–21. [DOI] [PubMed] [Google Scholar]
- 33. Li J, Karlsson MO, Brahmer J, Spitz A, Zhao M, Hidalgo M, Baker SD. CYP3A phenotyping approach to predict systemic exposure to EGFR tyrosine kinase inhibitors. J Natl Cancer Inst. 2006;98(23):1714–23. [DOI] [PubMed] [Google Scholar]
- 34. Kolesar J, Brahmer J, Lee J, Guaglianone P, Patel J, Keppen M, Hidalgo M, Carbone D, Schiller J. Final results of ECOG 3503: A pilot study to determine if downstream markers of EGFR linked signaling pathways predict response to erlotinib (OSI-774) in the first-line treatment of patients with advanced non-small cell lung cancer (NSCLC). ASCO Meet Abstr. 2007;25(18 Suppl):7588. [Google Scholar]
- 35. Steffens M, Paul T, Hichert V, Scholl C, Mallek D von, Stelzer C, Sölger F, Reiser B, Schumann C, Rüdiger S, Boeck S, Heinemann V, Kächele V, Seufferlein T, Stingl J. Dosing to rash?—The role of erlotinib metabolic ratio from patient serum in the search of predictive biomarkers for EGFR inhibitor-mediated skin rash. Eur J Cancer 2016;55:131–9. [DOI] [PubMed] [Google Scholar]
- 36. Thatcher N, Nicolson M, Groves RW, Steele J, Eaby B, Dunlop J, McPhelim J, Nijjar R, Ukachukwu I; U.K. Erlotinib Skin Toxicity Management Consensus Group. Expert consensus on the management of erlotinib-associated cutaneous toxicity in the U.K. Oncologist 2009;14(8):840–7. [DOI] [PubMed] [Google Scholar]
- 37. Tsimboukis S, Merikas I, Karapanagiotou EM, Saif MW, Syrigos KN. Erlotinib-induced skin rash in patients with non-small-cell lung cancer: Pathogenesis, clinical significance, and management. Clin Lung Cancer 2009;10(2):106–11. [DOI] [PubMed] [Google Scholar]
- 38. Kotteas EA, Charpidou AG, Syrigos KN. Targeted therapy for nonsmall cell lung cancer: Focusing on angiogenesis, the epidermal growth factor receptor and multikinase inhibitors. Anticancer Drugs 2010;21(2):151–68. [DOI] [PubMed] [Google Scholar]
- 39. Haspinger ER, Agustoni F, Torri V, Gelsomino F, Platania M, Zilembo N, Gallucci R, Garassino MC, Cinquini M. Is there evidence for different effects among EGFR-TKIs? Systematic review and meta-analysis of EGFR tyrosine kinase inhibitors (TKIs) versus chemotherapy as first-line treatment for patients harboring EGFR mutations. Crit Rev Oncol Hematol. 2015;94(2):213–27. [DOI] [PubMed] [Google Scholar]
- 40. Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer 2015;88(1):74–9. [DOI] [PubMed] [Google Scholar]
- 41. Agero ALC, Dusza SW, Benvenuto-Andrade C, Busam KJ, Myskowski P, Halpern AC. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55(4):657–70. [DOI] [PubMed] [Google Scholar]
- 42. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabárbara P, Seymour L. Erlotinib in previously treated non–small-cell lung cancer. N Engl J Med. 2005;353(2):123–32. [DOI] [PubMed] [Google Scholar]
- 43. Pérez-Soler R, Delord JP, Halpern A, Kelly K, Krueger J, Sureda BM, von Pawel J, Temel J, Siena S, Soulières D, Saltz L, Leyden J. HER1/EGFR inhibitor-associated rash: Future directions for management and investigation outcomes from the HER1/EGFR inhibitor rash management forum. Oncologist 2005;10(5):345–56. [DOI] [PubMed] [Google Scholar]
- 44. Wagner LI, Lacouture ME. Dermatologic toxicities associated with EGFR inhibitors: The clinical psychologist’s perspective. Impact on health-related quality of life and implications for clinical management of psychological sequelae. Oncology (Williston Park) 2007;21(11 Suppl 5):34–6. [PubMed] [Google Scholar]
- 45. Jacot W, Bessis D, Jorda E, Ychou M, Fabbro M, Pujol J-L, Guillot B. Acneiform eruption induced by epidermal growth factor receptor inhibitors in patients with solid tumours. Br J Dermatol. 2004;151(1):238–41. [DOI] [PubMed] [Google Scholar]