Abstract
Homeobox B5 (HOXB5), a member of the HOX gene family, has been shown to play an important role in tumor progression. However, the expression and functional role of HOXB5 in human non-small cell lung cancer (NSCLC) have not been defined. Thus, the purpose of this study was to elucidate the expression and functional role of HOXB5 in human NSCLC. Our results showed that HOXB5 expression was elevated in human NSCLC tissues and cell lines. The in vitro experiments demonstrated that knockdown of HOXB5 inhibited proliferation, migration, and invasion and prevented the EMT phenotype in NSCLC cells. In vivo experiments indicated that knockdown of HOXB5 attenuated the growth of NSCLC xenografts in vivo. Furthermore, knockdown of HOXB5 suppressed the protein expression levels of β-catenin and its downstream targets c-Myc and cyclin D1 in A549 cells. Taken together, for the first time we have shown that knockdown of HOXB5 significantly inhibited NSCLC cell proliferation, invasion, metastasis, and EMT, partly through the Wnt/β-catenin signaling pathway. These findings suggest that HOXB5 may be a novel therapeutic target for the treatment of NSCLC.
Key words: Homeobox B5 (HOXB5), Non-small cell lung cancer (NSCLC), Invasion, Wnt/β-catenin pathway
INTRODUCTION
Lung cancer is the leading cause of cancer-related mortality in the world. The incidence of lung cancer in China has rapidly increased in the past years. Non-small cell lung cancer (NSCLC) is the dominant type of lung cancer, which accounts for 80% of all types1. Despite recent advances in diagnosis and treatment strategies in early diagnosis and therapy, including surgery, radiation therapy, chemotherapy, and/or targeted therapies2–4, the prognosis of NSCLC is still unfavorable, and the 5-year overall survival rate is still less than 15%5,6. Therefore, it is urgent that we elucidate the molecular mechanisms underlying NSCLC development for improving the diagnosis, prevention, and treatment of NSCLC.
Homeobox (HOX) genes are the family of transcription factors that play a crucial role in modulating embryonic morphogenesis and cell differentiation in mammals, and a multistep process of carcinogenesis, including transformation, proliferation, angiogenesis, migration, and metastasis7–9. HOXB5, a member of the HOX gene family, has been demonstrated to play an important role in the survival and cell lineage differentiation of vagal and trunk neural tube cells during early development10,11. Recently, increasing evidence indicates a critical role for HOXB5 in the regulation of tumor progression12–15. For example, Hong et al. reported that the expression of HOXB5 was significantly increased in gastric cancer tissues compared with adjacent normal tissues, and overexpression of HOXB5 induced invasion and migration activities in gastric cancer cells16. However, the expression and functional role of HOXB5 in human NSCLC have not been defined. Thus, the purpose of this study was to elucidate the expression and functional role of HOXB5 in human NSCLC. Here we report a novel function of HOXB5 in promoting NSCLC cell growth and metastasis.
MATERIALS AND METHODS
Patients and Tissues
This study was approved by the Institute Research Ethics, The Second Affiliated Hospital of Zhejiang University, School of Medicine (P.R. China). A total of 12 pairs of NSCLC tissues and their matched adjacent normal lung tissues were obtained from patients who underwent surgery at The Second Affiliated Hospital of Zhejiang University, School of Medicine. Informed consent was written and obtained from all the subjects in our study.
Cell Culture
Human NSCLC cell lines (A549, H460, and H292) and a normal human bronchial epithelial cell line (HBE) were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). All cells were maintained at 37°C and 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco BRL), 100 U/ml penicillin, and 0.1 mg/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA).
Short Hairpin RNA and Cell Transfection
The specific short hairpin RNA targeting HOXB5 (sh-HOXB5) and its negative control (sh-NC) were purchased from Invitrogen (Carlsbad, CA, USA). A549 cells (5 × 104 cells/ml) and H460 cells (5 × 104 cells/ml) were seeded into 24-well plates and transfected with sh-HOXB5 or sh-NC using Lipofectamine 2000 (Invitrogen), respectively, according to the manufacturer’s instructions. The relative knockdown efficiency was evaluated using Western blot with the HOXB5 antibody.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from NSCLC cells using the TRIzol reagent (Invitrogen) and reversely transcribed into complementary DNA (cDNA) using the First-Strand cDNA Synthesis Kit (MBI Fermentas, Vilnius, Lithuania) according to the manufacturer’s instructions. The following primers were used: HOXB5, 5′-TGCATCGCTATAATTCATT-3′ (sense) and 5′-GCCTCGTCTATTTCGGTGA-3′ (antisense); β-actin, 5′-CCGTGAAAAGATGACCCAGATC-3′ (sense) and 5′-CACAGCCTGGATGGCTACGT-3′ (antisense). Aliquots of the PCR products were electrophoresed on 1.5% agarose gels, and PCR fragments were visualized by ethidium bromide staining. Expression levels of the relative genes were calculated using the 2−ΔΔCT method17.
Western Blot
The transfected cells were lysed in RIPA buffer consisting of 50 mM Tris (pH 7.4), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate. The cell lysates separated by 10% sodium dodecyl sulfate-PAGE and subsequently transferred to a PVDF membrane. The membranes were blocked with 5% skim milk for 1 h at room temperature and then incubated with primary antibodies as follows: HOXB5, N-cadherin, E-cadherin, β-catenin, c-Myc, cyclin D1, and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C. The blots were then incubated with HRP-conjugated secondary antibody for 1 h at room temperature. Finally, the bands in the membrane were visualized using enhanced chemiluminescence reagents (Pierce Biotechnology, Rockford, IL, USA).
Cell Proliferation Assay
Cell proliferation was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, the transfected cells (1 × 105 cells/well) were seeded into 96-well flat-bottom microtiter plates (Pierce) and then cultured at 24-h intervals for 4 days. MTT solutions (Sigma-Aldrich) dissolved in culture medium at a final concentration of 0.5 mmol/L were added to each well, and the plates were incubated for another 4 h; 100 μl of DMSO (Sigma-Aldrich) was added to dissolve the formazan crystals. Finally, the optical density was measured on a Bio-Rad microplate reader (Bio-Rad, Hercules, CA, USA) using a test wavelength of 490 nm.
Cell Migration and Invasion Assays
A Transwell migration assay was performed using 8-μm Transwell chambers (Costar, Cambridge, MA, USA). The transfected cells (1 × 105 cells/well) suspended in serum-free DMEM were added to the upper chamber. In the lower chamber, 500 μl of DMEM including 10% FBS was added. Twenty-four hours later, the cells on the lower surface of the membrane were fixed, stained with crystal violet, and then counted under a light microscope. A cell invasion assay was performed using a Transwell chamber precoated with Matrigel (8-μm pore size; BD Biosciences, France).
In Vivo Xenograft Tumor Assay
A549 cells (1 × 107 cells) transfected with sh-HOXB5 or sh-NC diluted in 200 μl of PBS were subcutaneously injected into the right flank of 4-week-old male BALB/c nude mice (n = 6 per group). The tumor volume was measured every week and calculated using the formula: volume = width2 × length × 0.5. Four weeks after injection, mice were euthanized, and tumors were dissected and weighed. All animal studies were approved by the Institutional Animal Care and Use Committee of The Second Affiliated Hospital of Zhejiang University, School of Medicine (P.R. China).
Statistical Analysis
Statistical analyses were performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Data were expressed as mean ± SD. The differences were analyzed by the Student’s t-test or one-way analysis of variance and Student’s t-test. A value of p < 0.05 was considered to be statistically significant.
RESULTS
Expression Profile of HOXB5 in NSCLC Tissues and Cell Lines
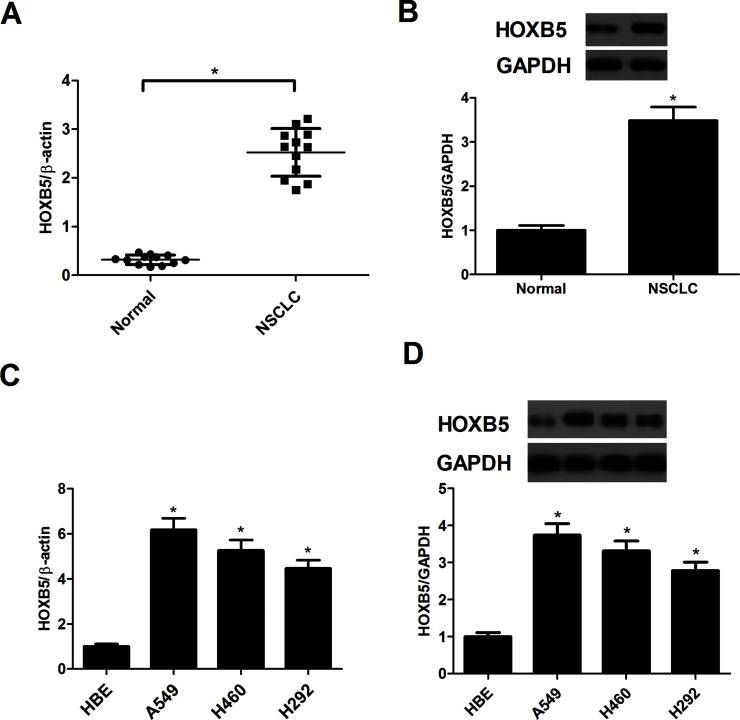
To determine whether HOXB5 was expressed in NSCLC, NSCLC tissues and paired normal tissues were used to detect the expression of HOXB5. The expression of HOXB5 at both mRNA and protein levels was significantly upregulated in NSCLC tissues, compared with the paired normal lung tissues (Fig. 1A and B). Furthermore, the mRNA level of HOXB5 was greatly increased in three human NSCLC cell lines (Fig. 1C). The protein expression of HOXB5 in the three NSCLC cell lines was in accordance with the mRNA expression (Fig. 1D).
Figure 1.
Expression profile of homeobox B5 (HOXB5) in non-small cell lung cancer (NSCLC) tissues and cell lines. (A) The mRNA expression of HOXB5 in 12 paired NSCLC tissues (NSCLC) and their corresponding normal tissues (Normal) was detected using quantitative real-time polymerase chain reaction (qRT-PCR). (B) The protein expression of HOXB5 in 12 paired NSCLC tissues (NSCLC) and their corresponding normal tissues (Normal) was evaluated by Western blot. *p < 0.05 versus the Normal group. (C) The mRNA expression of HOXB5 in the normal human bronchial epithelial cell line (HBE) and three NSCLC cell lines was measured by qRT-PCR. (D) The protein expression of HOXB5 in the normal human bronchial epithelial cell line (HBE) and three NSCLC cell lines was measured by Western blot. Results were obtained from three independent experiments. *p < 0.05 versus the HBE group.
Knockdown of HOXB5 Prevents the Proliferation of NSCLC Cells
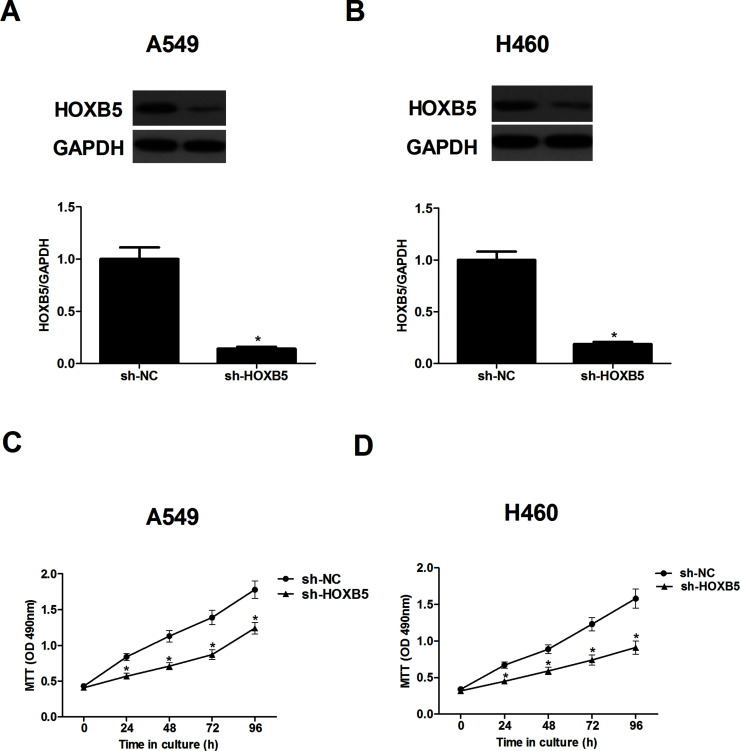
To better understand the biological functions of HOXB5 in NSCLC, we stably transfected A549 and H460 cells with sh-HOXB5, respectively. Compared with the sh-NC group, sh-HOXB5 evidently decreased HOXB5 protein expression in both A549 (Fig. 2A) and H460 cells (Fig. 2B). Furthermore, we examined the effect of sh-HOXB5 on NSCLC cell proliferation using the MTT assay. The results indicated that knockdown of HOXB5 significantly suppressed the proliferation rate of A549 (Fig. 2C) and H460 cells (Fig. 2D), respectively.
Figure 2.
Knockdown of HOXB5 prevents the proliferation of NSCLC cells. A549 and H460 cells were transfected with sh-HOXB5, respectively. (A) The protein expression of HOXB5 in A549 cells was detected using Western blot. (B) The protein expression of HOXB5 in H460 cells was detected using Western blot. (C) Cell proliferation was detected using the MTT assay in A549 cells. (D) Cell proliferation was detected using the MTT assay in H460 cells. Results were obtained from three independent experiments. *p < 0.05.
Knockdown of HOXB5 Prevents the Migration and Invasion of NSCLC Cells
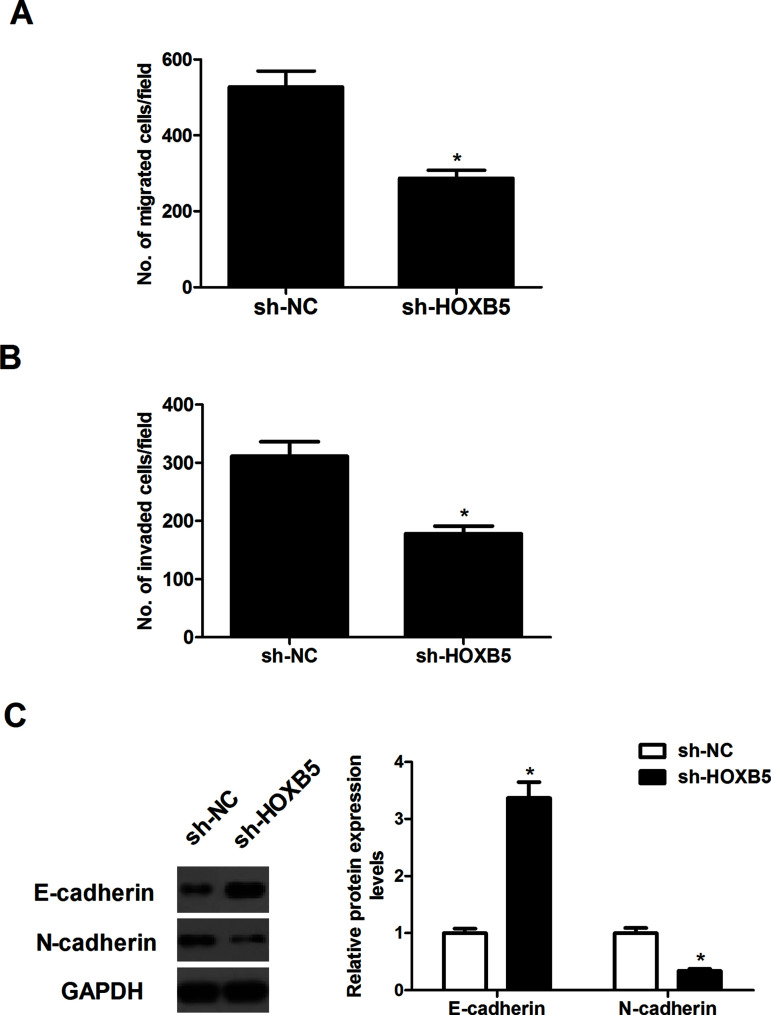
We investigated the potential impact of HOXB5 on cell migration using a Transwell migration assay. The number of cells that migrated was greatly reduced in A549 cells transfected with sh-HOXB5, compared with cells that were transfected with sh-NC (Fig. 3A). Similarly, Transwell invasion assays showed that knockdown of HOXB5 markedly suppressed the invasive ability of A549 cells (Fig. 3B). Furthermore, expression patterns of the EMT markers were evaluated in A549 cells transfected with sh-HOXB5. The results of the Western blot analysis demonstrated that knockdown of HOXB5 markedly upregulated the expression of E-cadherin while downregulating the expression of N-cadherin in A549 cells (Fig. 3C).
Figure 3.
Knockdown of HOXB5 prevents the migration and invasion of NSCLC cells. A549 cells were transfected with sh-HOXB5 for 24 h. (A) Cell migration was measured using the Transwell migration assay. (B) Cell invasion was measured using the Transwell invasion assay. (C) The expression patterns of E-cadherin and N-cadherin were measured using Western blot. Results were obtained from three independent experiments. *p < 0.05.
Knockdown of HOXB5 Suppresses Tumor Growth in a Xenograft Mouse Model
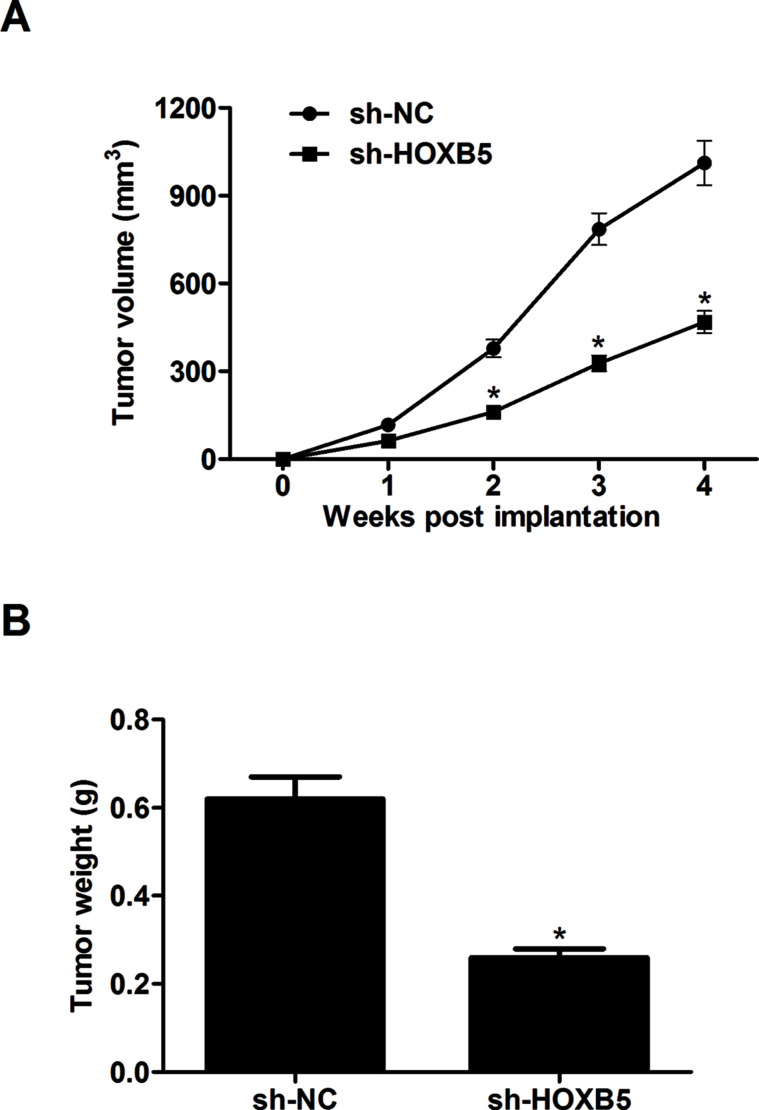
To further examine the effects of HOXB5 on tumor growth in vivo, A549 cells (1 × 107 cells) transfected with sh-HOXB5 or sh-NC diluted in 200 μl of PBS were subcutaneously injected into the right flank of nude mice. The average tumor volume was significantly decreased in HOXB5-silencing tumors compared to the controls (Fig. 4A). Analysis of the tumor weights showed that knockdown of HOXB5 markedly reduced tumor growth after 4 weeks compared to the control tumors (Fig. 4B).
Figure 4.
Knockdown of HOXB5 suppresses tumor growth in a xenograft mouse model. A549 cells (1 × 107 cells) transfected with sh-HOXB5 or sh-NC diluted in 200 μl of PBS were subcutaneously injected into the right flank of nude mice. (A) The average tumor volume was monitored every week. (B) Four weeks after injection, mice were euthanized, and tumors were dissected and weighed. Results were obtained from three independent experiments. *p < 0.05.
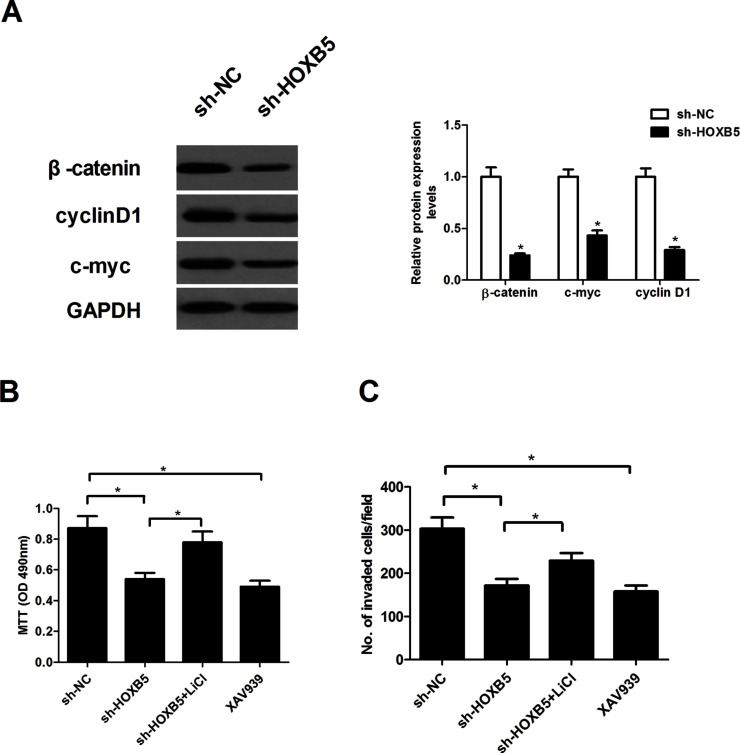
Knockdown of HOXB5 Suppresses the Proliferation and Invasion Through Inactivation of the Wnt/β-Catenin Pathway
To explore the molecular mechanism of HOXB5 in NSCLC growth and metastasis, we examined the effect of HOXB5 on the activation of the Wnt/β-catenin pathway in A549 cells in this study. Knockdown of HOXB5 dramatically suppressed the protein expression levels of β-catenin, cyclin D1, and c-Myc in A549 cells (Fig. 5A). We next examined whether the Wnt/β-catenin pathway activator LiCl could regulate the proliferation and invasion in HOXB5 knockdown A549 cells. The results showed that LiCl significantly reversed the HOXB5 knockdown effect on cell proliferation (Fig. 5B) and invasion (Fig. 5C) in A549 cells. Furthermore, we observed that an inhibitor of the β-catenin pathway (XAV939) greatly inhibited A549 cell proliferation (Fig. 5B) and invasion (Fig. 5C).
Figure 5.
Knockdown of HOXB5 suppresses proliferation and invasion through inactivation of the Wnt/β-catenin pathway. A549 cells were transfected with sh-HOXB5 for 24 h. (A) The protein expression levels of β-catenin, cyclin D1, and c-myc were detected using Western blot. (B) MTT assay evaluated the effects of LiCl in HOXB5 knockdown A549 cells. The effect of XAV939 on A549 cell proliferation was also detected. (C) Transwell invasion assay evaluated the effects of LiCl in HOXB5 knockdown A549 cells. The effect of XAV939 on A549 cell invasion was also detected. Results were obtained from three independent experiments. *p < 0.05.
DISCUSSION
In this study, we found that HOXB5 expression was elevated in human NSCLC tissues and cell lines. The in vitro experiments demonstrated that knockdown of HOXB5 inhibited the proliferation, migration, and invasion, and also prevented the EMT phenotype in NSCLC cells. In vivo experiments indicated that knockdown of HOXB5 attenuated the growth of NSCLC xenografts in vivo in NSCLC cells. Furthermore, knockdown of HOXB5 dramatically suppressed the protein expression levels of β-catenin, cyclin D1, and c-Myc in A549 cells, and the Wnt/β-catenin pathway activator LiCl significantly reversed the HOXB5 knockdown effect on cell proliferation and invasion in A549 cells, whereas an inhibitor of the β-catenin pathway (XAV939) greatly inhibited A549 cell proliferation and invasion.
A large body of evidence has indicated that HOXB5 is correlated to the progression of several cancers. A recent study showed that HOXB5 was highly expressed in breast cancer tissues, particularly from estrogen receptor-positive (ER+) breast cancer patients. In these studies, silencing of HOXB5 significantly decreased cell proliferation and anchorage-independent cell growth in ER+ cells13. HOXB5 was also shown to be overexpressed both in bladder cancer tissues and cell lines compared to normal bladder tissues and cell lines. Knockdown of HOXB5 markedly suppressed clonogenicity in vitro15. In line with these demonstrations, in the current study we observed that knockdown of HOXB5 markedly reduced the proliferation of NSCLC cells. Consistent with these in vitro data, we found that knockdown of HOXB5 in A549 cells also obviously attenuated tumor growth in a xenograft mouse mode, which further suggests a role for HOXB5 in NSCLC growth.
Metastasis is the major cause of death in patients with NSCLC18. Cancer cell migration and invasion are the critical steps in tumor progression and metastasis. EMT is considered to be a key element of cell migration and invasion in NSCLC19. During EMT, epithelial cells acquire mesenchymal, fibroblast-like properties including increased motility and decreased intercellular adhesion20. Lee et al. confirmed that overexpression of HOXB5 increased migration and invasion abilities, as well as reduced the protein levels of E-cadherin in breast cancer cells13. In agreement with these reports, our data showed that knockdown of HOXB5 inhibited migration and invasion, as well as prevented the EMT phenotype in NSCLC cells, suggesting that HOXB5 plays an important role in promoting NSCLC metastasis.
Increasing evidence has reported that the Wnt/β-catenin signaling pathway is crucial in the regulation of cell proliferation, metastasis, EMT, and apoptosis21–23. The Wnt/β-catenin pathway is frequently activated in human NSCLC, and abnormal expression and activation of the Wnt/β-catenin pathway can induce NSCLC cell proliferation, survival, and metastasis24,25. β-Catenin is a main downstream effector of the canonical Wnt signaling pathway, and accumulation of β-catenin activates the transcription of target pro-oncogenes, including c-Myc, cyclin D1, c-Jun, and fra-126. In the current study, we observed that knockdown of HOXB5 suppressed the protein expression level of β-catenin and its downstream targets c-Myc and cyclin D1 in A549 cells. In addition, the Wnt/β-catenin pathway activator LiCl reversed the HOXB5 knockdown effect on NSCLC cell proliferation and invasion. An inhibitor of the β-catenin pathway (XAV939) greatly inhibited A549 cell proliferation and invasion. These data imply that knockdown of HOXB5 inhibited NSCLC cell proliferation, invasion, metastasis, and EMT, at least in part, through inactivation of the Wnt/β-catenin signaling pathway.
In conclusion, we show for the first time that HOXB5 may act as an oncogene in human NSCLC, and knockdown of HOXB5 significantly inhibits NSCLC cell proliferation, invasion, metastasis, and EMT, partly through the inactivation of the Wnt/β-catenin signaling pathway. These findings suggested that HOXB5 may be a novel therapeutic target in the treatment of NSCLC.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Dragnev K, You M, Wang Y, Lubet R. Lung cancer chemoprevention: Difficulties, promise and potential agents? Expert Opin Investig Drugs 2013;22:35–47. [DOI] [PubMed] [Google Scholar]
- 2. Gettinger S, Lynch T. A decade of advances in treatment for advanced non-small cell lung cancer. Clin Chest Med. 2011;32:839–51. [DOI] [PubMed] [Google Scholar]
- 3. Ricciuti B, Mencaroni C, Paglialunga L, Paciullo F, Crinò L, Chiari R, Metro G. Long noncoding RNAs: New insights into non-small cell lung cancer biology, diagnosis and therapy. Med Oncol. 2016;33:18–27. [DOI] [PubMed] [Google Scholar]
- 4. Peters S, Adjei AA, Gridelli C, Reck M, Kerr K, Felip E. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23:vii56–64. [DOI] [PubMed] [Google Scholar]
- 5. Ll VDB, Klinkenberg TJ, Groen HJ, Widder J. Patterns of recurrence and survival after surgery or stereotactic radiotherapy for early stage NSCLC. J Thorac Oncol. 2015;10:826–31. [DOI] [PubMed] [Google Scholar]
- 6. Wang T, Nelson RA, Bogardus A, Grannis FW Jr. Five-year lung cancer survival: Which advanced stage nonsmall cell lung cancer patients attain long-term survival? Cancer 2010;116:1518–25. [DOI] [PubMed] [Google Scholar]
- 7. Mallo M, Alonso CR. The regulation of Hox gene expression during animal development. Development 2013;140:3951–63. [DOI] [PubMed] [Google Scholar]
- 8. Armat M, Ramezani F, Molavi O, Sabzichi M, Samadi N. Six family of homeobox genes and related mechanisms in tumorigenesis protocols. Tumori 2016;2016:236–43. [DOI] [PubMed] [Google Scholar]
- 9. Samuel S, Naora H. Homeobox gene expression in cancer: Insights from developmental regulation and deregulation. Eur J Cancer 2005;41:2428–37. [DOI] [PubMed] [Google Scholar]
- 10. Kam MKM, Lui VCH. Roles of Hoxb5 in the development of vagal and trunk neural crest cells. Dev Growth Differ. 2015;57:158–68. [DOI] [PubMed] [Google Scholar]
- 11. Kam MK, Cheung M, Zhu JJ, Cheng WW, Sat EW, Tam PK, Lui VC. Homeobox b5 (Hoxb5) regulates the expression of forkhead box D3 gene (Foxd3) in neural crest. Int J Biochem Cell B 2014;55:144–52. [DOI] [PubMed] [Google Scholar]
- 12. Sun KY, Tao P, Zhe C, Jing H, Zhou XH. MicroRNA-1275 suppresses cell growth, and retards G1/S transition in human nasopharyngeal carcinoma by down-regulation of HOXB5. J Cell Commun Signal. 2016;10:305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee JY, Hur H, Yun HJ, Kim Y, Yang S, Kim SI, Kim MH. HOXB5 promotes the proliferation and invasion of breast cancer cells. Int J Biol Sci. 2014;11:701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tucci R, Campos MS, Matizonkasantonio LF, Durazzo M, Pinto JDS, Nunes FD. HOXB5 expression in oral squamous cell carcinoma. J Appl Oral Sci. 2011;19:125–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luo J, Cai Q, Wang W, Huang H, Zeng H, Wang H, Deng W, Yu H, Chan E, Chifai NG. A microRNA-7 binding site polymorphism in HOXB5 leads to differential gene expression in bladder cancer. PLoS One 2012;7:e40127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hong CS, Jeong O, Piao Z, Guo C, Jung MR, Choi C, Park YK. HOXB5 induces invasion and migration through direct transcriptional upregulation of β-catenin in human gastric carcinoma. Biochem J. 2015;472:393–403. [DOI] [PubMed] [Google Scholar]
- 17. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 18. Zhang X, Xie J, Yu C, Yan L, Yang Z. mRNA expression of CK19, EGFR and LUNX in patients with lung cancer micrometastasis. Exp Ther Med. 2014;7:360–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim B, Sohn EJ, Jung JH, Shin EA, You OH, Im J, Kim SH. Inhibition of ZNF746 suppresses invasion and epithelial-to-mesenchymal transition in H460 non-small cell lung cancer cells. Oncol Rep. 2014;31:73–8. [DOI] [PubMed] [Google Scholar]
- 20. Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metast Rev. 2009;28:15–33. [DOI] [PubMed] [Google Scholar]
- 21. Libro R, Bramanti P, Mazzon E. The role of the Wnt canonical signaling in neurodegenerative diseases. Life Sci. 2016;158:78–88. [DOI] [PubMed] [Google Scholar]
- 22. Lustig B, Behrens J. The Wnt signaling pathway and its role in tumor development. J Cancer Res Clin Oncol. 2003;129:199–221. [DOI] [PubMed] [Google Scholar]
- 23. Huelsken J, Behrens J. The Wnt signalling pathway. J Cell Sci. 2002;115:3977–78. [DOI] [PubMed] [Google Scholar]
- 24. Stewart DJ. Wnt signaling pathway in non-small cell lung cancer. J Natl Cancer Inst. 2014;106:djt356. [DOI] [PubMed] [Google Scholar]
- 25. Xu X, Sun PL, Li JZ, Jheon S, Lee CT, Chung JH. Aberrant Wnt1/β-catenin expression is an independent poor prognostic marker of non-small cell lung cancer after surgery. J Thorac Oncol. 2011;6:716–24. [DOI] [PubMed] [Google Scholar]
- 26. Li Y-J, Wei Z-M, Meng Y-X, Ji X-R. β-Catenin up-regulates the expression of cyclinD1, c-myc and MMP-7 in human pancreatic cancer: Relationships with carcinogenesis and metastasis. World J Gastroenterol. 2005;11:2117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]