Abstract
Background
Second primary cancers (SPCs) are diagnosed in over 5% of patients after a first primary cancer (FPC). We explore here the impact of immune checkpoint inhibitors (ICIs) given for an FPC on the risk of SPC in different age groups, cancer types and treatments.
Patients and methods
The files of the 46 829 patients diagnosed with an FPC in the Centre Léon Bérard from 2013 to 2018 were analyzed. Structured data were extracted and electronic patient records were screened using a natural language processing tool, with validation using manual screening of 2818 files of patients. Univariate and multivariate analyses of the incidence of SPC according to patient characteristics and treatment were conducted.
Results
Among the 46 829 patients, 1830 (3.9%) had a diagnosis of SPC with a median interval of 11.1 months (range 0-78 months); 18 128 (38.7%) received cytotoxic chemotherapy (CC) and 1163 (2.5%) received ICIs for the treatment of the FPC in this period. SPCs were observed in 7/1163 (0.6%) patients who had received ICIs for their FPC versus 437/16 997 (2.6%) patients receiving CC and no ICIs for the FPC versus 1386/28 669 (4.8%) for patients receiving neither CC nor ICIs for the FPC. This reduction was observed at all ages and for all histotypes analyzed. Treatment with ICIs and/or CC for the FPC are associated with a reduced risk of SPC in multivariate analysis.
Conclusion
Immunotherapy with ICIs alone and in combination with CC was found to be associated with a reduced incidence of SPC for all ages and cancer types.
Key words: immunotherapy, immune checkpoints, second primary cancer, second malignant neoplasia
Highlights
-
•
From 2013 to 2018, 3.9% of the 46 829 patients diagnosed with a first cancer presented with an SPC.
-
•
Treatment of the first cancer with ICIs was associated with a major reduction of SPC.
-
•
CC given for an FPC was also associated with a lower magnitude of reduction of SPC.
-
•
There were no SPC in cancer patients treated with ICIs in the localized phase of their first cancer.
Introduction
Second primary cancers (SPCs) are increasingly diagnosed in the long term follow-up of children and adult patients cured of a first primary cancer (FPC).1, 2, 3, 4, 5, 6, 7, 8, 9, 10 In recent studies, over 10% of all incident cancers are SPCs. SPCs are among the important causes of early death in patients treated and cured of a first cancer, along with cardiovascular diseases and complications of the first cancer treatments.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 There are multiple risk factors for SPCs including genetic predispositions, carcinogens involved in the FPC (e.g. smoking, alcohol, sun exposure), lifestyle, overweight, low exercise. The cytotoxic treatments given for the first cancer may also contribute to an excess risk of SPCs for cured patients.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 This has been largely documented for secondary leukemias after alkylating agents, as well as for solid tumors in the irradiated field.1,2,5,9
In recent years, immunotherapy for cancer using immune checkpoint inhibitors (ICIs), PD-1 or PD-L1 Ab as well as anti-CTLA-4 have transformed the management of patients with advanced cancers. These antibodies against ICIs are now demonstrated to reduce the risk of relapse and to improve survival in localized and advanced phases of the disease, in particular in malignant melanomas and in lung cancer.11, 12, 13, 14
We recently reported that the administration of immunotherapy with ICIs for the FPC is associated with a major reduction risk of SPC.15 This was observed in the exhaustive population of cancer patients treated in the cancer center over a 6-year period, screening the patient records of the exhaustive database of patients with an FPC treated in a comprehensive cancer center, the Centre Léon Bérard (CLB).16
In the present work, we explore the impact of ICIs on the reduction of the risk of SPC according to patient characteristics, different age groups and histotypes, exploring also the interaction with the administration of cytotoxic chemotherapy (CC) for the FPC. The incidence of SPCs was reduced in all subgroups after exposure to immunotherapy with ICIs and/or CC in this series of 46 829 patients.
Patients and methods
Patients
All patients, adults and children, diagnosed with a primary tumor in the CLB, Lyon, France, from January 2013 to December 2018 were considered in this work. Data from patients with a newly diagnosed malignant tumor or locally aggressive, rarely metastasizing tumors (e.g. giant cell tumor or bone or desmoid tumors) between January 2013 and December 2018 were collected. Only patients not opposed to the re-analysis of their anonymized health data within internal academic studies were included according to the standard operating procedures of the CLB and national and European Union (EU) legislation. Specifically, the study was approved by the national commission [CNIL Délibération number 2016-331 of 10 November 2016 (authorization number 1773637)] and by the local institutional review board of the CLB in May 2019. A limited set of anonymous de-identified patient characteristics, specifically, sex, age, tumor site, stage and histology and treatments administered (surgery, radiotherapy, cytotoxic treatments and immunotherapy with ICIs) were collected and analyzed. Records from 46 863 patients matching these characteristics were extracted and analyzed; 34 patients with synchronous cancers were excluded for a total population of 46 829 patients for this study. In this time period, 1163 patients received immunotherapy as part of the treatment of the first cancer. The ICI antibodies used for the 1163 patients who received immunotherapy for their first cancer were the following treatments: nivolumab (n = 420, 36.6%), pembrolizumab (n = 221, 19.0%), atezolizumab (n = 92, 7.9%), durvalumab (n = 87, 7.4%), ipilimumab (n = 72, 6.1%) or a combination of one of these ICIs with CC, either synchronous or sequentially (n = 271, 23.4%).
Extracting electronic patient records with the ConSoRe tool
The electronic patient records (EPR) of the patients of the CLB matching the inclusion criteria and diagnosed from January 2013 to December 2018 were first extracted for structured data, e.g. age sex, histological types of cancers, dates of diagnosis. The EPR system of the CLB includes a standard set of data [e.g. tumor(s) characteristics, staging, first treatments, dates, follow-up], but other information such as subsequent lines of treatment may be present only in unstructured data formats. For these, the EPRs were extracted with the ConSoRe tool15 (https://www.sword-group.com/en/news/projet-consore). ConSoRe is an academic data analytics solution aggregating diverse forms of structured and unstructured data extracted from EPR and structuring cancer management for all patients. ConSoRe uses natural language processing to search aggregated data and perform advanced data mining. Both cancers and locally aggressive tumors that rarely (<5%) metastasized, typically desmoid tumors or giant cell tumors of the bones, were added. The characteristics extracted included: sex, age, dates, histotype and stages of first and second cancers, surgery, chemotherapy, radiotherapy (RT) and immunotherapy for the first cancer, relapse (and date) of the first cancer, date of last news and date of death. The extracted relevant structured data was integrated into Excel files. Of the 2119 patients, 1455 (68.7%) with SPCs identified by the ConSoRe program had their SPCs formally declared by their responsible physician in the EPR. In addition to this ConSoRe extraction, 2818 EPRs were manually screened to confirm immunotherapy use for the FPC and/or for the presence/absence of a diagnosis of SPC. Six additional second primary cancers were identified; 283 possible SPCs were reclassified as relapses of the FPC. A total of 1830 confirmed SPCs are thus documented in this series of 46 829 patients. All 1163 files of patients treated with ICIs were screened manually and seven SPCs were identified in this subgroup.
Analysis
A descriptive presentation of the patient characteristics is presented in Table 1. The event considered in this study was the diagnosis of an SPC. To describe the survival time from the FPC to the diagnosis of an SPC, which is the time to event used in the present work, the following methodology was used: survival without second cancer was defined as the time from the date of histological diagnosis of the first cancer to the date of the histological diagnosis of the second cancer. Patients who died without a diagnosis of second cancer were censored at the date of death. Other patients were censored at the date of last contact of the patient. To limit potential biases related to disease severity, which could have had an impact on decision of treatment with immunotherapy or chemotherapy, a landmark analysis was conducted with landmark thresholds of 6 months, reducing therefore the number of patients analyzed. All patients with a diagnosis of SPC or last news inferior to this threshold were removed from these specific analyses.
Table 1.
Description of the patients and treatments received for the first primary cancer before the second primary cancer
| Cytotoxics only |
Cytotoxics and immunotherapy |
Immunotherapy only |
Neither cytotoxics nor immunotherapy |
All patients |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
n = 16 997 |
n = 1131 |
n = 32 |
n = 28 669 |
N = 46 829 |
||||||
| n | % | n | % | n | % | n | % | n | % | |
| Sex | ||||||||||
| Female | 9657 | (56.8%) | 426 | (37.7%) | 14 | (43.8%) | 16 628 | (58.0%) | 26 725 | (57.1%) |
| Male | 7340 | (43.2%) | 705 | (62.3%) | 18 | (56.3%) | 12 041 | (42.0%) | 20 104 | (42.9%) |
| Age of cancer 1 | ||||||||||
| n | 16 997 | 1131 | 32 | 28 669 | 46 829 | |||||
| Mean (SE) | 57.7 (18.6) | 59.6 (12.6) | 50.8 (19.0) | 57.2 (18.8) | 57.5 (18.6) | |||||
| Median (min; max) | 60.9 (0; 100) | 60.9 (0; 90) | 54.9 (10; 77) | 60.0 (0; 115) | 60.4 (0; 115) | |||||
| Q1-Q3 | 48.1-70.4 | 52.0-67.8 | 43.8-65.1 | 46.1-70.5 | 47.1-70.4 | |||||
| Classes of age for first cancera | ||||||||||
| <30 | 1436 | (8.4%) | 24 | (2.1%) | 5 | (15.6%) | 2597 | (9.1%) | 4062 | (8.7%) |
| 30-60 | 6737 | (39.6%) | 509 | (45.0%) | 14 | (43.8%) | 11 717 | (40.9%) | 18 977 | (40.5%) |
| >60 | 8824 | (51.9%) | 598 | (52.9%) | 13 | (40.6%) | 14 355 | (50.1%) | 23 790 | (50.8%) |
| Cancer sites | ||||||||||
| Breast | 4809 | (28.3%) | 33 | (2.9%) | 1 | (3.1%) | 6857 | (23.9%) | 11700 | (25.0%) |
| Lung | 2125 | (12.5%) | 486 | (43.0%) | 11 | (34.4%) | 2084 | (7.3%) | 4706 | (10.0%) |
| Head & neck | 929 | (5.5%) | 167 | (14.8%) | 5 | (15.6%) | 2286 | (8.0%) | 3387 | (7.2%) |
| Gynecological | 1105 | (6.5%) | 62 | (5.5%) | 0 | (0.0%) | 1967 | (6.9%) | 3134 | (6.7%) |
| Digestive | 1445 | (8.5%) | 53 | (4.7%) | 1 | (3.1%) | 1498 | (5.2%) | 2997 | (6.4%) |
| Connective tissue | 646 | (3.8%) | 19 | (1.7%) | 0 | (0.0%) | 2253 | (7.9%) | 2918 | (6.2%) |
| Colorectal | 1099 | (6.5%) | 30 | (2.7%) | 1 | (3.1%) | 1545 | (5.4%) | 2675 | (5.7%) |
| Prostate | 643 | (3.8%) | 11 | (1.0%) | 0 | (0.0%) | 1732 | (6.0%) | 2386 | (5.1%) |
| Urothelial | 434 | (2.6%) | 142 | (12.6%) | 1 | (3.1%) | 837 | (2.9%) | 1414 | (3.0%) |
| Thyroid | 220 | (1.3%) | 7 | (0.6%) | 0 | (0.0%) | 1106 | (3.9%) | 1333 | (2.8%) |
| Skin cancer | 149 | (0.9%) | 57 | (5.0%) | 0 | (0.0%) | 713 | (2.5%) | 919 | (2.0%) |
| Others | 3393 | (20.0%) | 64 | (5.7%) | 12 | (37.5%) | 5791 | (20.2%) | 9260 | (19.8%) |
| Metastatic at diagnosis | ||||||||||
| No | 10 199 | (60.0%) | 492 | (43.5%) | 31 | (96.9%) | 28 669 | (100.0%) | 39 391 | (84.1%) |
| Yes | 6798 | (40.0%) | 639 | (56.5%) | 1 | (3.1%) | 0 | (0.0%) | 7438 | (15.9%) |
| Second primary cancer | ||||||||||
| No | 16 560 | (97.4%) | 1125 | (99.5%) | 31 | (96.9%) | 27 283 | (95.2%) | 44 999 | (96.1%) |
| Yes | 437 | (2.6%) | 6 | (0.5%) | 1 | (3.1%) | 1386 | (4.8%) | 1830 | (3.9%) |
| Histotypes (second cancer) | ||||||||||
| Head & neck | 47 | (0.3%) | 0 | (0.0%) | 0 | (0.0%) | 185 | (0.6%) | 232 | (0.5%) |
| Sarcoma | 43 | (0.3%) | 0 | (0.0%) | 0 | (0.0%) | 153 | (0.5%) | 196 | (0.4%) |
| Lung | 40 | (0.2%) | 0 | (0.0%) | 0 | (0.0%) | 140 | (0.5%) | 180 | (0.4%) |
| Breast | 54 | (0.3%) | 0 | (0.0%) | 0 | (0.0%) | 104 | (0.4%) | 158 | (0.3%) |
| Digestiveb | 36 | (0.2%) | 0 | (0.0%) | 1 | (3.1%) | 99 | (0.3%) | 136 | (0.3%) |
| Skin | 30 | (0.2%) | 1 | (0.1%) | 0 | (0.0%) | 83 | (0.3%) | 114 | (0.2%) |
| Colorectal | 22 | (0.1%) | 3 | (0.3%) | 0 | (0.0%) | 69 | (0.2%) | 94 | (0.2%) |
| Gynecological | 19 | (0.1%) | 0 | (0.0%) | 0 | (0.0%) | 70 | (0.2%) | 89 | (0.2%) |
| Urothelial | 12 | (0.1%) | 0 | (0.0%) | 0 | (0.0%) | 53 | (0.2%) | 65 | (0.1%) |
| Thyroid | 11 | (0.1%) | 0 | (0.0%) | 0 | (0.0%) | 38 | (0.1%) | 49 | (0.1%) |
| Prostate | 4 | (0.0%) | 0 | (0.0%) | 0 | (0.0%) | 31 | (0.1%) | 35 | (0.1%) |
| Other | 119 | (0.7%) | 2 | (0.2%) | 0 | (0.0%) | 361 | (1.3%) | 482 | (1.0%) |
SE, standard error.
Adults and children treated in the Centre Léon Bérard were all included in this analysis.
Digestive: non-colorectal carcinoma (CRC).
Since immunotherapy with ICIs was given mostly to patients with advanced disease, by definition at higher risk of mortality as compared with patients with localized disease and with a possibly shorter life expectancy, a competing risk survival method was used to estimate the risk of being alive without an SPC.
Risk of SPC was evaluated using the Cox proportional hazard model in a univariate and then multivariate analysis. Parameters tested were those published as risk factors for SPC,1, 2, 3, 4, 5, 6, 7, 8, 9, 10 age, sex, cancer types, metastatic disease at diagnosis as well as the application of immunotherapy and CC for the FPC. A backward selection procedure was used to determine the final model by removing non-significant variables (P > 0.05) one at a time. All statistical analyses were carried out using SAS software, v 9.4 (SAS Institute Inc., Cary, NC).
Results
All 46 829 patients with a newly diagnosed cancer treated in the CLB matching the criteria (see patients and methods) were analyzed. Their characteristics are presented in Table 1. At diagnosis, 7438 patients had metastasis (15.9%) and a total of 11 434 (24.4%) had diagnosed metastasis during the observation period (2013-2018); 8513 (18.2%) died during this period. In the same period, 1830 (3.9%) patients had a diagnosis of an SPC (Table 1). The median interval from FPC to SPC was 11.1 months (range 0-78 months). Median follow-up of the series was 19 months. During this period, 18 128 (38.7%) patients received CC for the treatment of the FPC and 1163 (2.5%) received immunotherapy with ICIs for the treatment of the FPC (Table 1).
Despite the large size of the series, the number of patients treated with ICIs only for their FPC is limited (n = 32) (Table 1). We therefore decided to pool them with the group of patients treated with immunotherapy and chemotherapy for their FPC hereunder. Seven SPCs were reported in the group of 1163 (0.6%) patients receiving ICIs (with or without CC) for their FPC versus 437 (2.6%) in the group of 16 997 patients receiving CC and no immunotherapy for the first cancer, versus 1386 of 28 669 (4.8%) for those receiving neither CC nor ICIs (Table 2) (P < 0.0001). The difference was also statistically significant when comparing individually the group receiving immunotherapy versus those receiving cytotoxics only (P < 0.0001) (Table 2).
Table 2.
Second primary cancers in the different age classes according to the treatment of the primary cancer
| Systemic treatment of the first primary cancer | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cytotoxics only |
Cytotoxics and immunotherapy |
Immunotherapy only |
No cytotoxics, no immunotherapy |
All patients |
||||||
| n = 16 997 | (%) | n = 1131 | (%) | n = 32 | (%) | n = 28 669 | (%) | N = 46 829 | (%) | |
| Age at first cancer | ||||||||||
| <30 | 38/1436 | 2.6% | 0/24 | 0.0% | 0/5 | 0.0% | 123/2597 | 4.7% | 161/4062 | 4.0% |
| 30-60 | 151/6737 | 2.2% | 1/509 | 0.2% | 0/14 | 0.0% | 496/11 717 | 4.2% | 648/18 977 | 3.4% |
| >60 | 248/8824 | 2.8% | 5/598 | 0.8% | 1/13 | 7.7% | 767/14 355 | 5.3% | 1021/23 790 | 4.3% |
| All | 437/16 997 | 2.6% | 6/1131 | 0.5% | 1/32 | 3.1% | 1386/28 669 | 4.8% | 1830/46 829 | 3.9% |
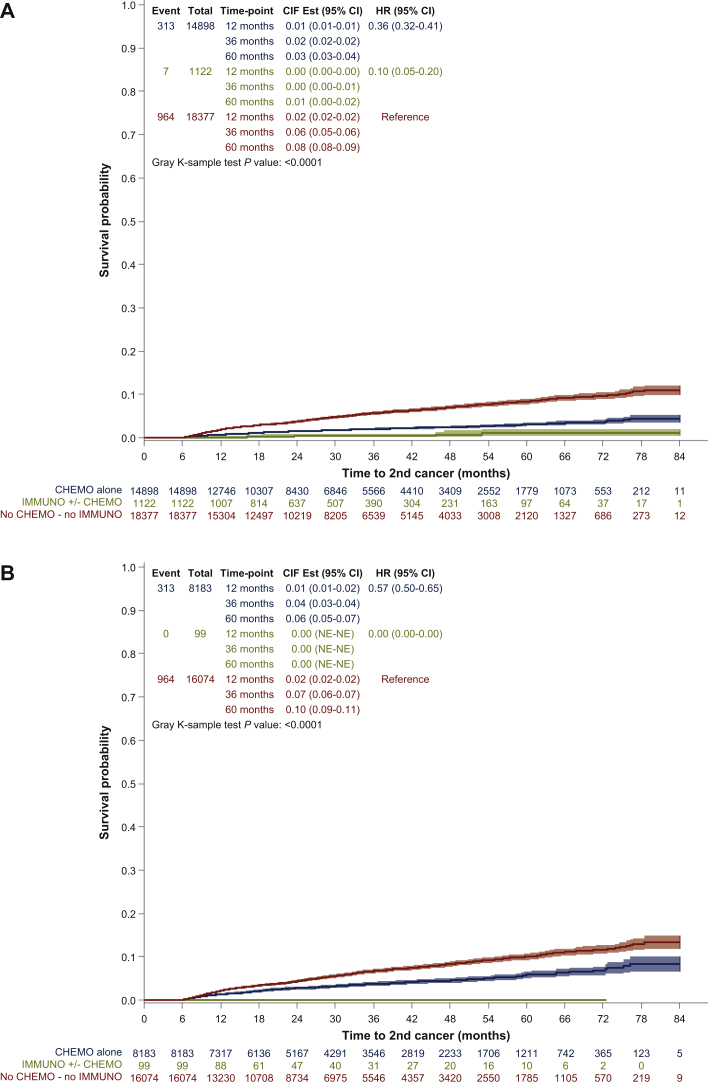
Figure 1A presents the time from the diagnosis of the FPC to the date of diagnosis of the second cancer in patients (i) who received immunotherapy with ICIs, with or without CC for the FPC, and (ii) who received CC without ICIs, and who received neither ICIs nor CC with a landmark analysis at 6 months (see patients and methods). The risk of developing an SPC was lower in patients treated with immunotherapy with or without chemotherapy [hazard ration (HR) = 0.07; confidence interval (CI) 95% = 0.05-0.20], and CC without immunotherapy (HR = 0.36; CI 95% = 0.32-0.41), as compared with patients receiving neither CC nor immunotherapy (Figure 1A and Tables 3 and 4).
Figure 1.
Time to second primary tumor according to the treatment given for the first primary tumor.
(A) Time to second primary tumor in patients treated with immunotherapy and/or cytotoxic chemotherapy or none for the first primary cancer (all patients). (B) Time to second primary tumor in patients treated with immunotherapy and/or cytotoxic chemotherapy or none for the first primary cancer in patients without documented metastasis. Survival analysis was carried out using competing risk analysis to estimate the risk of being alive without second primary cancer (SPC) and 6-months landmark analysis to limit analysis to subjects who have survived long enough for treatment to be initiated. Due to this last constraint, 12 432 patients (26.5%) with SPC or death before 6 months were excluded from the survival analysis. Thus, the population in this analysis consists of 34 397 patients. CI, confidence interval; CIF est., cumulative incidence function with competing risks data; FPC, first primary cancer; HR, hazard ratio; NE, not evaluable.
Table 3.
Multivariate analysis of predictive factors for second primary cancer: entire series
| Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|
| Parameter | Events/competing events/n (landmark population) | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value |
| Sex | <0.0001 | ||||
| Male | 654/4439/15 087 | 1 | NS | ||
| Female | 630/2157/19 310 | 0.791 (0.709-0.882) | |||
| Age at FPC | <0.0001 | <0.0001 | |||
| <30 | 93/239/3062 | 1 | 1 | ||
| 30-60 | 451/2223/14 069 | 1.056 (0.844-1.321) | 1.516 (1.200-1.913) | ||
| >60 | 740/4134/17 266 | 1.468 (1.182-1.822) | 1.887 (1.496-2.381) | ||
| Metastasis at diagnosis | <0.0001 | <0.0001 | |||
| No | 1280/3952/28 311 | 1 | 1 | ||
| Yes | 4/2644/6086 | 0.011 (0.005-0.024) | 0.01 (0.005-0.020) | ||
| Localization of FPC | <0.0001 | <0.0001 | |||
| Lung | 107/1447/3381 | 1 | 1 | ||
| Melanoma | 31/98/604 | 1.924 (1.290-2.869) | 0.919 (0.616-1.369) | ||
| Head & neck | 145/711/2730 | 1.685 (1.313-2.163) | 0.944 (0.736-1.211) | ||
| Prostate | 67/271/1828 | 1.143 (0.843-1.55) | 0.622 (0.459-0.843) | ||
| Colorectal | 81/504/2091 | 1.202 (0.901-1.604) | 1.070 (0.802-1.427) | ||
| Breast | 199/422/8619 | 0.717 (0.567-0.907) | 0.408 (0.321-0.518) | ||
| Sarcoma | 122/320/1976 | 2.044 (1.576-2.653) | 1.311 (0.999-1.720) | ||
| Gynecological | 79/386/2353 | 1.157 (0.865-1.548) | 0.886 (0.662-1.185) | ||
| Urological | 68/427/1072 | 1.889 (1.393-2.560) | 1.996 (1.474-2.702) | ||
| Gastrointestinal | 68/993/2172 | 1.007 (0.743-1.364) | 0.872 (0.644-1.182) | ||
| Thyroid | 17/97/796 | 0.771 (0.463-1.285) | 0.454 (0.272-0.759) | ||
| Other | 300/920/6775 | 1.386 (1.111-1.728) | 0.777 (0.621-0.972) | ||
| Treatment of the FPC | <0.0001 | <0.0001 | |||
| No chemo/no immuno | 964/2517/18 377 | 1 | 1 | ||
| Chemo alone | 313/3634/14 898 | 0.364 (0.320-0.413) | 0.698 (0.609-0.799) | ||
| Immuno +/− chemo | 7/545/1122 | 0.097 (0.046-0.203) | 0.770 (0.388-1.53) | ||
CI, confidence interval; FPC, first primary cancer; NS, non significant.
Table 4.
Multivariate analysis of predictive factors for second primary cancer: patients without metastasis of the first cancer during the observation period
| Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|
| Parameter | Events/competing events/n (landmark population) | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value |
| Sex | <0.0001 | ||||
| Male | 647/1762/9851 | 1 | NS | ||
| Female | 630/590/14 505 | 0.682 (0.611-0.761) | |||
| Age at FPC | <0.0001 | <0.0001 | |||
| <30 | 93/93/2506 | 1 | 1 | ||
| 30-60 | 450/557/9941 | 1.252 (1.000-1.566) | 1.522 (1.205-1.921) | ||
| >60 | 734/1702/11 909 | 1.773 (1.427-2.202) | 1.887 (1.495-2.381) | ||
| Anatomic site of FPC | <0.0001 | <0.0001 | |||
| Lung | 100/422/1396 | 1 | 1 | ||
| Melanoma | 31/53/336 | 1.016 (0.679-1.521) | 0.951 (0.635-1.424) | ||
| Head & neck | 145/336/2015 | 0.988 (0.766-1.275) | 0.978 (0.757-1.264) | ||
| Prostate | 67/79/1333 | 0.701 (0.515-0.954) | 0.639 (0.469-0.871) | ||
| Colorectal | 81/127/1061 | 1.106 (0.825-1.483) | 1.108 (0.826-1.487) | ||
| Breast | 199/88/7198 | 0.365 (0.287-0.464) | 0.416 (0.325-0.531) | ||
| Sarcoma | 122/86/1482 | 1.201 (0.920-1.567) | 1.349 (1.022-1.782) | ||
| Gynecological | 79/89/1408 | 0.891 (0.664-1.198) | 0.913 (0.679-1.229) | ||
| Urological | 68/88/462 | 2.140 (1.573-2.911) | 2.090 (1.536-2.844) | ||
| Gastrointestinal | 68/319/1074 | 0.912 (0.670-1.242) | 0.910 (0.669-1.240) | ||
| Thyroid | 17/34/606 | 0.451 (0.270-0.754) | 0.466 (0.278-0.781) | ||
| Other | 300/631/5816 | 0.680 (0.542-0.853) | 0.798 (0.632-1.006) | ||
| Treatment of the FPC | <0.0001 | <0.0001 | |||
| No chemo/no immuno | 964/1624/16 074 | 1 | 1 | ||
| Chemo alone | 313/694/8183 | 0.571 (0.502-0.648) | 0.706 (0.617-0.808) | ||
| Immuno +/− chemo | 0/34/99 | 0.000 (0.000-0.000) | 0.000 (0.000-0.000) | ||
CI, confidence interval; FPC, first primary cancer.
When focusing on patients with no diagnosis of metastasis in the observation period, which includes 24 356 patients with 1277 SPCs (Figure 1B), a significant reduction in the incidence of SPC was also observed in the group treated with CC only for the FPC (HR = 0.57; CI 95% = 0.50-0.65), and no second cancer was observed in the group treated with ICIs (Figure 1B, P < 0.0001).
Analyzing each individual year of study (from 2013 to 2018), the incidence of SPC was consistently smaller for patients treated with ICIs and/or cytotoxics versus cytotoxics alone versus patients not receiving cytotoxics or ICIs (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2020.100044). The reduction of the risk of SPC with immunotherapy versus cytotoxics only was observed for children as well as young and older adults (Table 2).
We then analyzed the impact of immunotherapy on the risk of SPCs in patients according to the primary site of the FPC (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2020.100044). Treatment with ICIs for the FPC was associated with a reduction of the incidence of SPCs in the different subgroups of FPC (e.g. lung, head and neck, colorectal, sarcoma, prostate, skin cancers). The sites and histotypes of the SPC identified in the group selected for the landmark analysis are presented in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2020.100044. The second cancers observed in the group of patients treated with immunotherapy for the FPC were colorectal adenocarcinoma (n = 3), upper digestive tract cancers (n = 1), melanoma (n = 1) and other sites (n = 2). Though the number of SPCs from the gastrointestinal (GI) tract was decreased overall, four of the seven patients treated with immunotherapy (57.2%) for the FPC had an SPC from the digestive tract versus 145/1270 (11.5%, P = 0.0001) of patients not receiving ICIs for the FPC.
A multivariate Cox model was conducted to explore the clinical characteristics and therapeutic actions for the FPC associated with the risk of developing an SPC. Of note, the numbers in Tables 3 and 4 are different from those of Table 2 because of the 6-month landmark analysis. Sex, age, stage (metastasis at diagnosis), tumor sites or histotypes (sarcoma) as well as immunotherapy and CC were introduced to the model. Young age, thyroid, breast and prostate cancer, presence of metastasis at diagnosis, as well as administration of chemotherapy and/or immunotherapy were correlated with a reduced risk of SPC (Table 3).
A second multivariate Cox model was conducted on the population of patients without documented metastasis of their FPC, introducing the same parameters (age, sex, tumor sites and histotypes, cytotoxic and/or immunotherapy administration for the FPC). Again, age, tumor type, as well as administration of chemotherapy and/or immunotherapy were correlated with the risk of SPC (Table 4). No SPCs were observed in patients treated with immunotherapy in this subgroup.
Discussion
SPCs are an important cause of death for children and adult patients treated for an FPC in long term remission.1, 2, 3 The etiological factors of SPCs are those of the FPC (e.g. smoking, alcohol, diet), but also the genotoxic treatments used for the first cancer. The hazard ratio associated with the risk of SPC as compared with the general population are reported to range from 1.2 up to 30 depending on the FPC and its treatment.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 We recently reported that the administration of ICIs, mostly PD-1/PD-L1 or CTLA4 antibodies for the FPC, was associated with a reduced risk of developing an SPC.15 Only seven of the 1163 patients treated with ICIs for a first cancer had a diagnosis of an SPC in this observation period.
In the present study, we report an analysis of the impact of ICIs on the risk of SPC in the different age groups, histotypes and according to the administration of cytotoxic treatments for the FPC.
The results presented here indicate that not only immunotherapy but also the administration of CC was associated with a reduced incidence of SPC in the population and period explored. Cytotoxic treatment of the FPC was also associated with a reduction in the incidence of SPC, even when immunotherapy was not administered, but the reduction observed was of lower magnitude. This is paradoxical given the abundant literature on the increased risk of SPC in survivors treated with alkylating agents, anthracyclines and others.1,3,17,18 Indeed, cytotoxic agents increase the risk of secondary myeloid malignancy,1, 2, 3, 4, 5,17,18 but their impact is less consistent for solid tumors, depending on the drug and organs. The dose of procarbazine correlates with the risk of second solid cancers, but this agent is now less used; cyclophosphamide exposure increases bladder cancer.18 An increased risk of sarcoma was reported after exposure to anthracyclines but not after other cytotoxic agents, including alkylating agents.17 These secondary solid tumors are however diagnosed later at a median of >10 years after the diagnosis of first cancer, i.e. much later than the observation period of the present study. Conversely, a 50% reduction of the risk of contralateral breast cancer is observed in patients at high risk who had received adjuvant CC for the first breast cancer, consistently with the present observations.19
The reduction of the number of SPC in patients treated with immunotherapy for their FPC is of larger magnitude in this series with a hazard ratio inferior to 0.1. With the limited follow-up, it remains unclear whether treatment with ICIs for the FPC may either delay or prevent the emergence of an SPC. It is actually striking to observe that the median time between the FPC and the SPC was 11 months (ranging from 0 to 78 months) but this must be considered in the context of a median follow-up of 19 months for this series. This follow-up is relatively short in view of the usual reported delays of the emergence of the second cancer in most series in the literature, frequently exceeding 10 years. The observed short-term reduction of the risk of SPC may involve the reactivation of an efficient antitumor immune response against an infra-clinical tumor, as shown for the adjuvant treatment of high-risk melanoma.12,13 The reduction associated with ICI administration was consistent for each of the individual years, from 2013 to 2018, of this study, and observed in all age groups and for all histotypes and primary cancer sites explored.
To our knowledge, the impact of immunotherapy for the FPC on the risk of developing an SPC has not been reported in another series. The analysis of the published literature on large clinical trials of immunotherapy with ICIs in the approved indications is not informative of the incidence of SPCs; often none are reported in these series.20,21 Even in cancers occurring in patients with germline mismatch repair deficiency, who are prone to developing multiple tumor types, and where immunotherapy is highly active, this has not been reported to our knowledge.22, 23, 24, 25, 26, 27, 28, 29 It will be important to study the incidence of SPC in published randomized clinical trials evaluating ICIs in the adjuvant setting of non-metastatic cancers.
Of note, a large proportion of patients in whom immunotherapy was applied for their FPC is known to be at risk of an SPC given the etiological factors of their FPC (e.g. lung and head and neck cancers or melanoma, with sun exposure, smoking, alcohol). We would have expected a higher risk of SPC in this population, even in the advanced phase. The opposite was seen, and actually no head and neck carcinomas, lung cancer, bladder nor renal cancers were observed as SPCs in the group treated with ICIs for the FPC. Actually, histotypes and sites of SPC observed in the group treated with immunotherapy for the FPC were different from those treated without ICIs for the FPC, with more digestive tract cancers in the rare SPCs observed in patients treated with ICIs. An analysis of the genomic and molecular characteristics of these SPCs occurring after immunotherapy is ongoing.
This study has several limitations. Part of the data was collected through an automatic extraction using natural language processing of the EPR. To ensure the quality of data extracted through this screening, a second, manual, screening of 2818 files was thus carried out by the team on all patients who received immunotherapy and on those where the diagnosis of SPC had not been formally declared by the physician in charge. Only six additional SPCs were identified by this procedure. Another limitation is that the follow-up of the series is 19 months; indeed further analysis will be required in the same series in the years to come to determine whether our results point to a reduction of the incidence or only to a prolonged delay to diagnosis. Treatment with radiotherapy for the FPC was not integrated in this report. It was not found to be associated with an increased risk of SPC over the observation period and did not impact the conclusion of the multivariate analysis (not shown). Another issue is that patients treated with ICIs often had advanced disease, receiving immunotherapy with a short life expectancy. For this reason, a second analysis was conducted on the subgroup of patients who never had a metastasis of their primary cancers. Both analyses are consistent in their results. Finally, different histological groups of tumors were grouped to ensure sufficient numbers, e.g. in ‘lung cancer’, which includes adenocarcinoma or squamous cell carcinoma. or ‘skin cancers’, which includes melanoma, Merkel cell carcinomas, epidermoid carcinoma and others. Specific analysis of given histological subtypes will be required in the future. Though consistent for each individual year here, this early reduction in the risk of SPC requires validation from other large series and datasets. This is a large, exhaustive, but single-center study that needs to be confirmed using other exhaustive databases of EPRs of comprehensive cancer centers. It must be stressed that this study is not a clinical trial and additional series are needed to confirm this observation.
These results have potentially important consequences, in particular in adolescents and young adults cured of the first cancer and in whom SPCs are important causes of death. Their long life expectancy may expose a high cumulative risk of second primary malignancy. Several studies have explored the immunological environment of premalignant lesions and indicate the presence of active immune responses.27,28,30,31
Altogether, these results collected in a large exhaustive database of 46 829-patient population treated in a comprehensive cancer center indicates that the risk of SPC is reduced in the years following the treatment of the cancer when immunotherapy with ICIs is used for the treatment of the first cancer. These results are consistent in all age groups, for all histotypes and in each of the different years of this study. A longer follow-up will be needed to determine whether this reduction of incidence of SPC is maintained over time. If these observations are confirmed, these results open a novel area of clinical research in tertiary prevention for cancer patients, which could test an active prevention of SPCs in a population of patients at high risk, such as those with germline predisposition to cancer or with long term exposure to carcinogens such as smoking. A randomized study is in preparation.
Acknowledgments
Funding
This work was supported by LYRICAN [grant number INCA-DGOS-INSERM 12563]; NetSARC (INCA & DGOS) (no grant number); TMRG (INCA & DGOS) (no grant number); InterSARC (INCA) (no grant number); DEvweCAN [grant number ANR-10-LABX-0061]; PIA Institut Convergence François Rabelais PLAsCAN [grant number PLAsCAN, 17-CONV-0002]; RHU4 DEPGYN [grant number ANR-18-RHUS-0009]; EURACAN [grant number EC 739521]; Association DAM's (no grant number); la Fondation ARC (no grant number); Infosarcome (no grant number); Ligue de L'Ain Contre le Cancer (no grant number) and La Ligue Contre le Cancer (no grant number).
Disclosure
DP: research grant: MSDAVENIR; honoraria: Roche, BMS, MSD, AstraZeneca; travel: AstraZeneca. AF: grants, personal fees and nonfinancial support from ROCHE, AZ, Pfizer, MSD, BMS. JF: grants, personal fees and nonfinancial support from AZ, MSD, BMS, Merck Serono, Biogen, Rakuten, Roche, Servier. OT: Roche, AstraZeneca, Novartis, Pfizer, MSD, BMS, Sandoz. HB received travel expenses from BMS, Pfizer and honoraria from BMS and Pfizer. MP: grants, personal fees and nonfinancial support from Roche, Genentech, Eli Lilly, Pfizer, Boehringer-Ingelheim, MSD, Bristol-Myers Squibb, Novartis, Pierre Fabre, AstraZeneca, Takeda, Chugai, Amgen (adboards and symposiums). TB: advisory board and travel from Roche, Novartis, AstraZeneca, Pfizer and Seattle Genetics. PC: Roche/Genentech, AZ, Amgen, EMD/Merck Serono, Novartis (symposiums/adboard). VA: honoraria for advisory boards from Bristol-Myers Squibb. E-MN-B received travel expenses and honoraria from MSD, BMS, Pierre Favre and Novartis. FN: grants, personal fees and nonfinancial support from Novartis, Incyte Biosciences, Sun Pharma Ltd., Ylang Pharma Inc. AS received honoraria for advisory boards from Roche, Bristol-Myers Squibb; symposiums: Roche, AstraZeneca, Bristol-Myers Squibb. PS: Research grant from BMS and Roche. PM declares to have interest in NETRIS Pharma as founder and minority shareholder. IR-C: honoria from AbbVie, Agenus, Advaxis, BMS, Pharma Mar, Genmab, Pfizer, AstraZeneca, Roche, GSK, MSD, Deciphera, Mersena, Merck Sereno, Novartis, Amgen, Tesaro and Clovis; advisory/consultancy from AbbVie, Agenus, Advaxis, BMS, Pharma Mar, Genmab, Pfizer, AstraZeneca, Roche/Genentech, GSK, MSD, Deciphera, Mersena, Merck Sereno, Novartis, Amgen, Tesaro and Clovis; research grant from MSD, Roche and BMS. JYB: research support and honoraria from Roche, MSD, BMS, Bayer, Novartis, GSK, AstraZeneca. The remaining authors declare no conflicts of interest.
Supplementary data
Time to second primary tumor according to cytotoxic and/or immunotherapy administration per year of the study period (from 2013 to 2018).
Subgroup analysis: risk of second cancer according to the treatment given to the first primary cancer in the different subgroups of primary sites.
Cumulative incidence of the different types of second cancer according to the treatment given to the first primary cancer.
Secondary cancers according to the type of first cancer and to the treatment given for the first cancer.
References
- 1.Travis L.B., Demark-Wahnefried W., Allan J.M., Wood M.E., Ng A.K. Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nat Rev Clin Oncol. 2013;10:289–301. doi: 10.1038/nrclinonc.2013.41. [DOI] [PubMed] [Google Scholar]
- 2.Newhauser W.D., Durante M. Assessing the risk of second malignancies after modern radiotherapy. Nat Rev Cancer. 2011;11:438–448. doi: 10.1038/nrc3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatia S., Sklar C. Second cancers in survivors of childhood cancer. Nat Rev Cancer. 2002;2:124–132. doi: 10.1038/nrc722. [DOI] [PubMed] [Google Scholar]
- 4.Eichenauer D.A., Becker I., Monsef I. Secondary malignant neoplasms, progression-free survival and overall survival in patients treated for Hodgkin lymphoma: a systematic review and meta-analysis of randomized clinical trials. Haematologica. 2017;102:1748–1757. doi: 10.3324/haematol.2017.167478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaapveld M., Aleman B.M., van Eggermond A.M. Second cancer risk up to 40 years after treatment for Hodgkin's lymphoma. N Engl J Med. 2015;373:2499–2511. doi: 10.1056/NEJMoa1505949. [DOI] [PubMed] [Google Scholar]
- 6.Chattopadhyay S., Zheng G., Sud A. Second primary cancers in non-Hodgkin lymphoma: family history and survival. Int J Cancer. 2020;146:970–976. doi: 10.1002/ijc.32391. [DOI] [PubMed] [Google Scholar]
- 7.Vakharia P.P., Kelm R.C., Orrell K.A. Risks for noncutaneous second primary malignancy in cutaneous malignant melanoma survivors: an analysis of data from the surveillance, epidemiology, and end results (SEER) program. Int J Dermatol. 2020;59:463–468. doi: 10.1111/ijd.14781. [DOI] [PubMed] [Google Scholar]
- 8.Li D., Weng S., Zhong C. Risk of second primary cancers among long-term survivors of breast cancer. Front Oncol. 2020;9:1426. doi: 10.3389/fonc.2019.01426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehtälä J., Zong J., Vassilev Z. Overall survival and second primary malignancies in men with metastatic prostate cancer. PLoS One. 2020;15(2):e0227552. doi: 10.1371/journal.pone.0227552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feller A., Matthes K.L., Bordoni A. The relative risk of second primary cancers in Switzerland: a population-based retrospective cohort study. BMC Cancer. 2020;20:51. doi: 10.1186/s12885-019-6452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Havel J.J., Chowell D., Chan T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19:133–150. doi: 10.1038/s41568-019-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber J., Mandala M., Del Vecchio M. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 13.Eggermont A.M.M., Blank C.U., Mandala M. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378:1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 14.Antonia S.J., Villegas A., Daniel D. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 15.Heudel P., Chabaud S., Perol D., Ray-Coquard I., Blay J.Y. Reduced risk of second primary cancer in patients treated with immune checkpoint inhibitors for a first cancer. Ann Oncol. 2020;31:1773–1775. doi: 10.1016/j.annonc.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Heudel P., Livartowski A., Arveux P. The ConSoRe project supports the implementation of big data in oncology. Bull Cancer. 2016;103:949–950. doi: 10.1016/j.bulcan.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Henderson T.O., Rajaraman P., Stovall M. Risk factors associated with secondary sarcomas in childhood cancer survivors: a report from the childhood cancer survivor study. Int J Radiat Oncol Biol Phys. 2012;84:224–230. doi: 10.1016/j.ijrobp.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morton L.M., Swerdlow A.J., Schaapveld M. Current knowledge and future research directions in treatment-related second primary malignancies. EJC Suppl. 2014;12:5–17. doi: 10.1016/j.ejcsup.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reding K.W., Bernstein J.L., Langholz B.M. Adjuvant systemic therapy for breast cancer in BRCA1/BRCA2 mutation carriers in a population-based study of risk of contralateral breast cancer. Breast Cancer Res Treat. 2010;123:491–498. doi: 10.1007/s10549-010-0769-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu C., Chen Y.P., Du X.J. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ. 2018;363:k4226. doi: 10.1136/bmj.k4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chai Q.-Q., Du J.-Y., Zhu J., Wu B. The differences in the safety and tolerability of immune checkpoint inhibitors as treatment for non-small cell lung cancer and melanoma: network meta-analysis and systematic review. Front Pharmacol. 2019;10:1260. doi: 10.3389/fphar.2019.01260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch H.T., Snyder C.L., Shaw T.G., Heinen C.D., Hitchins M.P. Milestones of Lynch syndrome: 1895-2015. Nat Rev Cancer. 2015;15:181–194. doi: 10.1038/nrc3878. [DOI] [PubMed] [Google Scholar]
- 23.Le D.T., Uram J.N., Wang H. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Overman M.J., Lonardi S., Wong K.Y.M. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36:773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 25.Marabelle A., Le D.T., Ascierto P.A. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38:1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le D.T., Kim T.W., Van Cutsem E. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol. 2020;38:11–19. doi: 10.1200/JCO.19.02107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendis S., Gill S. Cautious optimism-the current role of immunotherapy in gastrointestinal cancers. Curr Oncol. 2020;27(suppl 2):S59–S68. doi: 10.3747/co.27.5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spira A., Yurgelun M.B., Alexandrov L. Precancer atlas to drive precision prevention trials. Cancer Res. 2017;77:1510–1541. doi: 10.1158/0008-5472.CAN-16-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spira A., Disis M.L., Schiller J.T. Leveraging premalignant biology for immune-based cancer prevention. Proc Natl Acad Sci U S A. 2016;113:10750–10758. doi: 10.1073/pnas.1608077113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mascaux C., Angelova M., Vasaturo A. Immune evasion before tumour invasion in early lung squamous carcinogenesis. Nature. 2019;571:570–575. doi: 10.1038/s41586-019-1330-0. [DOI] [PubMed] [Google Scholar]
- 31.Foy J.P., Bertolus C., Ortiz-Cuaran S. Immunological and classical subtypes of oral premalignant lesions. Oncoimmunology. 2018;7(12):e1496880. doi: 10.1080/2162402X.2018.1496880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time to second primary tumor according to cytotoxic and/or immunotherapy administration per year of the study period (from 2013 to 2018).
Subgroup analysis: risk of second cancer according to the treatment given to the first primary cancer in the different subgroups of primary sites.
Cumulative incidence of the different types of second cancer according to the treatment given to the first primary cancer.
Secondary cancers according to the type of first cancer and to the treatment given for the first cancer.