Abstract
Background
Contact precautions for endemic methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) are under increasing scrutiny, in part due to limited clinical trial evidence.
Methods
We retrospectively analyzed data from the Strategies to Reduce Transmission of Antimicrobial Resistant Bacteria in Intensive Care Units (STAR*ICU) trial to model the use of contact precautions in individual intensive care units (ICUs). Data included admission and discharge times and surveillance test results. We used a transmission model to estimate key epidemiological parameters, including the effect of contact precautions on transmission. Finally, we performed multivariate meta-regression to identify ICU-level factors associated with contact precaution effects.
Results
We found that 21% of admissions (n = 2194) were placed on contact precautions, with most for MRSA and VRE. We found little evidence that contact precautions reduced MRSA transmission. The estimated change in transmission attributed to contact precautions was –16% (95% credible interval, –38% to 15%). VRE transmission was higher than MRSA transmission due to contact precautions, but not significantly. In our meta-regression, we did not identify associations between ICU-level factors and estimated contact precaution effects. Importation and transmission were higher for VRE than for MRSA, but clearance rates were lower for VRE than for MRSA.
Conclusions
We found little evidence that contact precautions implemented during the STAR*ICU trial reduced transmission of MRSA or VRE. We did find important differences in the transmission dynamics between MRSA and VRE. Differences in organism and healthcare setting may impact the efficacy of contact precautions.
Keywords: contact precautions, MRSA, VRE, transmission, effectiveness
We conducted a retrospective analysis of data from the Strategies to Reduce Transmission of Antimicrobial Resistant Bacteria in Intensive Care Units trial, finding no evidence that contact precautions reduced transmission of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci.>
Antibiotic-resistant pathogens are a major cause of morbidity and mortality in healthcare settings. In particular, intensive care units (ICUs) experience a higher burden of antibiotic-resistant pathogens, as patients are high-acuity and often require interventions that put patients at an increased risk for colonization or infection. Bundled interventions are often implemented to control resistant organisms and include various strategies, such as antibiotic stewardship, active surveillance and contact precautions, environmental decontamination, and decolonization [1–8]. This bundling makes it difficult to evaluate the efficacy of a specific strategy. Additionally, the generation of evidence on the effectiveness of interventions has been slow.
Of the strategies to control the spread of antibiotic-resistant healthcare-associated infections, contact precautions, which typically involve the use of gowns and gloves for detected carriers, remain among the most debated [9, 10]. This is primarily because the use of contact precautions has not been supported by a strong clinical trial evidence base. Existing clinical trials have not definitively determined whether contact precautions are effective at reducing the spread of antibiotic-resistant infections for endemic organisms such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) [11]. However, there is some evidence from a clinical trial to support a reduction in the acquisition rate of MRSA based on the use of universal gowns and gloves [5]. In addition, some observational studies have suggested that a bundled approach that includes contact precautions for detected carriers of MRSA has played a role in reducing MRSA infection rates across the Department of Veterans Affairs (VA) [4, 11–13]. A number of dynamic transmission-based studies have attempted to directly measure the effect of infection control practices on transmission of antibiotic-resistant bacteria with mixed results [14–16].
In a previous study that reevaluated the Strategies to Reduce Transmission of Antimicrobial Resistant Bacteria in Intensive Care Units (STAR*ICU) trial [3], we developed an explicit hierarchal model of transmission dynamics [17] and found that the intervention of contact precautions resulted in no difference in the estimated transmission rate. Our results were consistent with the conclusions from the original STAR*ICU trial, serving as a validation of the approach of dynamic modeling in evaluating interventional trials. However, one limitation that was noted in the STAR*ICU trial that has not been directly evaluated is the long turnaround time from sample collection to test result [3], resulting in fewer patients being placed on contact precautions than should have been.
We aimed to directly address this limitation by incorporating the patient-level data on implementation of contact precautions in order to generate estimates of the impact of contact precautions on transmission of MRSA and VRE. This work extends our recently published work [18] by incorporating a parameter for measuring contact precautions. Our approach accounts for the imperfect nature of surveillance tests and estimates the rate of clearance.
METHODS
Data
We performed a retrospective analysis of data from the original STAR*ICU trial [3], extending some of our previous work [17]. Data was collected from April 2005 through August 2006 and included patients admitted to 18 participating ICUs. Nasal and perianal surveillance swabs were collected at the time of ICU admission, weekly thereafter, and on discharge from the ICU. Surveillance swabs were not collected for short-stay patients (ICU stay <3 days) in the STAR*ICU trial, except for a random sample, which was used in the original study to estimate admission prevalence. Swabs were collected from all long-stay patients (ICU stay ≥3 days), but not all short-stay patients were tested. Approximately 60% of all admissions to the ICU had at least 1 swab for MRSA and VRE. Our transmission model used ICU identifier, ICU study arm (control vs intervention), patient identifier, admission and discharge dates, and dates of starting and stopping contact precautions; our data were organized by ICU.
Model
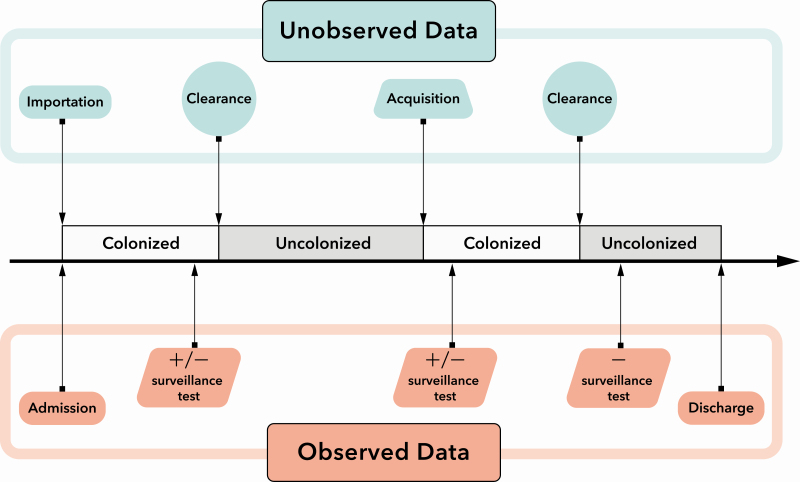
We modeled the effectiveness of contact precautions using a transmission model that integrates clinical parameters with mechanistic features that represent fundamental assumptions about the dynamics of transmission, illustrated in Figure 1. We modeled the admission and discharge processes in addition to unobserved data including both acquisition and clearance of MRSA and VRE. To model these dynamics, we included model parameters such as ICU-level importation probability, the transmission rate, and the clearance rate. In addition, we included a parameter to estimate the relative transmissibility of patients who are on contact precautions. We call this the “contact precautions effect” () (Figure 2). We ran the analysis for each ICU independently. We estimated parameters using Markov chain Monte Carlo (MCMC) [19], which is an iterative approach for obtaining parameter distributions. The transmission model incorporated 3 fundamental elements, namely, model parameters, observed data, and unobserved data. What we refer to as “unobserved data” represent imperfectly observed interval-censored data, informed by test results that consisted of variables that are not possible to observe directly but are critical for completely specifying the model likelihood, such as times of acquisition and clearance (Figure 1). We refer to the combined observed and unobserved data as the “augmented data.” A more detailed overview of the transmission model and its assumptions are provided in the following text; technical details can be found in the Supplementary Materials.
Figure 1.
The underlying transmission model showing the possible transitions for patient colonization and the relationship between the unobserved and observed data in the model.
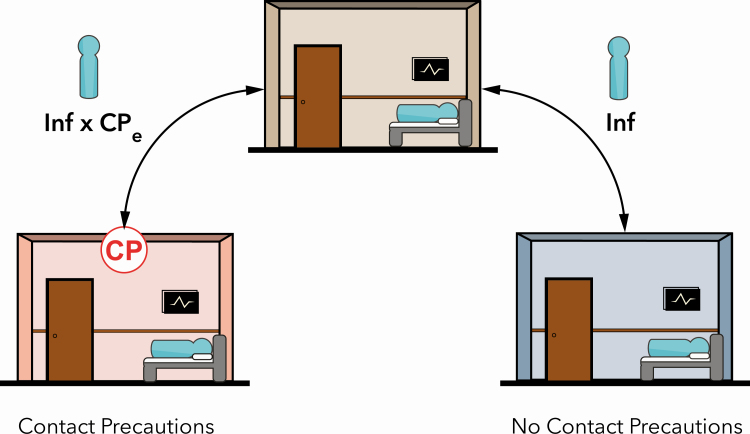
Figure 2.
Illustration of the differential transmissibility, given a baseline Inf and the CPe for patients who are on contact precautions (left) compared with those who are not on contact precautions (right). Abbreviations: CPe, effect of contact precautions; Inf, infectiousness.
Importation
At admission, we assumed patients were either colonized (an importation event) or uncolonized. Although patients may have multiple admissions to the ICU, we define the importation probability as the probability that an individual who is admitted for the first time is colonized at the time of admission. We account for colonization status between admissions through a separate parameter described below. Based on our model, importation is equivalent to the notion of a steady-state importation probability. In other words, given a sufficiently long period between consecutive admissions, the patient’s colonization status is almost independent.
We assumed that patients with a recent hospitalization have a different likelihood of importation compared with those in the general community. The times and results of patients’ previous surveillance tests at a prior ICU stay informed the probability of importation at the time of readmission for each individual. Thus, we assumed that patients could acquire and lose colonization between consecutive admissions. This between-admission model, which is described in more detail in the Supplementary Materials, leads to a simple formulaic relationship between the importation probability and the readmission probability of importation. This leads to a simple method for calculating the importation probability of readmissions based on the time since the previous discharge and colonization status at the previous discharge. For an individual who was colonized at a previous admission, as the time away from the ICU increases, the probability for that individual to remain colonized approaches the steady-state first-admission importation probability.
Transmission
The underlying model for transmission assumes frequency-dependent transmission, which means that the transmission rate parameter is a proportional constant that describes the intensity of the force of infection. In other words, for a given transmission rate parameter, as the proportion of colonized patients on the ward increases, so does the force of infection. This is a dynamic model analogue to colonization pressure. A consequence of this model for transmission is that the transmission rate parameter is a measure of transmission that is not confounded by prevalence; given 2 wards with the same prevalence, a higher transmission rate parameter in one ward suggests an increased level of transmission.
Contact Precautions Effect
To evaluate the effects of contact precautions, we defined 2 groups of patients, those on contact precautions and those not on contact precautions, with differing risk of transmission (Figure 2). Previously, we described how we modeled transmission from patients not on contact precautions. In contrast, for patients on contact precautions, we included an additional parameter, , which represents the differential risk of transmission posed by colonized patients while on contact precautions. Given our implementation, when , contact precautions have no effect on transmission; when , contact precautions reduce transmission (contact precautions are effective); and when , contact precautions result in increased transmission.
Clearance
We assumed that colonized patients lose colonization at a constant rate during their ICU stay. This assumption does not reflect a particular mechanism of clearance, rather the composite patient-care and ICU-specific factors that contribute to clearance. Once cleared, patients are immediately at risk of acquiring MRSA or VRE regardless of admission status.
Test Sensitivity
Due to the rarity of false positives, it is common to assume that false positives are negligible in statistical transmission models [15, 20–23]. We assumed that there were no false-positive surveillance tests but allowed for false negatives (Figure 1).
Estimation
Our MCMC algorithm consisted of an iterative process for obtaining posterior samples, or parameter distributions. Estimation within each iteration of the MCMC algorithm involved generating a new sample of both the augmented, or unobserved, data and the parameters. Conditional on the observed data and the set of parameter values, a new augmented dataset (or patient histories) that was consistent with the observed data and the parameter values was proposed as the new sample. This parameter proposal was accepted with a probability that depended on the relative likelihood of the model with the proposed and current augmented data. If the proposed augmented data were rejected, the current augmented data remained as the next augmented data sample until the next iteration through the MCMC; otherwise, the current augmented data was replaced with the proposal. Given the augmented dataset, parameter values were proposed using both the Gibbs sampler [24] and the Metropolis-Hastings [25] algorithm, based on the new augmented data. This process of updating the augmented data and parameter values was iterated and resulted in a collection of parameter values with a distribution that is consistent with the likelihood, conditioned on all observed and unobserved data. The posterior distributions were based on 20 000 samples with a burn-in of 1000 samples.
Analysis
We computed both posterior means and 95% credible intervals (CrIs) for the model parameters for each ICU and report median and range of each estimate across ICUs. We also computed the posterior mean-logs and variance-logs of the parameter samples for inclusion into pooled analyses. We obtained pooled estimates of the effect of contact precautions for both MRSA and VRE in 2 ways; we obtained pooled estimates separately by time period and also obtained a single estimate across time. For our model across time, we assumed that the covariance structure in ICU dependence over time was a heteroscedastic autoregressive structure for the estimated effect of contact precaution on transmission. Finally, we performed a meta-regression on estimates of for each ICU and included ICU-level moderators to identify potentially important factors that were associated with the effectiveness of contact precautions. The ICU-level moderators used in the meta-regression included model parameters, fixed ICU characteristics (eg, ICU type), ICU-specific estimates obtained through observation of the patient care environment (eg, healthcare worker–patient contact rate), and estimates related to infection control compliance (eg, percent of contacts with a gown, universal gloving). The MCMC algorithm was implemented in C++, and analysis of the posterior distributions used the rmeta [26], metaphor [27], and base [28] packages from the R Project for Statistical Computing. For additional technical details on the modeling assumptions and formulas, see the Supplementary Materials.
RESULTS
Data Summary
There were 10 579 admissions into 1 of the 18 ICUs. Of those admissions, 2194 (or 20.7%) were placed on contact precautions, some for more than 1 reason, resulting in 2332 different contact precautions initiated. A majority of precautions were classified as contact precautions (Table 1), although there was variation in the precaution types [29]. Additionally, organisms attributed for the use of contact precautions varied, but MRSA and VRE were the most common (Table 2).
Table 1.
Distribution of Precaution Types Classified as 1 of 4 Classes of Precautions
| Distribution of Precaution Types | |
|---|---|
| Precaution Type | Frequency (N = 5628) (%) |
| Contact | 4438 (78.9) |
| Other | 782 (13.9) |
| Droplet | 260 (4.6) |
| Airborne | 148 (2.6) |
Table 2.
Distribution of Organisms Attributed as the Reason for Initiating Precautions
| Distribution of Reasons for Precautions | |
|---|---|
| Organism | Frequency (N = 5628) (%) |
| MRSA | 2063 (36.7) |
| VRE | 1483 (26.4) |
| Other | 847 (15.0) |
| MRSA and VRE | 548 (9.7) |
| Clostridioides difficile | 322 (5.7) |
| Multidrug-resistant gram-negative rod | 219 (3.9) |
| Missing | 142 (2.5) |
| Vancomycin-resistant Staphylococcus aureus | 4 (0.0) |
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci.
Contact Precautions Effect
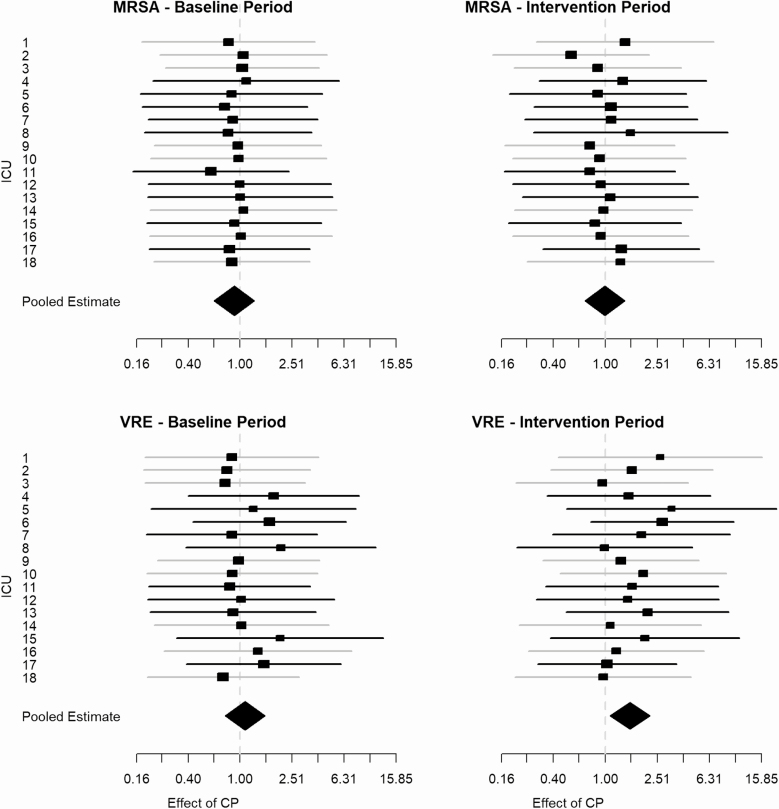
We found little evidence of an effect of contact precautions on transmission of MRSA or VRE in any of the individual ICUs (Figure 3). The pooled estimates of the effectiveness of contact precautions on transmission of either MRSA or VRE and in either time period across all ICUs resulted in a slightly elevated but not statistically significant effect (1.11; 95% confidence interval, .93–1.32), suggesting no benefit of contact precautions for preventing transmission. If we account for the time period (baseline vs intervention) and the 2 organisms (MRSA vs VRE) and incorporate them as model moderators, we find that the estimated effect of contact precautions on MRSA transmission during the baseline period is reduced to 0.84 (95% CrI, 0.62–1.15). Transmission of VRE during the intervention period results in a slight increase in the effectiveness of contact precautions on transmission, but neither estimate is significant. The Omnibus test for the parameters suggests that neither variable changes the overall estimated effectiveness of contact precautions. Similarly, accounting for the dependence between the baseline and intervention phases of the study, we find no evidence for effectiveness of contact precautions to reduce transmission, and the estimated impact on transmission of VRE is slightly elevated. The random effects component of the pooled analysis in each of the models had an estimated variance of zero (P = 1), suggesting no evidence of heterogeneity across ICUs. The temporal correlation across ICUs from the autoregressive component of the model between the 2 periods was 0.79 for the effectiveness of contact precautions on transmission.
Figure 3.
Forest plots showing the estimated CPe, which represents the relative rate of transmissibility attributed to contact precautions compared with transmissibility attributed to no contact precautions. Results are organized by ICU and pooled (diamond shape) for MRSA (top) and VRE (bottom) during the baseline period (left) and intervention period (right). The gray lines represent the control ICUs, and the black lines represent the intervention ICUs. Abbreviations: CPe, effect of contact precautions; ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci.
Model Parameters
For the remaining model parameters, the pooled estimates suggest that the importation probability and transmission rate are higher for VRE than for MRSA and that clearance rate estimates are lower for VRE than for MRSA (Table 3). Additionally, we found that the estimated test sensitivity parameters were similar but slightly higher for the VRE culture.
Table 3.
Parameter Estimates and Confidence Intervals for Model Parameters Obtained from Pooling Across Intensive Care Unit–Specific Estimates
| Pooled Parameter Estimatesa | ||
|---|---|---|
| Pooled Estimate (95% Confidence Interval) | ||
| Model Parameter | Methicillin-Resistant Staphylococcus aureus | Vancomycin- Resistant Enterococci |
| Transmission rate | 0.05 (.04–.06) | 0.07 (.07–.08) |
| Importation probability | 0.18 (.16–.20) | 0.22 (.19–.24) |
| Clearance rate | 0.03 (.03–.04) | 0.02 (.02–.03) |
| Surveillance sensitivity | 0.66 (.62–.71) | 0.68 (.65–.71) |
aAlthough primary analysis was done using Bayesian methods, secondary analysis included meta-regression, which provided confidence intervals (CIs) rather than credible intervals. Additionally, rounding of the estimates and CIs resulted in the point estimates occasionally taking the same value as an endpoint of the CI.
Discussion
We found that although 21% of admissions were placed on contact precautions, there was little evidence that contact precautions reduced transmission of MRSA or VRE in these ICUs. Additionally, there was some evidence of increased transmissibility of VRE for patients on contact precautions relative to MRSA transmission, but this was not statistically significant. Our other estimated model parameters were similar to what we estimated previously using the STAR*ICU study data [17], although our previous estimates were based on a slightly different variation of mass action, assuming density-dependent transmission.
We included a variety of ICU measures in a meta-regression model to look for variables that were associated with our estimated contact precaution effect estimates. These included ICU type, compliance with hand hygiene, compliance with wearing gowns and gloves, staffing ratios, and an indicator for universal gloving that was done at the intervention ICUs during the intervention phase of the study. We did not identify associations of these measures with the estimated contact precautions effect.
Given that our results do not provide evidence that the use of contact precautions during the STAR*ICU trial helped to reduce transmission, questions remain regarding why contact precautions do not seem to reduce transmission of MRSA and VRE. It is particularly surprising that our analysis suggests that the use of contact precautions during the intervention period seemed to result in elevated transmission of VRE. In our previous work [17], we found a slight increase in the overall transmission rate for VRE between the baseline and intervention periods, independent of the intervention effect on transmission. Similarly, in the original STAR*ICU trial, although colonization or infection incidence rates increased for both MRSA and VRE from the baseline period to the intervention period, the increase was larger for VRE, which is consistent with what we have observed. More generally, in our previous work, we noted differences in the transmission dynamics between MRSA and VRE, with VRE having a slightly higher importation probability and transmission rate and a lower clearance rate compared with MRSA [17]. Although we have observed these differences in the epidemiology of MRSA and VRE, the increase in transmission due to contact precautions remains puzzling.
Others have also observed differences between MRSA and VRE on the impact of contact precautions on transmission [5]. They found that the use of gowns and gloves for all patient contacts compared with usual care among patients in medical and surgical ICUs did not result in a difference in acquisition of VRE, but that there was a lower risk of acquisition for MRSA (P = .046). Additionally, using a dynamic model, Wei et al evaluated the impact of contact precautions on transmission of VRE in a US hospital with 8 ICUs and found no compelling evidence to support the effectiveness of the precaution measures [14]. In a separate analysis, Kypraios et al evaluated the effectiveness of isolation precautions on MRSA transmission at the same hospital during the same time period and found that in 5 of the 8 wards, there was weak evidence indicating that contact precautions were associated with reduced transmissibility [15], and pooled estimates across all of the wards suggested the same.
Not only is it possible that there are differences between organisms that result in differences in the efficacy of contact precautions, it may also be possible that the healthcare setting could play a role [30]. Many large studies have been conducted in ICUs, which are complex environments typically filled with high-acuity patients. Under the assumption that contact precautions are effective at reducing transmission, it is still possible that estimating this effect in an environment with so many additional factors that also play a role in transmission (eg, antibiotics, procedures, devices) makes the effect difficult to isolate. The study discussed previously that found weak evidence associating isolation with reduced transmission was in ICUs [15]. However, in another study, Worby et al used similar methods and found a larger reduction in general wards due to the combination of isolation and decolonization in reducing MRSA transmission [16]. It is difficult to separate the effectiveness of contact precautions from those of decolonization, yet there is some evidence of no benefit with the addition of decolonization for MRSA when contact precautions were used for patients colonized with MRSA in acute care [31]. We recently found that using these same methods that pooled estimates of the effectiveness of contact precautions across more than 100 hospitals in the VA demonstrate a significant reduction in the transmission rate (K Khader, manuscript in preparation). Given that these estimates are hospital-wide, the influence of ICUs is likely limited.
This study had some limitations. Although we looked for variables that could help explain the estimated impact of contact precautions on transmission using meta-regression, we were unable to explicitly include these data in the model. The relatively small number of ICUs included in the study presents challenges for reliably estimating moderator effects through meta-regression. We plan to perform similar evaluations in a larger group of ICUs moving forward.
Given the disparate results from studies that evaluated the impact of contact precautions, it is important to consider a more nuanced view of the entire suite of infection control strategies. As others have suggested, it is possible that the effectiveness of a given infection control strategy depends on additional factors, including the pathogen, healthcare setting, patient mix, and patient care factors that are being used [11, 32]. Given this uncertainty, it is important to move forward with high-quality studies to address this question in order to better inform infection control practices [9, 33].
Conclusions
We found little evidence that contact precautions implemented during the STAR*ICU trial reduced transmission of MRSA or VRE. However, we found that during the intervention period of the study, contact precautions seem to have slightly increased transmission of VRE compared with MRSA. We also identified important differences in the transmission dynamics between MRSA and VRE. In particular, transmission and importation were higher for VRE than for MRSA and clearance of VRE was lower than clearance of MRSA. These estimates suggest that overall prevalence in the ICUs was much higher for VRE than MRSA.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors acknowledge Carrie Edlund, MS, for her many iterations reviewing and editing this manuscript and Jeanette Young for her time and expertise on the development of the model figures (Figure 1 and Figure 2).
Disclaimer. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
Financial support. This work was supported by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (project REA-08-264); the Centers for Disease Control and Prevention (Prevention Epicenters award number U54CK000456 and MIND-Healthcare Program award number U01CK000538); and the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (grant 8UL1TR000105).
Supplement sponsorship. This supplement is sponsored by the Informatics, Decision-Enhancement and Analytic Sciences (IDEAS) Center.
Potential conflicts of interest. W. C. H. reports contract N01-AI-15440 for the Strategies to Reduce Transmission of Antimicrobial Resistant Bacteria in Intensive Care Units (STAR*ICU) trial from the National Institute of Allergy and Infectious Diseases; a donation of gloves and gowns to be used in the STAR*ICU trial from Kimberly Clark during the conduct of the study; consulting fees from ADMA Biologics, Inc; and personal fees from Pfizer outside the submitted work. All remaining authors: No potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Schultsz C, Bootsma MC, Loan HT, et al. Effects of infection control measures on acquisition of five antimicrobial drug-resistant microorganisms in a tetanus intensive care unit in Vietnam. Intensive Care Med 2013; 39:661–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Madaras-Kelly KJ, Remington RE, Lewis PG, Stevens DL. Evaluation of an intervention designed to decrease the rate of nosocomial methicillin-resistant Staphylococcus aureus infection by encouraging decreased fluoroquinolone use. Infect Control Hosp Epidemiol 2006; 27:155–69. [DOI] [PubMed] [Google Scholar]
- 3. Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med 2011; 364:1407–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med 2011; 364:1419–30. [DOI] [PubMed] [Google Scholar]
- 5. Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA 2013; 310:1571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harbarth S, Fankhauser C, Schrenzel J, et al. Universal screening for methicillin-resistant Staphylococcus aureus at hospital admission and nosocomial infection in surgical patients. JAMA 2008; 299:1149–57. [DOI] [PubMed] [Google Scholar]
- 7. Dancer SJ. Importance of the environment in meticillin-resistant Staphylococcus aureus acquisition: the case for hospital cleaning. Lancet Infect Dis 2008; 8:101–13. [DOI] [PubMed] [Google Scholar]
- 8. Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med 2013; 368:2255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rubin MA, Samore MH, Harris AD. The importance of contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. JAMA 2018; 319:863–4. [DOI] [PubMed] [Google Scholar]
- 10. Morgan DJ, Wenzel RP, Bearman G. Contact precautions for endemic MRSA and VRE: time to retire legal mandates. JAMA 2017; 318:329–30. [DOI] [PubMed] [Google Scholar]
- 11. Fätkenheuer G, Hirschel B, Harbarth S. Screening and isolation to control meticillin-resistant Staphylococcus aureus: sense, nonsense, and evidence. Lancet 2015; 385:1146–9. [DOI] [PubMed] [Google Scholar]
- 12. Evans ME, Kralovic SM, Simbartl LA, et al. Nationwide reduction of health care-associated methicillin-resistant Staphylococcus aureus infections in Veterans Affairs long-term care facilities. Am J Infect Control 2014; 42:60–2. [DOI] [PubMed] [Google Scholar]
- 13. Kralovic SM, Evans ME, Simbartl LA. Zeroing in on methicillin-resistant Staphylococcus aureus: US Department of Veterans Affairs’ MRSA Prevention Initiative. Am J Infect Control 2013. Available at: http://www.sciencedirect.com/science/article/pii/S0196655312008772. Accessed 28 October 2015. [DOI] [PubMed]
- 14. Wei Y, Kypraios T, O ’Neill PD, Huang SS, Rifas-Shiman SL, Cooper BS. Evaluating hospital infection control measures for antimicrobial-resistant pathogens using stochastic transmission models: application to vancomycin-resistant enterococci in intensive care units. Stat Methods Med Res 2016; 0:1–20. [DOI] [PubMed] [Google Scholar]
- 15. Kypraios T, O’Neill PD, Huang SS, Rifas-Shiman SL, Cooper BS. Assessing the role of undetected colonization and isolation precautions in reducing methicillin-resistant Staphylococcus aureus transmission in intensive care units. BMC Infect Dis 2010; 10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Worby CJ, Jeyaratnam D, Robotham JV, et al. Estimating the effectiveness of isolation and decolonization measures in reducing transmission of methicillin-resistant Staphylococcus aureus in hospital general wards. Am J Epidemiol 2013; 177:1306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khader K, Thomas A, Huskins WC, et al. A dynamic transmission model to evaluate the effectiveness of infection control strategies. Open Forum Infect Dis 2016; 195:ofw247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thomas A, Khader K, Redd A, et al. Extended models for nosocomial infection: parameter estimation and model selection. Math Med Biol 2017; 00:1–21. Available at: https://watermark.silverchair.com/dqx010.pdf?token=AQECAHi208BE49Ooan9kkhW_Ercy7Dm3ZL_9Cf3qfKAc485ysgAAAhswggIXBgkqhkiG9w0BBwagggIIMIICBAIBADCCAf0GCSqGSIb3DQEHATAeBglghkgBZQMEAS4wEQQMtNBCLf51StolodvzAgEQgIIBzmlPUP6jhu3Xx2AtCOfDcF5utTwXnpSSwhhC0bgypKhR95RI. Accessed 7 January 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kass RE, Gilks WR, Richardson S, Spiegelhalter DJ. Markov chain Monte Carlo in practice. J Am Stat Assoc 1997; 92:1645. [Google Scholar]
- 20. Haverkate MR, Bootsma MCJ, Weiner S, et al. Modeling spread of KPC-producing bacteria in long-term acute care hospitals in the Chicago region, USA. Infect Control Hosp Epidemiol 2015; 36:1148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Forrester M, Pettitt AN. Use of stochastic epidemic modeling to quantify transmission rates of colonization with methicillin-resistant Staphylococcus aureus in an intensive care unit. Infect Control Hosp Epidemiol 2005; 26:598–606. [DOI] [PubMed] [Google Scholar]
- 22. Pettitt AN, Forrester ML, Gibson GJ. Bayesian inference of hospital-acquired infectious diseases and control measures given imperfect surveillance data. Biostatistics 2007; 8:383–401. [DOI] [PubMed] [Google Scholar]
- 23. Cooper BS, Medley GF, Bradley SJ, Scott GM. An augmented data method for the analysis of nosocomial infection data. Am J Epidemiol 2008; 168:548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Geman S, Geman D. Stochastic relaxation, Gibbs distributions, and the Bayesian restoration of images. IEEE Trans Pattern Anal Mach Intell 1984; 6:721–41. [DOI] [PubMed] [Google Scholar]
- 25. Metropolis N, Rosenbluth AW, Rosenbluth MN, Teller AH, Teller E. Equation of state calculations by fast computing machines. J Chem Phys 1953; 21:1087–91. [Google Scholar]
- 26. Lumley T. rmeta: meta-analysis.2018. Available at: https://cran.r-project.org/package=rmeta. Accessed 14 May 2020.
- 27. Viechtbauer W. Conducting meta-analyses in {R} with the {metafor} package. J Stat Softw 2010; 36:1–48. [Google Scholar]
- 28. R Core Team. R: a language and environment for statistical computing.2019. Available at: https://www.r-project.org/. Accessed 20 May 2020.
- 29. Siegel JD, Rhinehart E, Jackson M, Chiarello L; Health Care Infection Control Practices Advisory Committee 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control 2007; 35:S65–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Robicsek A, Beaumont JL, Paule SM, et al. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Intern Med 2008; Available at: https://www.acpjournals.org/doi/10.7326/0003-4819-148-6-200803180-00003. Accessed 14 May 2020. [DOI] [PubMed] [Google Scholar]
- 31. Peterson LR, Wright MO, Beaumont JL, et al. Nonimpact of decolonization as an adjunctive measure to contact precautions for the control of methicillin-resistant Staphylococcus aureus transmission in acute care. Antimicrob Agents Chemother 2016; 60:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Malani PN. Preventing infections in the ICU: one size does not fit all. JAMA 2013; 310:1567–8. [DOI] [PubMed] [Google Scholar]
- 33. Morgan DJ, Wenzel RP, Bearman G. Contact precautions for multidrug-resistant organisms-reply. JAMA 2017; 318:2258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.