Abstract
Accumulated studies have strongly implicated aberrantly expressed microRNAs (miRNAs) in carcinogenesis and cancer progression of various cancers, including colorectal cancer (CRC). Hence, a comprehensive study of miRNAs and their association with CRC may be a promising therapeutic method for patients with this malignancy. MicroRNA-744 (miR-744) is abnormally expressed in several types of human cancer. Thus far, little is known about the expression, biological roles, and exact mechanisms of miR-744 in CRC. Thus, the present study measured the expression level of miR-744 and investigated its roles and associated molecular mechanisms in CRC. This study demonstrated that miR-744 expression was significantly underexpressed in CRC tissues and cell lines. Low miR-744 expression was positively associated with lymphatic metastasis and TNM stage. Functional experiments revealed that miR-744 overexpression obviously inhibited the proliferation and invasion of CRC cells. Furthermore, Notch1 was identified as a direct target of miR-744 in CRC. Moreover, the inhibition of Notch1 phenocopied the inhibitory effects of miR-744 overexpression on CRC cells. Restored Notch1 expression markedly rescued the tumor-suppressive effects of miR-744 overexpression on CRC cells. Overall, miR-744 exhibits an essential role in CRC progression, and the miR-744/Notch1 axis may provide novel insights into future treatments for patients with CRC.
Key words: Colorectal cancer (CRC), MicroRNA-744 (miR-744), Notch1, Proliferation, Invasion
INTRODUCTION
Colorectal cancer (CRC) ranks as the third most common cancer and the leading cause of cancer-related deaths globally1. The incidence of CRC varies significantly in different regions worldwide due to differences in lifestyle, environment, and genetics2,3. Approximately 1.2 million new cases and 600,000 deaths from CRC are estimated each year around the world4. Currently, the main modalities for the treatment of patients with CRC are surgery resection, followed by radiotherapy and chemotherapy5. Despite tremendous achievements in diagnosis and therapeutic methods, the prognosis for patients with CRC remains poor6. This is mainly because about two thirds of patients with CRC exhibit local recurrence or distant metastasis after surgical resection7. The diagnosis at an advanced stage of the majority of patients with CRC is also responsible for the poor therapeutic outcome8. Therefore, the molecular and cellular mechanisms underlying CRC formation and progression should be analyzed in depth, and novel therapeutic strategies should be developed.
MicroRNAs (miRNAs) are a large group of endogenously expressed, noncoded, single-stranded RNA molecules that are 18–25 nucleotides long9. miRNAs have been validated as a novel class of gene regulators via directly binding to the 3′-untranslated regions (3′-UTRs) of their target genes in a sequence-specific way and degrading mRNA or inhibiting translation10. Until now, over 1,400 miRNAs have been identified, which accounts for 2%–5% of the entire human genome and regulates 30% of all human genes11. A number of studies indicated the expression alterations of miRNAs in nearly all types of human neoplasm12–14. miRNA dysregulation possibly plays important roles in tumor occurrence and development by regulating a wide variety of biological and cellular processes, including cell proliferation, cell cycle, apoptosis, differentiation, migration, invasion, metastasis, and angiogenesis15–17. miRNAs may serve tumor-suppressive or oncogenic roles depending on the biological characteristic of their target genes18,19. Therefore, cancer-related miRNAs should be systematically investigated to identify novel therapeutic targets for anticancer therapy.
miR-744 is abnormally expressed in several types of human cancer20–22. Thus far, little is known about the expression, biological roles, and exact mechanisms of miR-744 in CRC. Thus, the present study measured the expression level of miR-744 and investigated its roles and associated molecular mechanisms in CRC.
MATERIALS AND METHODS
Patients and Tissue Specimens
A total of 53 paired CRC tissues and adjacent nontumor tissues were obtained from patients who received surgical resection at Beijing Chao-Yang Hospital (Beijing, P.R. China) between August 2014 and May 2016. None of the patients had been treated with chemotherapy, radiotherapy, or other treatments prior to surgery. All tissues were immediately frozen in liquid nitrogen and stored at −80°C until use. This study was approved by the Ethics Committee of the Beijing Chao-Yang Hospital. In addition, written informed consent was obtained from all CRC patients who participated in this research.
Cell Lines and Culture Condition
A normal human colon epithelium cell line (FHC) was ordered from the American Type Culture Collection (Manassas, VA, USA). Five human CRC cell lines (LoVo, SW480, SW620, HCT116, and HT29) were all acquired from Shanghai Institute of Biochemistry and Cell Biology (Shanghai, P.R. China). All cell lines were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 mg/ml streptomycin (all from Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The cells were maintained at 37°C under a humidified incubator containing 5% CO2.
Total RNA Isolation and Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA of tissue samples and cells was prepared using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer’s instructions. To quantify miR-744 expression, the extracted total RNA was reverse transcribed to complementary DNA (cDNA) using a TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA) in accordance with the manufacturer’s protocol. A TaqMan MicroRNA PCR Kit (Applied Biosystems) was used to detect miR-744 expression with U6 snRNA as an internal control. For the detection of Notch1 mRNA, cDNA was achieved with a PrimeScript RT Reagent Kit (Takara Bio, Dalian, P.R. China). qPCR was performed with SYBR Premix Ex Taq™ Kit (Takara Bio). GAPDH was used as an endogenous control for Notch1 mRNA expression. The relative expression levels of each gene were quantified using the 2−ΔΔCt method23.
Cell Transfection
miR-744 mimic, corresponding negative control miRNA mimic (miR-NC), small interfering RNA (siRNA) against the expression of Notch1 (Notch1 siRNA), and negative control siRNA (NC siRNA) were all purchased from GenePharma (Shanghai, P.R. China). In order to induce overexpression of endogenous Notch1, Notch1 overexpression plasmid (pcDNA3.1-Notch1) and empty plasmid pcDNA3.1 were synthesized and purified by the Chinese Academy of Sciences (Changchun, P.R. China). Cell transfection was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. The culture medium was discarded and replaced with fresh DMEM supplemented with 10% FBS at 6 h posttransfection.
Cell Counting Kit-8 (CCK-8) Assay
Cell proliferation was determined using CCK-8 assay. Transfected cells were collected and plated into 96-well plates with a density of 3,000 cells per well in 150 μl of culture medium. Following incubation for 0, 24, 48, and 72 h, CCK-8 assay was conducted. A total of 10 μl of CCK-8 solution (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added into each well, and the cells were incubated at 37°C for an additional 2 h. The optical density (OD) value was detected at a wavelength of 450 nm using a microplate reader (Bio-Rad, Laboratories, Inc., Hercules, CA, USA). All experiments were performed in triplicate.
Matrigel Invasion Assay
Transfected cells were grown at 37°C with 5% CO2. At 48 h after transfection, cell invasion ability was evaluated with an 8-μm pore size Corning chambers (Corning Costar, Corning, NY, USA) coated with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). Transfected cells were collected and suspended in DMEM without FBS. A total of 5 × 104 cells were seeded into upper Transwell chambers. The lower chambers were loaded with 500 μl of DMEM containing 20% FBS to serve as a chemoattractant. Following incubation at 37°C for 24 h, the remaining noninvaded cells on the upper surface of Transwell chambers were gently removed by cotton swabs. The invaded cells were fixed with 100% methanol, stained with 0.5% crystal violet solution, photographed, and then counted under an IX71 inverted microscope (Olympus Corporation, Tokyo, Japan) in five randomly selected visual fields per chamber.
Bioinformatics Prediction and Luciferase Reporter Assay
TargetScan (www.targetscan.org/) and miRanda (www.microrna.org) were applied to predict the potential targets of miR-744. Notch1 was predicted as a potential target of miR-744 and was selected for experimental confirmation. The 3′-UTR of human Notch1 containing the wild type or mutant predicted that binding sequences of miR-744 was cloned by GenePharma, inserted into the p-MIR-reporter vector (Promega, Manheim, Germany), and named as p-MIR-WT-Notch1-3′-UTR and p-MIR-MUT-Notch1-3′-UTR, respectively. For luciferase reporter assay, cells were plated into 24-well plates and then cotransfected with miR-744 mimic or miR-NC and p-MIR-WT-Notch1-3′-UTR or p-MIR-MUT-Notch1-3′-UTR using Lipofectamine 2000 following the manufacturer’s instructions. Luciferase activity was determined at 48 h posttransfection using the Dual-Luciferase Reporter Assay System (Promega) in accordance with the manufacturer’s protocol. Renilla luciferase activity was normalized to firefly luciferase activity.
Western Blot Analysis
Tissue specimens or cells were lysed in a radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Haimen, P.R. China). Total protein concentration was assessed using a Bicinchoninic Acid Assay Kit (Beyotime Institute of Biotechnology). Equal amounts of proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subsequently transferred onto PVDF membranes (Millipore, Billerica, MA, USA). Subsequent to blocking with 5% nonfat milk in TBS containing 0.1% Tween 20 (TBST), the membranes were incubated overnight at 4°C with mouse anti-human monoclonal Notch1 antibody (1:1,000 dilution; sc-373944; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or mouse anti-human monoclonal β-actin antibody (1:1,000 dilution; sc-69879; Santa Cruz Biotechnology). After washing with TBST, the membranes were probed with a goat anti-mouse horseradish peroxidase-conjugated secondary antibody (1:5,000 dilution; sc-2005; Santa Cruz Biotechnology), and protein signals were visualized with an ECL detection kit (GE Healthcare Life Sciences, Chalfont, UK). β-Actin was used as a loading control.
Statistical Analysis
SPSS software ver. 17.0 (SPSS, Inc., Chicago, IL, USA) was utilized to perform all the statistical analyses. Data were expressed as mean ± standard deviation and compared with Student’s t-test, or one-way analysis of variance (ANOVA). Student–Newman–Keuls test was used as a post hoc test following ANOVA. A value of p < 0.05 was considered to indicate a statistically significant difference.
RESULTS
Downregulation of miR-744 in CRC and its Association With CRC Development
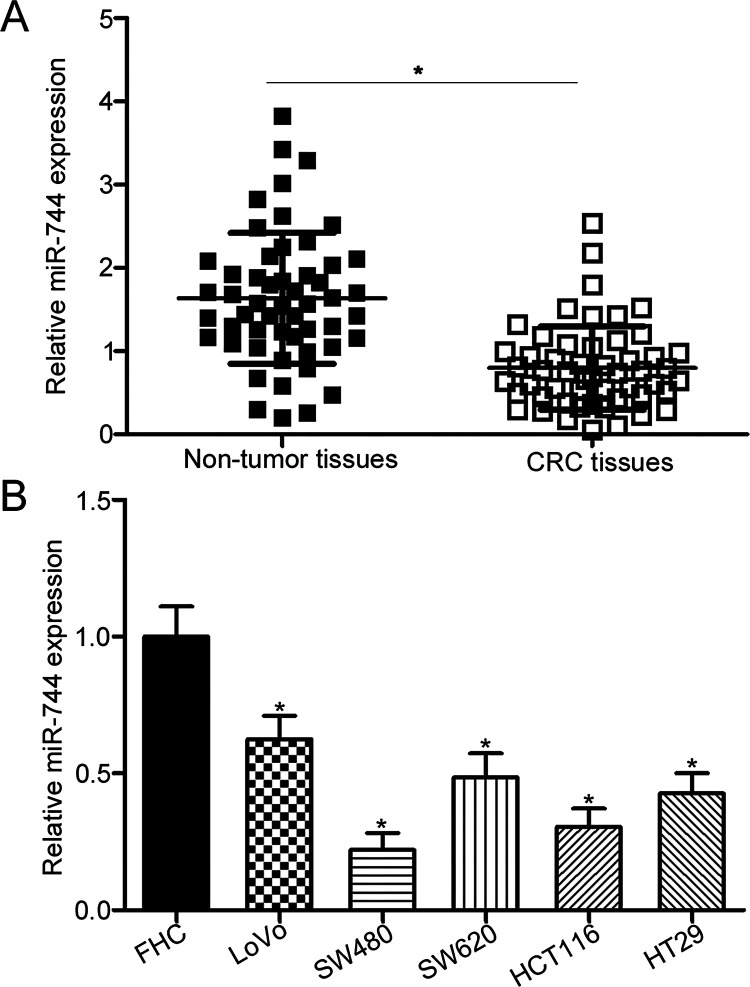
To elucidate the expression pattern of miR-744 in CRC, its expression was measured in 53 paired CRC tissues and adjacent nontumor tissues using RT-qPCR. Results showed that the expression levels of miR-744 were significantly lower in CRC tissues than in adjacent nontumor tissues (p < 0.05) (Fig. 1A). We divided all CRC patients into the miR-744 low-expression group (n = 27) and miR-744 high-expression group (n = 26) on the basis of the median expression level of miR-744 and then investigated the association between miR-744 and clinicopathological factors of CRC patients. The analysis indicated that decreased expression level of miR-744 was strongly correlated with lymphatic metastasis (p = 0.002) and TNM stage (p = 0.001). However, nonsignificant association was observed with other clinicopathological features, including gender (p = 0.696), age (p = 0.501), tumor differentiation (p = 0.685), and tumor size (p = 0.695). We measured miR-744 expression in five human CRC cell lines and a normal human colon epithelium cell line (FHC). As illustrated in Figure 1B, miR-744 expression was significantly downregulated in all CRC cell lines relative to that in FHC (p < 0.05). These results suggest that downregulation of miR-744 is correlated with CRC progression.
Figure 1.
Relative expression of microRNA-744 (miR-744) is downregulated in colorectal cancer (CRC) tissues and cell lines. (A) Total RNA was extracted from 53 paired CRC tissues and adjacent nontumor tissues, and subjected to reverse transcription quantitative polymerase chain reaction (RT-qPCR) analysis for the detection of miR-744 expression. *p < 0.05 versus nontumor tissues. (B) RT-qPCR analysis was performed to measure miR-744 expression in five human CRC cell lines (LoVo, SW480, SW620, HCT116, and HT29) and a normal human colon epithelium cell line (FHC). *p < 0.05 versus FHC.
miR-744 Suppresses CRC Cell Proliferation and Invasion In Vitro
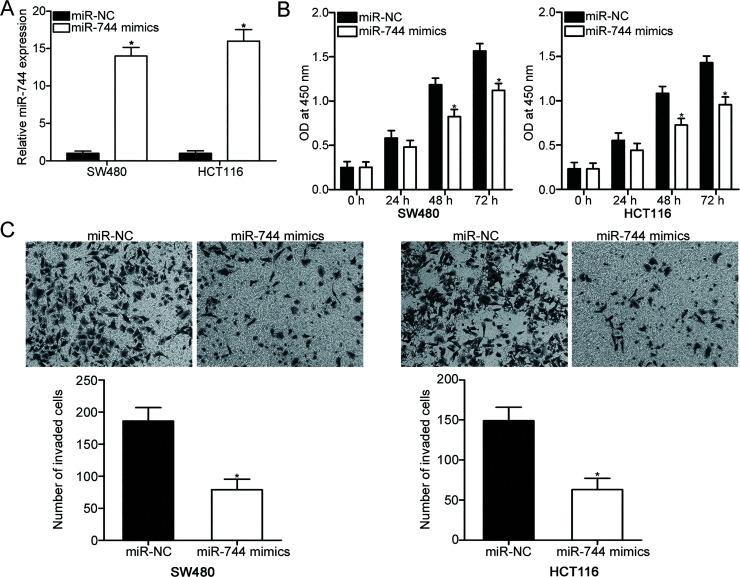
To determine whether miR-744 is involved in the regulation of malignant phenotype in CRC cells, SW480 and HCT116 cells with relatively low endogenous miR-744 levels were selected for subsequent experiments and transfected with miR-744 mimic or miR-NC. RT-qPCR was performed to evaluate the transfection efficiency and recognized that miR-744 expression was markedly overexpressed in the SW480 and HCT116 cells transfected with miR-744 mimics (p < 0.05) (Fig. 2A). CCK-8 assays were employed to investigate the effect of miR-744 overexpression on CRC cell proliferation. The results of the CCK-8 assays showed that upregulation of miR-744 inhibited the proliferation of SW480 and HCT116 cells (p < 0.05) (Fig. 2B). Furthermore, the effect of miR-744 overexpression on cell invasion capability was evaluated using Matrigel invasion assay. The results revealed that the resumption of expression of miR-744 reduced the invasion capabilities of SW480 and HCT116 cells compared with the cells transfected with miR-NC (p < 0.05) (Fig. 2C). These results suggest that miR-744 plays tumor-suppressive roles in CRC progression.
Figure 2.
Upregulation of miR-744 prohibits proliferation and invasion of SW480 and HCT116 cells. (A) SW480 and HCT116 cells were transfected with miR-744 mimic or miR-negative control (NC). At 48 h after transfection, total RNA was isolated, and RT-qPCR was performed to determine miR-744 expression. *p < 0.05 versus miR-NC. (B) Cell proliferation was evaluated using cell counting kit-8 (CCK-8) assay in SW480 and HCT116 cells transfected with miR-744 mimics or miR-NC. (C) Matrigel invasion assay was employed to determine invasion capacity in SW480 and HCT116 cells transfected with miR-744 mimics or miR-NC. *p < 0.05 versus miR-NC.
miR-744 Directly Targeted Notch1 by Interaction With the Binding Sites in the 3′-UTR
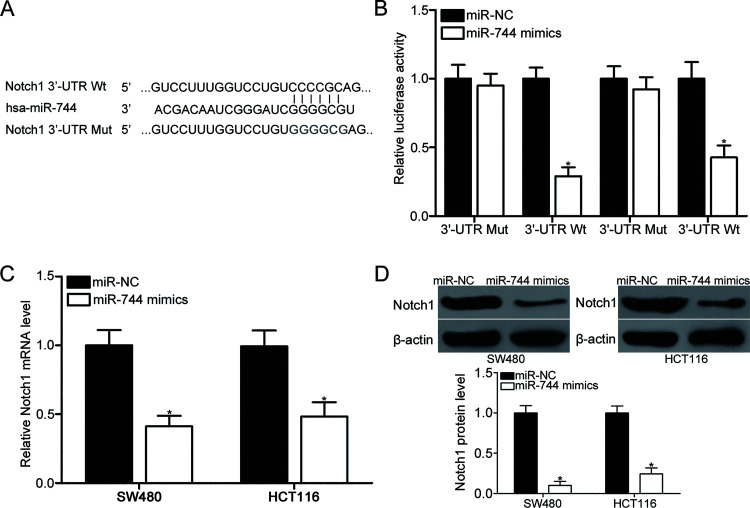
To elucidate the molecular mechanisms responsible for the inhibitory effects of miR-744 in CRC, bioinformatics prediction was conducted to predict the potential targets of miR-744. Notch1 (Fig. 3A) has attracted our attention because of its roles in CRC occurrence and development24–28. To validate the prediction, luciferase reporter assays were conducted in the SW480 and HCT116 cells cotransfected with miR-744 mimic or miR-NC and p-MIR-WT-Notch1-3′-UTR or p-MIR-MUT-Notch1-3′-UTR. As shown in Figure 3B, miR-744 overexpression obviously decreased the relative luciferase activity with WT Notch1 3′-UTR plasmid (p < 0.05) but did not affect the luciferase activity of the MUT Notch1 3′-UTR plasmid in SW480 and HCT116 cells. To determine whether miR-744 regulates Notch1 expression at the transcriptional and translational levels, Notch1 expression in the SW480 and HCT116 cells transfected with miR-744 mimics or miR-NC was measured using RT-qPCR and Western blot analysis. After exogenetically expressing miR-744 in SW480 and HCT116 cells, the expression of Notch1 was suppressed at the mRNA (p < 0.05) (Fig. 3C) and protein (p < 0.05) (Fig. 3D) levels. These results suggest that miR-744 directly targets Notch1 in CRC by interacting with the binding sites in its 3′-UTR.
Figure 3.
Notch1 is a direct target of miR-744 in CRC. (A) Predicted and mutated miR-744 binding sequences in the 3′-untranslated region (3′-UTR) of Notch1. (B) SW480 and HCT116 cells were cotransfected with miR-744 mimic or miR-NC and p-MIR-WT-Notch1-3′-UTR or p-MIR-MUT-Notch1-3′-UTR. At 48 h after transfection, cells were harvested and subjected to the analysis of luciferase activity using the Dual-Luciferase Reporter Assay System. *p < 0.05 versus miR-NC. miR-744 mimics or miR-NC was transfected into SW480 and HCT116 cells. (C) RT-qPCR and (D) Western blot analysis were applied to measure Notch1 mRNA and protein levels, respectively. *p < 0.05 versus miR-NC.
Silencing of Notch1 Inhibits Cell Proliferation and Invasion in CRC
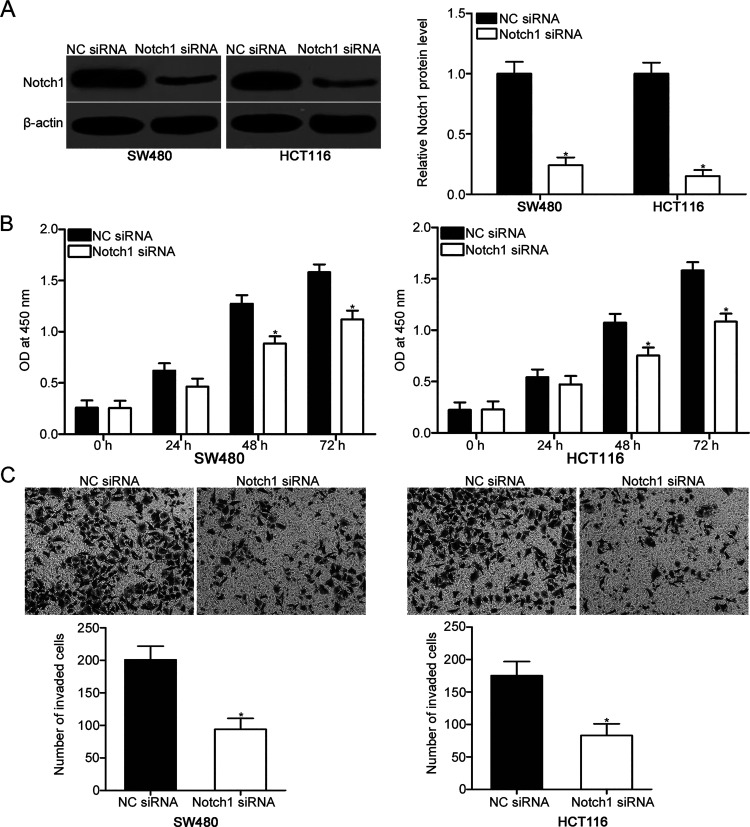
To investigate the biological roles of Notch1 in CRC, we knocked down Notch1 expression in SW480 and HCT116 cells. Western blot analysis revealed that Notch1 was significantly downregulated in the Notch1 siRNA-transfected SW480 and HCT116 cells compared with the NC siRNA group (p < 0.05) (Fig. 4A). Subsequent functional assays indicated that downregulation of Notch1 attenuated the proliferation (p < 0.05) (Fig. 4B) and invasion (p < 0.05) (Fig. 4C) of SW480 and HCT116 cells, which was similar to those mediated by miR-744 overexpression. These results further suggest that Notch1 is a direct target of miR-744 in CRC.
Figure 4.
Notch1 knockdown reduces the proliferation and invasion of SW480 and HCT116 cells. (A) Notch1 protein expression was measured in SW480 and HCT116 cells transfected with Notch1 small interfering RNA (siRNA) or NC siRNA using Western blot analysis. *p < 0.05 versus NC siRNA. (B) Cell proliferation activity was measured by CCK-8 assay in SW480 and HCT116 cells transfected with Notch1 siRNA or NC siRNA. *p < 0.05 versus NC siRNA. (C) Cell invasion capacity was analyzed with Matrigel invasion assay in SW480 and HCT116 cells transfected with Notch1 siRNA or NC siRNA. *p < 0.05 versus NC siRNA.
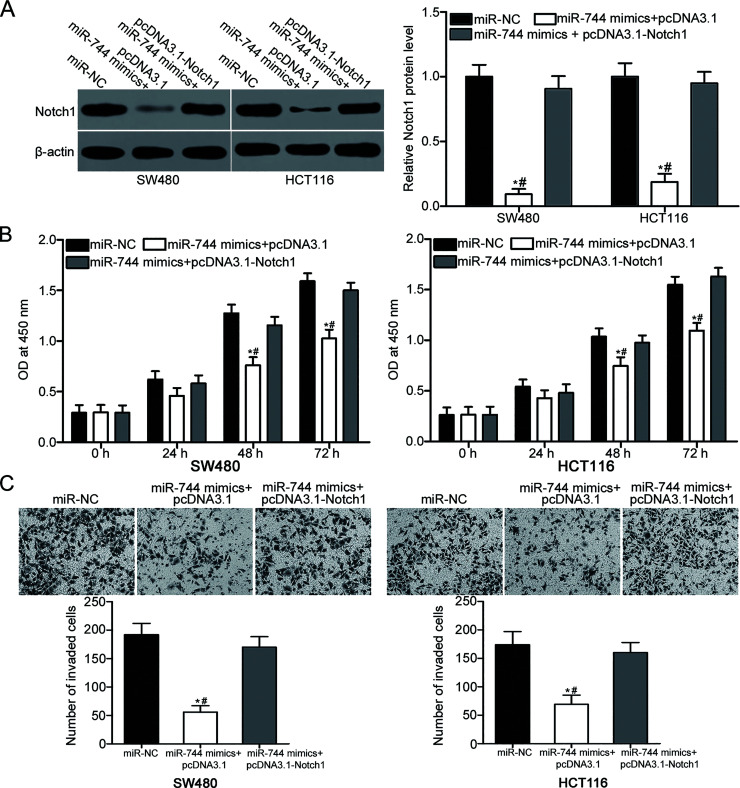
Restored Notch1 Expression Partially Rescues the Suppressive Effects of miR-744 in CRC Cells
To determine whether Notch1 could mediate the suppressive effects of miR-744 in CRC cells, rescue experiments were conducted. SW480 and HCT116 cells were cotransfected with miR-744 mimic and pcDNA3.1 or pcDNA3.1-Notch1 (without 3′-UTR). At 72 h after transfection, Western blot analysis revealed that downregulation of Notch1 in the SW480 and HCT116 cells transfected with miR-744 mimic was restored by cotransfection with pcDNA3.1-Notch1 (p < 0.05) (Fig. 5A). In addition, CCK-8 and Matrigel invasion assays demonstrated that the restoration expression of Notch1 partially reversed the inhibitory effects caused by miR-744 overexpression in SW480 and HCT116 cell proliferation (p < 0.05) (Fig. 5B) and invasion (p < 0.05) (Fig. 5C). Collectively, our present results suggest that miR-744 exerts its suppressive roles in CRC by targeting and inhibiting Notch1.
Figure 5.
Restoration of Notch1 expression reverses the suppressive effects of miR-744 mimics in CRC cells. miR-744 mimic or miR-NC was introduced into SW480 and HCT116 cells in combination with pcDNA3.1 or pcDNA3.1-Notch1. (A) Western blot analysis was conducted at 72 h posttransfection to determine Notch1 protein level. *p < 0.05 versus miR-NC. #p < 0.05 versus miR-744 mimic < pcDNA3.1-Notch1. (B) CCK-8 and (C) Matrigel invasion assays were used to detect the proliferation and invasion of the above-mentioned treated cells. *p < 0.05 versus miR-NC. #p < 0.05 versus miR-744 mimic < pcDNA3.1-Notch1.
DISCUSSION
Accumulated studies have strongly implicated aberrantly expressed miRNAs in carcinogenesis and cancer progression in various cancers, including CRC29–31. Hence, a comprehensive study of miRNAs and their association with CRC may be a promising therapeutic method for patients with this malignancy. The present study demonstrated that the expression level of miR-744 was decreased in CRC tissues and cell lines, and this dysregulation was markedly correlated with lymphatic metastasis and TNM stage. In addition, the upregulation of miR-744 reduced the proliferation and invasion of CRC cells. Furthermore, Notch1 was validated as a direct target gene of miR-744 in CRC. Moreover, the inhibition of Notch1 could mimic the inhibitory effects of miR-744 overexpression on CRC. Restoration of Notch1 expression partially rescued the suppressive effects of miR-744 on CRC. Therefore, miR-744 may be highlighted as a novel therapeutic target for the treatment of patients with CRC.
miR-744 is frequently dysregulated in various cancers. For example, miR-744 is overexpressed in prostate cancer tissues and cell lines. Prostate cancer patients with high miR-744 levels have shorter survival period than those with low miR-744 levels20. In laryngeal squamous cell carcinoma, the expression level of miR-744 is upregulated and positively correlated with regional lymph node metastasis21. miR-744 is also increased in the serum of nasopharyngeal carcinoma. A high level of plasma miR-744 is obviously correlated with the N stage, clinical stage, and grade of patients with nasopharyngeal carcinoma. In addition, nasopharyngeal carcinoma patients with low miR-744 level have higher 5-year overall and relapse-free survival rates. The expression level of miR-744 in serum is an independent factor for predicting the prognosis of nasopharyngeal carcinoma22. In pancreatic cancer, miR-744 expression is high in tissues, cell lines, and plasma. Upregulation of miR-744 in tissue specimens is correlated with clinical stage, and its expression level in plasma is associated with lymph node metastasis and recurrence. miR-744 level is an independent poor prognostic factor for patients with pancreatic cancer32,33. However, miR-744 expression is underexpressed in cervical cancer34 and hepatocellular carcinoma35. Hepatocellular carcinoma patients with low miR-744 level exhibit lower recurrence-free survival and overall survival rates than those with high miR-744 levels. These conflicting findings suggest that the expression pattern of miR-744 has tissue specificity and may be a potential biomarker for their prognosis.
Dysregulation of miR-744 is closely correlated with tumor onset and development. For instance, the upregulation of miR-744 promotes prostate cancer cell growth and metastasis in vitro and increases tumor growth in vivo20. Li et al. reported that miR-744 inhibition prohibits in vitro cell migration, invasion, and in vivo lung metastasis in laryngeal squamous cell carcinoma21. Zhou et al. found that miR-744 downregulation suppresses the stemness of the cancer stem cell-like phenotype and decreases the tumorigenicity of pancreatic cancer cells in vivo33. Fang et al. revealed that miR-744 promotes nasopharyngeal carcinoma progression through regulation of cell growth and metastasis in vitro and in vivo36. However, miR-744 serves as a tumor suppressor in cervical cancer via inhibiting cell proliferation and inducing apoptosis34. Lin et al. revealed that resumption of miR-744 expression attenuates cell proliferation and elevates cell cycle arrest in hepatocellular carcinoma37. These findings suggest that miR-744 plays key roles in the carcinogenesis and progression of these specific cancer types and might be developed as an effective novel target for their treatment.
Numerous targets of miR-744 have been identified, including naked cuticle homolog 1 (NKD1)20 in prostate cancer, programmed cell death 4 (PDCD4)21 and phosphatize and tensin homolog (PTEN)21 in laryngeal squamous cell carcinoma, secreted frizzled-related protein 1 (SFRP1)33, glycogen synthase kinase 3β (GSK3β)33, transducin-like enhance of split 3 (TLE3)33 in pancreatic cancer, Rho GTPase-activating protein 5 (ARHGAP5)36 in nasopharyngeal carcinoma, B-cell lymphoma 2 (Bcl-2)34 in cervical cancer, and c-myc37 in hepatocellular carcinoma. In the current study, Notch1 was confirmed as a novel target of miR-744 in CRC. Notch1 is aberrantly and highly expressed in various types of human cancer, such as ovarian cancer38, gastric cancer39, thyroid cancer40, breast cancer41, and bladder cancer42. Notch1 is also upregulated in CRC and is negatively associated with lymph node metastasis, tumor stage, depth of infiltration, and histological differentiation24. CRC patients with higher Notch1 levels exhibit shorter survival time than those patients with low Notch1 levels. Multivariate analysis identified Notch1 level as an independent predictor of prognosis in CRC25. Notch1 serves important roles in CRC by modulating different cancer-related biological processes26–28. Hence, targeting Notch1 may be a novel promising therapeutic strategy in treating patients with CRC.
In conclusion, miR-744 was lowly expressed in CRC tissues and cell lines. The low expression of miR-744 was obviously correlated with the lymphatic metastasis and TNM stage of patients with CRC. miR-744 plays tumor-suppressive roles in CRC partly by directly targeting Notch1. These results suggest that the miR-744/Notch1 pathway is a potential therapeutic target for the treatment of patients with CRC.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Andrews L. Dietary flavonoids for the prevention of colorectal cancer. Clin J Oncol Nurs. 2013;17:671–2. [DOI] [PubMed] [Google Scholar]
- 3. Altobelli E, Lattanzi A, Paduano R, Varassi G, di Orio F. Colorectal cancer prevention in Europe: Burden of disease and status of screening programs. Prev Med. 2014;62:132–41. [DOI] [PubMed] [Google Scholar]
- 4. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2014;383:1490–502. [DOI] [PubMed] [Google Scholar]
- 5. Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haggar FA, Boushey RP. Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22:191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davies RJ, Miller R, Coleman N. Colorectal cancer screening: Prospects for molecular stool analysis. Nat Rev Cancer 2005;5:199–209. [DOI] [PubMed] [Google Scholar]
- 8. Dueland S, Hagness M, Line PD, Guren TK, Tveit KM, Foss A. Is liver transplantation an option in colorectal cancer patients with nonresectable liver metastases and progression on all lines of standard chemotherapy? Ann Surg Oncol. 2015;22:2195–200. [DOI] [PubMed] [Google Scholar]
- 9. Hutvagner G, Zamore PD. A microRNA in a multiple-turnover RNAi enzyme complex. Science 2002;297:2056–60. [DOI] [PubMed] [Google Scholar]
- 10. Towler BP, Jones CI, Newbury SF. Mechanisms of regulation of mature miRNAs. Biochem Soc Trans. 2015;43:1208–14. [DOI] [PubMed] [Google Scholar]
- 11. Forman JJ, Legesse-Miller A, Coller HA. A search for conserved sequences in coding regions reveals that the let-7 microRNA targets Dicer within its coding sequence. Proc Natl Acad Sci USA 2008;105:14879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun Y, Wang L, Guo SC, Wu XB, Xu XH. High-throughput sequencing to identify miRNA biomarkers in colorectal cancer patients. Oncol Lett. 2014;8:711–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iorio MV, Croce CM. MicroRNAs in cancer: Small molecules with a huge impact. J Clin Oncol. 2009;27:5848–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004;116:281–97. [DOI] [PubMed] [Google Scholar]
- 15. Ambros V. The functions of animal microRNAs. Nature 2004;431:350–5. [DOI] [PubMed] [Google Scholar]
- 16. Ye J, Wang Z, Zhao J, Chen W, Wu D, Wu P, Huang J. MicroRNA-141 inhibits tumor growth and minimizes therapy resistance in colorectal cancer. Mol Med Rep. 2017;15:1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li B, Xie Z, Li Z, Chen S, Li B. MicroRNA-613 targets FMNL2 and suppresses progression of colorectal cancer. Am J Transl Res. 2016;8:5475–84. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Yang T, Thakur A, Chen T, Yang L, Lei G, Liang Y, Zhang S, Ren H, Chen M. MicroRNA-15a induces cell apoptosis and inhibits metastasis by targeting BCL2L2 in non-small cell lung cancer. Tumour Biol. 2015;36:4357–65. [DOI] [PubMed] [Google Scholar]
- 19. Wu G, Liu J, Wu Z, Wu X, Yao X. MicroRNA-184 inhibits cell proliferation and metastasis in human colorectal cancer by directly targeting IGF-1R. Oncol Lett. 2017;14:3215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guan H, Liu C, Fang F, Huang Y, Tao T, Ling Z, You Z, Han X, Chen S, Xu B, Chen M. MicroRNA-744 promotes prostate cancer progression through aberrantly activating Wnt/beta-catenin signaling. Oncotarget 2017;8:14693–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li JZ, Gao W, Lei WB, Zhao J, Chan JY, Wei WI, Ho WK, Wong TS. MicroRNA 744-3p promotes MMP-9-mediated metastasis by simultaneously suppressing PDCD4 and PTEN in laryngeal squamous cell carcinoma. Oncotarget 2016;7:58218–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu Q, Zhang F, Du Z, Xiang Y. Up-regulation of serum miR-744 predicts poor prognosis in patients with nasopharyngeal carcinoma. Int J Clin Exp Med. 2015;8:13296–302. [PMC free article] [PubMed] [Google Scholar]
- 23. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 24. Li G, Zhou Z, Zhou H, Zhao L, Chen D, Chen H, Zou H, Qi Y, Jia W, Pang L. The expression profile and clinicopathological significance of Notch1 in patients with colorectal cancer: A meta-analysis. Future Oncol. 2017;13:2103–18. [DOI] [PubMed] [Google Scholar]
- 25. Chu D, Zhou Y, Zhang Z, Li Y, Li J, Zheng J, Zhang H, Zhao Q, Wang W, Wang R, Ji G. Notch1 expression, which is related to p65 status, is an independent predictor of prognosis in colorectal cancer. Clin Cancer Res. 2011;17:5686–94. [DOI] [PubMed] [Google Scholar]
- 26. Zhang H, Jiang H, Chen L, Liu J, Hu X, Zhang H. Inhibition of Notch1/Hes1 signaling pathway improves radiosensitivity of colorectal cancer cells. Eur J Pharmacol. 2017;818:364–70. [DOI] [PubMed] [Google Scholar]
- 27. Song M, Yin Y, Zhang J, Zhang B, Bian Z, Quan C, Zhou L, Hu Y, Wang Q, Ni S, Fei B, Wang W, Du X, Hua D, Huang Z. MiR-139-5p inhibits migration and invasion of colorectal cancer by downregulating AMFR and NOTCH1. Protein Cell 2014;5:851–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fender AW, Nutter JM, Fitzgerald TL, Bertrand FE, Sigounas G. Notch-1 promotes stemness and epithelial to mesenchymal transition in colorectal cancer. J Cell Biochem. 2015;116:2517–27. [DOI] [PubMed] [Google Scholar]
- 29. Castro D, Moreira M, Gouveia AM, Pozza DH, De Mello RA. MicroRNAs in lung cancer. Oncotarget 2017;8:81679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wald P, Liu XS, Pettit C, Dillhoff M, Manilchuk A, Schmidt C, Wuthrick E, Chen W, Williams TM. Prognostic value of microRNA expression levels in pancreatic adenocarcinoma: A review of the literature. Oncotarget 2017;8:73345–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yi R, Li Y, Wang FL, Miao G, Qi RM, Zhao YY. MicroRNAs as diagnostic and prognostic biomarkers in colorectal cancer. World J Gastrointest Oncol. 2016;8:330–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miyamae M, Komatsu S, Ichikawa D, Kawaguchi T, Hirajima S, Okajima W, Ohashi T, Imamura T, Konishi H, Shiozaki A, Morimura R, Ikoma H, Ochiai T, Okamoto K, Taniguchi H, Otsuji E. Plasma microRNA profiles: Identification of miR-744 as a novel diagnostic and prognostic biomarker in pancreatic cancer. Br J Cancer 2015;113:1467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou W, Li Y, Gou S, Xiong J, Wu H, Wang C, Yan H, Liu T. MiR-744 increases tumorigenicity of pancreatic cancer by activating Wnt/beta-catenin pathway. Oncotarget 2015;6:37557–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen XF, Liu Y. MicroRNA-744 inhibited cervical cancer growth and progression through apoptosis induction by regulating Bcl-2. Biomed Pharmacother. 2016;81:379–87. [DOI] [PubMed] [Google Scholar]
- 35. Tan YL, Bai ZG, Zou WL, Ma XM, Wang TT, Guo W, Liu J, Li JS, Jie Y, Zang YJ, Zhang ZT. miR-744 is a potential prognostic marker in patients with hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2015;39:359–65. [DOI] [PubMed] [Google Scholar]
- 36. Fang Y, Zhu X, Wang J, Li N, Li D, Sakib N, Sha Z, Song W. MiR-744 functions as a proto-oncogene in nasopharyngeal carcinoma progression and metastasis via transcriptional control of ARHGAP5. Oncotarget 2015;6:13164–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lin F, Ding R, Zheng S, Xing D, Hong W, Zhou Z, Shen J. Decrease expression of microRNA-744 promotes cell proliferation by targeting c-Myc in human hepatocellular carcinoma. Cancer Cell Int. 2014;14:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu L, Jiao YJ, Zhou L, Song WQ, Wu SW, Wang DN. [Expressions of OCT4, Notch1 and DLL4 and their clinical implications in epithelial ovarian cancer]. Nan Fang Yi Ke Da Xue Xue Bao 2016;37:444–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang H, Wang X, Xu J, Sun Y. Notch1 activation is a poor prognostic factor in patients with gastric cancer. Br J Cancer 2014;110:2283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Park HS, Jung CK, Lee SH, Chae BJ, Lim DJ, Park WC, Song BJ, Kim JS, Jung SS, Bae JS. Notch1 receptor as a marker of lymph node metastases in papillary thyroid cancer. Cancer Sci. 2012;103:305–9. [DOI] [PubMed] [Google Scholar]
- 41. Zhong Y, Shen S, Zhou Y, Mao F, Lin Y, Guan J, Xu Y, Zhang S, Liu X, Sun Q. NOTCH1 is a poor prognostic factor for breast cancer and is associated with breast cancer stem cells. Onco Targets Ther. 2016;9:6865–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sima J, Zhu MY, Ai Q, Zhang C, Yao ZY, Huang QB, Zhang X. [Expression analysis of NOTCH1/HES1/PTEN signaling pathway in invasive bladder transitional cell carcinoma]. Zhonghua Yi Xue Za Zhi. 2012;92:964–7. [PubMed] [Google Scholar]