Abstract
Glioblastoma multiforme (GBM), a malignant tumor of the central nervous system, has a high mortality rate. No curative treatment is presently available, and the most commonly used chemotherapeutic drug, the alkylating agent temozolomide (TMZ), is only able to increase life expectancy and is often associated with drug resistance. Therefore, an urgent need does exist for novel drugs aimed at treating gliomas. In the present study, we obtained three major results using corilagin: (a) demonstrated that it inhibits the growth of U251 glioma cells through activation of the apoptotic pathway; (b) demonstrated that it is also active on TMZ-resistant T98G glioma cells; and (c) demonstrated that when used in combination with TMZ on T98G glioma cells, a higher level of proapototic and antiproliferative effects is observed. Our study indicates that corilagin should be investigated in more detail to determine whether it can be developed as a potential therapeutic agent. In addition, our results suggest that corilagin could be used in combination with low doses of other standard anticancer chemotherapeutic drugs against gliomas (such as TMZ) with the aim of obtaining enhanced anticancer effects.
Key words: Glioma, Temozolomide (TMZ), Corilagin (CORL), Apoptosis
INTRODUCTION
Glioblastoma multiforme (GBM) is a lethal malignant tumor accounting for 42% of central nervous system tumors, with median survival of 15 months1. At present, no curative treatment is available, and the most frequently used drug, the alkylating agent temozolomide (TMZ), is not satisfactory, being only able to cause an increase in life expectancy for the treated patients2. Therefore, new drugs are urgently needed for validation and possible use in therapeutic protocols for antiglioma treatments3. Moreover, a high proportion of gliomas become resistant to TMZ over time4, making the search for new drugs or drugs to be used in combination on TMZ-resistant glioma cells very significant, with several reports being published recently. For example, Lan et al. recently demonstrated that sulforaphane reverses chemoresistance to TMZ5, while others have shown that combined treatments with lobarstin6, bortezomib7, quercetin8, methoxyamine9, and resveratrol10 sensitize resistant glioblastoma cell lines to TMZ. In addition, nucleic acid-based strategies such as AKT3 and PI3KCA siRNAs11 were developed to enhance the antitumor effects of TMZ.
One of the most interesting low-molecular weight drugs recently developed is corilagin [CORL, beta-1-O-galloyl-3,6-(R)-hexahydroxydiphenoyl-d-glucose]12–14, a gallotannin found in several extracts from medicinal plants (such as Phyllanthus urinaria), which was shown to have versatile medicinal activities, including hepatoprotective effects on male Sprague–Dawley rats induced with galactosamine and lipopolysaccharide15. Recently, it was also reported that corilagin inhibits radiation-induced microglia activation through suppression of the nuclear factor κB (NF-κB) pathway, suggesting that this compound is a potential agent for the treatment of radiation-induced brain injury16. These putative effects on NF-κB are relevant in the context of the search for novel compounds in glioma therapy17–19. In fact, NF-κB inhibitors are expected to exert potent apoptosis-inducing effects, which are the basis of the antitumor effects of antiglioma agents, including TMZ20–23.
The aims of the present study were to determine the activity of CORL on cell growth and apoptosis of the human U251 and T98G glioma cell lines, in order to verify whether CORL exerts cell growth inhibitory and proapoptotic activities. Furthermore, we aimed to determine whether CORL and TMZ can act synergistically and induce higher levels of apoptosis when administered in combination to glioma cell lines, with the objective to verify the possibility of a combined treatment protocol for gliomas.
MATERIALS AND METHODS
Glioma Cell Lines and Culture Conditions
U251 and T98G glioma cell lines24,25 were cultured in a humidified atmosphere of 5% CO2/air in RPMI-1640 medium (Life Technologies, Monza, Italy) supplemented with 10% fetal bovine serum (FBS; Celbio, Milan, Italy), 100 U/ml penicillin, and 100 mg/ml streptomycin. To determine the effect on proliferation, cell growth was monitored by determining the cell number/ml using a Z2 Beckman Coulter Counter (Beckman Coulter, Pasadena, CA, USA)26.
Computational Studies
All computational studies were carried out on a 4 CPU (Intel Core2 Quad CPU Q9550, 2.83 GHz) ACPI x64 Linux workstation with the Ubuntu 12.04 operating system. The 3D structure of NF-κB was obtained from the Protein Data Bank (PDB code: 1NFK), and the structure of CORL was obtained with MarvinSketch 5.5 software (Marvin, version 5.5.0.1, Program B; ChemAxon: Budapest, Hungary; www.chemaxon.com/products). The lowest energy conformation and the degree of protonation (pH 7.2) were determined with the OpenBabel 2.2.3 software (DOI: 10.1186/1758-2946-3-33), using the MMFF94s force field. The docking simulation was performed with AutoDock 4.227–31. The grid box for AutoGrid was centered at x = −11.11, y = 18.23, z = 15.33 (Zhang et al.31) with 0.375 grid spacing and 40 × 40 ×40 point dimensions. The Lamarkian Genetic Algorithm (LGA) was used as a conformational search engine, and 10 runs with a maximum of 2,500,000 energy evaluations were carried out. The lowest energy conformation was retained and analyzed. The images were rendered with Pymol (The PyMOL Molecular Graphics System, Version 1.2r3pre, Schrödinger, LLC)28.
Analysis of Apoptosis
Annexin V and Dead Cell assay on U251 and T98G cells, untreated and treated for 48 h with TMZ (400 μM) and CORL (35 μM), was performed with the “Muse” (Millipore Corporation, Billerica, MA, USA) method, following the manufacturer’s instructions. This procedure uses annexin V to detect phosphatidylserine (PS) on the external membrane of apoptotic cells. A dead cell marker is also used as an indicator of cell membrane structural integrity. Briefly, cells were washed with sterile 1× phosphate-buffered saline (PBS), trypsinized, resuspended, and diluted (1:2) with the one-step addition of the Muse Annexin V & Dead Cell reagent. After a 20-min incubation at room temperature in the dark, samples were analyzed. Data from samples were acquired utilizing the Annexin V and Dead Cell Software Module (Millipore)26,29. The same culture conditions were followed for the caspase 3/7 assay. This analysis was performed after 48 h of treatment using the two-step Muse Caspase 3/7 reagent. Briefly, 50 μl of cell suspension, corresponding to about 1 × 105 cells/ml, was centrifuged (1,200 rpm × 5 min) and resuspended in 25 μl of 1× assay buffer BA. The Muse Caspase 3/7 reagent was added to each sample and incubated for 30 min at 37°C in the dark. Muse caspase 7-AAD was subsequently added, and, after 5 min, the assay was run using the Muse Cell Analyzer. Four populations of cells could be distinguished in both assays: live, apoptotic, late apoptotic, and dead cells.
Scratch Wound Assay
T98G cells were seeded into a 24-well plate at a confluence of 80%; 24 h later, a vertical wound was created in the T98G cell monolayer using a 200-μl pipette tip. After washing three times with PBS to eliminate cellular debris, fresh medium was added back, and the T98G cells were either untreated or treated with 35 μM COR, 400 μM TMZ, and the two drugs together. Images were captured with a camera connected to a Nikon Eclipse TS100 microscope at designated times (0, 16, 24, and 48 h) to assess the rate of gap closure30.
Fluorometric TUNEL Assay
T98G apoptotic cells were detected and quantified using the DeadEnd Fluorometric TUNEL System (Promega, Madison, WI, USA) that measures the fragmented DNA of apoptotic cells by catalytically incorporating fluorescein-12-dUTP at 3′-OH DNA ends using terminal deoxynucleotidyl transferase (TdT), which forms a polymeric tail. T98G cells were cultured in poly-l-lysine-coated culture slides; about 1 × 104 cells were seeded into each slide chamber, and after 24 h they were treated with different compounds. Fluorometric TUNEL assay was performed after 48 h according to the manufacturer’s protocol. Briefly, T98G cells were fixed with formaldehyde and permeabilized, and, after an equilibration step, the TdT reaction mix (containing equilibration buffer, nucleotide mix, and TdT) was added to each sample, which was further incubated for 60 min, after which the cells were washed with PBS. The reaction was then stopped, the cells washed with PBS, and then stained with propidium iodide. Finally, the fluorescein-12-dUTP-labeled DNA that identifies apoptotic cells could be visualized by fluorescence microscopy using a Nikon Eclipse 80i microscope.
Statistics
All data are presented as mean ± SD. Statistical differences between groups were compared using one-way ANOVA (analyses of variance between groups). The p values were obtained using the paired t-test of the GraphPad Prism Software. Statistical differences were considered significant with a value of p < 0.05 and highly significant with a value of p < 0.01.
RESULTS
Computational Studies
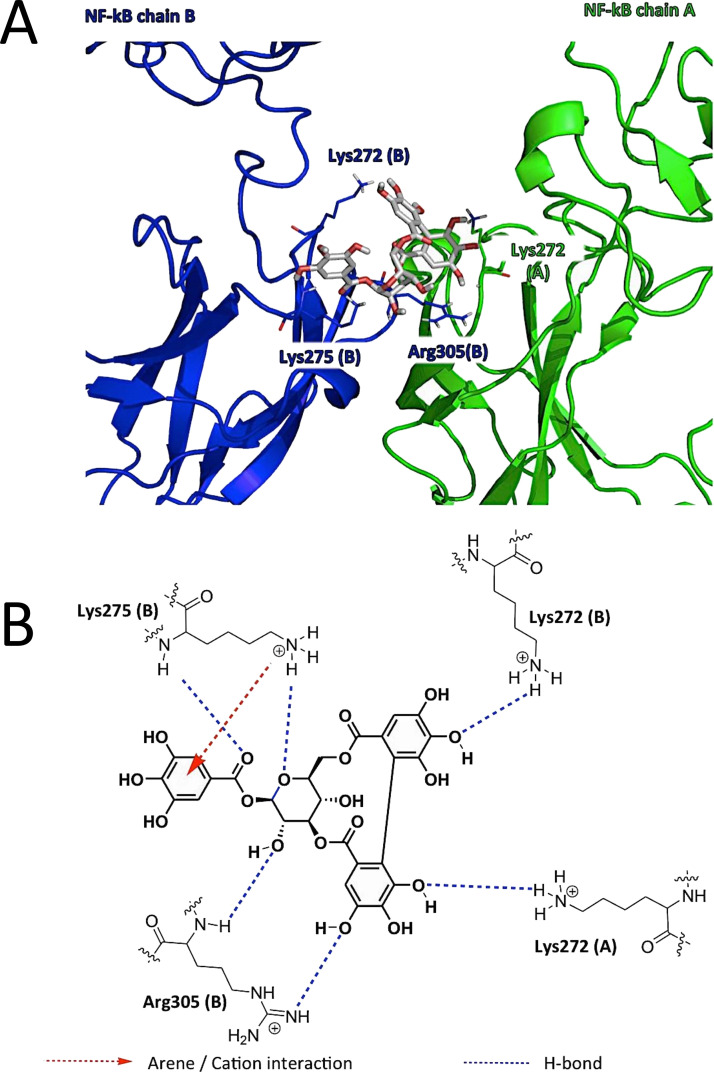
Following the hypothesis of a possible interaction with NF-κB, docking studies were performed to identify a possible binding mode for CORL (see the experimental section for a detailed description of the docking experiments). The CORL was proposed to establish H bonds and one arene–cation interactions with the positively charged amino acid residues (Lys and Arg), abundant in the DNA-binding region of NF-κB, thus impairing the interaction between the transcription factor and the nucleic acids (Fig. 1A; summarized in Fig. 1B). This possible mechanism of action of CORL is clearly different to that proposed for TMZ, which has been hypothesized to methylate the DNA31.
Figure 1.
(A) Proposed binding site of corilagin (CORL) in the DNA-binding region of NF-κB: detail of the amino acid residues involved in the proposed binding mode of CORL (atom colored stick) with NF-κB (green and blue). (B) Schematic representation of the interactions revealed by molecular docking simulation between CORL and NF-κB.
Corilagin Inhibits Cell Growth of Glioma U251 and the TMZ-Resistant T98G Cells
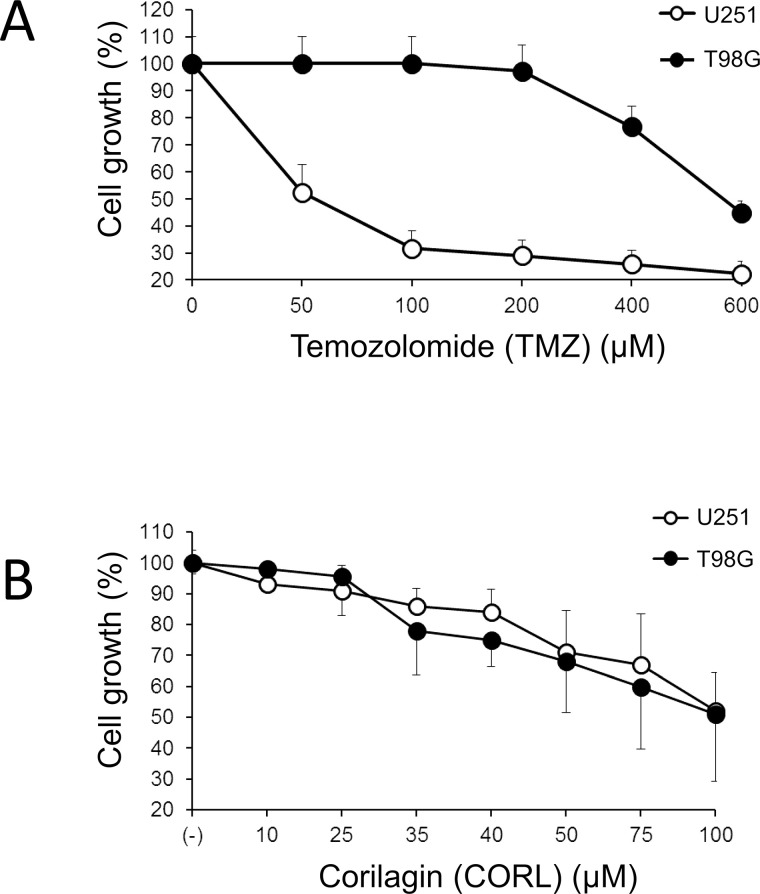
Cell lines U251 and T98G display a significantly different response to in vitro treatment with TMZ (Fig. 2A). The IC50 for U251 was 25–50 μM, while for T98G it was about 560 μM. On the contrary, both U251 and T98G exhibited a similar response to CORL treatment, with both cell lines having IC50 values close to 70–85 μM (Fig. 2B). The results of this experiment support the concept that CORL might be proposed as an antiproliferative agent also for drug-resistant glioma cell lines.
Figure 2.
Effects of treatment of U251 and T98G glioma cells with the indicated concentrations of (A) temozolomide (TMZ) and (B) corilagin (CORL). Cells were seeded at the concentration of 3.5 × 104 cells/ml and cultured for 48 h in the presence of TMZ or CORL, as indicated. Each experiment was performed three times and the percent mean ± SD of cell growth was calculated.
Corilagin Induces Apoptosis of Glioma U251 and T98G Cell Lines
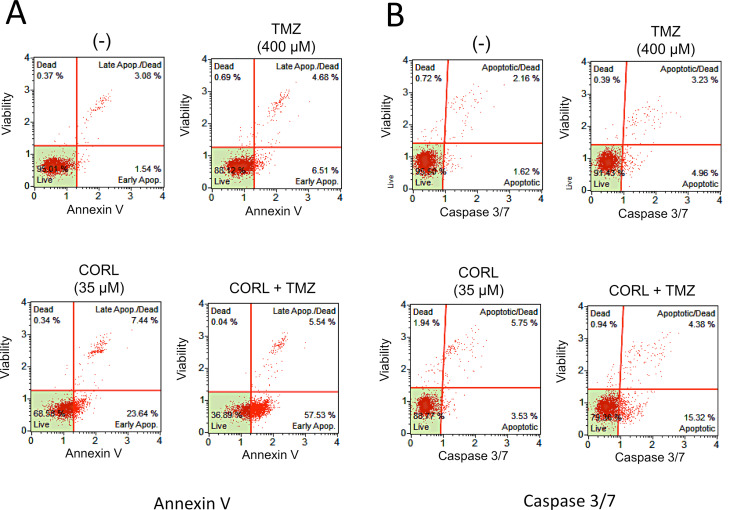
Taking into consideration the fact that a possible target of CORL is the antiapoptotic factor NF-κB, the effects of CORL on apoptosis were studied. The antiproliferative activity of CORL was associated with the activation of the apoptotic pathway in both the U251 and T98G cell lines, as judged by Annexin V (Fig. 3A and C) and Caspase 3/7 assays (Fig. 3B and C). Only a minor difference in the dose–response activity was apparent when CORL was used on U251 and T98G cells. In both cases, a high percentage of U251 and T98G cells were positive in the Annexin V and Caspase 3/7 assays when CORL was administered. This result supports the concept that the antiproliferative effects of CORL are associated with activation of the apoptotic pathway.
Figure 3.
Representative analysis of apoptosis of T98G glioma cells cultured for 48 h in the absence or in the presence of the indicated concentrations of CORL. Apoptosis was assessed by the Annexin V (A) or Caspase 3/7 (B) assays. (C) Increase in apoptotic cells in U251 and T98G glioma cell lines treated with the indicated concentrations of CORL. Annexin V (left) and Caspase 3/7 (right) assays were performed after 48 h of cell culture. The results represent the average ± SD of four independent experiments.
Effects of Combined Treatment of TMZ and CORL in the TMZ-Resistant T98G Glioma Cell Line
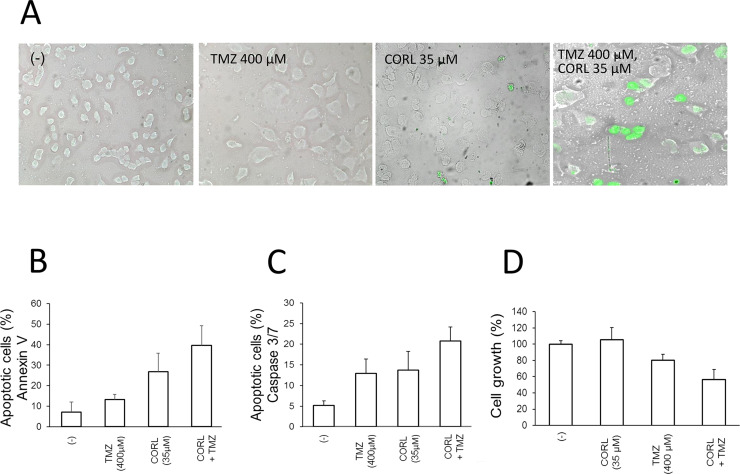
A representative experiment demonstrated that combined treatment of TMZ-resistant glioma T98G cells with both CORL and TMZ leads to a significant early apoptotic effect, evaluated by the Annexin V (Fig. 4A) and Caspase 3/7 (Fig. 4B) assays. This effect is synergistic and higher than that obtained by the single administrations of either TMZ or CORL. The summary of three independent experiments is shown in Figure 5. Increased apoptosis in cells treated with both TMZ and CORL (Fig. 5B and C) is associated with a decrease in cell growth rate (Fig. 5D). The significant effects on apoptosis of the combined treatment with TMZ + CORL were also confirmed by the TUNEL assay shown in Figure 5A.
Figure 4.
Representative analysis of apoptosis of T98G glioma cells cultured for 48 h in the absence or in the presence of the indicated concentrations of TMZ and CORL. Apoptosis was assessed by the Annexin V (A) or Caspase 3/7 (B) assays.
Figure 5.
(A) Increase in apoptotic T98G glioma cells after treatment with CORL or TMZ or a combination of both TMZ + CORL. Effects of combined CORL + TMZ treatment of T98G cells on apoptosis (B, C) and cell proliferation (D). Apoptosis was analyzed with Annexin V (B) and Caspase 3/7 (C) assays performed after 48 h of cell culture. The effects on cell growth were determined after 48 h (D). The results show the mean ± SD of four independent experiments.
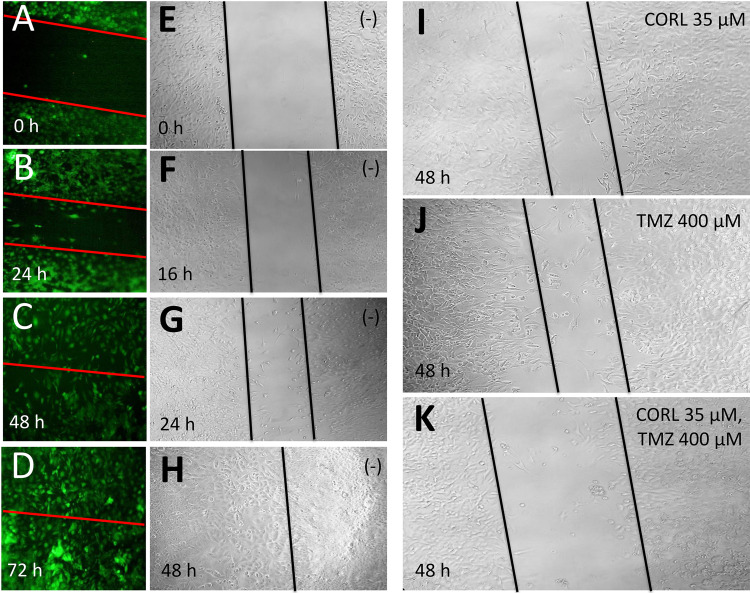
Figure 6 shows a strong inhibition of T98G cell growth when a scratch wound healing assay was performed. T98G cells were cultured in a 24-well plate in adherent monolayer. After 24 h, when T98G monolayer cells were confluent, a 200-μl pipette tip was used to scratch the cells in the plates, and the cells were then washed three times with PBS. The cells were then placed in fresh medium and divided as follows: the control (untreated T98G cells) (Fig. 6A–H), TMZ-treated T98G cells, CORL-treated T98G cells, and cells treated with both TMZ and CORL. Samples were taken at the beginning and after 48 h of cell culture with 5% CO2 at 37°C. Each treatment was compared with the control (untreated T98G cells). The experiments were repeated three times. The results obtained demonstrated that the scratch wounds were almost the same size in each experimental group at 0 h; however, the healing and cell migration rate was significantly reduced (p < 0.05) in the CORL + TMZ-treated cells after 48 h (Fig. 6K) compared with the control (Fig. 6H) and cells treated with singular administration of TMZ (Fig. 6J) and CORL (Fig. 6I).
Figure 6.
Effects of the treatments with CORL and TMZ (either added alone or in combination, as indicated in I–K) on T98G cell growth, determined by the scratch wound assay. (A–H) untreated cells (−).
DISCUSSION
The major conclusions of our study are the following: (a) CORL inhibits cell growth of U251 glioma cells through activation of the apoptotic pathway; (b) CORL is active also on TMZ-resistant T98G glioma cells; and (c) when T98G glioma cells are treated with both TMZ and CORL, a higher level of proapototic and antiproliferative effects was obtained. Our study highlights the important biological effects of CORL and indicates that it deserves further attention, particularly in the development of effective therapeutic protocols, as suggested for other low-molecular weight molecules demonstrated to be capable of increasing the effects of TMZ5–10.
The inhibitory activity of CORL on tumor cell growth has been the object of several investigations32–34. For instance, we have demonstrated the potential in vivo antitumor activity of CORL using the Hep3B hepatocellular carcinoma cell line and an athymic nude mice xenograft model32. CORL was administrated intraperitoneally for a continuous period of 7 days at a concentration of 15 mg/kg of body weight per day. A significant inhibition of tumor growth was observed when treated mice are compared with control groups32. In addition, we have recently reported the potential sensitization of Hep3B hepatoma cells to cisplatin and doxorubicin by CORL. Our results showed that CORL is able to enhance the cytotoxicity of both cisplatin and doxorubicin on Hep3B hepatoma cells33. Other interesting effects of CORL, both in vitro and in vivo, have been reported. For example, Jia et al. demonstrated that corilagin inhibits ovarian cancer cell growth by inducing an arrest at the G2/M cell cycle stage and enhancing apoptosis13. Interestingly, weaker effects were found on normal ovarian surface epithelial cells. Furthermore, Gu et al. reported a suppression of cholangiocarcinoma (CCA) progression in vivo after CORL administration30.
As far as mechanism of action is concerned, our results, combined with other reports, suggest that CORL induces the observed biological effects by interfering with the antiapoptotic NF-κB transcription factor14,35. Nevertheless, it is likely that other biochemical pathways may be operating, including the CORL-mediated block of the TGF-β signaling pathway, as suggested by Jia et al.13 The latter authors found that CORL inhibits TGF-β secretion by ovarian cancer cell lines and blocks the TGF-β-induced stabilization of Snail. In addition, an effect of CORL on the Notch signaling pathway was recently reported30, leading to inhibition of CCA development. In the studied experimental model, CORL significantly inhibited Notch1 and mTOR protein expression in vivo30.
Our present study should be considered as a proof of principle, supporting the possible use of CORL for the induction of high levels of apoptosis and inhibition of in vitro tumor cell growth in TMZ-resistant glioma cells.
Before proposing a possible therapeutic use of CORL in combination with low dosages of other anticancer chemotherapeutic standard drugs against gliomas (such as TMZ), further studies should be undertaken, based on the use of primary glioma cells, in vivo model systems mimicking gliomas, and in vivo pilot clinical trials on selected glioblastoma patients. These studies will allow other investigators in the field to determine the real relevance and impact on clinical settings of our data.
ACKNOWLEDGMENTS
This work is granted by CIB, by COFIN-2009, and by AIRC (IG 13575: Peptide nucleic acids targeting oncomiR and tumor-suppressor miRNAs: cancer diagnosis and therapy). We thank Dr. Susan Treves (Department of Biomedicine, University Hospital, Basel, CH) for checking the English style of the paper.
REFERENCES
- 1. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–20. [DOI] [PubMed] [Google Scholar]
- 2. Wei W, Chen X, Ma X, Wang D, Guo Z. The efficacy and safety of various dose-dense regimens of temozolomide for recurrent high-grade glioma: A systematic review with meta-analysis. J Neurooncol. 2015;125:339–49. [DOI] [PubMed] [Google Scholar]
- 3. Seystahl K, Wick W, Weller M. Therapeutic options in recurrent glioblastoma—An update. Crit Rev Oncol Hematol. 2016;99:389–408. [DOI] [PubMed] [Google Scholar]
- 4. Messaoudi K, Clavreul A, Lagarce F. Toward an effective strategy in glioblastoma treatment. Part I: Resistance mechanisms and strategies to overcome resistance of glioblastoma to temozolomide. Drug Discov Today 2015;20:899–905. [DOI] [PubMed] [Google Scholar]
- 5. Lan F, Yang Y, Han J, Wu Q, Yu H, Yue X. Sulforaphane reverses chemo-resistance to temozolomide in glioblastoma cells by NF-κB-dependent pathway downregulating MGMT expression. Int J Oncol. 2016;48:559–68. [DOI] [PubMed] [Google Scholar]
- 6. Kim S, Jo S, Lee H, Kim TU, Kim IC, Yim JH, Chung H. Lobarstin enhances chemosensitivity in human glioblastoma T98G cells. Anticancer Res. 2013;33:5445–51. [PubMed] [Google Scholar]
- 7. Vlachostergios PJ, Hatzidaki E, Befani CD, Liakos P, Papandreou CN. Bortezomib overcomes MGMT-related resistance of glioblastoma cell lines to temozolomide in a schedule-dependent manner. Invest New Drugs 2013;31:1169–81. [DOI] [PubMed] [Google Scholar]
- 8. Jakubowicz-Gil J, Langner E, Bądziul D, Wertel I, Rzeski W. Apoptosis induction in human glioblastoma multiforme T98G cells upon temozolomide and quercetin treatment. Tumour Biol. 2013;34:2367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Montaldi AP, Sakamoto-Hojo ET. Methoxyamine sensitizes the resistant glioblastoma T98G cell line to the alkylating agenttemozolomide. Clin Exp Med. 2013;13:279–88. [DOI] [PubMed] [Google Scholar]
- 10. Huang H, Lin H, Zhang X, Li J. Resveratrol reverses temozolomide resistance by downregulation of MGMT in T98G glioblastoma cells by the NF-κB-dependent pathway. Oncol Rep. 2012;27:2050–6. [DOI] [PubMed] [Google Scholar]
- 11. Paul-Samojedny M, Pudełko A, Kowalczyk M, Fila-Daniłow A, Suchanek-Raif R, Borkowska P, Kowalski J. Combination therapy with AKT3 and PI3KCA siRNA enhances the antitumor effect of temozolomide and carmustine in T98G glioblastoma multiforme cells. BioDrugs 2016;30:129–44. [DOI] [PubMed] [Google Scholar]
- 12. Dhanani T, Shah S, Kumar S. A validated high-performance liquid chromatography method for determination of tannin-related marker constituents gallic acid, corilagin, chebulagic acid, ellagic acid and chebulinic acid in four Terminalia species from India. J Chromatogr Sci. 2015;53:625–32. [DOI] [PubMed] [Google Scholar]
- 13. Jia L, Jin H, Zhou J, Chen L, Lu Y, Ming Y, Yu Y. A potential anti-tumor herbal medicine, Corilagin, inhibits ovarian cancer cell growth through blocking the TGF-β signaling pathways. BMC Complement Altern Med. 2013;13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gambari R, Borgatti M, Lampronti I, Fabbri E, Brognara E, Bianchi N, Piccagli L, Yuen MC, Kan CW, Hau DK, Fong WF, Wong WY, Wong RS, Chui CH. Corilagin is a potent inhibitor of NF-kappaB activity and downregulates TNF-alpha induced expression of IL-8 gene in cystic fibrosis IB3-1 cells. Int Immunopharmacol. 2012;13:308–15. [DOI] [PubMed] [Google Scholar]
- 15. Kinoshita S, Inoue Y, Nakama S, Ichiba T, Aniya Y. Antioxidant and hepatoprotective actions of medicinal herb, Terminalia catappa L. from Okinawa Island and its tannin corilagin. Phytomedicine 2007;14:755–62. [DOI] [PubMed] [Google Scholar]
- 16. Tong F, Zhang J, Liu L, Gao X, Cai Q, Wei C, Dong J, Hu Y, Wu G, Dong X. Corilagin attenuates radiation-induced brain injury in mice. Mol Neurobiol. 2016;53:6982–96. [DOI] [PubMed] [Google Scholar]
- 17. Dong XR, Luo M, Fan L, Zhang T, Liu L, Dong JH, Wu G. Corilagin inhibits the double strand break-triggered NF-kappaB pathway in irradiated microglial cells. Int J Mol Med. 2010;25:531–6. [PubMed] [Google Scholar]
- 18. Friedmann-Morvinski D, Narasimamurthy R, Xia Y, Myskiw C, Soda Y, Verma IM. Targeting NF-κB in glioblastoma: A therapeutic approach. Sci Adv. 2016;2:e1501292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gray GK, McFarland BC, Nozell SE, Benveniste EN. NF-κB and STAT3 in glioblastoma: Therapeutic targets coming of age. Expert Rev Neurother. 2014;14:1293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun P, Mu Y, Zhang S. A novel NF-κB/MMP-3 signal pathway involves in the aggressivity of glioma promoted by Bmi-1. Tumour Biol. 2014;35:12721–7. [DOI] [PubMed] [Google Scholar]
- 21. Su J, Liu F, Xia M, Xu Y, Li X, Kang J, Li Y, Sun L. p62 participates in the inhibition of NF-κB signaling and apoptosis induced by sulfasalazine in human glioma U251 cells. Oncol Rep. 2015;34:235–43. [DOI] [PubMed] [Google Scholar]
- 22. Sai K, Li WY, Chen YS, Wang J, Guan S, Yang QY, Guo CC, Mou YG, Li WP, Chen ZP. Triptolide synergistically enhances temozolomide-induced apoptosis and potentiates inhibition of NF-κB signaling in glioma initiating cells. Am J Chin Med. 2014;42:485–503. [DOI] [PubMed] [Google Scholar]
- 23. Li J, Tang H, Zhang Y, Tang C, Li B, Wang Y, Gao Z, Luo P, Yin A, Wang X, Cheng G, Fei Z. Saponin 1 induces apoptosis and suppresses NF-κB-mediated survival signaling in glioblastoma multiforme (GBM). PLoS One 2013;8:e81258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cao X, Gu Y, Jiang L, Wang Y, Liu F, Xu Y, Deng J, Nan Y, Zhang L, Ye J, Li Q. A new approach to screening cancer stem cells from the U251 human glioma cell line based on cell growth state. Oncol Rep. 2013;29:1013–8. [DOI] [PubMed] [Google Scholar]
- 25. Pen A, Durocher Y, Slinn J, Rukhlova M, Charlebois C, Stanimirovic DB, Moreno MJ. Insulin-like growth factor binding protein 7 exhibits tumor suppressive and vessel stabilization properties in U87MG and T98G glioblastoma cell lines. Cancer Biol Ther. 2011;12:634–46. [DOI] [PubMed] [Google Scholar]
- 26. Brognara E, Fabbri E, Montagner G, Gasparello J, Manicardi A, Corradini R, Bianchi N, Finotti A, Breveglieri G, Borgatti M, Lampronti I, Milani R, Dechecchi MC, Cabrini G, Gambari R. High levels of apoptosis are induced in human glioma cell lines by co-administration of peptide nucleic acids targeting miR-221 and miR-222. Int J Oncol. 2016;48:1029–38. [DOI] [PubMed] [Google Scholar]
- 27. Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marzaro G, Guiotto A, Borgatti M, Finotti A, Gambari R, Breveglieri G, Chilin A. Psoralen derivatives as inhibitors of NF-κB/DNA interaction: Synthesis, molecular modeling, 3D-QSAR, and biological evaluation. J Med Chem. 2013;56:1830–42. [DOI] [PubMed] [Google Scholar]
- 29. Brognara E, Fabbri E, Bazzoli E, Montagner G, Ghimenton C, Eccher A, Cantù C, Manicardi A, Bianchi N, Finotti A, Breveglieri G, Borgatti M, Corradini R, Bezzerri V, Cabrini G, Gambari R. Uptake by human glioma cell lines and biological effects of a peptide-nucleic acids targeting miR-221. J Neurooncol. 2014;118:19–28. [DOI] [PubMed] [Google Scholar]
- 30. Gu Y, Xiao L, Ming Y, Zheng Z, Li W. Corilagin suppresses cholangiocarcinoma progression through Notch signaling pathway in vitro and in vivo. Int J Oncol. 2016;48:1868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang J, Stevens MF, Bradshaw TD. Temozolomide: Mechanisms of action, repair and resistance. Curr Mol Pharmacol. 2012;5:102–14. [DOI] [PubMed] [Google Scholar]
- 32. Hau DK, Zhu GY, Leung AK, Wong RS, Cheng GY, Lai PB, Tong SW, Lau FY, Chan KW, Wong WY, Lam KH, Cheng CH, Cheung F, Chui CH, Gambari R, Fong DW. In vivo anti-tumour activity of corilagin on Hep3B hepatocellular carcinoma. Phytomedicine 2010;18:11–5. [DOI] [PubMed] [Google Scholar]
- 33. Gambari R, Hau DK, Wong WY, Chui CH. Sensitization of Hep3B hepatoma cells to cisplatin and doxorubicin by corilagin. Phytother Res. 2014;28:781–3. [DOI] [PubMed] [Google Scholar]
- 34. Yang WT, Li GH, Li ZY, Feng S, Liu XQ, Han GK, Zhang H, Qin XY, Zhang R, Nie QM, Jin F. Effect of corilagin on the proliferation and NF-κB in U251 glioblastoma cells and U251 glioblastoma stem-like cells. Evid Based Complement Alternat Med. 2016;2016:1418309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao L, Zhang SL, Tao JY, Pang R, Jin F, Guo YJ, Dong JH, Ye P, Zhao HY, Zheng GH. Preliminary exploration on anti-inflammatory mechanism of corilagin (beta-1-O-galloyl-3,6-(R)-hexahydroxydiphenoyl-D-glucose) in vitro. Int Immunopharmacol. 2008;8:1059–64. [DOI] [PubMed] [Google Scholar]