Abstract
Liver injury is often observed in various pathological conditions including posthepatectomy state and cancer chemotherapy. It occurs mainly as a consequence of the combined necrotic and apoptotic types of cell death. In order to study liver/hepatocyte injury by the necrotic type of cell death, we studied signal-regulated necrosis (necroptosis) by developing a new optic probe for detecting receptor-interacting protein kinase 1 (RIP)/RIP3 binding, an essential process for necroptosis induction. In the mouse hepatocyte cell line, TIB-73 cells, TNF-α/cycloheximide (T/C) induced RIP1/3 binding only when caspase activity was suppressed by the caspase-specific inhibitor z-VAD-fmk (zVAD). T/C/zVAD-induced RIP1/3 binding was inhibited by necrostatin-1 (Nec-1), an allosteric inhibitor of RIP1. The reduced cell survival by T/C/zVAD was improved by Nec-1. These facts indicate that T/C induces necroptosis of hepatocytes when the apoptotic pathway is inhibited/unavailable. FasL also induced cell death, which was only partially inhibited by zVAD, indicating the possible involvement of necroptosis rather than apoptosis. FasL activated caspase 3 and, similarly, induced RIP1/3 binding when the caspases were inactivated. Interestingly, FasL-induced RIP1/3 binding was significantly suppressed by the antioxidants Trolox and N-acetyl cysteine (NAC), suggesting the involvement of reactive oxygen species (ROS) in FasL-induced necroptotic cellular processes. H2O2, by itself, induced RIP1/3 binding that was suppressed by Nec-1, but not by zVAD. Hypoxia induced RIP1/3 binding after reoxygenation, which was suppressed by Nec-1 or by the antioxidants. Cell death induced by hypoxia/reoxygenation (H/R) was also improved by Nec-1. Similar to H2O2, H/R did not require caspase inhibition for RIP1/3 binding, suggesting the involvement of a caspase-independent mechanism for non-ligand-induced and/or redox-mediated necroptosis. These data indicate that ROS can induce necroptosis and mediate the FasL- and hypoxia-induced necroptosis via a molecular mechanism that differs from a conventional caspase-dependent pathway. In conclusion, necroptosis is potentially involved in liver/hepatocyte injury induced by oxidative stress and FasL in the absence of apoptosis.
Key words: Regulated cell death, Necroptosis, Reactive oxygen species (ROS), Fas ligand, Hypoxia/reoxygenation
INTRODUCTION
Liver injury reflects various pathological conditions within the liver and is a consequence of hepatocyte destruction1–8. Hepatocyte destruction occurs in various liver diseases, liver surgery, and following chemotherapy against cancer as a consequence of necrotic and apoptotic types of cell death1–8. Chemotherapeutic drugs often injure hepatic parenchymal cells as well as cancer cells, and surgical resection of the liver is commonly accompanied with ischemia/reperfusion (I/R)-induced injury during the postoperative period. Although the transcatheter chemoembolization therapy is commonly applied for liver tumors, this causes injury to both tumorous and nontumorous liver cells. Therefore, understanding the pathogenesis of liver injury in terms of hepatic cell death may lead to clinical benefits, such as decreased morbidity and mortality due to liver injury.
Recently, some different types of cell death have been identified to occur as the result of signal-regulated and redox-dependent events6–10. To date, apoptotic cell death is well known to occur in various hepatic pathological conditions such as acute and chronic hepatitis and postischemic liver6,11. For example, Fas ligand (FasL) primarily induces apoptosis both in a caspase- and redox-dependent manner7 and is involved in many pathological situations, such as postoperative liver injury, hepatitis B/C, and alcoholic hepatitis12–15. Fas antigen (CD95) is constitutively expressed on hepatocytes in noncancerous and even cancerous states16, so FasL/Fas is relevant to various kinds of pathological cell death in both cancerous and noncancerous states.
In recent years, it has been confirmed that some apoptosis-inducing proteins, such as tumor necrosis factor-α (TNF-α), can induce necrosis under specific conditions17. This programmed type of necrosis is called necroptosis. TNF-α activates two signaling pathways: cell death and cell survival. The cell death signal branches further into apoptotic or necrotic cell death signaling pathways. When TNF-α binds to the TNF-α receptor of the cell, the Complex I composed of TRADD (TNF receptor-associated death domain), TRAF2 (TNF receptor-associated factor 2), TRAF5, and RIP1 (receptor-interacting protein kinase 1) is formed. These proteins are usually polyubiquitinated, but when deubiquitinated by the actions of proteins such as A20/TNF-α-induced protein 3 (TNFAIP3), cezanne/OUT deubiquitinase 7B (OTUD7B), or ubiquitin-specific peptidase 21 (USP21), RIP1 shifts to the Complex II formation. Complex II consists of RIP1, RIP3, Fas-associated protein with a death domain (FADD), TRADD, and caspase 8. When caspase 8 in Complex II is activated by self-digestion, RIP1 and RIP3 lose activity, and caspase-dependent cell death “apoptosis” occurs as a result of activated caspase 8 switching on the downstream effector caspases (caspases 3, 6, and 7)17. If cells are treated with z-VAD-fmk (zVAD), a reagent that inhibits caspases, the cells cause necrosis-like cell death “necroptosis.” Activation of RIP1 has been shown to be important for this process18. It has previously been reported that when RIP1 is activated and autophosphorylated, RIP1 then phosphorylates and activates RIP319,20. Many of the mechanisms from the activation of RIP1/RIP3 to the occurrence of necroptosis are still unknown.
Evidence for the pathological involvement of necroptosis in renal I/R injury, myocardial infarction, and acute pancreatitis has recently been reported21–25. However, liver necroptosis has not been well studied, and its physiopathological relevance has not been completely elucidated. Recent reports and reviews describe how necroptosis is induced by TNF-associated mechanisms, which has high relevance for many types of inflammatory and cancerous liver diseases, including I/R injury26–28. Necroptosis is often suppressed during tumorigenesis states and therefore is considered as one of the targets for pharmacological anticancer approaches29. One of the most extensively investigated systems for induction of necroptosis is the one induced by the ligand/receptor stimulus such as TNF-α/TNF receptor (TNFR). TNF-α/TNFR induces the physical interaction of RIP1 and RIP3, which is an essential process for necroptosis induction. Another death receptor includes the FasL and Fas (CD95) system, which has also been reported to trigger a form of nonapoptotic cell death consistent with necroptosis29. Necroptosis has also been postulated to act as an apoptotic “backup” cell death mechanism since it functions to enable cell death under conditions where apoptosis cannot occur21,27.
In the present study, we developed a new optic probe for detecting cellular necroptosis by sensing physical interaction of RIP1 and RIP3. By using the optic probes for necroptosis and apoptosis, we first demonstrated that FasL induces necroptosis as well as apoptosis in a redox-dependent manner in hepatocytes. Necroptosis was also induced directly by reactive oxygen species (ROS) and by hypoxia/reoxygenation (H/R) in a redox-dependent manner. ROS and H/R induced necroptosis by a mechanism independent of caspase activity, differently from FasL. These findings will provide important perspectives on the potential pathological and therapeutic roles of necroptosis in liver cancer and other diseases.
MATERIALS AND METHODS
Constructing cDNA Vectors Encoding the Fusion Proteins for Monitoring mRIP1–mRIP3 Interaction
DNA-modifying enzymes were purchased from Takara Bio (Otsu, Japan). Mammalian expression vectors pcDNA3.1(+) and pcDNA3.1/Hygro(+) encoding a neomycin-resistant gene (neor) and a hygromycin-resistant gene (hygr), respectively, were purchased from Invitrogen (Carlsbad, CA, USA). The cDNA of a red-emitting click beetle luciferase (CBR), murine RIP1 (mRIP1), and murine RIP3 (mRIP3) were obtained from Promega (Madison, WI, USA). A transfection reagent TransIT-LT1 was purchased from Takara Bio. An Escherichia coli strain, DH5α, was used as the bacterial host for construction of all vectors. The cDNAs encoding mRIP1, mRIP3, epitope-tagged CBR fragments, neor, and hygr were generated by polymerase chain reaction (PCR). All PCR fragments were sequenced using a genetic analyzer ABI310 (Applied Biosystems, Carlsbad, CA, USA). The cDNA fragments were subcloned into a retroviral vector pMX30.
Cell Culture and Establishment of Stable Clones
Murine liver-derived TIB-73 cells established from normal mouse hepatocytes, mouse embryonic fibroblasts (MEFs; American Type Culture Collection, Manassas, VA, USA), and retrovirus packaging cells Platinum-E (Plat-E)31 were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in an atmosphere of 5% CO2 (v/v). Plat-E cells were transfected with the constructed retroviral vectors using TransIT-LT1 reagent and left at 37°C in an atmosphere of 5% (v/v) CO2. After 48 h, retroviruses were collected and used to infect the cells, TIB-73 cells, and MEFs. The cells stably expressing the fusion proteins were obtained after selection with G418 (Life Technologies, Carlsbad, CA, USA) and hygromycin B (Wako, Osaka, Japan) for 2 weeks.
Cell Culture and Reagents
TIB-73 cells and MEFs were maintained at 37°C in 5% CO2 in DMEM supplemented with 10% FBS. The reagents, sodium azide (NaN3), and cycloheximide, and the antioxidants Trolox and N-acetyl-l-cysteine (NAC) were from Sigma-Aldrich, while TNF-α and zVAD were obtained from R&D Systems (Minneapolis, MN, USA). Necrostatin-1 was purchased from Merck Millipore (Darmstadt, Germany), and Jo2 (FasL) from BD Biosciences (Franklin Lakes, NJ, USA). These reagents were added at the concentrations described in the Results section to the culture media to determine their effects on the cells with regard to caspases and RIP1/3 (apoptosis and necroptosis).
Hypoxia/Reoxygenation
Cellular hypoxic conditions were created and maintained in a modular incubator chamber (Billups–Rothenberg, San Diego, CA, USA) by flushing with a 95% N2/5% CO2 gas mixture for 10 min and then sealing the chamber. This method has been shown to achieve a pO2 of 10 ± 5 Torr32,33. Following 6 h of hypoxia, TIB-73 cells were reoxygenated by opening the chamber and replacing the hypoxic medium with oxygenated medium.
Monitoring Caspase 3 Activity in Live Cells
An optical probe, termed pcFirefly luciferase (pcFluc)-DEVD, was used to detect caspase 3 activity in live cells5,34. Replication-deficient recombinant adenovirus-encoding pcFluc-DEVD (AdpcFluc) was transfected into TIB-73 cells at five multiplicities of infection, 48 h prior to the experiment. After adding d-luciferin substrate (1 mM), bioluminescence for caspase 3 activity stimulated by FasL was measured chronologically with a luminometer (Kronos Dio; Atto Corp., Tokyo, Japan).
Monitoring Live Cells and Biochemical Evaluation of Necrotic Cells
Growth curves were determined by plating the cells (20%–30% confluence) in an xCELLigence System (Roche, Basel, Switzerland), which allows for automated, noninvasive, and real-time monitoring of live cells in culture. Biochemical analysis for necrotic cell death was performed by measuring lactate dehydrogenase (LDH) activity in culture media (LDH Detection Kit; Takara Bio).
In Situ Proximity Ligation Assay for RIP1/3 Binding in Hepatocytes
In order to visualize and quantify intracellular RIP1/3 binding by immunocytochemistry, a Duolink In Situ Proximity Ligation Assay (Sigma-Aldrich) was used35,36. The proximity of RIP1 and RIP3 was detected using two primary antibodies: mouse anti-RIP1 (BD Biosciences) and rabbit anti-RIP3 (Abcam, Cambridge, UK). A pair of oligonucleotide-labeled secondary antibodies generated a fluorescent spot signal only when these two probes bound in close proximity, showing physical binding of RIP1 and RIP3 in cells. These signals were assigned to a specific subcellular location based on microscopy images and were quantified using ImageJ software for image analysis (NIH, Bethesda, MD, USA).
Western Blot Analysis
Whole-cell protein extracts (25 μg) were separated by 10% sodium dodecyl sulfate-polyacrylamide electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes. Western blot analysis of PVDF membranes was performed with appropriate antibodies specific for RIP1, RIP3, mixed lineage kinase domain-like protein (MLKL; Abcam), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Cell Signaling Technology Inc., Danvers, MA, USA).
Statistical Analysis
All results were expressed as mean ± standard error of the mean (SEM). Statistical analyses were performed with Fisher’s test, and a value of p < 0.05 was considered significant.
RESULTS AND DISCUSSION
Newly Developed Optic Probe to Monitor Murine (m)RIP1–mRIP3 Interaction in Live Cells
The structures of the constructed cDNAs encoding the fusion proteins for monitoring the interaction of mRIP1 and mRIP3 on the basis of protein–fragment complementation (split luciferase reconstitution) are shown in Figure 1A 37. The cells infected with the cDNAs express chimeric proteins, mRIP3 fused to the N-terminal fragments of CBR [CBR (1–413); CBRN], and mRIP1 linked with the C-terminal fragment of CBR [CBR (395–542); CBRC]. When the fusion proteins interact mutually, CBRN and CBRC refold correctly, and their bioluminescence activity is recovered38. The principle of mRIP1–mRIP3 interaction, namely, the reconstitution of split luciferase (CBRN and CBRC), is schematically shown in Figure 1B and can be evaluated by measuring the recovered bioluminescence intensity.
Figure 1.
The newly developed optic probe for murine receptor-interacting protein kinase 1/3 (RIP1/3) binding and the detection of cell death by tumor necrosis factor-α (TNF-α)/cycloheximide and sodium azide (NaN3). (A) Schematic structures of cDNA constructs of the optic probe. The click beetle luciferase N- and C-terminal fragments (CBRN and CBRC) indicate the cDNAs encoding the N- and C-terminal ends of CBR, CBR (1–413), and CBR (395–542), respectively. mRIP1, murine RIP1; mRIP3, murine RIP3; GGGGS, five-amino-acid flexible linker of -Gly-Gly-Gly-Gly-Ser-; myc, c-myc epitope; V5, V5 epitope; IRES, internal ribosome entry site; neor, neomycin-resistant gene; hygr, hygromycin-resistant gene; LTR, long terminal repeat; ψ, retroviral package signal. (B) The principle of protein complementation assay (split luciferase reconstitution) for monitoring the interaction of mRIP1–mRIP3. mRIP1–mRIP3 interaction brings CBRN and CBRC, resulting in bioluminescence recovery. (C) TNF-α/cycloheximide (T/C) induced dose-dependent RIP1/3 binding and marked cell death (detachment of cells) in mouse embryonic fibroblasts (MEFs) (TNF-α: 25 or 50 ng/ml, cycloheximide: 1 μg/ml). (D) Sodium azide (NaN3) induced RIP1/3 binding transiently that became rapidly reduced. Photomicrographs show massive cell death (cell aggregation and detachment) in MEFs treated with NaN3 (2.5 or 5.0 mM). Data from the optic probe are representative of at least three independent experiments (C, D).
RIP1/3 Binding Was Increased by TNF-α/Cycloheximide or Sodium Azide (NaN3) Treatment
We first tried TNF-α/cycloheximide (T/C) or NaN3 in MEFs stably transfected with the probe. T/C and NaN3 are known to induce regulated cell death, at least partly through caspase-dependent apoptosis, in the ligand- and non-ligand-mediated manners, respectively39,40. In MEFs, T/C induced a rapid increase in RIP1/3 binding within 4 h after the treatment and subsequent cell death (detachment of cells) within 24 h (Fig. 1C). NaN3 also immediately induced a robust increase in RIP1/3 binding and massive MEF cell death 24 h after the treatment (Fig. 1D). The newly developed optic probe succeeded in demonstrating RIP1/3 binding (i.e., necroptosis) causing cell death in T/C- and NaN3-treated MEF.
TNF-α/Cycloheximide Induced RIP1/3 Binding in TIB-73 Mouse Liver Cells
Next, we challenged this probe to TIB-73 cells treated with T/C. T/C on its own did not induce RIP1/3 binding (Fig. 2A), but induced a prompt and marked increase in RIP1/3 binding after the addition of zVAD (Fig. 2A). This increase was significantly suppressed and delayed in a dose-dependent manner by Nec-1, an allosteric inhibitor of RIP1 (Fig. 2A and B). Photomicrographs of TIB-73 cells revealed that Nec-1 also suppressed T/C/zVAD-induced cell death (Fig. 2A). The in situ proximity ligation assay confirmed T/C/zVAD-induced RIP1/3 binding immunocytochemically in TIB-73 cells (Fig. 2C). Such T/C/zVAD-induced signals were also clearly and significantly suppressed by Nec-1 treatment.
Figure 2.
TNF-α/cycloheximide induced RIP1/3 binding and cell death in TIB-73 mouse liver cells. (A) T/C induced RIP1/3 binding only when caspase activity was inhibited by z-VAD-fmk (zVAD) in TIB-73 cells, which was suppressed by necrostatin-1 (Nec-1), an allosteric inhibitor of RIP1. Photomicrographs show that cell death in TIB-73 cells induced by T/C/zVAD was improved by Nec-1 treatment. (B) RIP1/3 binding induced by T/C/zVAD was inhibited dose-dependently by Nec-1 in TIB-73 cells. (C) RIP1/3 binding and inhibition by Nec-1 were confirmed immunocytochemically (in situ proximity ligation assay). Red color [4,6-diamidino-2-phenylindole (DAPI), pseudocolor] and green color indicate nucleus and interacted RIP1/3, respectively. (D) The cell survival rate dropped rapidly after T/C treatment, which was partially improved by zVAD (12 h after the treatment). T/C/zVAD- (top) and T/C-induced (bottom) cell death were both significantly improved by the addition of Nec-1 (24–30 h after the treatment), suggesting that T/C treatment induced necroptosis at later time points, especially when caspases were inactivated/unavailable. The concentrations of T/C (TNF-α, cycloheximide), zVAD, and Nec-1 used were 25 ng/ml, 1 μg/ml, 20 μM, and 20 μM, respectively (A, C, and D). Results are expressed as mean ± standard error of the mean (SEM) of five independent experiments, with p < 0.05 considered significant (A, C, and D). Data from the optic probe and photomicrographs are representative of at least three independent experiments.
T/C/zVAD-induced cell death was accompanied by RIP1/3 binding, so we studied the TIB-73 cell survival chronologically after T/C, zVAD, and Nec-1 treatments (Fig. 2D, top). The cell survival rate rapidly dropped after T/C treatment, which was partially improved by zVAD. This protective effect by zVAD was evident at early time points after the treatment but became less evident with time. This indicates that T/C induced mainly apoptosis at early time points. At later time points when cell survival was not sufficiently improved by zVAD, Nec-1 improved cell survival in a marked and more effective manner than zVAD. Comparing the independent effects of zVAD and Nec-1 on T/C-induced damage, Nec-1 improved cell survival more than zVAD, especially at later time points (Fig. 2D, bottom). These facts indicate the possibility that T/C primarily induces apoptosis and then necroptosis at later time points when caspases are inactivated/unavailable in hepatocytes.
FasL Induced Both Apoptosis and Necroptosis in TIB-73 Mouse Liver Cells
Next, we studied the induction of regulated cell death by FasL in mouse liver cells (TIB-73 cells). FasL markedly induced cell death, as assessed by the elevation of LDH in culture media and by microscopic observation, which was only partially inhibited by zVAD (Fig. 3A). This indicates that another type of cell death is involved other than apoptosis in FasL-mediated hepatocyte cell death. Therefore, we studied two types of cell death (apoptosis and necroptosis) in FasL-mediated cell death using the optic probes for caspase 3 activity34 and RIP1/3 binding representing apoptosis and necroptosis, respectively (Fig. 3B). FasL rapidly induced caspase 3 activity 4–6 h after the treatment but did not induce RIP1/3 binding (Fig. 3B, left and right, respectively). However, by the further addition of zVAD, RIP1/3 binding promptly increased (Fig. 3B, right). These observations provide evidence for the conventional hypothesis that necroptosis functions as an apoptotic “backup”21,27 to enable cell death in case the caspase-dependent pathway is not available (or inactivated).
Figure 3.
Fas ligand (FasL; Jo2) induced necrotic cell death and RIP1/3 binding in a redox-dependent manner in TIB-73 cells. (A) A caspase inhibitor (zVAD) only partially suppressed FasL (Jo2)-induced necrotic cell death in TIB-73 cells. (B) Optic probes for caspase 3 activity (left) and RIP1/3 binding (right) were used to evaluate apoptosis and necroptosis, respectively. FasL (Jo2) induced activation of caspase 3, and RIP1/3 binding occurred only when the caspases were inactivated. (C) Antioxidants [Trolox and N-acetyl cysteine (NAC)] significantly suppressed FasL (Jo2; 2 μg/ml)-induced RIP1/3 binding. The concentration of zVAD used was 20 μM (A, B, and C). Results are expressed as mean ± SEM of five independent experiments, with p < 0.05 considered significant (A, C). Data from the optic probes and photomicrographs are representative of at least three independent experiments.
TNF-α and FasL are both known to induce ROS-mediated cell death in liver cells5,7,41,42. In order to confirm the involvement of ROS in the ligand-induced necroptosis in hepatocytes, we examined the effect of the antioxidants upon FasL-induced necroptosis. Pretreatment of cells with the antioxidants Trolox and NAC significantly suppressed FasL-induced RIP1/3 binding (Fig. 3C), suggesting ROS are involved in necroptosis-inducing processes in hepatocytes.
H/R Induced Necroptotic Cell Death in a Redox-Dependent Manner in TIB-73 Cells
We next studied whether H/R induces necroptosis in mouse hepatocytes. H/R increased RIP1/3 binding, with a maximal peak 16–18 h after reoxygenation, and induced necrotic cell death (Fig. 4A), which were both suppressed significantly by Nec-1. RIP1/3 binding was also inhibited dose dependently and significantly by the antioxidants Trolox and NAC (Fig. 4B). These data indicate that H/R induces RIP1/3 binding (i.e., necroptosis) in a redox-dependent manner, similar to FasL. ROS (H2O2) by itself induced RIP1/3 binding, which was inhibited by Nec-1 but not affected by zVAD (Fig. 4C).
Figure 4.
Hypoxia/reoxygenation (H/R) induced RIP1/3 binding and necrotic cell death in a redox-dependent manner in TIB-73 mouse liver cells. (A) H/R induced RIP1/3 binding and, accordingly, necrotic cell death, which were both inhibited by Nec-1 (20 μM). (B) H/R-induced RIP1/3 binding was inhibited by the antioxidants Trolox and NAC in a dose-dependent manner. (C) H2O2 (500 μM)-induced RIP1/3 binding was significantly inhibited by Nec-1 and was not augmented by caspase inhibition in TIB-73 cells, in contrast to FasL. (D) The expressions of necroptosis-associated proteins [RIP1, RIP3, and mixed lineage kinase domain-like protein (MLKL)] were transiently reduced and gradually recovered over time after reoxygenation. (E) H/R-induced RIP1/3 binding was not augmented by caspase inhibition in TIB-73 cells, similar to H2O2 treatment. The concentration of zVAD used was 20 μM. Data from the optic probe (A, E) and photomicrographs (A) are expressed as representative of at least three independent experiments. The experiments of Western blot were performed independently in duplicate (D). The results are expressed as mean ± SEM of five independent experiments, with p < 0.05 considered significant (B).
The expression levels of the endogenous necroptosis-associated proteins (RIP1, RIP3, and MLKL) were reduced after hypoxia (Fig. 4D). The expressions of RIP1 and MLKL began to markedly recover 16 h post-H/R and onward. However, RIP3 recovered transiently 16 h post-H/R but decreased thereafter. H/R-induced RIP1/3 binding seems to require the reexpressions of RIP1, RIP3, and MLKL, though MLKL is known to be essential for necroptosis43. The induction of RIP1/3 binding by H/R occurred later than that by FasL but was not affected/augmented by caspase inhibition differently from FasL (Figs. 3B and 4E), suggesting that there are different molecular mechanisms in H/R- and FasL/Fas-induced necroptosis. FasL- and H/R-induced necroptosis in hepatocytes occurred in a redox-dependent molecular mechanism, which were dependent and independent from caspase activity, respectively.
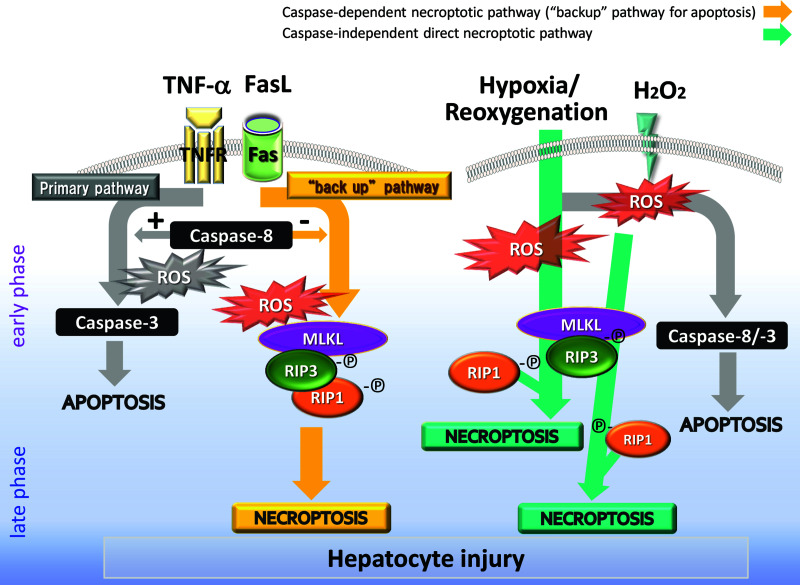
In conclusion, T/C and FasL induced necroptosis if caspases were inactivated. FasL- and H/R-induced necroptosis occurred in a redox-dependent manner. H/R induced necroptosis in the mechanism dependent of ROS and independent of caspase activity (Fig. 5). The precise mechanisms involved in necroptotic cell death in hepatocytes are still unknown and require further study. However, our findings advance the understanding of the physiopathology of regulated cell death causing hepatocyte/liver injury.
Figure 5.
Redox-mediated regulated cell death (apoptosis and necroptosis) in hepatocytes. TNF-α/FasL induces primarily redox-dependent apoptosis via a caspase 8/3 pathway. When caspase 8 is not available/inactivated, the alternative pathway is activated to form a necrosome (MLKL/RIP1/RIP3) to induce necroptosis, also in a redox-dependent manner. Necroptosis occurs as “a backup for apoptosis” at a later phase. Reactive oxygen species (ROS; e.g., H2O2) primarily induce apoptosis and, thereafter, necroptosis in the presence of MLKL. The induction of necroptosis by H/R is affected by the reexpression of RIP1, RIP3, and MLKL after reoxygenation but is independent of caspase activity. The precise molecular mechanisms are still unknown, but oxidative stress definitely plays a pivotal role in liver injury via regulated cell death.
ACKNOWLEDGMENTS
This work was supported by the JSPS Grants-in-Aid for Scientific Researches (KAKENHI) [Grant Nos. 26220805 (to M.O.), and 26670573 and 15H05659 (to S.H.)]. This work was also supported in part by the Grants-in-Aid for Regional R&D Proposal-Based Program from the Northern Advancement Center for Science and Technology of Hokkaido Japan (to M.O.), the Takeda Science Foundation (to S.H.), a research grant from The Akiyama Life Science Foundation (to S.H.), and a donation from Mr. and Mrs. Fujikawa (to M.O.).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Dunn W, Shah VH. Pathogenesis of alcoholic liver disease. Clin Liver Dis. 2016;20:445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khoury T, Rmeileh AA, Yosha L, Benson AA, Daher S, Mizrahi M. Drug induced liver injury: Review with a focus on genetic factors, tissue diagnosis, and treatment options. J Clin Transl Hepatol. 2015;3:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oh IS, Park SH. Immune-mediated liver injury in hepatitis B virus infection. Immune Netw. 2015;15:191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Suhail M, Abdel-Hafiz H, Ali A, Fatima K, Damanhouri GA, Azhar E, Chaudhary AG, Qadri I. Potential mechanisms of hepatitis B virus induced liver injury. World J Gastroenterol. 2014;20:12462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haga S, Ozawa T, Yamada Y, Morita N, Nagashima I, Inoue H, Inaba Y, Noda N, Abe R, Umezawa K, Ozaki M. p62/SQSTM1 plays a protective role in oxidative injury of steatotic liver in a mouse hepatectomy model. Antioxid Redox Signal. 2014;21:2515–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haga S, Remington SJ, Morita N, Terui K, Ozaki M. Hepatic ischemia induced immediate oxidative stress after reperfusion and determined the severity of the reperfusion-induced damage. Antioxid Redox Signal. 2009;11:2563–72. [DOI] [PubMed] [Google Scholar]
- 7. Haga S, Terui K, Zhang HQ, Enosawa S, Ogawa W, Inoue H, Okuyama T, Takeda K, Akira S, Ogino T, Irani K, Ozaki M. Stat3 protects against Fas-induced liver injury by redox-dependent and -independent mechanisms. J Clin Invest. 2003;112:989–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bahirwani R, Reddy KR. Drug-induced liver injury due to cancer chemotherapeutic agents. Semin Liver Dis. 2014;34:162–71. [DOI] [PubMed] [Google Scholar]
- 9. Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J Gastroenterol Hepatol. 2011;26:173–9. [DOI] [PubMed] [Google Scholar]
- 10. Wang K. Molecular mechanisms of liver injury: Apoptosis or necrosis. Exp Toxicol Pathol. 2014;66:351–6. [DOI] [PubMed] [Google Scholar]
- 11. Gao WY, Li D, Cai DE, Huang XY, Zheng BY, Huang YH, Chen ZX, Wang XZ. Hepatitis B virus X protein sensitizes HL-7702 cells to oxidative stress-induced apoptosis through modulation of the mitochondrial permeability transition pore. Oncol Rep. 2017;37:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pinkoski MJ, Brunner T, Green DR, Lin T. Fas and Fas ligand in gut and liver. Am J Physiol Gastrointest Liver Physiol. 2000;278:G354–6. [DOI] [PubMed] [Google Scholar]
- 13. Galle PR, Hofmann WJ, Walczak H, Schaller H, Otto G, Stremmel W, Krammer PH, Runkel L. Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J Exp Med. 1995;182:1223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kanzler S, Galle PR. Apoptosis and the liver. Semin Cancer Biol. 2000;10:173–84. [DOI] [PubMed] [Google Scholar]
- 15. Faubion WA, Guicciardi ME, Miyoshi H, Bronk SF, Roberts PJ, Svingen PA, Kaufmann SH, Gores GJ. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J Clin Invest. 1999;103:137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Castaneda F, Kinne RK. Ethanol treatment of hepatocellular carcinoma: High potentials of low concentrations. Cancer Biol Ther. 2004;3:430–3. [DOI] [PubMed] [Google Scholar]
- 17. Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–14. [DOI] [PubMed] [Google Scholar]
- 18. Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–9. [DOI] [PubMed] [Google Scholar]
- 19. Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 1996;4:387–96. [DOI] [PubMed] [Google Scholar]
- 20. Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–95. [DOI] [PubMed] [Google Scholar]
- 21. Saeed WK, Jun DW. Necroptosis: An emerging type of cell death in liver diseases. World J Gastroenterol. 2014;20:12526–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shen B, He Y, Zhou S, Zhao H, Mei M, Wu X. TRPC6 may protect renal ischemia-reperfusion injury through inhibiting necroptosis of renal tubular epithelial cells. Med Sci Monit. 2016;22:633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Linkermann A, De Zen F, Weinberg J, Kunzendorf U, Krautwald S. Programmed necrosis in acute kidney injury. Nephrol Dial Transplant. 2012;27:3412–9. [DOI] [PubMed] [Google Scholar]
- 24. Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv F, Liu Y, Zheng W, Shang H, Zhang J, Zhang M, Wu H, Guo J, Zhang X, Hu X, Cao CM, Xiao RP. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat Med. 2016;22:175–82. [DOI] [PubMed] [Google Scholar]
- 25. Sendler M, Mayerle J, Lerch MM. Necrosis, apoptosis, necroptosis, pyroptosis: It matters how acinar cells die during pancreatitis. Cell Mol Gastroenterol Hepatol. 2016;2:407–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: Mechanisms and clinical relevance. Gastroenterology 2014;147:765–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Linkermann A, Green DR. Necroptosis. N Engl J Med. 2014;370:455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Naomoto Y, Tanaka N, Fuchimoto S, Orita K. In vitro synergistic effects of natural human tumor necrosis factor and natural human interferon-alpha. Jpn J Cancer Res. 1987;78:87–92. [PubMed] [Google Scholar]
- 29. Fulda S. The mechanism of necroptosis in normal and cancer cells. Cancer Biol Ther. 2013;14:999–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Onishi M, Kinoshita S, Morikawa Y, Shibuya A, Phillips J, Lanier LL, Gorman DM, Nolan GP, Miyajima A, Kitamura T. Applications of retrovirus-mediated expression cloning. Exp Hematol. 1996;24:324–9. [PubMed] [Google Scholar]
- 31. Morita S, Kojima T, Kitamura T. Plat-E: An efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–6. [DOI] [PubMed] [Google Scholar]
- 32. Crawford LE, Milliken EE, Irani K, Zweier JL, Becker LC, Johnson TM, Eissa NT, Crystal RG, Finkel T, Goldschmidt-Clermont PJ. Superoxide-mediated actin response in post-hypoxic endothelial cells. J Biol Chem. 1996;271:26863–7. [DOI] [PubMed] [Google Scholar]
- 33. Ozaki M, Deshpande SS, Angkeow P, Suzuki S, Irani K. Rac1 regulates stress-induced, redox-dependent heat shock factor activation. J Biol Chem. 2000;275:35377–83. [DOI] [PubMed] [Google Scholar]
- 34. Ozaki M, Haga S, Ozawa T. In vivo monitoring of liver damage using caspase-3 probe. Theranostics 2012;2:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sorokina EM, Feinstein SI, Zhou S, Fisher AB. Intracellular targeting of peroxiredoxin 6 to lysosomal organelles requires MAPK activity and binding to 14-3-3ε. Am J Physiol Cell Physiol. 2011;300:C1430–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Petri MK, Koch P, Stenzinger A, Kuchelmeister K, Nestler U, Paradowska A, Steger K, Brobeil A, Viard M, Wimmer M. PTPIP51, a positive modulator of the MAPK/Erk pathway, is upregulated in glioblastoma and interacts with 14-3-3β and PTP1B in situ. Histol Histopathol. 2011;26:1531–43. [DOI] [PubMed] [Google Scholar]
- 37. Michnick SW, Ear PH, Manderson EN, Remy I, Stefan E. Universal strategies in research and drug discovery based on protein-fragment complementation assays. Nat Rev Drug Discov. 2007;6:569–82. [DOI] [PubMed] [Google Scholar]
- 38. Hida N, Awais M, Takeuchi M, Ueno N, Tashiro M, Takagi C, Singh T, Hayashi M, Ohmiya Y, Ozawa T. High-sensitivity real-time imaging of dual protein-protein interactions in living subjects using multicolor luciferases. PLoS One 2009;4:e5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jin S, Ray RM, Johnson LR. TNF-alpha/cycloheximide-induced apoptosis in intestinal epithelial cells requires Rac1-regulated reactive oxygen species. Am J Physiol Gastrointest Liver Physiol. 2008;294:G928–37. [DOI] [PubMed] [Google Scholar]
- 40. Ji D, Kamalden TA, del Olmo-Aguado S, Osborne NN. Light- and sodium azide-induced death of RGC-5 cells in culture occurs via different mechanisms. Apoptosis 2011;16:425–37. [DOI] [PubMed] [Google Scholar]
- 41. Schwabe RF, Brenner DA. Mechanisms of liver injury. I. TNF-alpha-induced liver injury: Role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583–9. [DOI] [PubMed] [Google Scholar]
- 42. Kastl L, Sauer SW, Ruppert T, Beissbarth T, Becker MS, Süss D, Krammer PH, Gülow K. TNF-α mediates mitochondrial uncoupling and enhances ROS-dependent cell migration via NF-κB activation in liver cells. FEBS Lett. 2014;588:175–83. [DOI] [PubMed] [Google Scholar]
- 43. Wu J, Huang Z, Ren J, Zhang Z, He P, Li Y, Ma J, Chen W, Zhang Y, Zhou X, Yang Z, Wu SQ, Chen L, Han J. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 2013;23:994–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]