Abstract
Melanoma is characterized by aggressive invasion, early metastasis, and resistance to existing chemotherapeutic agents. Accumulated studies have reported that microRNA (miRNA) is a potentially robust molecular tool for developing future therapeutic technologies. Therefore, examining the expression patterns, biological roles, and associated mechanisms of cancer-related miRNAs in melanoma is essential for developing novel therapeutic targets for patients with this disease. In this study, miRNA-331 (miR-331) was underexpressed in melanoma tissues and cell lines. Functional assays revealed that the enforced expression of miR-331 inhibited cell proliferation and invasion. In addition, astrocyte-elevated gene-1 (AEG-1) was identified as a novel target of miR-331 through bioinformatics analysis, reverse transcription quantitative polymerase chain reaction analysis, Western blot analysis, dual-luciferase reporter assay, and Spearman’s correlation analysis. Furthermore, reintroduction of AEG-1 partially abrogated the inhibitory effects of miR-331 overexpression on the proliferation and invasion of melanoma cells. Moreover, miR-331 suppressed the activation of the PTEN/AKT signaling pathway in melanoma by inhibiting AEG-1. In short, miR-331 may play tumor-suppressive roles in melanoma by directly targeting AEG-1 and regulating the PTEN/AKT signaling pathway, suggesting that miR-331 could be investigated as a therapeutic strategy for patients with this malignancy.
Key words: MicroRNA-331 (miR-331), Melanoma, Astrocyte-elevated gene-1 (AEG-1), Proliferation, Invasion
INTRODUCTION
Melanoma, the most aggressive form of skin cancer, accounts for the most common cause of skin malignancy-related deaths1. It is the seventh most common malignancy in females and the fifth most common cancer in males worldwide1,2. In recent years, the morbidity of melanoma has increased more rapidly compared with those of other malignancies3. Moreover, approximately 200,000 new cases and 46,000 deaths due to melanoma annually occur around the world4. Currently, wide surgical resection is considered as the main option in treating melanoma diagnosed at the early stage. However, combined treatments for patients at the advanced stage, including surgery followed by molecular-targeted therapy, radiotherapy, and immunotherapy, are not currently satisfactory5. Prognosis for melanoma patients with local and distant metastases is poor, with 10-year survival rates of only 64% and 16%, respectively6. Therefore, further investigation into the detailed molecular mechanisms involved in the tumorigenesis and tumor development of melanoma is required to develop novel effective therapeutic methods for patients with this malignancy.
MicroRNAs (miRNAs) represent a group of endogenous, noncoding, short RNA molecules 20–25 nucleotides in length7. miRNAs silence gene expression via direct interaction with the 3′-untranslated regions (3′-UTRs) of their target genes in a sequence-specific manner, resulting in mRNA degradation or translation suppression8. Existing evidence suggests that miRNAs participate in the regulation of over half of all human protein-coding genes and perform specific roles in various physiological and pathological processes, including early embryonic development, metabolism, cell proliferation, cell cycle, apoptosis, differentiation, and metastasis9–11. An increasing number of studies have reported that miRNA expression is altered in almost all types of human malignancy and that miRNAs may be involved in tumorigenesis and tumor development as either oncogenes or tumor suppressors12–14. Therefore, miRNAs may be utilized as potential therapeutic targets for cancer therapy.
miR-331 is abnormally expressed in several types of human cancer15–18. However, the expression and biological roles of miR-331 in melanoma remain to be elucidated. In the present study, miR-331 expression was decreased in melanoma tissues and cell lines. Enforced expression of miR-331 prohibited the cell proliferation and invasion of melanoma. In addition, astrocyte-elevated gene-1 (AEG-1), also known as metadherin, was identified as a direct target of miR-331 in melanoma. Hence, the restoration of miR-331 expression could be a new therapeutic method for patients with melanoma.
MATERIALS AND METHODS
Tissue Samples and Cell Lines
A total of 22 pairs of human primary melanoma tissues and adjacent nontumorous skin specimens were obtained from patients who received surgical resections in Heilongjiang Provincial Hospital (Harbin, P.R. China) from January 2015 to November 2016. None of these patients underwent chemotherapy or radiotherapy prior to surgery. The resected tissues were immediately snap frozen in liquid nitrogen and stored at −80°C in a cryogenic refrigerator prior to further use. This study was approved by the Ethics Committee of Heilongjiang Provincial Hospital. Written informed consent was obtained from all participants.
Human epidermal melanocytes (HEMs) obtained from ScienCell Research Laboratories, Inc. (San Diego, CA, USA) were grown in a melanocyte medium (ScienCell Research Laboratories, Inc.) under conditions recommended by the manufacturer. Five melanoma cell lines, namely, A375, A2058, HT144, SK-MEL-1, and SK-MEL-28, were purchased from the American Type Culture Collection (Manassas, VA, USA). These cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) and supplemented with 10% fetal bovine serum (FBS), 100 mg/ml penicillin, and 100 mg/ml streptomycin (all Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). All the cell lines were cultured at 37°C in a humidified incubator with 5% CO2.
RNA Extraction and Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA of tissue samples and cells was extracted with TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). For the detection of miR-331 expression, total RNA was reverse transcribed into cDNA using a TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA). Subsequent qPCR was conducted using a TaqMan MicroRNA PCR Kit (Applied Biosystems) with U6 snRNA as an internal control. To quantify the mRNA level of AEG-1, cDNA was synthesized using a PrimeScript RT Reagent Kit (Takara Bio, Dalian, P.R. China) followed by qPCR with SYBR Premix Ex Taq™ Kit (Takara Bio). GAPDH served as an internal reference for AEG-1 mRNA expression. Relative gene expression was evaluated using the 2−ΔΔCt method19.
Transfection of miRNA Mimics and Plasmids
Synthetic miR-331 mimic and negative control miRNA mimic (miR-NC) were acquired from Genechem (Shanghai, P.R. China). AEG-1 overexpression plasmid pcDNA3.1-AEG-1 and empty pcDNA3.1 plasmid were purchased from GeneCopoeia (Guangzhou, P.R. China). Cells in log-phase growth were plated into six-well plates with a density of 60–70% confluence 24 h prior to transfection. Cells were transfected with miRNA mimics or plasmids using Lipofectamine™ 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer’s instructions.
Cell Counting Kit-8 (CCK-8) Assay
CCK-8 assay was utilized to detect cellular proliferation in accordance with the manufacturer’s instructions. At 24 h after transfection, cells were collected and plated into 96-well plates with a density of 3,000 cells per well. CCK-8 assay was carried out daily for 3 days. Subsequently, 10 μl of CCK-8 solution (Beyotime, Hangzhou, P.R. China) was added into each well and continued to incubate at 37°C with 5% CO2 for another 2 h. Afterward, optical density (OD) was detected at a wavelength of 450 nm with a microplate reader (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Transwell Invasion Assay
Transfected cells were harvested after 24 h of incubation and suspended into FBS-free DMEM. A total of 1 × 105 cells were seeded into the top chamber of Transwell chambers (Corning Incorporated, Corning, NY, USA) coated with Matrigel (BD Biosciences, San Jose, CA, USA). The bottom chambers were filled with 600 μl of DMEM supplemented with 20% FBS. After subsequent incubations at 37°C with 5% CO2 for 24 h, the noninvaded cells that remained on the upper surface of the membrane were gently removed using a cotton swab. The invaded cells were fixed with 100% methanol and stained using 0.1% crystal violet solution. Finally, the invaded cells were subjected to microscopic inspection and counted under an inverted microscope (Olympus Corporation, Tokyo, Japan) with five randomly selected visual fields.
miRNA Target Prediction
The target prediction of miRNA was performed with online miRNA target prediction algorithms TargetScan (http://www.targetscan.org/) and miRanda (http://www.microrna.org/).
Dual-Luciferase Reporter Assay
Luciferase reporter plasmids containing the wild type (Wt) and mutant (Mut) seed regions of miR-331 in the 3′-UTR of AEG-1 were chemically synthesized by GenePharma (Shanghai, P.R. China) and named as pMIR-AEG-1-3′-UTR Wt and pMIR-AEG-1-3′-UTR Mut, respectively. For the dual-luciferase reporter assay, cells were plated into 24-well plates 1 day before transfection. Cells were cotransfected with miR-331 mimic or miR-NC and luciferase reporter recombinant plasmid using Lipofectamine™ 2000 reagent. Following cultivation for 48 h, the cells were harvested, and luciferase activity was determined using a dual-luciferase reporter assay system (Promega, Madison, WI, USA) based on the manufacturer’s instructions. Renilla luciferase activity was used as an internal reference for Firefly luciferase activity.
Western Blot Analysis
The primary antibodies mouse anti-human AEG-1 antibody (sc-517220), mouse anti-human phosphatase and tensin homolog (PTEN) antibody (sc-7974), mouse anti-human AKT antibody (sc-56878), mouse anti-human p-AKT antibody (sc-271966), and mouse anti-human GAPDH antibody (sc-81545) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Protein was isolated from tissue samples or cells using ice-cold lysis buffer (Beyotime Biotechnology, Jiangsu, P.R. China). A BCA Protein Assay Kit (Pierce; Thermo Fisher Scientific, Inc.) was applied to examine the concentration of total protein. Equal amounts of protein were separated using 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and the fractionated proteins were transferred onto polyvinylidene difluoride membranes (Millipore, Boston, MA, USA). After blocking with TBS/0.1% Tween (TBST) containing 5% skimmed milk at room temperature for 2 h, the membranes were incubated with the primary antibodies at 4°C overnight. The membranes were washed three times with TBST and then probed with goat anti-mouse horseradish peroxidase-conjugated secondary antibody for 2 h (Santa Cruz Biotechnology) at the dilution ratio of 1:5,000. The protein signals were visualized using an ECL prime Western blot substrate (GE, Amersham, UK). Relative protein expression was presented as the density ratio versus GAPDH.
Statistical Analysis
All data were expressed as the mean ± standard deviation. Differences between groups were analyzed using Student’s t-test or one-way ANOVA with the Student–Newman–Keuls test as a post hoc test. Spearman’s correlation analysis was used to determine the association between miR-331 and AEG-1 mRNA in melanoma tissues. Statistical significance was considered at a value of p < 0.05.
RESULTS
miR-331 Is Underexpressed in Melanoma Tissues and Cell Lines
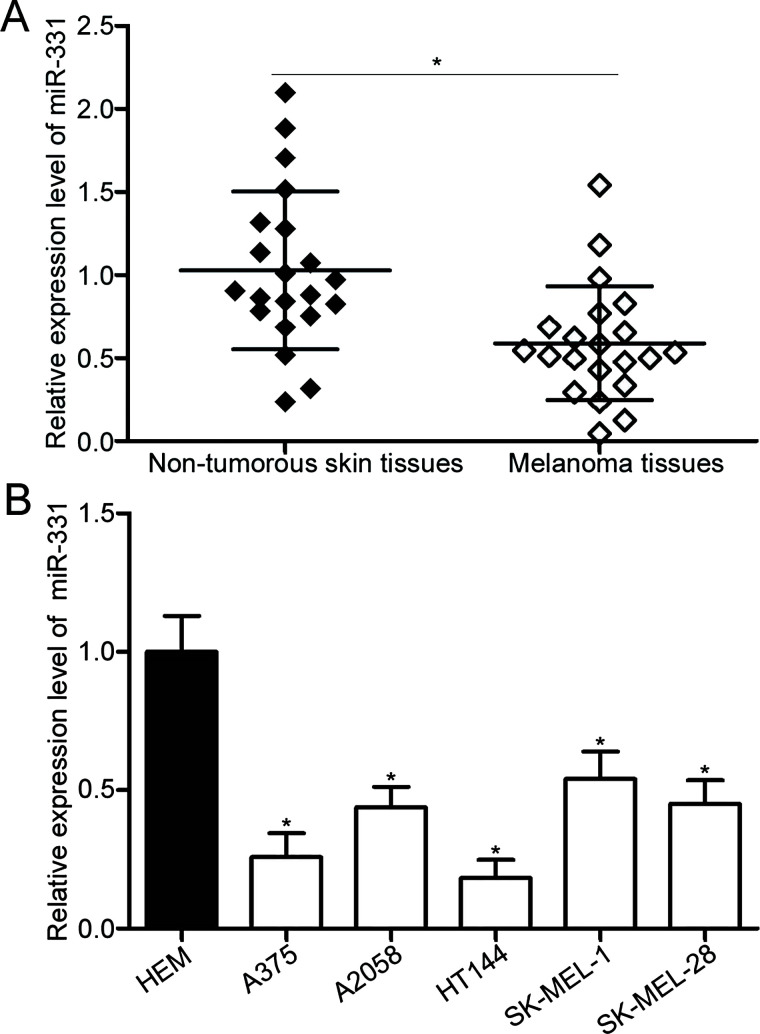
To determine miR-331 expression in melanoma, we first measured miR-331 expression in 22 pairs of human primary melanoma tissues and adjacent nontumorous skin specimens. The results of the RT-qPCR revealed that miR-331 expression was downregulated in melanoma tissues relative to that in adjacent nontumorous skin specimens (p < 0.05) (Fig. 1A). The expression level of miR-331 in five melanoma cell lines and HEMs was further detected using RT-qPCR. Results showed that the expression level of miR-331 was lower in all melanoma cell lines than in HEMs (p < 0.05) (Fig. 1B). In addition, miR-331 expression was relatively lower in the A375 and HT144 cell lines among the five cell lines. Thus, A375 and HT144 were selected for subsequent experiments.
Figure 1.
MicroRNA-331 (miR-331) is underexpressed in melanoma tissues and cell lines. (A) Reverse transcription quantitative polymerase chain reaction (RT-qPCR) detection of miR-331 expression in 22 pairs of human primary melanoma tissues and adjacent nontumorous skin specimens. *p < 0.05 compared with nontumorous skin specimens. (B) RT-qPCR was performed to detect miR-331 expression in five melanoma cell lines (A375, A2058, HT144, SK-MEL-1, and SK-MEL-28) and in human epidermal melanocytes (HEMs). *p < 0.05 compared with HEM.
miR-331 Overexpression Inhibits Cell Proliferation and Invasion in Melanoma
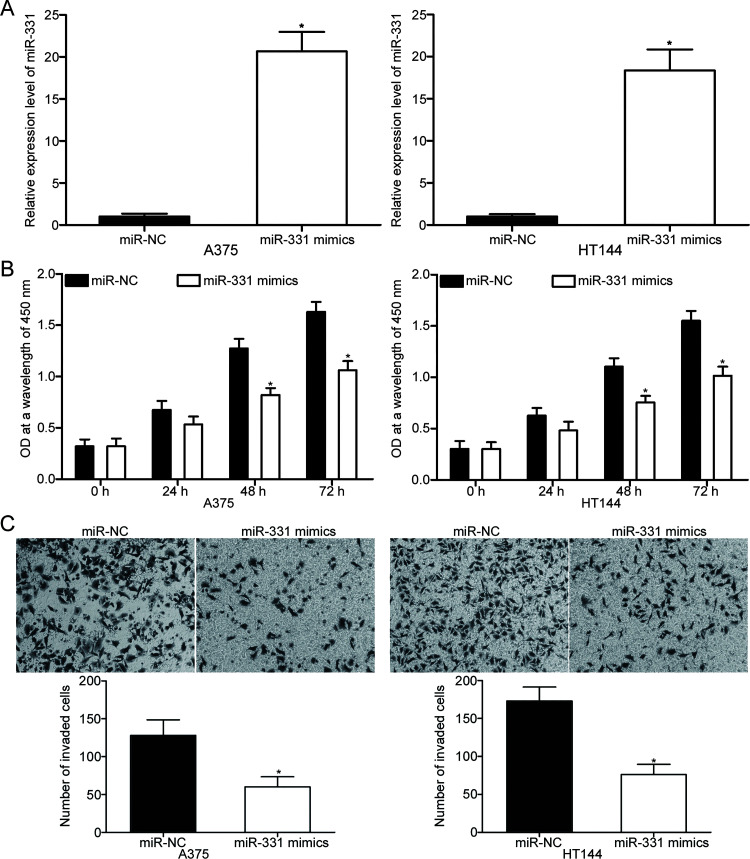
To investigate the effects of miR-331 on oncogenic melanoma phenotypes, miR-331 mimic was transfected into A375 and HT144 cells to increase miR-331 endogenous expression. RT-qPCR confirmed that miR-331 was markedly upregulated in the A375 and HT144 cells transfected with miR-331 mimic relative to the cells transfected with miR-NC (p < 0.05) (Fig. 2A). CCK-8 assay was then carried out in the miR-331 mimic-transfected A375 and HT144 cells at different time points to examine the effect of miR-331 overexpression on melanoma cell proliferation. As shown in Figure 2B, the ectopic expression of miR-331 suppressed the proliferation of A375 and HT144 cells (p < 0.05). In addition, Transwell invasion assay was performed to explore whether miR-331 overexpression affects the invasion ability of melanoma cells. Results revealed that enforced miR-331 expression significantly reduced the invasion capacities of A375 and HT144 cells compared with the miR-NC group (p < 0.05) (Fig. 2C). These results suggest that miR-331 could serve as a tumor suppressor in melanoma progression.
Figure 2.
miR-331 inhibits the proliferation and invasion of A375 and HT144 cells. (A) The relative expression of miR-331 in A375 and HT144 cells after transfection with miR-331 mimic or negative control miRNA mimic (miR-NC) was examined using RT-qPCR. *p < 0.05 compared with miR-NC. (B) The effect of miR-331 overexpression on A375 and HT144 cell proliferation was determined using cell counting kit-8 (CCK-8) assay. *p < 0.05 compared with miR-NC. (C) Transwell invasion assay was applied to evaluate the invasion ability of A375 and HT144 cells after transfection with miR-331 mimics or miR-NC. *p < 0.05 compared with miR-NC.
AEG-1 Is Characterized as a Direct Target of miR-331 and Is Negatively Correlated With miR-331 Expression in Melanoma Tissues
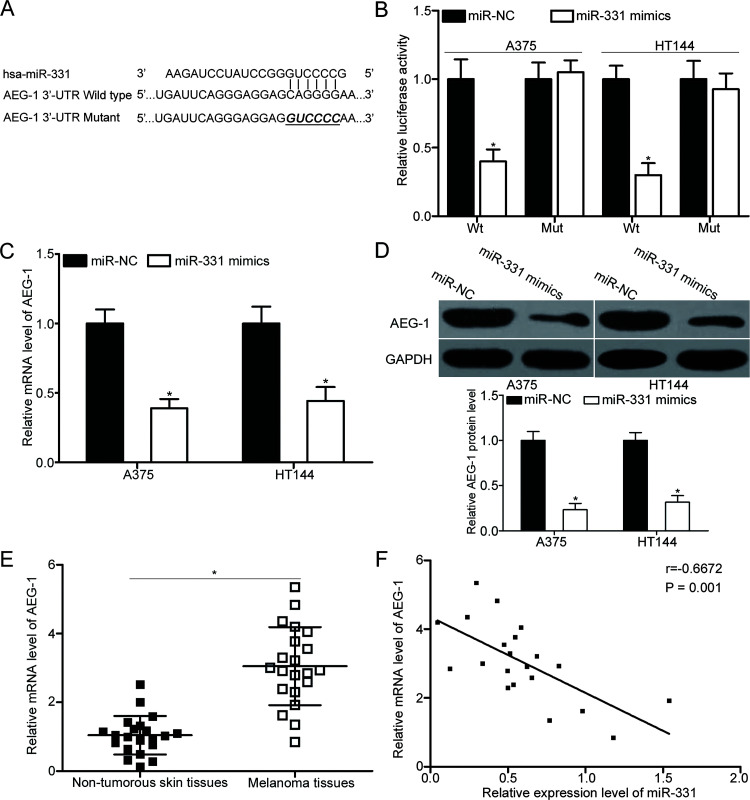
To delineate the molecular mechanism underlying the tumor-suppressive roles of miR-331 in melanoma, candidate targets were predicted using bioinformatics analysis. AEG-1 was predicted as a potential target of miR-331 (Fig. 3A). To confirm whether AEG-1 is a direct target of miR-331, dual-luciferase reporter assays were conducted in A375 and HT144 cells transfected with miR-331 mimics or miR-NC together with the luciferase reporter plasmid. As illustrated in Figure 3B, luciferase activity was significantly reduced in the A375 and HT144 cells cotransfected with miR-331 mimic and pMIR-AEG-1-3′-UTR Wt (p < 0.05). By contrast, the luciferase activity of pMIR-AEG-1-3′-UTR Mut was not altered in the cells cotransfected with miR-331 mimic. AEG-1 expression in A375 and HT144 cells after transfection with miR-331 mimic or miR-NC was measured using RT-qPCR and Western blot to verify the regulatory effect of miR-331 on endogenous AEG-1 expression. In comparison with the miR-NC group, miR-331 upregulation significantly decreased AEG-1 expression in A375 and HT144 cells at the mRNA (p < 0.05) (Fig. 3C) and protein (p < 0.05) (Fig. 3D) levels.
Figure 3.
Astrocyte-elevated gene-1 (AEG-1) is a direct target of miR-331 in melanoma. (A) Predicted binding sites of wild-type (Wt) and mutated (Mut) sequences of miR-331 in the 3′-untranslated region (3′-UTR) of AEG-1. (B) Wt or Mut AEG-1 3′-UTR luciferase reporter plasmid was transfected into A375 and HT144 cells together with miR-331 mimic or miR-NC. Luciferase activity was analyzed with a dual-luciferase reporter assay system. *p < 0.05 compared with miR-NC. A375 and HT144 cells were transfected with miR-331 mimic or miR-NC. The mRNA and protein levels of AEG-1 were detected by (C) RT-qPCR and (D) Western blot analysis, respectively. *p < 0.05 compared with miR-NC. (E) RT-qPCR was carried out to measure AEG-1 expression in 22 pairs of human primary melanoma tissues and adjacent nontumorous skin specimens. *p < 0.05 compared with nontumorous skin specimens. (F) The association between miR-331 and AEG-1 mRNA level was evaluated in melanoma tissues using Spearman’s correlation analysis. r = −0.6672, p = 0.001.
We further determined AEG-1 mRNA expression in 22 pairs of human primary melanoma tissues and adjacent nontumorous skin specimens using RT-qPCR. Results revealed that the mRNA level of AEG-1 was higher in melanoma tissues than in adjacent nontumorous skin specimens (p < 0.05) (Fig. 3E). Moreover, a significant negative association was found between miR-331 and AEG-1 mRNA level in melanoma tissues (r = −0.6672, p = 0.001) (Fig. 3F), suggesting that the upregulation of AEG-1 in melanoma may, at least in part, be due to the downregulation of miR-331. Collectively, AEG-1 is a direct target of miR-331 in melanoma.
Restored AEG-1 Expression Reverses the Suppressive Effects of miR-331 on the Proliferation and Invasion of Melanoma Cells
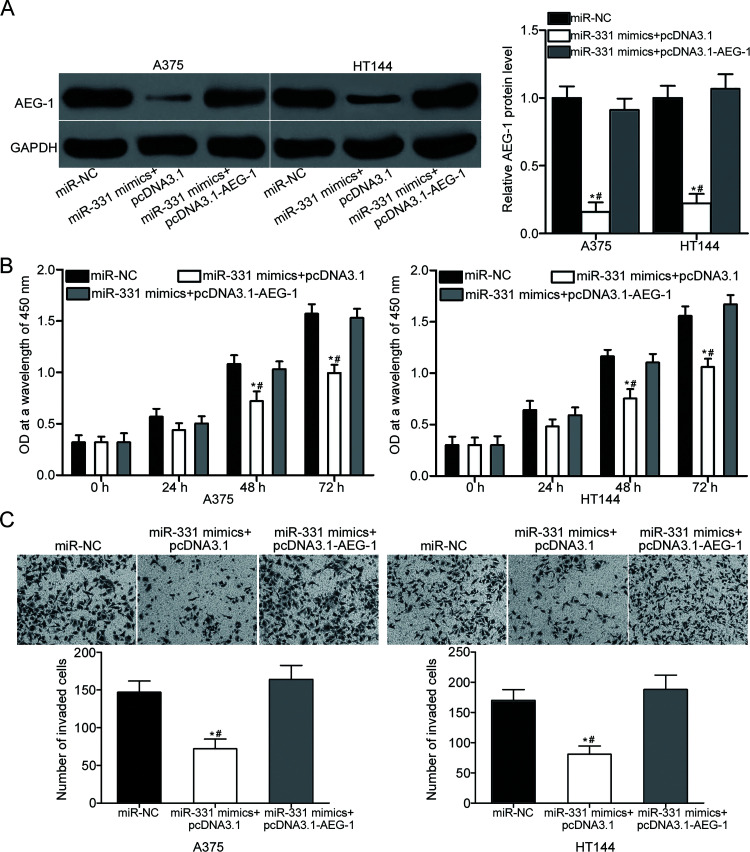
Rescue experiments were employed to confirm whether the tumor-suppressing roles of miR-331 on the proliferation and invasion of melanoma cells are mediated by AEG-1 downregulation. A375 and HT144 cells were cotransfected with miR-331 mimics and AEG-1 overexpression plasmid pcDNA3.1-AEG-1 or empty pcDNA3.1 plasmid. Following transfection, Western blot confirmed that the cotransfection with pcDNA3.1-AEG-1 restored the miR-331 overexpression-induced decrease in AEG-1 protein expression in A375 and HT144 cells (p < 0.05) (Fig. 4A). Furthermore, functional assays revealed that the restoration of AEG-1 expression in A375 and HT144 cells rescued the inhibition of cell proliferation (p < 0.05) (Fig. 4B) and invasion (p < 0.05) (Fig. 4C) caused by miR-331 mimic. These results demonstrate that miR-331 plays tumor-suppressive roles in melanoma progression, at least in part, by inhibiting AEG-1 expression.
Figure 4.
Reintroduction of AEG-1 partially abrogates the tumor-suppressive effects of miR-331 overexpression on the proliferation and invasion of A375 and HT144 cells. (A) Western blot analysis of AEG-1 protein levels in A375 and HT144 cells transfected with miR-331 mimics in the presence of pcDNA3.1 or pcDNA3.1-AEG-1. *p < 0.05 compared with miR-NC. #p < 0.05 compared with miR-331 mimics + pcDNA3.1-AEG-1. A375 and HT144 cells were cotransfected with miR-331 mimic and pcDNA3.1 or pcDNA3.1-AEG-1. Then CCK-8 and Transwell invasion assays were used to determine (B) cell proliferation and (C) invasion abilities. *p < 0.05 compared with miR-NC. #p < 0.05 compared with miR-331 mimics + pcDNA3.1-AEG-1.
miR-331 Suppresses the PTEN/AKT Signaling Pathway in Melanoma via AEG-1
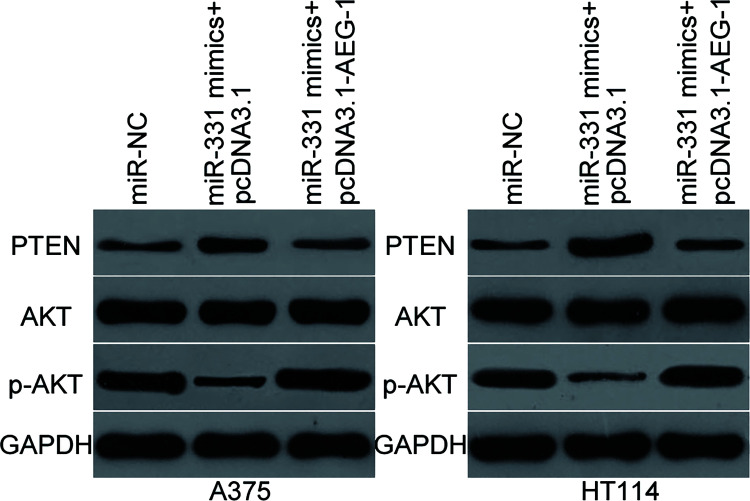
Previous studies reported that AEG-1 contributes to the regulation of the PTEN/AKT signaling pathway20,21. Hence, Western blot was utilized to detect PTEN, AKT, and p-AKT expression in the A375 and HT144 cells cotransfected with miR-331 mimic and pcDNA3.1 or pcDNA3.1-AEG-1. Results indicated that resumed expression of miR-331 increased PTEN expression and suppressed p-AKT expression. However, the total expression of AKT was unaltered compared with those in the cells transfected with miR-NC. Furthermore, the expression levels of p-AKT were recovered in the A375 and HT144 cells cotransfected with pcDNA3.1-AEG-1 (Fig. 5). These results suggest that miR-331 inhibits the activation of the PTEN/AKT signaling pathway through downregulation of AEG-1.
Figure 5.
miR-331 targets AEG-1 to inhibit the activation of the phosphatase and tensin homolog (PTEN)/AKT signaling pathway in melanoma. A375 and HT144 cells were cotransfected with miR-331 mimic and pcDNA3.1 or pcDNA3.1-AEG-1. At 72 h after transfection, total protein was extracted and subjected to Western blot analysis to detect PTEN, AKT, and p-AKT expression levels.
DISCUSSION
Melanoma is characterized by aggressive invasion, early metastasis, and resistance to existing chemotherapeutic agents22. Accumulated studies have reported that miRNA is a potentially robust molecular tool for developing future therapeutic technologies23–25. Therefore, examining the expression patterns, biological roles, and associated mechanisms of cancer-related miRNAs in melanoma is essential for developing novel therapeutic targets for patients with this disease. In the present work, miR-331 was significantly downregulated in melanoma tissues and cell lines. Enforced expression of miR-331 attenuated the proliferation and invasion of melanoma cells. In addition, AEG-1 was validated as a direct target of miR-331 through bioinformatics analysis, RT-qPCR, Western blot, dual-luciferase reporter assay, and Spearman’s correlation analysis. Furthermore, restored AEG-1 expression rescued the suppressive effects of miR-331 on the proliferation and invasion of melanoma cells. Moreover, miR-331 inhibited the activation of the PTEN/AKT signaling pathway through inhibition of AEG-1. Collectively, these data suggest that miR-331 inhibits the progression of melanoma by regulating the AEG-1/PTEN/AKT pathway.
Deregulation of miR-331 has been implicated in multiple types of human cancer. For example, miR-331 is upregulated in hepatocellular carcinoma tissues, cell lines, and serum15,16. Increased expression of miR-331 in serum is strongly correlated with the TNM stage of patients with hepatocellular carcinoma15. Hepatocellular carcinoma patients with high miR-331 expression have shorter long-term survival than those with low miR-331 level16. miR-331 expression is overexpressed in the bone marrow specimens of patients with acute myeloid leukemia than in those of healthy subjects. Upregulation of miR-331 in acute myeloid leukemia is significantly correlated with a worse response to therapy and short survival17. Nevertheless, miR-331 is reportedly downregulated in prostate cancer. Decreased miR-331 expression was associated with Gleason Score, tumor stage, lymph node involvement, and prostate-specific antigen value18. Underexpressed miR-331 was also observed in glioblastoma26, gastric cancer27, and colorectal cancer28. These conflicting results indicate that the expression pattern of miR-331 in human malignancy is tissue specific.
miR-331 is reportedly involved in tumorigenesis. For instance, previous research found that the ectopic expression of miR-331 promotes cell growth and metastasis in vitro and in vivo16,29. However, miR-331 serves as a tumor suppressor in prostate cancer through inhibiting cell growth and metastasis in vitro, as well as xenograft tumor initiation, growth, and survival in vivo18. Epis et al. verified that the resumption expression of miR-331 represses glioblastoma cell proliferation and clonogenic growth26. Guo et al. revealed that miR-331 overexpression suppresses cell proliferation and colony formation, and induces G1/S transition in gastric cancer27. Zhao et al. showed that the restoration of miR-331 expression attenuates the proliferation and promotes the apoptosis of colorectal cancer cells28. These conflicting studies suggest that the biological roles of miR-331 in cancer carcinogenesis and progression are tissue specific and that miR-331 could serve as a therapeutic target for the treatment of these specific cancer types.
Several targets of miR-331 have been identified, including PH domain and leucine-rich repeat protein phosphatase (PHLPP)16 and inhibitor of growth family member 5 (ING5)29 in hepatocellular carcinoma; Ras-like proto-oncogene A(RALA)18, phospholipase C γ 1 (PLCγ1)18, and erythroblastic oncogene-B2 receptor tyrosine kinase 2 (ERBB-2)30 in prostate cancer; neuropilin-2 (NRP-2) in glioblastoma26; E2F127 in gastric cancer; and human epidermal growth factor receptor 2 (HER2)28 in colorectal cancer. In the present study, AEG-1 was confirmed as a novel target of miR-331 in melanoma. AEG-1, located on chromosome 8q22, is overexpressed in numerous types of human cancer, such as breast cancer31, laryngeal squamous cell carcinoma32, gastric cancer33, glioma,34 and cervical cancer35. AEG-1 serves important roles in tumor development and maintenance through the regulation of cancer-related biological processes, including cell proliferation, apoptosis, cell cycle, invasion, metastasis, and epithelial-to-mesenchymal transition32–34. AEG-1 is also highly expressed in melanoma. AEG-1 knockdown reduces melanoma cell proliferation, migration, and invasion; blocks cell cycle progression in vitro; and decreases tumor growth in vivo35. These findings suggest that targeting AEG-1 shows a potential to be developed as a therapeutic strategy for patients with melanoma.
In conclusion, miR-331 was underexpressed in melanoma tissues and cell lines. Upregulation of miR-331 repressed melanoma progression by directly targeting AEG-1 and regulating the PTEN/AKT signaling pathway. Therefore, miR-331 may be a therapeutic target in the treatment of patients with this malignancy.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Trotter SC, Sroa N, Winkelmann RR, Olencki T, Bechtel M. A global review of melanoma follow-up guidelines. J Clin Aesthet Dermatol. 2013;6:18–26. [PMC free article] [PubMed] [Google Scholar]
- 2. Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129:1666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. [DOI] [PubMed] [Google Scholar]
- 4. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- 5. Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature 2007;445:851–7. [DOI] [PubMed] [Google Scholar]
- 6. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. [DOI] [PubMed] [Google Scholar]
- 7. Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004;116:281–97. [DOI] [PubMed] [Google Scholar]
- 8. Treiber T, Treiber N, Meister G. Regulation of microRNA biogenesis and function. Thromb Haemost. 2012;107:605–10. [DOI] [PubMed] [Google Scholar]
- 9. Xing F, Wu K, Watabe K. MicroRNAs in cancer stem cells: New regulators of stemness. Curr Pharm Des. 2014;20:5319–27. [DOI] [PubMed] [Google Scholar]
- 10. Davis-Dusenbery BN, Hata A. Mechanisms of control of microRNA biogenesis. J Biochem. 2010;148:381–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spizzo R, Nicoloso MS, Croce CM, Calin GA. SnapShot: MicroRNAs in Cancer. Cell 2009;137:586e581. [DOI] [PubMed] [Google Scholar]
- 12. Assumpcao MB, Moreira FC, Hamoy IG, Magalhaes L, Vidal A, Pereira A, Burbano R, Khayat A, Silva A, Santos S, Demachki S, Ribeiro-Dos-Santos A, Assumpcao P. High-throughput miRNA sequencing reveals a field effect in gastric xancer and suggests an epigenetic network mechanism. Bioinform Biol Insights 2015;9:111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen W, Zhang J, Xu H, Dai J, Zhang X. The negative regulation of miR-149-5p in melanoma cell survival and apoptosis by targeting LRIG2. Am J Transl Res. 2017;9:4331–40. [PMC free article] [PubMed] [Google Scholar]
- 14. Bu P, Luo C, He Q, Yang P, Li X, Xu D. MicroRNA-9 inhibits the proliferation and migration of malignant melanoma cells via targeting sirituin 1. Exp Ther Med. 2017;14:931–8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15. Chen L, Chu F, Cao Y, Shao J, Wang F. Serum miR-182 and miR-331-3p as diagnostic and prognostic markers in patients with hepatocellular carcinoma. Tumour Biol. 2015;36:7439–47. [DOI] [PubMed] [Google Scholar]
- 16. Chang RM, Yang H, Fang F, Xu JF, Yang LY. MicroRNA-331-3p promotes proliferation and metastasis of hepatocellular carcinoma by targeting PH domain and leucine-rich repeat protein phosphatase. Hepatology 2014;60:1251–63. [DOI] [PubMed] [Google Scholar]
- 17. Butrym A, Rybka J, Baczynska D, Tukiendorf A, Kuliczkowski K, Mazur G. Expression of microRNA-331 can be used as a predictor for response to therapy and survival in acute myeloid leukemia patients. Biomark Med. 2015;9:453–60. [DOI] [PubMed] [Google Scholar]
- 18. Epis MR, Giles KM, Beveridge DJ, Richardson KL, Candy PA, Stuart LM, Bentel J, Cohen RJ, Leedman PJ. miR-331-3p and aurora kinase inhibitor II co-treatment suppresses prostate cancer tumorigenesis and progression. Oncotarget 2017;8:55116–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 20. Xu C, Kong X, Wang H, Zhang N, Kong X, Ding X, Li X, Yang Q. MTDH mediates estrogen-independent growth and tamoxifen resistance by down-regulating PTEN in MCF-7 breast cancer cells. Cell Physiol Biochem. 2014;33:1557–67. [DOI] [PubMed] [Google Scholar]
- 21. Li WF, Ou Q, Dai H, Liu CA. Lentiviral-mediated short hairpin RNA knockdown of MTDH inhibits cell growth and induces apoptosis by regulating the PTEN/AKT pathway in hepatocellular carcinoma. Int J Mol Sci. 2015;16:19419–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. [DOI] [PubMed] [Google Scholar]
- 23. Vaes E, Khan M, Mombaerts P. Statistical analysis of differential gene expression relative to a fold change threshold on NanoString data of mouse odorant receptor genes. BMC Bioinformatics 2014;15:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ballester M, Cordon R, Folch JM. DAG expression: High-throughput gene expression analysis of real-time PCR data using standard curves for relative quantification. PLoS One 2013;8:e80385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Iterson M, t Hoen PA, Pedotti P, Hooiveld GJ, den Dunnen JT, van Ommen GJ, Boer JM, Menezes RX. Relative power and sample size analysis on gene expression profiling data. BMC Genomics 2009;10:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Epis MR, Giles KM, Candy PA, Webster RJ, Leedman PJ. miR-331-3p regulates expression of neuropilin-2 in glioblastoma. J Neurooncol. 2014;116:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guo X, Guo L, Ji J, Zhang J, Zhang J, Chen X, Cai Q, Li J, Gu Q, Liu B, Zhu Z, Yu Y. miRNA-331-3p directly targets E2F1 and induces growth arrest in human gastric cancer. Biochem Biophys Res Commun. 2010;398:1–6. [DOI] [PubMed] [Google Scholar]
- 28. Zhao D, Sui Y, Zheng X. MiR-331-3p inhibits proliferation and promotes apoptosis by targeting HER2 through the PI3K/Akt and ERK1/2 pathways in colorectal cancer. Oncol Rep. 2016;35:1075–82. [DOI] [PubMed] [Google Scholar]
- 29. Cao Y, Chen J, Wang D, Peng H, Tan X, Xiong D, Huang A, Tang H. Upregulated in hepatitis B virus-associated hepatocellular carcinoma cells, miR-331-3p promotes proliferation of hepatocellular carcinoma cells by targeting ING5. Oncotarget 2015;6:38093–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Epis MR, Giles KM, Barker A, Kendrick TS, Leedman PJ. miR-331-3p regulates ERBB-2 expression and androgen receptor signaling in prostate cancer. J Biol Chem. 2009;284:24696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu Y, Su Z, Li G, Yu C, Ren S, Huang D, Fan S, Tian Y, Zhang X, Qiu Y. Increased expression of metadherin protein predicts worse disease-free and overall survival in laryngeal squamous cell carcinoma. Int J Cancer 2013;133:671–9. [DOI] [PubMed] [Google Scholar]
- 32. Wang J, Chen X, Tong M. Knockdown of astrocyte elevated gene-1 inhibited cell growth and induced apoptosis and suppressed invasion in ovarian cancer cells. Gene 2017;616:8–15. [DOI] [PubMed] [Google Scholar]
- 33. Tong L, Chu M, Yan B, Zhao W, Liu S, Wei W, Lou H, Zhang S, Ma S, Xu J, Wei L. MTDH promotes glioma invasion through regulating miR-130b-ceRNAs. Oncotarget 2017;8:17738–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gnosa S, Capodanno A, Murthy RV, Jensen LD, Sun XF. AEG-1 knockdown in colon cancer cell lines inhibits radiation-enhanced migration and invasion in vitro and in a novel in vivo zebrafish model. Oncotarget 2016;7:81634–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang Y, Peng G, Wang Y, Cui L, Wu W, Wang L, Liu C, Han X. Silencing of astrocyte elevated gene-1 inhibits proliferation and migration of melanoma cells and induces apoptosis. Clin Exp Pharmacol Physiol. 2017;44:815–26. [DOI] [PubMed] [Google Scholar]