Abstract
FMS-like tyrosine kinase-3 fragments from exon 14 to the end without any mutations or deletions have been reported to fuse to ETV6 (TEL) in a few cases of myeloid/lymphoid neoplasms with eosinophilia carrying a translocation t(12;13)(p13;q12). This fusion protein confers constitutive activation on the FLT3 fragment and induces factor-independent growth in transfected Ba/F3 cells, indicating that it is an oncoprotein. However, the mechanism controlling the stability of this oncoprotein is unknown. In this study, we focus on finding factors controlling the stability of ETV6/FLT3. We have shown that the stability of ETV6/FLT3 is regulated by the Hsp90 chaperone. ETV6/FLT3 fusion protein forms a complex with Hsp90 by coimmunoprecipitation analyses using an Hsp90 antibody. The association between ETV6/FLT3 fusion protein and Hsp90 was impaired after treating ETV6/FLT3 transient transfection cos7 cells with 17-allylamino-17-demethoxygeldanamycin (17-AAG). 17-AAG induced a time- and dose-dependent downregulation of ectopically expressed ETV6/FLT3 protein in cos7 and HeLa-transfected cells. By using cycloheximide to block new protein translation, we found that 17-AAG accelerated the decay of ETV6/FLT3. Our findings could contribute to more understanding of the ETV6/FLT3 regulation through Hsp90 chaperone and open the way to finding effective treatment strategies for this rare disease.
Key words: ETS-translocation variant 6 (ETV6)/FMS-like tyrosine kinase-3 (FLT3), Tel/FLT3, Heat shock protein 90 (Hsp90)
INTRODUCTION
FMS-like tyrosine kinase-3 (FLT3) is one of the many single genes mutated in hematologic malignancies1. In a small number of myeloid/lymphoid neoplasms with eosinophilia (MLN-eo) and carrying the t(12;13)(p13;q12) translocation, FLT3 is detected to be fused to ETS-translocation variant 6 (ETV6 or TEL)2–5.
ETV6/FLT3 acts as a constitutively active tyrosine kinase by inducing interleukin-3 (IL-3)-independent growth of murine hematopoietic Ba/F3 cells2,6. Moreover, mice injected with Ba/F3 cells expressing ETV6/FLT3 developed leukemia4,7. In another study, ETV6/FLT3 has been reported to possess more oncogenic activity than FLT3 with internal tandem duplication4. These studies support the importance of ETV6/FLT3 in oncology research.
Effective treatment for patients expressing ETV6/FLT3 has not been shown. FLT3 inhibitor treatment does not respond well in patients with this fusion; relapse and resistance occurred in both patients studied3. Allogeneic stem cell transplantation seems to be the best option to achieve long-term survival5. Therefore, more effective treatment strategies are needed for this subgroup of leukemia.
The importance of ETV6/FLT3 as an oncoprotein has been shown, as indicated above. However, the mechanism to control the stability of this protein is unknown. Recently, heat shock protein 90 (Hsp90), a chaperone protein that stabilizes other proteins against heat stress, has been demonstrated to stabilize a number of proteins required for tumor growth, making it a potential target as an anticancer drug.
In this study, we investigate whether ETV6/FLT3 stabilization is controlled by Hsp90. We report for the first time that ETV6/FLT3 is a novel client of Hsp90. Inhibition of the chaperone Hsp90 by 17-(allylamino)-17-demethoxygeldanamycin (17-AAG) or (−)-epigallocatechin gallate (EGCG) could suppress or decrease the expression of ETV6/FLT3 and the growth of 32D cells stably expressing ETV6/FLT3. Our finding could contribute to a better understanding of ETV6/FLT3 regulation through Hsp90 chaperone and open the way to finding effective treatment strategies for this rare disease.
MATERIALS AND METHODS
Cell Lines and Culture Conditions
Parental 32D cells were cultured in RPMI-1640 medium (Sigma-Aldrich, Japan K.K., Tokyo, Japan). 293FT, cos7, and HeLa cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich) as described in detail previously8.
Generation of 32D Cells Expressing ETV6/FLT3
32D cells stably expressing ETV6/FLT3 (32D-EF1) were generated as described previously6. 32D-EF1 cells were maintained in RPMI-1640 medium containing 10% FBS in the absence of IL-3, mouse recombinant (rmIL-3).
Transient Transfection
For transient expression in 293FT, cos7, and HeLa cells, cells were transfected with pcDNA3.1-EF1 plasmid using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Briefly, cells were seeded in six-well plates at a density of 1.5 × 105 cells/well in 2 ml of complete medium and grown for 24 h until they were 70%–80% confluent. DNA was diluted in 250 μl of Opti-MEM I (Reduced Serum Medium; Invitrogen). Lipofectamine 2000 was diluted in 250 μl of Opti-MEM I. Mixtures were incubated for 5 min and then combined together for a further 20 min. Complexes were added to the cells containing 2 ml of complete medium and incubated. Cells were harvested 48 h after transfection for immunoprecipitation and immunoblotting.
Cell Proliferation Assays
Cell proliferation was determined by trypan blue dye exclusion test as described previously8. Briefly, 32D-EF1 cells at a density of 1 × 105 cells/ml were treated with 17-AAG (12.5, 25, and 50 nM) or dimethyl sulfoxide (DMSO) alone as control for 72 h. After the treatment, 10 μl of the cell suspension was mixed with 10 μl of 0.4% trypan blue, and live cells were counted manually using a hemacytometer. Results were calculated as the percentage of the values measured when cells were grown in the absence of 17-AAG.
Reagents
EGCG was a gift from Dr. Yukihiko Hara (Japan), and 17-AAG was purchased from Calbiochem (Darmstadt, Germany). All reagents were dissolved in DMSO (Wako Pure. Chemical Industries, Osaka, Japan). Controlled cells were cultured with the same concentration of carrier DMSO as used in the highest dose of reagents. The concentration of DMSO was kept under 0.1% throughout all the experiments to avoid cytotoxicity.
Western Blot Analysis
Cells were plated onto 10-cm dishes at a density of 1 × 105 cells/ml in the presence of various concentrations of reagents. After incubation for indicated durations, cells were collected and washed twice with PBS(−). Cells were then dissolved in a protein lysis buffer containing 5 mM EDTA, 50 mM NaF, 10 mM Na2H2P2O7, 0.01% Triton X-100, 5 mM HEPES, 150 mM NaCl, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, and 75 μg/ml aprotinin on ice for 30 min with a brief vortex of four times every 10 min. After centrifugation at 13,000 rpm at 4°C for 10 min, total cell lysates were collected. Protein samples were electrophoresed through a polyacrylamide gel and transferred to a Hypond-P membrane (Amersham, Buckinghamshire, UK) by electroblotting. After washing, the membrane was probed with antibodies, and antibody binding was detected using the BCIP/NBT substrate (Promega, Tokyo, Japan). The following antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA): FLT3/FLK-2 (S-18) (sc-480), anti-actin (AC-15) (sc-69879), and Hsp90α/β (F-8) (sc-13119). FLT3 (8F2) and normal rabbit IgG (#2729S) antibodies were purchased from Cell Signaling Technology Japan (Tokyo, Japan). Phosphotyrosine clone 4G10 antibody was purchased from Millipore (Tokyo, Japan). IgG from murine serum (I5381-5MG) antibody was obtained from Sigma-Aldrich.
Immunoprecipitation (IP) Analysis
For IP, cells were lysed as indicated above. Cell lysates were then precleared for reducing the amount of nonspecific contaminants with 50 μl of protein G-Sepharose 4 fast flow (Amersham Pharmacia Biosciences, Tokyo, Japan) to a total volume of 500 μl. After incubation on a rotator for 1 h at 4°C, supernatants were collected by centrifuging at 2,500 × g for 2–3 min at 4°C. A portion of the lysed samples was immunoprecipitated with FLT3 (F-8) or Hsp90 antibodies in an incubation buffer (10 mM Tris, pH 7.5, 5 mM MgCl2; 50 mM KCl and 0.01% Nonidet P-40) for 1 h or overnight at 4°C. Protein G-Sepharose 4 fast flow (Amersham Pharmacia Biosciences) was then added for 1 h. The immunoprecipitates were washed five times with Tris-buffered saline-Tween. The bound proteins were resolved by SDS-PAGE and analyzed by Western blotting.
Statistical Analysis
All data were expressed as the mean ± standard deviation. Statistical analyses were done using Student’s t-test, in which a value of p < 0.05 was the minimum requirement for a statistically significant difference.
RESULTS AND DISCUSSION
ETV6/FLT3 Is a Novel Client of Hsp90
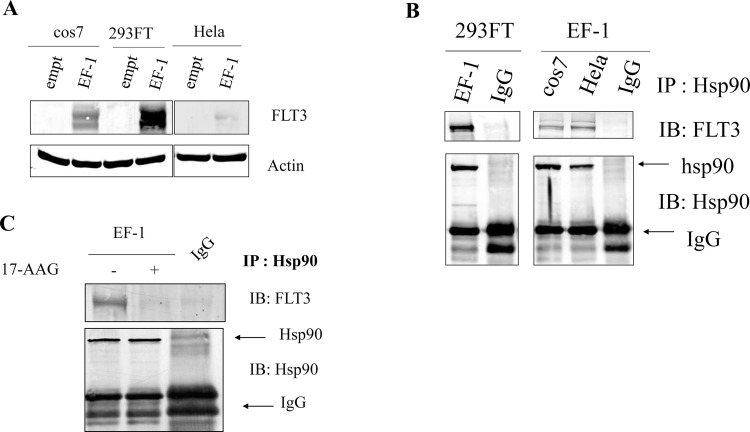
Because FLT3-ITD has been demonstrated to be a client protein of Hsp90 in cell models and primary AML cells8–12, we aimed to determine whether ETV6/FLT3 requires Hsp90 to maintain its structural stability. To test this hypothesis, the cell lysates of the 293FT, cos7, and HeLa cell lines expressing ETV6/FLT3 (EF-1) were isolated and coimmunoprecipitated (co-IP) with Hsp90 antibody; the precipitate was then immunoblotted (IB) with FLT3 antibody.
Parental 293FT, cos7, and HeLa cells do not express FLT3. In contrast, cells transfected with EF-1 strongly expressed EF-1 (Fig. 1A). To demonstrate a protein being a client of Hsp90, it is important to show that the protein forms a complex with Hsp90. In this study, we found that EF-1 could bind to Hsp90 (co-IP results) (Fig. 1B). These first data suggest that EF-1 could be a client of Hsp90 and prompted us to investigate whether inhibition of Hsp90 could affect EF-1 expression.
Figure 1.
ETS-translocation variant 6 (ETV6)/FMS-like tyrosine kinase-3 (FLT3) could coimmunoprecipitate with heat shock protein 90 (Hsp90) in 293FT, cos7, and HeLa cells transiently expressing ETV6/FLT3. 293FT, cos7, and HeLa cells were transfected with the ETV6/FLT3 (EF-1) plasmids using Lipofectamine 2000 according to the manufacturer’s instructions. Cells were harvested after 48-h transfection. (A) Total cell lysates were tested for the expression of ETV6/FLT3 by immunoblotting (IB) using FLT3 (F-8) antibody. (B) Total cell lysates were immunoprecipitated with Hsp90 antibody. Precipitated proteins were subjected to IB analysis with FLT3 antibody and Hsp90 antibody. (C) Effect of 17-allylamino-17-demethoxygeldanamycin (17-AAG) on the expression of ETV6/FLT3. cos7-EF1 cells were treated with or without 17-AAG (1 μM) for 5 h. Cell lysates were prepared and subjected to immunoprecipitation with Hsp90 antibody. Then IB was performed using FLT3 antibody to detect ETV6/FLT3 and Hsp90 antibody to detect total Hsp90.
Inhibition of Hsp90 Chaperone Activity by 17-AAG Results in Suppression of ETV6/FLT3 Expression
17-AAG, an Hsp90 inhibitor, has been used to inhibit Hsp90 function, alter the composition of the chaperone complex, and induce recruitment of E3 ubiquitin ligases, thereby targeting client protein to proteasome degradation. In Figure 1C, we have tested the effect of 17-AAG on the expression of ETV6/FLT3. After treatment with 17-AAG (1 μM) for 5 h in cos7 cells expressing EF-1, we found that the expression of EF-1 in 17-AAG-treated cells was significantly lower than that in the control cells. However, the expression of Hsp90 in the treated and control cells was not different (Fig. 1C). This suggests that the suppression of EF-1 expression is due to inhibition of Hsp90 function.
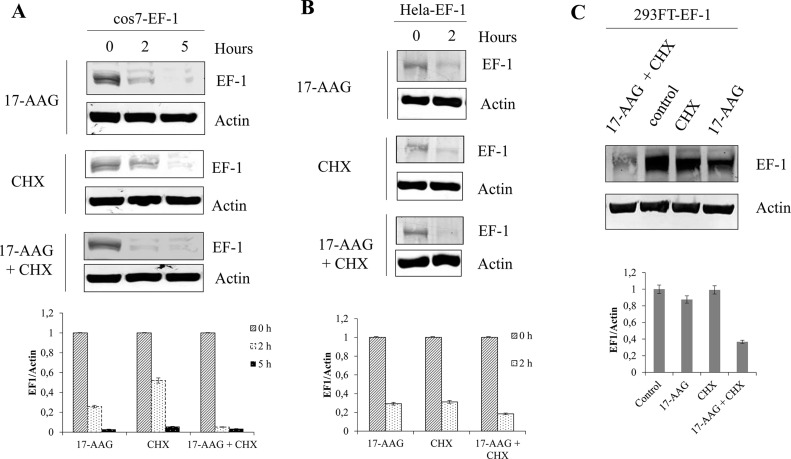
In order to confirm the hypothesis that EF-1 is a client of Hsp90, cell lines including 293FT, cos7, and HeLa expressing EF-1 were exposed to 17-AAG or the protein synthesis inhibitor cycloheximide (CHX) alone or 17-AAG combined with CHX for 2 h. The expression of EF-1 was detected by Western blotting. In all the cell lines tested, 17-AAG treatment reduced the expression of EF-1 (Fig. 2). Treatment of cells with CHX combined with 17-AAG revealed that 17-AAG facilitated degradation of EF-1 (Fig. 2). These data could support the fact that EF-1 is a client of Hsp90.
Figure 2.
17-AAG accelerated the decay of ETV6/FLT3. 293FT, cos7, and HeLa cells were transfected with the EF-1 plasmids using Lipofectamine 2000 according to the manufacturer’s instructions. After 24 h of transfection, cells at a density of 1 × 105 cells/ml were treated with 17-AAG (1 μM) or cycloheximide (CHX; 100 μg/ml) or both of these agents or dimethyl sulfoxide (DMSO) alone as control for 2 h in cos7-EF-1 cells (A), in HeLa-EF-1 cells (B), and in 293FT-EF-1 cells (C). Total cell lysates were subjected to Western blot analysis with indicated antibodies. Relative expression levels of ETV6/FLT3, determined by densitometric analysis, are shown (lower rows).
To date, hundreds of proteins have been shown to be clients of Hsp90. As mentioned previously, FLT3-ITD has been demonstrated to be a client of Hsp908–12. However, whether EF-1 is also a client of Hsp90 has not been demonstrated. In this study, we demonstrate that Hsp90 could interact with EF-1 (Fig. 1B) and inhibition of Hsp90 activity causes degradation of EF-1 (Figs. 1C and 2). It is important to note that most Hsp90 clients are involved in oncogenesis; therefore, targeting Hsp90 appears to be a novel strategy for treatment. Since EF-1 was discovered a decade ago, an effective therapy (targeted therapy) for the treatment of leukemic patients with EF-1 has not been found. Using FTL3 inhibitors for the treatment of patients harboring EF-1 seems to be not effective3. Based on our finding, Hsp90 could be considered as a novel target for the development of an effective treatment in patients with ETV6/FLT3.
Hsp90 Inhibitors Suppressed Proliferation of 32D Cells Stably Expressing ETV6/FLT3
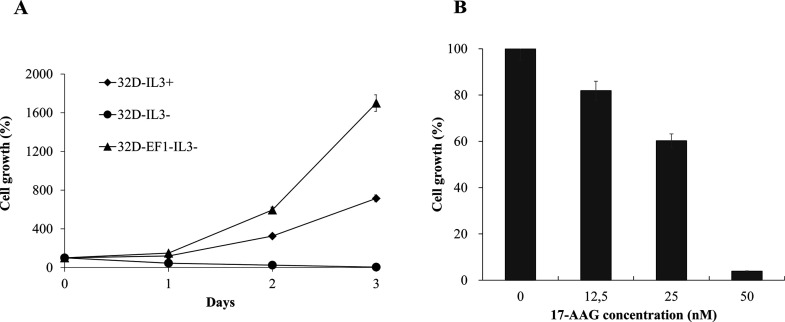
Other reports have demonstrated that EF-1 is an oncoprotein2–5. We have confirmed again that EF-1 could transform 32D cells (Fig. 3A). Parental 32D cells without expression of EF-1 need to provide IL-3 supplementation to grow. However, 32D cells stably expressing EF-1 could survive without IL-3 supplementation, suggesting the importance of EF-1 on oncogenesis.
Figure 3.
17-AAG and (−)-epigallocatechin gallate (EGCG) suppressed proliferation of 32D-EF1 cells. 32D cells were stably transfected with EF-1 construct, and the proliferation of 32D cells expressing EF-1 was compared with parental 32D cells cultured in medium with or without interleukin-3 (IL-3) supplementation (A). 32D-EF1 cells at a density of 1 × 105 cells/ml were treated with an indicated concentration of 17-AAG or DMSO alone as control for 72 h. The number of live cells was counted after trypan blue exclusion test. Results were calculated as the percentage of the control values (B).
In this study, we tested the inhibitory effect of 17-AAG on the growth of 32D cells stably expressing EF-1 (32D-EF1) (Fig. 3B). The cells were incubated with either the carrier DMSO alone (control) or different concentrations of 17-AAG for 72 h. Cell proliferations were evaluated using the trypan blue exclusion test. The result showed that the growth of 32D-EF1 cells was decreased when treated with 17-AAG (Fig. 3B). Increasing the concentration of 17-AAG caused more growth inhibitory effect on 32D-EF1 cells (Fig. 3B). However, we observed that parental 32D cells stimulated by IL-3 are not sensitive to 17-AAG treatment even at 50 nM (data not shown). It suggests the usefulness of 17-AAG (Hsp90 inhibitor) on targeting growth inhibition.
Recently it was reported that sunitinib and sorafenib, tyrosine kinase inhibitors with multiple targets including FLT3, had therapeutic efficacy in two patients with ETV6/FLT3+ MLN-eo3. Unfortunately, similar to most of the patients with FLT3-ITD+ AML, relapse and resistance occurred in both patients3. Therefore, our finding could provide additional choices for the treatment of the rare ETV6/FLT3-related leukemia using Hsp90 inhibitors.
ACKNOWLEDGMENTS
We thank Dr. Hoang Anh Vu (Ho Chi Minh City, Vietnam) and Professor Yuko Sato (Tokyo, Japan) for providing the ETV6/FLT3 construct utilized in this study. This work was funded by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 106.02-2017.347.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer 2003;3(9):650–65. [DOI] [PubMed] [Google Scholar]
- 2. Vu HA, Xinh PT, Masuda M, Motoji T, Toyoda A, Sakaki Y, Tokunaga K, Sato Y. FLT3 is fused to ETV6 in a myeloproliferative disorder with hypereosinophilia and a t(12;13)(p13;q12) translocation. Leukemia 2006;20(8):1414–21. [DOI] [PubMed] [Google Scholar]
- 3. Walz C, Erben P, Ritter M, Bloor A, Metzgeroth G, Telford N, Haferlach C, Haferlach T, Gesk S, Score J, Hofmann WK, Hochhaus A, Cross NC, Reiter A. Response of ETV6-FLT3-positive myeloid/lymphoid neoplasm with eosinophilia to inhibitors of FMS-like tyrosine kinase 3. Blood 2011;118(8):2239–42. [DOI] [PubMed] [Google Scholar]
- 4. Chonabayashi K, Hishizawa M, Kawamata S, Nagai Y, Ohno T, Ishikawa T, Uchiyama T, Takaori-Kondo A. Direct binding of Grb2 has an important role in the development of myeloproliferative disease induced by ETV6/FLT3. Leukemia 2013;27(6):1433–6. [DOI] [PubMed] [Google Scholar]
- 5. Chonabayashi K, Hishizawa M, Matsui M, Kondo T, Ohno T, Ishikawa T, Takaori-Kondo A. Successful allogeneic stem cell transplantation with long-term remission of ETV6/FLT3-positive myeloid/lymphoid neoplasm with eosinophilia. Ann Hematol. 2014;93(3):535–7. [DOI] [PubMed] [Google Scholar]
- 6. Vu HA, Xinh PT, Kano Y, Tokunaga K, Sato Y. The juxtamembrane domain in ETV6/FLT3 is critical for PIM-1 up-regulation and cell proliferation. Biochem Biophys Res Commun. 2009;383(3):308–13. [DOI] [PubMed] [Google Scholar]
- 7. Tse KF, Mukherjee G, Small D. Constitutive activation of FLT3 stimulates multiple intracellular signal transducers and results in transformation. Leukemia 2000;14(10):1766–76. [DOI] [PubMed] [Google Scholar]
- 8. Ly BT, Chi HT, Yamagishi M, Kano Y, Hara Y, Nakano K, Sato Y, Watanabe T. Inhibition of FLT3 expression by green tea catechins in FLT3 mutated-AML cells. PLoS One 2013;8(6):e66378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oshikawa G, Nagao T, Wu N, Kurosu T, Miura O. c-Cbl and Cbl-b ligases mediate 17-allylaminodemethoxygeldanamycin-induced degradation of autophosphorylated Flt3 kinase with internal tandem duplication through the ubiquitin proteasome pathway. J Biol Chem. 2011;286(35):30263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taipale M, Krykbaeva I, Koeva M, Kayatekin C, Westover KD, Karras GI, Lindquist S. Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell 2012;150(5):987–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yao Q, Nishiuchi R, Li Q, Kumar AR, Hudson WA, Kersey JH. FLT3 expressing leukemias are selectively sensitive to inhibitors of the molecular chaperone heat shock protein 90 through destabilization of signal transduction-associated kinases. Clin Cancer Res. 2003;9(12):4483–93. [PubMed] [Google Scholar]
- 12. Minami Y, Kiyoi H, Yamamoto Y, Yamamoto K, Ueda R, Saito H, Naoe T. Selective apoptosis of tandemly duplicated FLT3-transformed leukemia cells by Hsp90 inhibitors. Leukemia 2002;16(8):1535–40. [DOI] [PubMed] [Google Scholar]