Abstract
Although cisplatin has been shown to be an integral part of chemotherapy regimen in osteosarcoma (OS) treatment, toxicity issues and chemoresistance have hindered therapeutic development for OS. Exploring novel combination therapy methods is needed to circumvent the limitations of cisplatin alone. The proteasome inhibitor MG132 has shown antitumor effects in many solid tumors. However, little is known about its effects in combination with cisplatin in OS cells. In this study, we examined the effects of MG132 in combination with cisplatin in human OS cells (MG-63 and HOS). MG132 and cisplatin were applied to OS cells, respectively or jointly. The results demonstrated that MG132 markedly inhibited cell viability in a dose- and time-dependent manner, whereas viability of osteoblast cells was not affected, suggesting a selective toxicity of MG132 to cancerous cells. Mechanistically, MG132 arrested cells in the G2/M phase in association with increased p21waf1 and induced cell apoptosis, which was accompanied by cleaved PARP. In addition to its apoptotic effect alone, MG132 significantly enhanced cisplatin-induced apoptosis in OS cells. Furthermore, cell viability of the combined application of 10 μM MG132 and 5 μg/ml cisplatin was markedly inhibited compared to that of the individual application. These events were accompanied by the downregulation of NF-κB, mitochondrial antiapoptotic protein Bcl-xL, and PI3K/Akt, which play a key role in cell survival. Finally, combination treatment of MG132 and cisplatin showed more antiproliferative effect than the single treatment in OS xenograft models. In summary, we concluded that MG132 interacted synergistically with cisplatin, which raised the possibility that combining the two drugs may represent a novel strategy in OS.
Key words: MG132, Osteosarcoma (OS), Cisplatin, Synergistic efficacy, Cell viability, Apoptosis
INTRODUCTION
Osteosarcoma (OS) is the most prevalent malignant bone tumor, mainly accounting for 56% of malignant bone cancers and 6% of all cancers in children and young adults1. The 5-year survival rate has increased to approximately 70% with standard treatment, including a combination of resection of the primary tumor, radiotherapy, and multiple chemotherapeutic agents2,3. Cisplatin is a DNA damage-inducing agent that is widely used for the treatment of solid tumors4. Most tumor cells are sensitive to the apoptotic effects induced by cisplatin. However, toxicity and drug resistance associated with chemotherapy are major impediments affecting its efficacy. Induction of apoptosis requires additional treatment with other chemotherapeutic agents that may damage normal cells. Therefore, novel, safe, and more effective adjuvant treatments are needed to complement current treatments and improve overall survival.
The ubiquitin–proteasome pathway is involved in the degradation of regulatory proteins that govern DNA repair, signal transduction, cell differentiation, and apoptosis5. Therefore, the proteasome represents a novel target for cancer therapy. OS cells have been reported to undergo apoptosis when treated with proteasome inhibitors6–8. Recent studies have reported that a variety of tumor cells can be sensitized to cisplatin-induced apoptosis by combining with proteasome inhibitors such as bortezomib9,10. In contrast to bortezomib, sensitivity of OS cells to MG132 and its synergistic effect with other agents have not been extensively studied.
In our study, we investigated the sensitivity of OS cells to MG132 and examined the efficacy of combination therapy with cisplatin and MG132. We demonstrated that MG132 potently inhibited OS cell proliferation, whereas viability of osteoblast cells was not affected. Mechanistically, MG132 induced G2/M arrest and cell apoptosis in OS cells. Furthermore, we found that MG132 significantly enhanced cisplatin-induced cell apoptosis and markedly inhibited cell viability when combined with cisplatin. We then demonstrated that the synergistic effects were accompanied by the downregulation of NF-κB and PI3K/Akt, which play a key role in cell survival. Furthermore, we found a synergistic antitumor effect of combined treatment with MG132 and cisplatin in xenograft models.
MATERIALS AND METHODS
Cell Culture
Human OS cell lines (MG-63 and HOS) and noncancerous osteoblast hFOB 1.19 cells were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, P.R. China). All cell lines were maintained at 37°C in a humidified incubator with 5% CO2 in Eagle’s minimum essential medium (Gibco Life Technologies, Grand Island, NY, USA), supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco, Rockville, MD, USA), 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA).
Reagents
Cisplatin was obtained from MCE (HaoYuan Chemexpress, Shanghai, P.R. China) and was dissolved in dimethyl sulfoxide (DMSO) at 1 mM. MG132 was purchased from Sigma Chemical Co. (St. Louis, MO, USA) and was dissolved in DMSO at 1 mM.
Western Blot Analysis
Proteins were extracted from cells using radioimmunoprecipitation assay (RIPA) lysis buffer containing protease inhibitor cocktail tablets (Roche, Mannheim, Germany) for 30 min on ice. Equivalent amounts of 20 μg of proteins were separated using 10% SDS-PAGE electrophoresis and transferred to nitrocellulose membranes. After blocking with 5% skim milk in Tween–Tris-buffered saline (TBST) for 1 h, the membranes were incubated with specific primary antibodies against poly(ADP-ribose) polymerase (PARP), p21waf1, Bcl-xL, Akt, p-Akt, β-actin, or GAPDH (Cell Signaling Technology, San Jose, CA, USA) at 4°C overnight. Subsequently, the membranes were washed with TBST three times and incubated with horseradish peroxidase-conjugated secondary antibodies (1:5,000; Cell Signaling Technology) for 1 h at room temperature. Protein bands were visualized by enhanced SuperSignal WestPico chemiluminescent (Pierce, Rockford, IL, USA); the relative protein expression levels were evaluated using Quantity One software.
CCK-8 Assay
The sensitivity of OS and osteoblast cells to MG132 and cell proliferation were detected using cell counting kit-8 (CCK-8; Dojindo Molecular Technologies, Kumamoto, Japan). Briefly, cells were seeded into 96-well plates at a density of 5 × 103 cells/well in 100 μl of medium in septuple and exposed to varying concentrations of MG132 and/or cisplatin or DMSO for the indicated times. At each time point, 10 μl of CCK-8 was added into each well. After 1 h of incubation, the absorbance at 450 nm was measured using a microplate reader (iMark; Bio-Rad Laboratories, Inc., Hercules, CA, USA), and a cell growth curve was produced. Sensitivity of cells was calculated as a percentage: (A MG132/A DMSO) × 100.
Flow Cytometry
Cell cycle distribution was evaluated by DNA content analysis using propidium iodide (PI) staining. After treating with DMSO or 10 μM MG132 for 24 h, cells were detached by trypsin digestion, washed with phosphate-buffered saline (PBS), and fixed with ice-cold 70% ethanol at −20°C overnight, and then stained with 50 μg/ml PI in the presence of RNase A at room temperature for 1 h. Intracellular DNA content was analyzed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). For each sample, 10,000 events were counted. The fractions of the cells in the G1, S, and G2 phases were analyzed using CELL Quest software. The experiment was repeated three times.
Treated cells were digested into suspension of single cells with EDTA-free trypsin and then washed twice with cold PBS. Cell apoptosis was evaluated by flow cytometry using an Annexin-V–FITC Apoptosis Detection Kit (KeyGen Biotech Co. Roche, Nanjing, P.R. China) according to the manufacturer’s instructions. FACSCalibur flow cytometer was used to quantify the level of cell apoptosis.
Morphology of Apoptotic Cells
The morphology of OS cells treated with DMSO, 10 μM MG132, and/or 5 μg/ml cisplatin for 24 h was directly observed with optical microscopy.
NF-κB–p65 ELISA Assay
For nuclear extraction, cells were washed with cold PBS and suspended in hypotonic lysis buffer for 15 min. The cells were then lysed with 10% NP-40 and centrifuged; the supernatants containing the cytoplasmic extracts were removed and stored. The nuclear extracts were resuspended in hypertonic extraction buffer (10 mM HEPES, 0.42 M NaCl, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol plus cocktail) and mixed intermittently for 40 min. Subsequently, the extracts were centrifuged, and the supernatants containing nuclear proteins were obtained. The protein concentration was measured by bicinchoninic acid (BCA) assay. A total of 10 μg of nuclear extracts was used to determine nuclear factor κB (NF-κB) activity using the NF-κB p65 ELISA Kit (Enzo-Stressgen, Farmingdale, NY, USA) according to the manufacturer’s instructions. Unlabeled wild-type consensus NF-κB oligonucleotide (50-fold) was used as a competitor for NF-κB binding to monitor the specificity of the assay. The mutated consensus oligonucleotide should have no effect on NF-κB binding and served as the negative control.
In Vivo Antitumor Efficacy Study
Male BALB/c nude mice (aged 4 to 6 weeks old) were maintained under specific pathogen-free conditions. The animal study was approved by the Institutional Animal Ethics Committee of the Affiliated Hospital of Hubei Polytechnic University. HOS cells (1 × 107) were suspended in 200 μl of PBS and subcutaneously injected into the right flanks of nude mice. When the tumors were visible, the mice were randomly divided into four groups (n = 5): control (0.2 ml of PBS), MG132 (2 mg/kg), cisplatin (2 mg/kg), and combination group (MG132: 2 mg/kg + 2 mg/kg). The mice were intraperitoneally injected with the above reagents every 3 days for 15 days. Mice body weights were measured once a week and were used as an indicator of the systemic toxicity of the treatment. Tumor volume was measured every 2 days using a caliper and calculated by the formula volume = (length × width2)/2. At the end, mice were sacrificed, and tumors were removed and weighed.
Statistical Analysis
Statistical analysis was performed using the SPSS 13.0 software (IBM, Armonk, NY, USA). Student’s t-tests were carried out to make a comparison between different groups. Data are expressed as mean ± SD. A value of p < 0.05 was considered statistically significant.
RESULTS
MG132 Inhibited Cell Survival and Proliferation of OS Cells
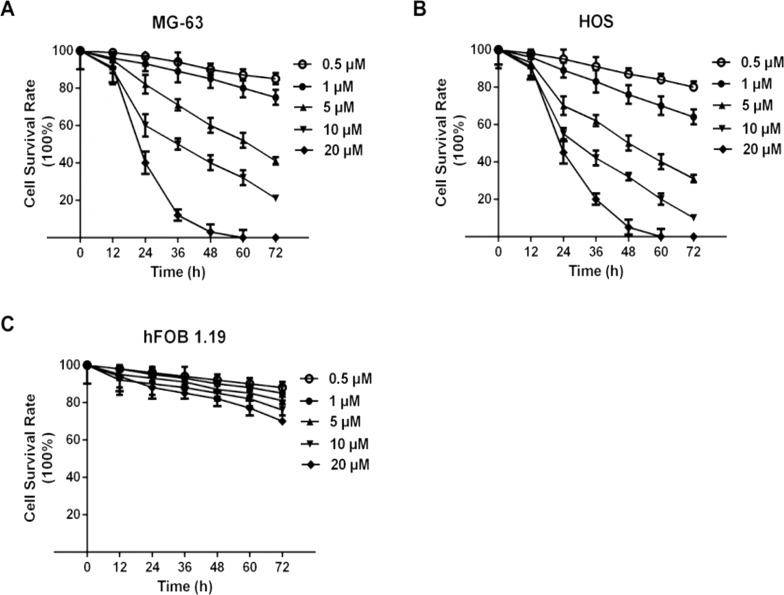
To investigate if MG132 had any cytotoxic activity against OS (MG-63 and HOS) and osteoblast cells (hFOB 1.19), cells were treated with 0.5, 1, 5, 10, and 20 μM MG132 for 12, 24, 36, 48, 60, and 72 h. As shown in Figure 1, MG132 markedly reduced the viability of MG-63 (Fig. 1A) and HOS (Fig. 1B) cells in a dose- and time-dependent manner. MG132 did not have a significant effect on the survival of osteoblast hFOB 1.19 cells (Fig. 1C). Next, we examined whether inhibition of cell proliferation was also accompanied with MG132-induced cell apoptosis and cell cycle arrest.
Figure 1.
MG132 selectively inhibited cell viability in a dose- and time-dependent manner in osteosarcoma (OS) cells but not in osteoblast cells. OS cell lines MG-63 (A) and HOS (B) and osteoblast hFOB 1.19 cells (C) were treated with different concentrations (0.5, 1, 5, 10, and 20 μM) of MG132 for different times (12, 24, 36, 48, 60, and 72 h), and cell counting kit-8 (CCK-8) assays were carried out. Cell survival rate was calculated as a percentage: (A MG132/A DMSO).
MG132 Induced Cell G2/M Arrest and Apoptosis in OS Cells
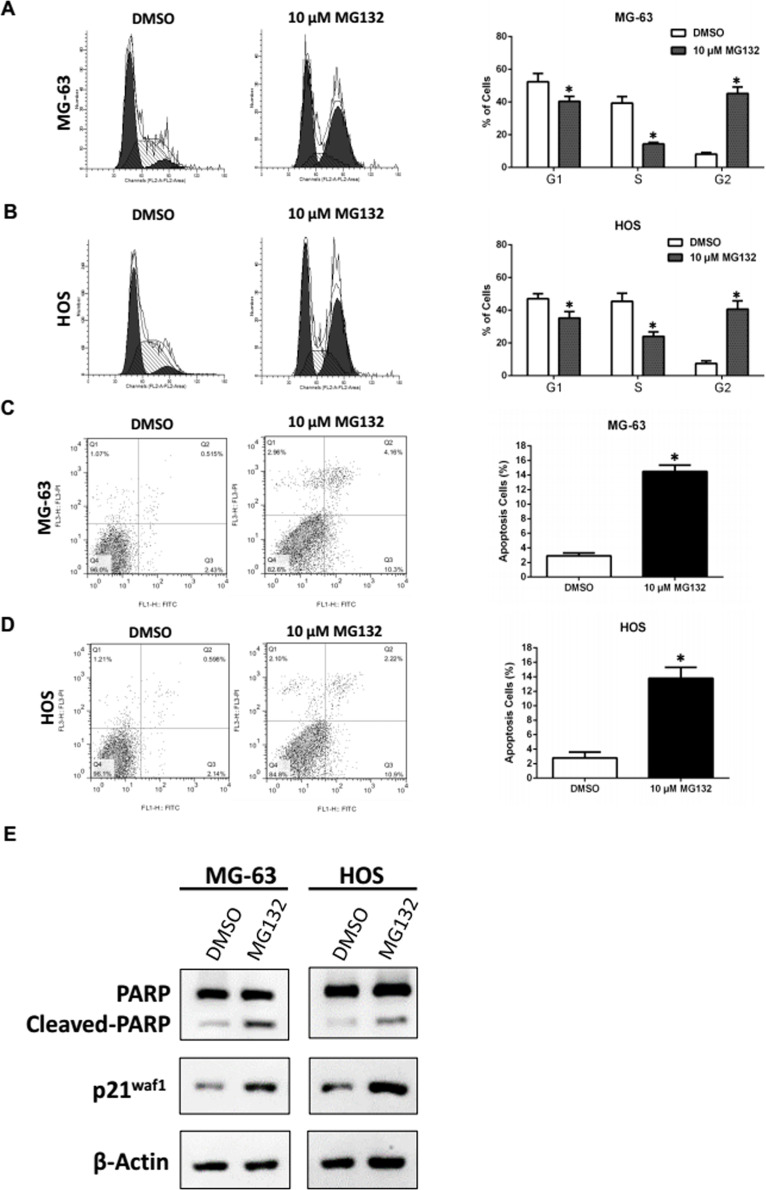
To explore the potential mechanism by which MG132 suppressed OS cell growth, we conducted flow cytometry analysis to monitor the alteration of the cell cycle distribution. The results showed that MG132 elicited an increase in OS cells at the G2 phase (8.2% to 45.3% in MG-63; 7.5% to 40.7% in HOS) and a decrease at the S phase (39.4% to 14.4% in MG-63; 45.4% to 24.1% in HOS) and the G1 phase (52.4% to 40.4% in MG-63; 47.1% to 35.2% in HOS) compared with DMSO (Fig. 2A and B). Furthermore, the apoptotic rate of OS cells increased significantly to 15% compared with the control 3% in MG-63 and 2.8% to 14% in HOS cells (Fig. 2C and D). To confirm the induction of MG132-mediated cell cycle arrest and apoptosis in OS cells, we carried out Western blot to examine the expression of p21waf1 and cleaved PARP. The accumulation of p21waf1 and cleaved forms of PARP were detected in cell lines treated with MG132 (Fig. 2E). These results indicated that suppression of cell growth by MG132 is partially attributable to increased G2 phase arrest and cell apoptosis. We next examined the effect of MG132 on cisplatin-induced cytotoxicity in OS cells.
Figure 2.
MG132 induced cell cycle arrest at the G2/M phase and apoptosis in OS cells. MG-63 and HOS cells were treated with dimethyl sulfoxide (DMSO) or 10 μM MG132 for 24 h. (A, B) Cell cycle distribution was analyzed by flow cytometry. *p < 0.05 versus control (DMSO) denotes a significant difference. (C, D) Apoptotic rates of the treated cells were detected by flow cytometry. *p < 0.05 versus control (DMSO) denotes a significant difference. (E) Expression of PARP and p21waf1 proteins in treated OS cells was detected by Western blot.
MG132 Increased Cisplatin-Induced Cytotoxicity and Growth Inhibition
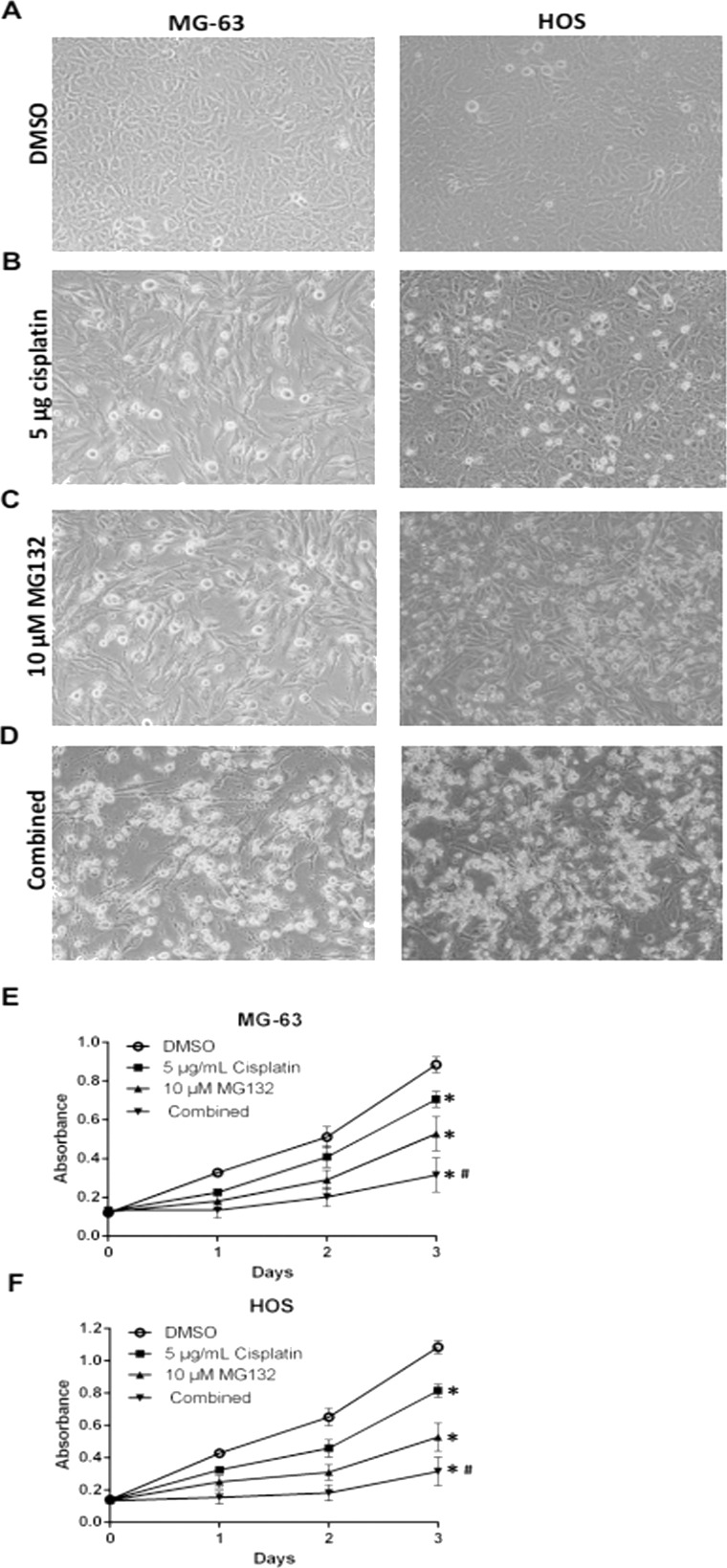
DMSO-treated MG-63 and HOS cells were attached to a dish under the inverted phase-contrast microscope. The cells appeared fusiform and angular (Fig. 3A). Parts of the cells became round and small with 10 μM MG132 or 5 μg/ml cisplatin (Fig. 3B and C). With the combination of MG132 and cisplatin, most of the cells became nonadherent and suspended in the culture medium (Fig. 3D). We then conducted a cell viability assay to evaluate the synergistic effects of MG132 with cisplatin. As shown in Figure 3E and F, more inhibition of cell growth was induced by the combination of MG132 and cisplatin than by either one alone.
Figure 3.
MG132 increased cisplatin-induced apoptosis and proliferation inhibition in OS cells. MG-63 and HOS cells were treated with DMSO/5 μg/ml cisplatin/10 μM MG132/combined (5 μg/ml cisplatin + 10 μM MG132) for 24 h. (A–D) Morphological appearances of the cells were observed under an inverted phase-contrast microscope. (E, F) The CKK-8 assay was performed to determine the effect of MG132/cisplatin/combination on cell proliferation. *p < 0.05 versus control (DMSO) denotes a significant difference, #p < 0.05 versus MG132 or cisplatin denotes a significant difference.
MG132 Inhibited NF-κB, Bcl-xL, and the PI3K/Akt Pathway in OS Cells
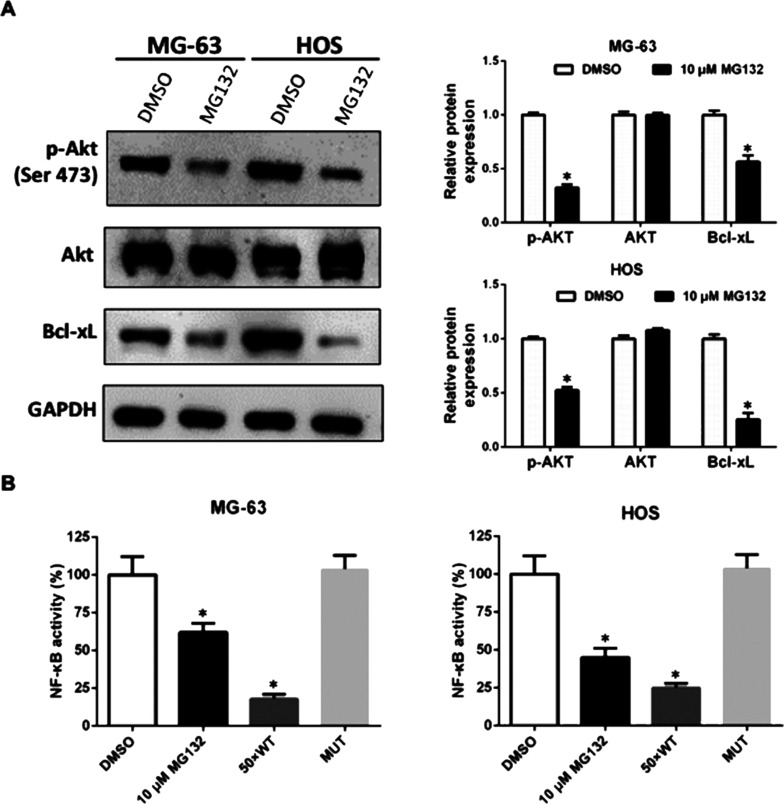
NF-κB increases expression of diverse antiapoptotic molecules such as Bcl-xL. One of the major effects of proteasome inhibitors is the reduction of NF-κB activity. Besides NF-κB, the PI3K/Akt pathway also plays a critical role in cancer cell survival. To assess the mechanism of the synergistic effects, we explored the effect of MG132 on these pathways. Using Western blot analysis, we detected a significant decrease in Bcl-xL and PI3K/Akt in OS cells 24 h after MG132 treatment (Fig. 4A). As assessed by the ELISA assay, MG132 also inhibited nearly 50% of the NF-κB activity (Fig. 4B).
Figure 4.
MG132 inhibited nuclear factor κB (NF-κB), Bcl-xL, and PI3K/Akt activities in OS cells. (A) MG-63 and HOS cells were treated with DMSO or 10 μM MG132 for 24 h, and the expression of p-Akt, Akt, and Bcl-xL was detected by Western blot and was normalized to GAPDH. *p < 0.05 versus control (DMSO) denotes a significant difference. (B) NF-κB activity in nuclear extracts of the treated cells was determined by ELISA. A 50-fold of unlabeled wild-type (WT) consensus NF-kB oligonucleotide (50 × WT) was used as a competitor for NF-kB binding to monitor the specificity of the assay. The mutated consensus oligonucleotide (MUT) should have no effect on NF-kB binding and served as negative control. *p < 0.05 versus control (DMSO) denotes a significant difference.
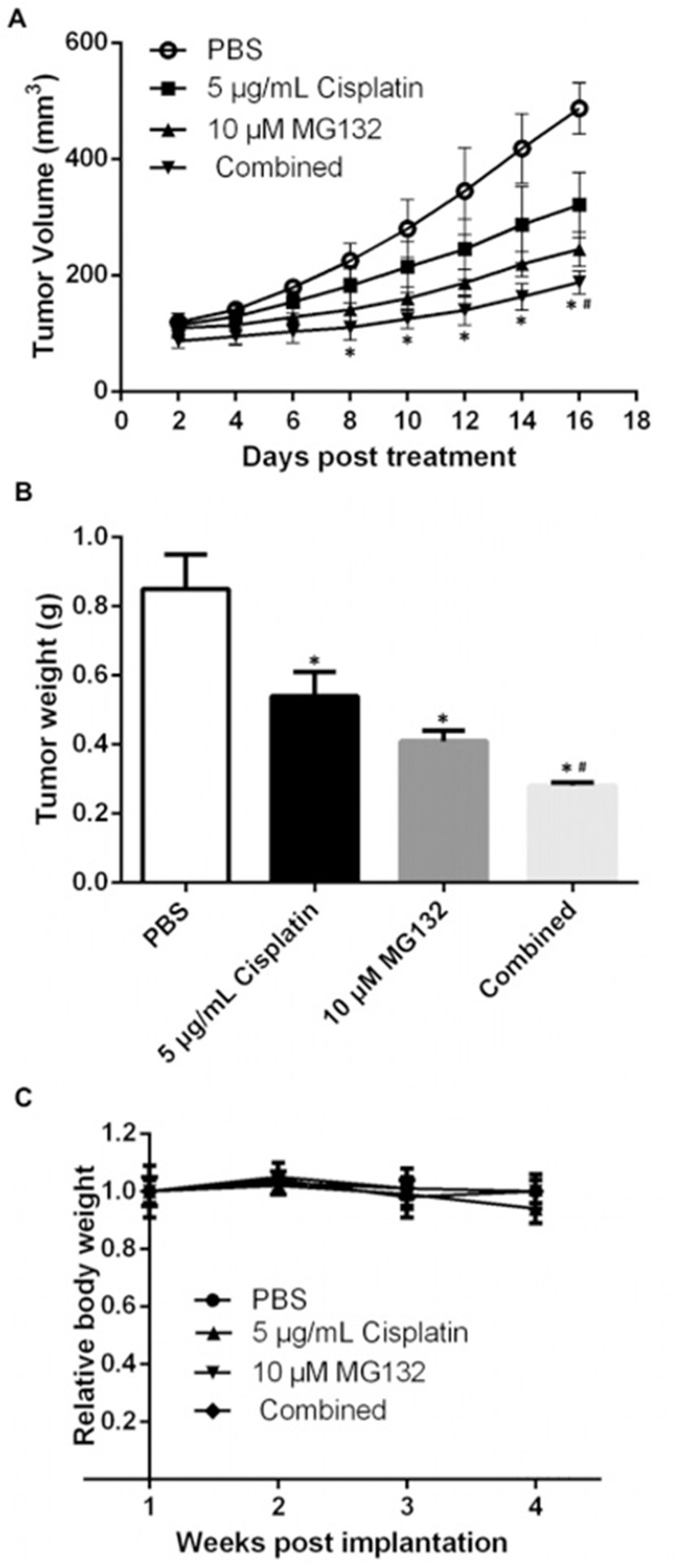
MG132 Exerted a Synergistically Inhibitory Effect With Cisplatin in OS Xenografts
Following the in vitro experiments, we performed HOS xenografts in nude mice. As shown in Figure 5A, treatment with MG132 or cisplatin alone resulted in a modest but suppressed tumor growth. These inhibitory events became more significant when we applied combined injection of MG132 and cisplatin. We also discovered a significantly decreased tumor weight in the combined treatment group compared with mice in the MG132/cisplatin/PBS treatment group (Fig. 5B). In this model, none of the treatment regimens produced any obvious loss of body weight, which may serve as a sign of low toxicity (Fig. 5C).
Figure 5.
Combined treatment with MG132 and cisplatin inhibited tumor growth in HOS xenograft mouse model. HOS cells (1 × 107) were suspended in 200 μl of PBS and subcutaneously injected into the right flanks of the nude mice. When the tumors were visible, the mice were randomly divided into four groups (n = 5): control (0.2 ml of PBS), MG132 (2 mg/kg), cisplatin (2 mg/kg), and combination group (MG132: 2 mg/kg + 2 mg/kg). The mice were intraperitoneally injected with the above reagents every 3 days for 15 days. (A) Tumor volume was measured every 2 days using a caliper. *p < 0.05 versus control (PBS) denotes a significant difference, #p < 0.05 versus MG132 or cisplatin denotes a significant difference. (B) At the end, mice were sacrificed, and tumors were removed and weighed. *p < 0.05 versus control (PBS) denotes a significant difference, #p < 0.05 versus MG132 or cisplatin denotes a significant difference. (C) Mice body weights were measured once a week and were used as an indicator of the systemic toxicity of the treatment.
DISCUSSION
The use of surgery and adjuvant chemotherapy has proven successful for the treatment of OS. Despite a high rate of long-term cure (65%–70%), OS is still the main reason for cancer-related mortality in children and adolescents. With its positive effects against numerous types of solid tumors, cisplatin has been one of the most commonly used first-line chemotherapy drugs for the regular treatment of OS11. However, occurrence of drug resistance and considerable side effects make it imperative to develop less toxic and more effective approaches to overcome these limitations12,13. Herein we presented the first study on the combined treatment of MG132 and cisplatin in OS.
Identification of signals and effective agents that promote cell apoptosis and sensitize resistant cells may provide clues for developing novel therapeutic strategies for OS therapy14,15. Ubiquitin–proteasome-mediated intracellular protein degradation is involved in numerous cellular processes including cell cycle, signal transduction, apoptosis, and transcription. Pharmacological inhibition of proteasome activity by small-molecule inhibitors exerts antitumor efficacy in vivo and induces apoptosis in tumor cells in vitro16,17. Bortezomib (also known as PS-341) has been reported as an antitumor reagent for hematological malignancies and numerous tumors18 by inducing MAPK pathway activity and apoptosis7,19. Gene expression signatures associated with sensitivity to bortezomib, impact of bortezomib on cancer stem cells, and its potent role in inducing endoplasmic reticulum (ER) stress responses have also been widely studied20–22. Proteasome inhibitors not only have antitumor effects but can also be combined with other chemotherapy drugs for better outcomes. Previous studies reported the effects of bortezomib on cisplatin-induced apoptosis in various types of tumor cells9,10. Epoxomicin, another proteasome inhibitor, was shown to sensitize resistant OS cells to TRAIL-induced apoptosis and significantly increase caspase 3, 8, and 9 activities23.
MG132 is a peptide aldehyde proteasome inhibitor extracted from a Chinese medicinal plant. By covalently binding to the active site of the β subunits, MG132 inhibits 20S proteasome activity24,25. Yang et al provided convincing evidence to suggest an essential role for MG132 in inducing cell cycle arrest and apoptosis26. Recently, it was shown that MG132 enhances TRAIL-induced apoptosis and inhibits the invasion of OS cells27. Findings of previous studies have indicated that MG132 enhances cisplatin-induced apoptosis in esophageal squamous cancer cells and ovarian carcinoma cells in vitro and in vivo28,29. However, the effects of MG132-related lethality and its synergistic effect with cisplatin in OS cells are not fully defined. In this present study, we indicated that MG132 exhibited significant inhibitory effects on the growth of OS cells MG-63 and HOS in a dose- and time-dependent manner, whereas it had no toxicity to osteoblast hFOB 1.19 cells. MG132 also induced G2/M cell cycle arrest, which was associated with accumulation of the CDK inhibitor p21waf1, and cell apoptosis in MG-63 and HOS cells.
Some studies had reported that combination of proteasome inhibitors and chemotherapy drugs could improve sensitivity to chemo drugs, not including the combination of MG132 and cisplatin in OS. We then addressed these issues in the present study. Exposure of OS cells to MG132 combined with cisplatin resulted in a marked increase in the cell apoptosis compared with a single agent. Cell viability assay showed that the combination of MG132 and cisplatin may have stronger inhibitory effect than the single application group. The results from the OS xenograft studies also showed that MG132 exhibited synergistic inhibitory effects with cisplatin on the growth of OS cells in vivo.
NF-κB and PI3K/Akt pathways play pivotal roles in tumor cell survival30. NF-κB activation depends on phosphorylation and ubiquitination of its inhibitory protein IκB-α. Proteasome inhibitors can stabilize IκB-α by inhibiting the proteasome activity and ultimately inhibit the activity of NF-κB. In agreement, we found that MG132 decreased the nuclear activity of NF-κB, causing a reduction in Bcl-xL, which is a classical NF-κB-regulated mitochondrial antiapoptosis protein. Furthermore, Akt phosphorylation was also significantly inhibited by M132. However, there are two main mechanisms of cytotoxicity due to proteasome activity inhibition in cancer cells, including NF-κB and a terminal unfolded protein response (UPR) due to enhanced ER stress. More studies will be needed to determine the precise molecular mechanism involved.
In conclusion, we demonstrated that the proteasome inhibitor MG132 has important effects on OS cells by inhibiting cell growth and inducing cell apoptosis. In addition to the inhibitory activity, MG132 synergized with cisplatin in OS cells by inducing more cell apoptosis and suppressing cell/tumor growth in vivo and in vitro. This was mediated by the inhibition of NF-κB and PI3K/Akt. Considering that side effects and chemoresistance are the main obstacles in OS treatment, MG132 might be an effective adjuvant agent of classic chemotherapeutics.
ACKNOWLEDGMENTS
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Topkas E, Cai N, Cumming A, Hazar-Rethinam M, Gannon OM, Burgess M, Saunders NA, Endo-Munoz L. Auranofin is a potent suppressor of osteosarcoma metastasis. Oncotarget 2016;7(1):831–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown HK, Tellez-Gabriel M, Heymann D. Cancer stem cells in osteosarcoma. Cancer Lett. 2017;386:189–95. [DOI] [PubMed] [Google Scholar]
- 3. Bacci G, Balladelli A, Palmerini E, Alberghini M, Pollastri P, Galletti S, Mercuri M, Picci P. Neoadjuvant chemotherapy for osteosarcoma of the extremities in preadolescent patients: The Rizzoli Institute experience. J Pediatr Hematol Oncol. 2008;30(12):908–12. [DOI] [PubMed] [Google Scholar]
- 4. Kim M, Jung JY, Choi S, Lee H, Morales LD, Koh JT, Kim SH, Choi YD, Choi C, Slaga TJ, Kim WJ, Kim DJ. GFRA1 promotes cisplatin-induced chemoresistance in osteosarcoma by inducing autophagy. Autophagy 2017;13(1):149–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suh KS, Tanaka T, Sarojini S, Nightingale G, Gharbaran R, Pecora A, Goy A. The role of the ubiquitin proteasome system in lymphoma. Crit Rev Oncol Hematol. 2013;87(3):306–22. [DOI] [PubMed] [Google Scholar]
- 6. Fujita M, Sugama S, Nakai M, Takenouchi T, Wei J, Urano T, Inoue S, Hashimoto M. Alpha-synuclein stimulates differentiation of osteosarcoma cells: Relevance to down-regulation of proteasome activity. J Biol Chem. 2007;282(8):5736–48. [DOI] [PubMed] [Google Scholar]
- 7. Shapovalov Y, Benavidez D, Zuch D, Eliseev RA. Proteasome inhibition with bortezomib suppresses growth and induces apoptosis in osteosarcoma. Int J Cancer 2010;127(1):67–76. [DOI] [PubMed] [Google Scholar]
- 8. Yan XB, Yang DS, Gao X, Feng J, Shi ZL, Ye Z. Caspase-8 dependent osteosarcoma cell apoptosis induced by proteasome inhibitor MG132. Cell Biol Int. 2007;31(10):1136–43. [DOI] [PubMed] [Google Scholar]
- 9. Fribley AM, Evenchik B, Zeng Q, Park BK, Guan JY, Zhang H, Hale TJ, Soengas MS, Kaufman RJ, Wang CY. Proteasome inhibitor PS-341 induces apoptosis in cisplatin-resistant squamous cell carcinoma cells by induction of Noxa. J Biol Chem. 2006;281(42):31440–7. [DOI] [PubMed] [Google Scholar]
- 10. Konac E, Varol N, Kiliccioglu I, Bilen CY. Synergistic effects of cisplatin and proteasome inhibitor bortezomib on human bladder cancer cells. Oncol Lett. 2015;10(1):560–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang C, Hong CS, Hu X, Yang C, Wang H, Zhu D, Moon S, Dmitriev P, Lu J, Chiang J, Zhuang Z, Zhou Y. Inhibition of protein phosphatase 2A with the small molecule LB100 overcomes cell cycle arrest in osteosarcoma after cisplatin treatment. Cell Cycle 2015;14(13):2100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keremu A, Aini A, Maimaitirexiati Y, Liang Z, Aila P, Xierela P, Tusun A, Moming H, Yusufu A. Overcoming cisplatin resistance in osteosarcoma through the miR-199a-modulated inhibition of HIF-1alpha. Biosci Rep. 2017. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Y, Teng JS. Increased multi-drug resistance and reduced apoptosis in osteosarcoma side population cells are crucial factors for tumor recurrence. Exp Ther Med. 2016;12(1):81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baranski Z, Booij TH, Cleton-Jansen AM, Price LS, van de Water B, Bovee JV, Hogendoorn PC, Danen EH. Aven-mediated checkpoint kinase control regulates proliferation and resistance to chemotherapy in conventional osteosarcoma. J Pathol. 2015;236(3):348–59. [DOI] [PubMed] [Google Scholar]
- 15. Sevelda F, Mayr L, Kubista B, Lotsch D, van Schoonhoven S, Windhager R, Pirker C, Micksche M, Berger W. EGFR is not a major driver for osteosarcoma cell growth in vitro but contributes to starvation and chemotherapy resistance. J Exp Clin Cancer Res. 2015;34:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Navon A, Ciechanover A. The 26 S proteasome: From basic mechanisms to drug targeting. J Biol Chem. 2009;284(49):33713–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sheridan C. Drug makers target ubiquitin proteasome pathway anew. Nat Biotechnol. 2015;33(11):1115–7. [DOI] [PubMed] [Google Scholar]
- 18. Mitsiades CS, McMillin D, Kotoula V, Poulaki V, McMullan C, Negri J, Fanourakis G, Tseleni-Balafouta S, Ain KB, Mitsiades N. Antitumor effects of the proteasome inhibitor bortezomib in medullary and anaplastic thyroid carcinoma cells in vitro. J Clin Endocrinol Metab. 2006;91(10):4013–21. [DOI] [PubMed] [Google Scholar]
- 19. Lou Z, Ren T, Peng X, Sun Y, Jiao G, Lu Q, Zhang S, Lu X, Guo W. Bortezomib induces apoptosis and autophagy in osteosarcoma cells through mitogen-activated protein kinase pathway in vitro. J Int Med Res. 2013;41(5):1505–19. [DOI] [PubMed] [Google Scholar]
- 20. Obeng EA, Carlson LM, Gutman DM, Harrington WJ Jr, Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood 2006;107(12):4907–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chng WJ, Kumar S, Vanwier S, Ahmann G, Price-Troska T, Henderson K, Chung TH, Kim S, Mulligan G, Bryant B, Carpten J, Gertz M, Rajkumar SV, Lacy M, Dispenzieri A, Kyle R, Greipp P, Bergsagel PL, Fonseca R. Molecular dissection of hyperdiploid multiple myeloma by gene expression profiling. Cancer Res. 2007;67(7):2982–9. [DOI] [PubMed] [Google Scholar]
- 22. Matsui W, Wang Q, Barber JP, Brennan S, Smith BD, Borrello I, McNiece I, Lin L, Ambinder RF, Peacock C, Watkins DN, Huff CA, Jones RJ. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res. 2008;68(1):190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hanikoglu F, Cort A, Ozben H, Hanikoglu A, Ozben T. Epoxomicin sensitizes resistant osteosarcoma cells to TRAIL induced apoptosis. Anticancer Agents Med Chem. 2015;15(4):527–33. [DOI] [PubMed] [Google Scholar]
- 24. Wong VK, Cheung SS, Li T, Jiang ZH, Wang JR, Dong H, Yi XQ, Zhou H, Liu L. Asian ginseng extract inhibits in vitro and in vivo growth of mouse Lewis lung carcinoma via modulation of ERK-p53 and NF-kappaB signaling. J Cell Biochem. 2010;111(4):899–910. [DOI] [PubMed] [Google Scholar]
- 25. Ji L, Chen Y, Liu T, Wang Z. Involvement of Bcl-xL degradation and mitochondrial-mediated apoptotic pathway in pyrrolizidine alkaloids-induced apoptosis in hepatocytes. Toxicol Appl Pharmacol. 2008;231(3):393–400. [DOI] [PubMed] [Google Scholar]
- 26. Yang H, Chen D, Cui QC, Yuan X, Dou QP. Celastrol, a triterpene extracted from the Chinese “Thunder of God Vine,” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006;66(9):4758–65. [DOI] [PubMed] [Google Scholar]
- 27. Li X, Huang T, Jiang G, Gong W, Qian H, Zou C. Proteasome inhibitor MG132 enhances TRAIL-induced apoptosis and inhibits invasion of human osteosarcoma OS732 cells. Biochem Biophys Res Commun. 2013;439(2):179–86. [DOI] [PubMed] [Google Scholar]
- 28. Dang L, Wen F, Yang Y, Liu D, Wu K, Qi Y, Li X, Zhao J, Zhu D, Zhang C, Zhao S. Proteasome inhibitor MG132 inhibits the proliferation and promotes the cisplatin-induced apoptosis of human esophageal squamous cell carcinoma cells. Int J Mol Med. 2014;33(5):1083–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guo N, Peng Z, Zhang J. Proteasome inhibitor MG132 enhances sensitivity to cisplatin on ovarian carcinoma cells in vitro and in vivo. Int J Gynecol Cancer 2016;26(5):839–44. [DOI] [PubMed] [Google Scholar]
- 30. Chitra S, Nalini G, Rajasekhar G. The ubiquitin proteasome system and efficacy of proteasome inhibitors in diseases. Int J Rheum Dis. 2012;15(3):249–60. [DOI] [PubMed] [Google Scholar]