Abstract
MicroRNAs (miRs) have been found to play promoting or suppressive roles in different human cancers. However, the exact regulatory mechanism of miR-30b in glioblastoma remains unknown. Here we have shown that the expression of miR-30b is significantly increased in glioblastoma tissues and cell lines. Moreover, a high expression of miR-30b is significantly associated with a shorter survival time for glioblastoma patients. Knockdown of miR-30b caused a significant reduction in the proliferation, migration, and invasion of U87 and A172 cells. Proline-rich transmembrane protein 2 (PRRT2) was further identified as a novel target gene of miR-30b, and its protein expression is negatively regulated by miR-30b in U87 and A172 cells. Furthermore, PRRT2 is significantly downregulated in glioblastoma tissues and cell lines, and we found an inverse correlation between miR-30b and PRRT2 expression in glioblastoma tissues. In addition, inhibition of PRRT2 reversed the suppressive effect of miR-30b downregulation on the malignant phenotypes of U87 and A172 cells. Accordingly, we demonstrated that miR-30b promotes glioblastoma cell proliferation, migration, and invasion via targeting PRRT2. Therefore, miR-30b may be used as a promising therapeutic target for glioblastoma.
Key words: Glioblastoma, MicroRNA, Proline-rich transmembrane protein 2 (PRRT2), Oncogene, Tumor suppressor
INTRODUCTION
Glioblastoma is one of the most common malignant tumors of the brain1,2. In recent decades, despite great efforts that have been made on surgical resection combined with radiotherapy and chemotherapy, the overall survival time for glioblastoma patients has not improved1,2. Increasing evidence has demonstrated that some oncogenes or tumor suppressors are significantly deregulated during glioblastoma development and progression, some of which have been suggested to be used as promising therapeutic targets for this disease3–5.
MicroRNAs (miRs), a class of noncoding RNAs that are 18–25 nucleotides in length, have been demonstrated to regulate gene expression by directly targeting the 3′-untranslated region (3′-UTR) of their target mRNAs, causing translation inhibition or mRNA degradation6–8. Through inhibiting the expression of their target genes, many miRs participate in the regulation of various physiological and pathological biological processes, such as cell proliferation and differentiation, survival and apoptosis, cell cycle progression, migration and invasion, and tumorigenesis8–10. Recently, deregulation of miRs has been observed in glioblastoma, and some miRs have been identified as oncogenes or tumor suppressors, such as miR-520b11, miR-42312, miR-499a13, and miR-66314.
Previous studies have identified miR-30a as an oncogene in glioma. The expression levels of miR-30a are significantly higher in glioma tissues and cell lines when compared to normal brain tissue, and its expression is positively correlated with tumor grade of malignancy15. Moreover, inhibition of miR-30a could suppress glioma cell growth and invasion by targeting SEPT7 and NCAM16,17. These findings suggest that miR-30a may become a potential molecular target in the treatment of glioma. Recently, miR-30b has been demonstrated to play an oncogenic role in some common human cancers. Amplification and overexpression of miR-30b in medulloblastoma have been observed18. Quintavalle et al. reported that miR-30b could inhibit TRAIL-induced apoptosis in glioma cells via targeting caspase 3 and TAp6319. However, the exact role of miR-30b in glioblastoma has not been studied.
Accordingly, our study was to explore the molecular mechanism of miR-30b underlying the malignant progression of glioblastoma.
MATERIALS AND METHODS
Tissue Collection
The study was approved by the ethics committee of Xiangya Hospital (Central South University, Changsha, P.R. China), and written informed consents were obtained from all patients. A total of 77 glioblastoma tissues and 18 normal brain tissues were collected immediately during the surgery between April 2012 and December 2014 and frozen in liquid nitrogen. The tissue samples were stored at −80°C until use.
Cell Culture and Transfection
Human glioblastoma cell lines including A172, U87, U251, M059J, and M059K were purchased from the American Tissue Culture Collection (Manassas, VA, USA). All cell lines were cultured in DMEM (Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine serum (FBS; Thermo Fisher Scientific). Cells were cultured at 37°C with 5% CO2.
For cell transfection, U87 and A172 cells were transfected with a negative control miR inhibitor (NC inhibitor), an miR-30b inhibitor, a scramble miR mimic (miR-NC), miR-30b mimics (miR-30b), a blank pcDNA3.1 vector (NC), and pcDNA3.1-PRRT2 ORF plasmid (PRRT2), or cotransfected with a miR-30b inhibitor and PRRT2 siRNA (miR-20b in + siPRRT2), or miR-30b inhibitor and NC siRNA (miR-30b in + siNC) (all purchased from Yearthbio, Changsha, P.R. China) using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s instructions. Cells were then cultured for 48 h before the following assays.
Real-Time qPCR
Total RNA was extracted from tissues and cell lines using TRIzol reagent (Thermo Fisher Scientific). RNA was converted into cDNA using RevertAid™ First-Strand cDNA Synthesis Kit (Fermentas, Thermo Fisher Scientific) according to the manufacturer’s instructions. To examine the expression of miR, real-time qPCR was conducted using PrimeScript® miRNA RT-PCR Kit (Takara, Dalian, P.R. China) on an ABI 7300 Plus thermocycler (Thermo Fisher Scientific). To examine the expression of mRNA, real-time qPCR was conducted using the standard SYBR Green RT-PCR Kit (Takara). U6 and GAPDH were used as internal references. The relative expression was analyzed by the 2−ΔΔCt method.
Western Blot
Cells were lysed with cold radioimmunoprecipitation assay buffer (Thermo Fisher Scientific), and 50 μg of protein was separated with 10% SDS-PAGE. Afterward, the protein was transferred onto the polyvinylidene difluoride membrane (Thermo Fisher Scientific). The membrane was incubated with PBS (Thermo Fisher Scientific) containing 5% nonfat milk (Mengniu, Beijing, P.R. China) at 4°C overnight, and then washed with PBST (Thermo Fisher Scientific) for 10 min. The membrane was then incubated with rabbit antibodies against PRRT2 and GAPDH (Abcam, Cambridge, MA, USA) at room temperature for 3 h. After washing with PBST for 10 min, the membrane was incubated with goat anti-rabbit secondary antibody (Abcam) at room temperature for 1 h. Chemiluminescence detection was conducted using the Pierce ECL Western Blotting Kit (Pierce, Thermo Fisher Scientific). The protein expression was analyzed by Image-Pro Plus software 6.0 (Media Cybernetics, Rockville, MD, USA), represented as the density ratio versus GAPDH.
MTT Assay
U87 and A172 cells were plated at a density of 10,000 cells per well in 96-well plates. After being cultured for 24, 48, and 72 h, cells were incubated with 0.5 mg/ml of MTT (Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 4 h. The medium was then removed, and 150 mM of DMSO (Sigma-Aldrich) was added. The absorbance was read at 570 nm using the Multiskan Spectrum Microplate reader (Thermo Fisher Scientific).
Wound Healing Assay
U87 and A172 cells were cultured in DMEM with 10% FBS to full confluence. A wound was generated using a plastic scriber, and cells were washed with PBS once. Afterward, U87 and A172 cells were cultured in DMEM with 10% FBS added at 37°C for 48 h, and the wound was observed and photographed under an inverted microscope (Olympus, Tokyo, Japan).
Transwell Assay
U87 and A172 cell suspension (106 cells/ml) was prepared in DMEM, 300 μl of which was added into the upper chamber (BD, Franklin Lakes, NJ, USA) that had been precoated with Matrigel (BD). Afterward, 300 μl of DMEM with 10% FBS was added into the lower chamber. Cells were incubated at 37°C for 24 h. The cells that did not migrate through the pores were wiped out using a cotton-tipped swab. The filters were stained with crystal violet (Sigma-Aldrich), and invading cells were counted under an inverted microscope (Olympus).
Bioinformatics Analysis
TargetScan (www.targetscan.org), PicTar (pictar.mdc-berlin.de), and miRanda (www.microrna.org) were used to analyze the putative target genes of miR-30b according to the manufacturer’s instructions.
Dual-Luciferase Reporter Assay
The wild type (WT) of PRRT2 3′-UTR was constructed by PCR and inserted into the MCS in the psiCHECK vector (Promega, Madison, WI, USA). The mutant type (Mut) of PRRT2 3′-UTR was constructed by a QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA) according to the manufacturer’s instructions. This was also inserted into the MCS in the psiCHECK vector.
For the luciferase reporter assay, U87 and A172 cells were cotransfected with WT-PRRT2-3′-UTR or MT-PRRT2-3′-UTR plasmid, with miR-30b mimics or miR-NC, respectively, using Lipofectamine 2000. After incubation for 6 h, the medium was refreshed with DMEM added to 10% FBS. After transfection for 48 h, the dual-luciferase reporter assay system (Promega) was used to determine the luciferase activities. Renilla luciferase activity was normalized to firefly luciferase activity.
Statistical Analysis
Statistical analysis was performed using SPSS 2.0 (SPSS, Armonk, NY, USA). Data are expressed as the mean ± SD. The differences between two groups were analyzed using the Student’s t-test. The association of gene expression with clinical characteristics was analyzed using the chi-square test. A value of p < 0.05 was considered to be significantly different.
RESULTS
miR-30b Is Upregulated in Glioblastoma Tissues and Cell Lines
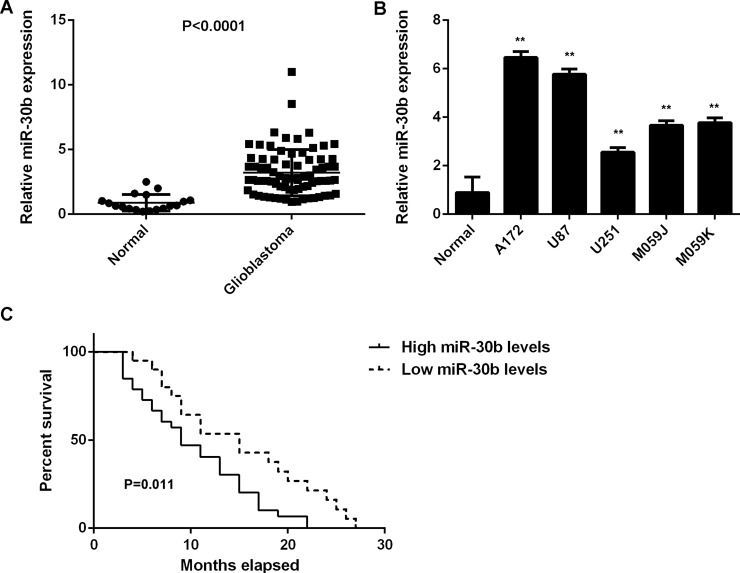
In this study, we first showed that miR-30b was significantly upregulated in glioblastoma cell lines compared with normal brain tissues (Fig. 1A). To confirm these findings, we then examined the miR-30b levels in glioblastoma tissues. Our data indicated that the expression levels of miR-30b were also significantly increased in glioblastoma tissues compared with normal brain tissues (Fig. 1B). Therefore, we demonstrated that miR-30b is upregulated in glioblastoma. Moreover, we found that glioblastoma patients with a high expression of miR-30b showed a shorter overall survival time when compared with those having low miR-30b levels (Fig. 1C).
Figure 1.
Upregulation of miR-30b in glioblastoma. (A) Real-time PCR was used to examine the miR-30b levels in glioblastoma tissues compared with normal brain tissues. (B) Real-time PCR was used to examine the miR-30b levels in glioblastoma cell lines compared with normal brain tissues. **p < 0.01 versus Normal. (C) Glioblastoma patients with a high miR-30b expression showed a shorter survival time compared with those having a low miR-30b expression.
Knockdown of miR-30b Expression Decreases the Proliferation, Migration, and Invasion of Glioblastoma Cells
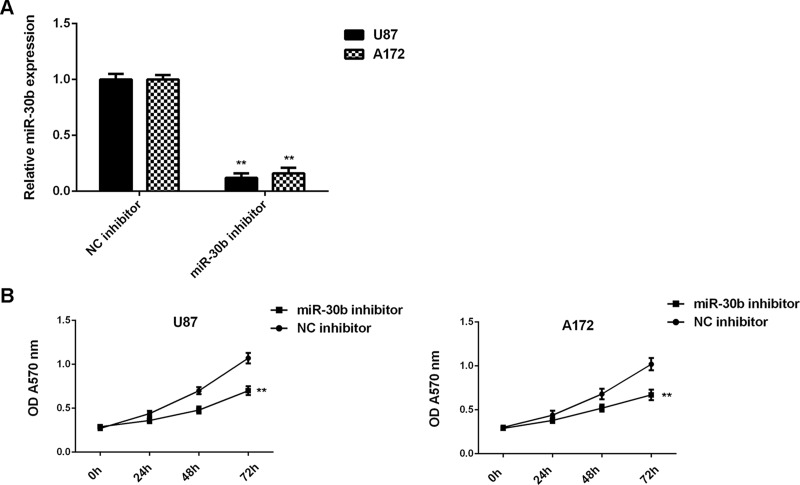
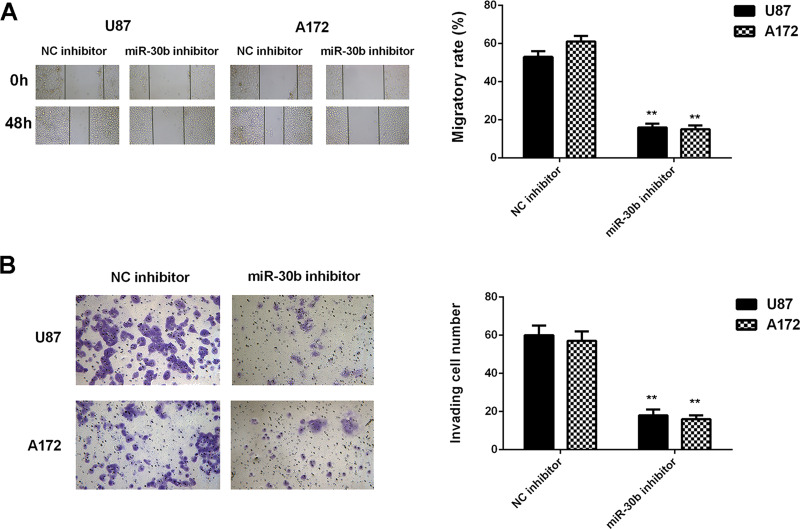
Because we found that miR-30b was significantly upregulated in glioblastoma cells, U87 and A172 cells were transfected with an miR-30b inhibitor to knock down its expression. After transfection, the miR-30b levels were notably reduced in the miR-30b inhibitor group compared with the NC inhibitor group (Fig. 2A). MTT, wound healing, and Transwell assays were then conducted to examine cell proliferation, migration, and invasion, respectively. Inhibition of miR-30b caused a significant reduction in U87 and A172 cell proliferation, migration, and invasion (Figs. 2B and 3). These findings suggest that miR-30b plays a promoting role in glioblastoma growth and metastasis.
Figure 2.
Knockdown of miR-30b expression decreases the proliferation of glioblastoma cells. U87 and A172 cells were transfected with the miR-30b inhibitor or negative control (NC) inhibitor. (A) Real-time PCR was used to examine the miR-30b levels. (B) The MTT assay was conducted to examine cell proliferation. **p < 0.01 versus NC inhibitor.
Figure 3.
Knockdown of miR-30b expression decreases the migration and invasion of glioblastoma cells. U87 and A172 cells were transfected with the miR-30b inhibitor or negative control (NC) inhibitor. (A) Wound healing assay and (B) Transwell assay were conducted to examine cell migration and invasion, respectively. **p < 0.01 versus NC inhibitor.
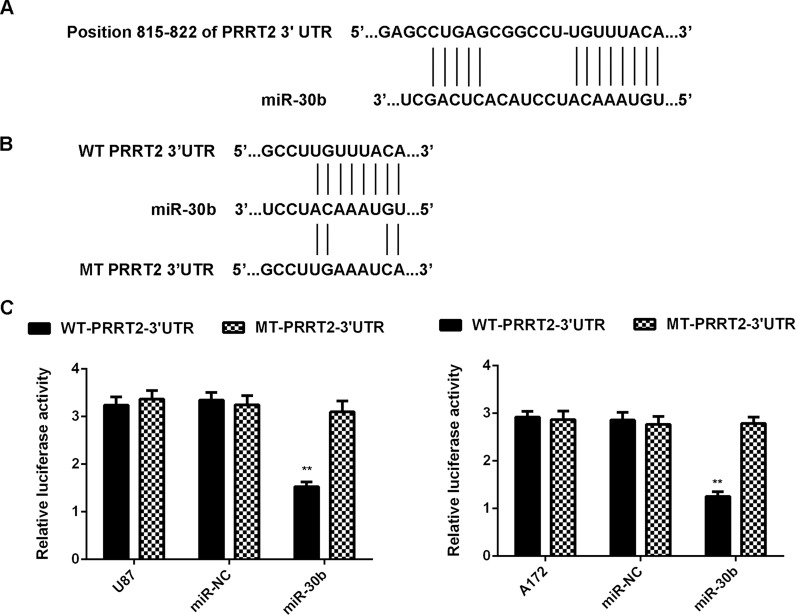
PRRT2 Is a Novel Target of miR-30b in Glioblastoma Cells
The potential targets of miR-30b were further investigated in glioblastoma cells. Bioinformatics analysis data showed that PRRT2 was a putative target gene of miR-30b (Fig. 4A) and that the targeting relationship between PRRT2 and miR-30b was evolutionally conserved (data not shown). To confirm their targeting relationship, we generated luciferase reporter plasmids containing WT or MT of PRRT2 3′-UTR (Fig. 4B). A luciferase reporter gene assay was further conducted in U87 and A172 cells. Luciferase activity was significantly reduced in cells cotransfected with the WT-PRRT2-3′-UTR luciferase reporter plasmid and the miR-30b mimic, which was eliminated when transfected with the MT-PRRT2-3′-UTR luciferase reporter plasmid (Fig. 4C). Therefore, PRRT2 is identified as a novel target gene of miR-30b in U87 and A172 cells.
Figure 4.
PRRT2 is a novel target of miR-30b in glioblastoma cells. (A) PRRT2 was predicted to be a potential target of miR-30b. (B) The wild-type (WT)-PRRT2-3′-UTR and mutant-type (MT)-PRRT2-3′-UTR luciferase reporter plasmids were generated. (C) Luciferase reporter gene assay was further conducted in U87 and A172 cells. **p < 0.01 versus U87 or A172.
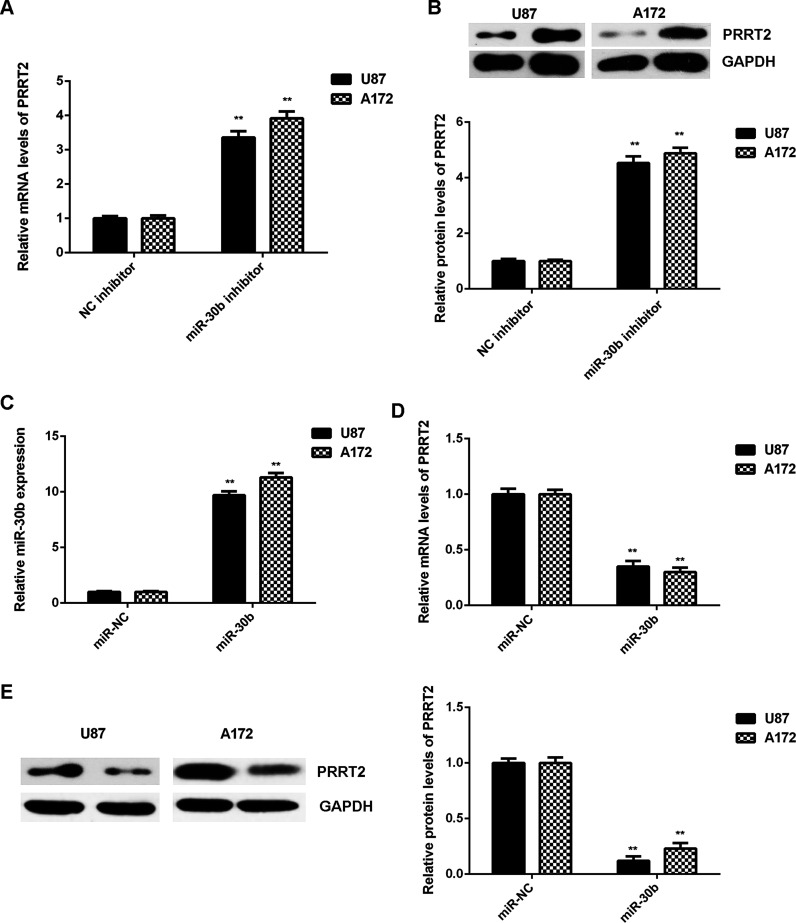
The effect of miR-30b on PRRT2 expression was then studied in U87 and A172 cells. The mRNA and protein levels of PRRT2 were significantly higher in the miR-30b inhibitor group compared with the NC inhibitor group, indicating that knockdown of miR-30b promotes the expression of PRRT2 in U87 and A172 cells (Fig. 5A and B). Afterward, U87 and A172 cells were transfected with the miR-30b mimic or miR-NC, respectively. After transfection, the miR-30b levels were significantly increased in the miR-30b group compared with the miR-NC group (Fig. 5C). Further investigation showed that overexpression of miR-30b significantly reduced the mRNA and protein expressions of PRRT2 in U87 and A172 cells (Fig. 5D and E). Taking the above data together, we demonstrated that miR-30b negatively regulates the expression of PRRT2 in U87 and A172 cells through directly binding to the 3′-UTR of PRRT2 mRNA.
Figure 5.
PRRT2 was negatively regulated by miR-30b in glioblastoma cells. (A) Real-time PCR and (B) Western blot were used to examine the mRNA and protein expression of PRRT2 in U87 and A172 cells transfected with the miR-30b inhibitor or negative control (NC) inhibitor, respectively. **p < 0.01 versus NC inhibitor. Afterward, U87 and A172 cells were transfected with the miR-30b mimic or miR-NC, respectively. (C) Real-time PCR was used to examine the miR-30b levels. (D) Real-time PCR and (E) Western blot were used to examine the mRNA and protein expression of PRRT2. **p < 0.01 versus miR-NC.
PRRT2 Is Downregulated in Glioblastoma, With an Inverse Correlation to miR-30b Expression
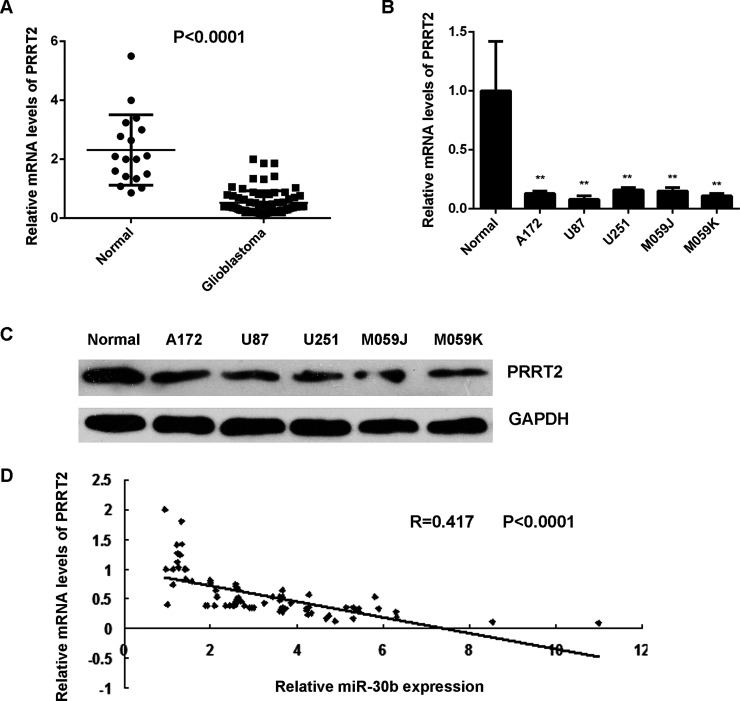
The expression levels of PRRT2 were further examined in glioblastoma tissues and cell lines. The mRNA levels of PRRT2 were significantly decreased in glioblastoma tissues compared with normal brain tissues (Fig. 6A). Consistently, it was also downregulated in glioblastoma cell lines compared with normal brain tissues (Fig. 6B and C). An inverse correlation was observed between the PRRT2 and miR-30b levels in glioblastoma tissues (Fig. 6D). These data suggest that the decreased expression of PRRT2 may be due to the increased expression of miR-30b in glioblastoma.
Figure 6.
Downregulation of PRRT2 in glioblastoma. (A) Real-time PCR was used to examine the mRNA expression of PRRT2 in glioblastoma tissues compared with normal brain tissues. (B) Real-time PCR and (C) Western blot were used to examine the mRNA and protein expression of PRRT2 in glioblastoma cell lines compared with normal brain tissues. **p < 0.01 versus Normal. (D) An inverse correlation was observed between the PRRT2 and miR-30b levels in glioblastoma tissues.
Overexpression of PRRT2 Shows a Similar Effect on Glioblastoma Cells as the Inhibition of miR-30b
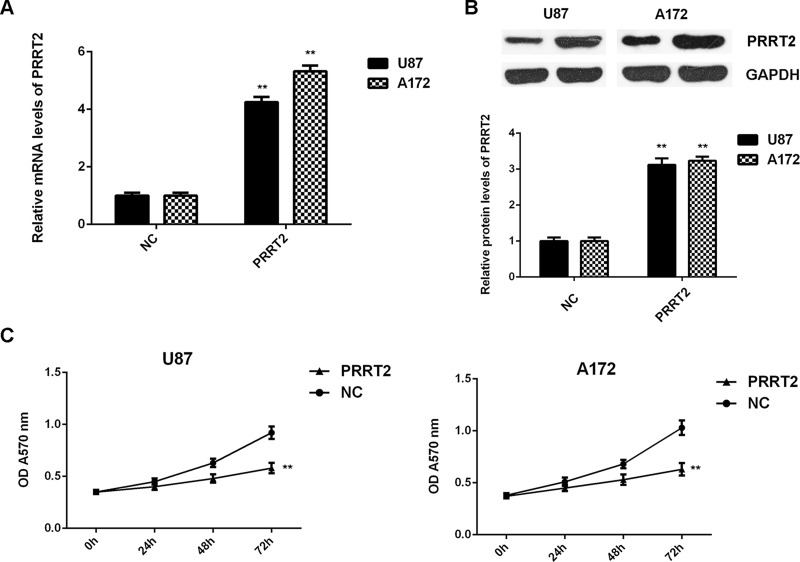
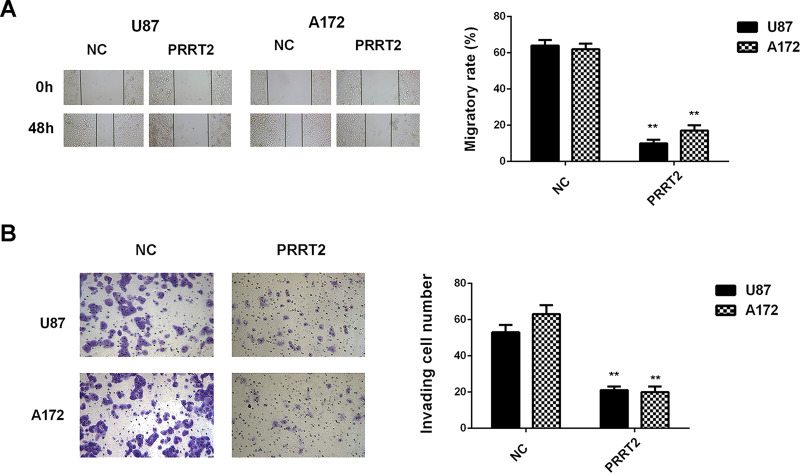
As PRRT2 was significantly downregulated in glioblastoma tissues and cell lines, U87 and A172 cells were transfected with PRRT2 ORF plasmid to increase its expression levels. The PRRT2 levels were increased in the PRRT2 ORF plasmid group compared with the NC group (Fig. 7A and B). Further investigation revealed that overexpression of PRRT2 also significantly inhibited the proliferation, migration, and invasion of U87 and A172 cells (Figs. 7C and 8). Therefore, overexpression of PRRT2 shows a similar effect on glioblastoma cells as the inhibition of miR-30b.
Figure 7.
Overexpression of PRRT2 reduces glioblastoma cell proliferation. U87 and A172 cells were transfected with pcDNA3. 1-PRRT2 ORF plasmid or blank pcDNA3.1 vector (NC), respectively. (A) Real-time PCR and (B) Western blot were used to examine the mRNA and protein expression of PRRT2. (C) MTT assay was conducted to examine cell proliferation. **p < 0.01 versus NC.
Figure 8.
Overexpression of PRRT2 reduces glioblastoma cell migration and invasion. U87 and A172 cells were transfected with pcDNA3.1-PRRT2 ORF plasmid or blank pcDNA3.1 vector (NC), respectively. (A) Wound healing assay and (B) Transwell assay were conducted to examine cell migration and invasion, respectively. **p < 0.01 versus NC.
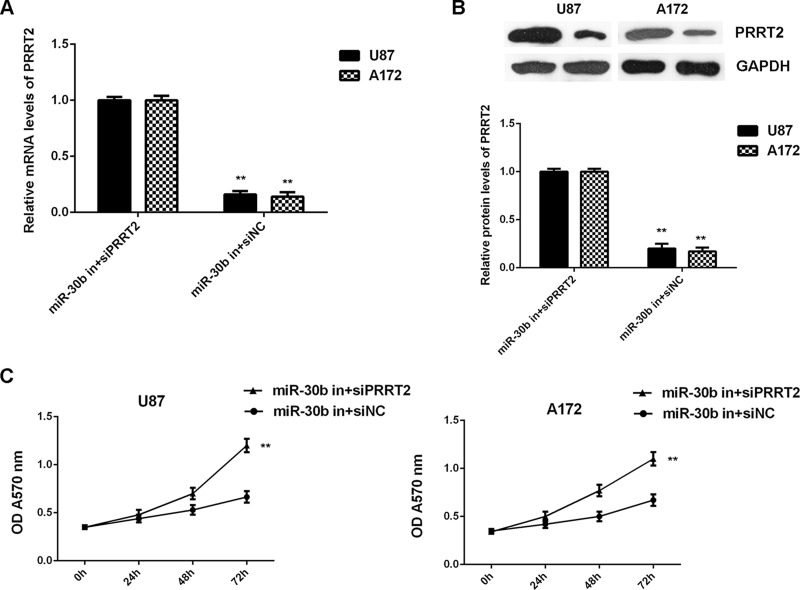
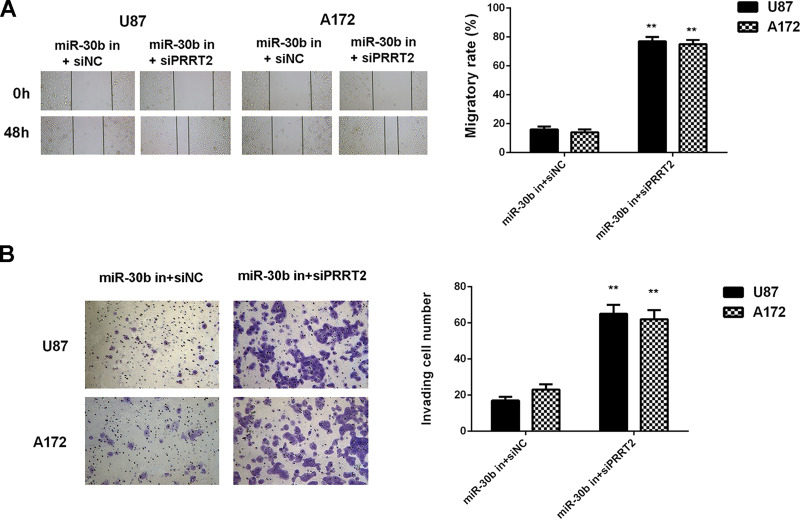
Inhibition of PRRT2 Rescues the Suppressive Effect of miR-30b Downregulation on Glioblastoma Cell Proliferation, Migration, and Invasion
Based on the findings above, we speculated that PRRT2 probably acts as a downstream effector of miR-30b in U87 and A172 cells. To clarify this speculation, U87 and A172 cells were cotransfected with the miR-30b inhibitor and PRRT2 siRNA. Transfection with the miR-30b inhibitor and NC siRNA was used as the control group. After transfection, the expression of PRRT2 was significantly reduced in the miR-30b in + siPRRT2 group when compared with the miR-30b in + siNC group (Fig. 9A and B). Afterward, we found that the proliferation, migration, and invasion of U87 and A172 cells were significantly upregulated in the miR-30b inhibitor + PRRT2 siRNA group when compared with the miR-30b inhibitor + NC siRNA group (Figs. 9C and 10). Accordingly, knockdown of PRRT2 rescued the suppressive effect of miR-30b inhibition on the proliferation, migration, and invasion of glioblastoma cells.
Figure 9.
Inhibition of PRRT2 rescues the suppressive effect of miR-30b downregulation on glioblastoma cell proliferation. U87 and A172 cells were cotransfected with the miR-30b inhibitor and NC siRNA (miR-30b in + siNC), or the miR-30b inhibitor and PRRT2 siRNA (miR-30b in+siPRRT2), respectively. (A) Real-time PCR and (B) Western blot were used to examine the mRNA and protein expression of PRRT2. (C) MTT assay was conducted to examine cell proliferation. **p < 0.01 versus miR-30b in + siNC.
Figure 10.
Inhibition of PRRT2 rescues the suppressive effect of miR-30b downregulation on glioblastoma cell migration and invasion. U87 and A172 cells were cotransfected with the miR-30b inhibitor and NC siRNA (miR-30b in + siNC), or the miR-30b inhibitor and PRRT2 siRNA (miR-30b in + siPRRT2), respectively. (A) Wound healing assay and (B) Transwell assay were conducted to examine cell migration and invasion, respectively. **p < 0.01 versus miR-30b in + siNC.
DISCUSSION
Recently, miR-30a was found to play a promoting role in glioblastoma, but the exact role of miR-30b in glioblastoma has not been previously studied. In this study, we observed that miR-30b is significantly upregulated in glioblastoma, which is significantly associated with a poor prognosis for patients. Knockdown of miR-30b significantly inhibited glioblastoma cell proliferation, migration, and invasion. PRRT2 was significantly downregulated in glioblastoma and was identified as a novel target gene of miR-30b in glioblastoma cells. An inverse correlation was observed between miR-30b and PRRT2 expression in glioblastoma tissues. In addition, PRRT2 overexpression showed a similar effect on glioblastoma cells as the inhibition of miR-30b, and knockdown of PRRT2 rescued the suppressive effect of miR-30b inhibition on the malignant phenotypes of glioblastoma cells.
In recent years, miR-30b has been demonstrated to play oncogenic or tumor-suppressive roles in some common human cancers20–22. For instance, miR-30b could promote the metastatic behavior of melanoma cells by directly targeting the GalNAc transferase GALNT7, causing increased IL-10 synthesis and reduced immune cell activation and recruitment20. It was also significantly upregulated in bladder cancer tissues compared with adjacent nontumor tissues21. On the contrary, Zhao et al. reported that miR-30b was downregulated in colorectal cancer tissues compared with normal tissues, and the forced expression of miR-30b inhibited the migration and invasion of colorectal cancer cells via targeting SIX123. Zhu et al. showed that miR-30b, downregulated in gastric cancer, promoted apoptosis and suppressed tumor growth by targeting plasminogen activator inhibitor-122. The dual roles of miR-30b may be due to its different target genes in different cancer types. Moreover, miR-30b was found to be amplified and overexpressed in medulloblastoma18. Xu and Li also found that the EGFR expression levels were lower in glioblastoma microvascular proliferation when compared with those in glioblastoma tumor cells, and suggested that the upregulation of miR-30b targeting EGFR contributed to the lack of EGFR expression in glioblastoma microvascular proliferation24. In this study, we also found that miR-30b was frequently upregulated in glioblastoma tissues and cell lines when compared with normal brain tissues, and its high expression was associated with a shorter survival time for glioblastoma patients. Afterward, we found that inhibition of miR-30b significantly reduced the proliferation, migration, and invasion of glioblastoma U87 and A172 cells.
We further identified PRRT2 as a novel target gene of miR-30b in glioblastoma cells. PRRT2, a transmembrane protein containing a proline-rich domain in its N-terminal half, is predominantly expressed in the brain and spinal cord in embryonic and postnatal stages25,26. Previous studies have suggested that mutations in PRRT2 cause paroxysmal kinesigenic dyskinesia25,26. In recent years, Alves et al. identified PRRT2 to be frequently mutated in DNA mismatched repair-deficient cell lines from colorectal and endometrial cancer patients27. Moreover, the suppressive effect of PRRT2 on glioma has also been suggested. Bi et al. found that the expression of PRRT2 was reduced in glioma tissues compared with normal brain tissues, consistent with our findings28. Moreover, they showed that restoration of PRRT2 expression suppressed glioma cell viability and promoted glioma cell apoptosis, probably through inhibition of the three branches of unfolded protein response pathways, including the PERK axis, the IRE1 axis, and the ATF6 axis28. In addition, they found that miR-30a could reverse the effects of PRRT2 on glioma cells28. In our study, we found that the expression of PRRT2 was inversely correlated with the miR-30b expression in glioblastoma tissues, suggesting that downregulation of miR-30b may contribute to PRRT2 upregulation.
As we found that the expression of PRRT2 was negatively regulated by miR-30b in glioblastoma cells, and overexpression of PRRT2 showed a similar effect on the malignant phenotypes of glioblastoma cells as the inhibition of miR-30b, we speculated that PRRT2 might act as a downstream effector in the miR-30b-mediated glioblastoma cells. To clarify this speculation, U87 and A172 cells were cotransfected with the miR-30b inhibitor and PRRT2 siRNA, and our data indicated that knockdown of PRRT2 rescued the inhibitory effect of miR-30b inhibition on the proliferation, migration, and invasion of glioblastoma cells. These findings demonstrate that miR-30b plays a promoting role in the malignant phenotypes of glioblastoma cells, partly at least, through directly targeting PRRT2, highlighting the significance of the miR-30b/PRRT2 axis in glioblastoma.
In conclusion, our study demonstrates that miR-30b plays a promoting role in the regulation of proliferation, migration, and invasion of glioblastoma cells, partly at least via targeting PRRT2. These findings suggest that miR-30b may be used as a potential therapeutic target for glioblastoma.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Brower JV, Clark PA, Lyon W, Kuo JS. MicroRNAs in cancer: Glioblastoma and glioblastoma cancer stem cells. Neurochem Int. 2014;77:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang Y, Jiang T. Understanding high grade glioma: Molecular mechanism, therapy and comprehensive management. Cancer Lett. 2013;331(2):139–46. [DOI] [PubMed] [Google Scholar]
- 3. Xu F, Li F, Zhang W, Jia P. Growth of glioblastoma is inhibited by miR-133-mediated EGFR suppression. Tumour Biol. 2015;36(12):9553–8. [DOI] [PubMed] [Google Scholar]
- 4. Xin J, Zhang XK, Xin DY, Li XF, Sun DK, Ma YY, Tian LQ. FUS1 acts as a tumor-suppressor gene by upregulating miR-197 in human glioblastoma. Oncol Rep. 2015;34(2):868–76. [DOI] [PubMed] [Google Scholar]
- 5. Sun J, Zheng G, Gu Z, Guo Z. MiR-137 inhibits proliferation and angiogenesis of human glioblastoma cells by targeting EZH2. J Neurooncol. 2015;122(3):481–9. [DOI] [PubMed] [Google Scholar]
- 6. Wang CY, Zhang JJ, Hua L, Yao KH, Chen JT, Ren XQ. MicroRNA-98 suppresses cell proliferation, migration and invasion by targeting collagen triple helix repeat containing 1 in hepatocellular carcinoma. Mol Med Rep. 2016;13(3):2639–44. [DOI] [PubMed] [Google Scholar]
- 7. Zhang X, Cai D, Meng L, Wang B. MicroRNA-124 inhibits proliferation, invasion, migration and epithelial-mesenchymal transition of cervical carcinoma cells by targeting astrocyte-elevated gene-1. Oncol Rep. 2016;36(4):2321–8. [DOI] [PubMed] [Google Scholar]
- 8. John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human microRNA targets. PLoS Biol. 2004;2(11):e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ambros V. The functions of animal microRNAs. Nature 2004;431(7006):350–5. [DOI] [PubMed] [Google Scholar]
- 10. Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004;116(2):281–97. [DOI] [PubMed] [Google Scholar]
- 11. Liu X, Wang F, Tian L, Wang T, Zhang W, Li B, Bai YA. MicroRNA-520b affects the proliferation of human glioblastoma cells by directly targeting cyclin D1. Tumour Biol. 2016;37(6):7921–8. [DOI] [PubMed] [Google Scholar]
- 12. Li S, Zeng A, Hu Q, Yan W, Liu Y, You Y. miR-423-5p contributes to a malignant phenotype and temozolomide chemoresistance in glioblastomas. Neuro Oncol. 2017;19(1):55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yao Y, Ma J, Xue Y, Wang P, Li Z, Hu Y, Shang X, Liu Y. MiR-449a exerts tumor-suppressive functions in human glioblastoma by targeting Myc-associated zinc-finger protein. Mol Oncol. 2015;9(3):640–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shi Y, Chen C, Yu SZ, Liu Q, Rao J, Zhang HR, Xiao HL, Fu TW, Long H, He ZC, Zhou K, Yao XH, Cui YH, Zhang X, Ping YF, Bian XW. MiR-663 suppresses oncogenic function of CXCR4 in glioblastoma. Clin Cancer Res. 2015;21(17):4004–13. [DOI] [PubMed] [Google Scholar]
- 15. Wang K, Jia Z, Zou J, Zhang A, Wang G, Hao J, Wang Y, Yang S, Pu P. Analysis of hsa-miR-30a-5p expression in human gliomas. Pathol Oncol Res. 2013;19(3):405–11. [DOI] [PubMed] [Google Scholar]
- 16. Wang Z, Dai X, Chen Y, Sun C, Zhu Q, Zhao H, Liu G, Huang Q, Lan Q. MiR-30a-5p is induced by Wnt/beta-catenin pathway and promotes glioma cell invasion by repressing NCAM. Biochem Biophys Res Commun. 2015;465(3):374–80. [DOI] [PubMed] [Google Scholar]
- 17. Jia Z, Wang K, Wang G, Zhang A, Pu P. MiR-30a-5p antisense oligonucleotide suppresses glioma cell growth by targeting SEPT7. PLoS One 2013;8(1):e55008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Lu Y, Ryan SL, Elliott DJ, Bignell GR, Futreal PA, Ellison DW, Bailey S, Clifford SC. Amplification and overexpression of Hsa-miR-30b, Hsa-miR-30d and KHDRBS3 at 8q24.22-q24.23 in medulloblastoma. PLoS One 2009;4(7):e6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Quintavalle C, Donnarumma E, Iaboni M, Roscigno G, Garofalo M, Romano G, Fiore D, De Marinis P, Croce CM, Condorelli G. Effect of miR-21 and miR-30b/c on TRAIL-induced apoptosis in glioma cells. Oncogene 2013;32(34):4001–8. [DOI] [PubMed] [Google Scholar]
- 20. Gaziel-Sovran A, Segura MF, Di Micco R, Collins MK, Hanniford D, Vega-Saenz de Miera E, Rakus JF, Dankert JF, Shang S, Kerbel RS, Bhardwaj N, Shao Y, Darvishian F, Zavadil J, Erlebacher A, Mahal LK, Osman I, Hernando E. miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis. Cancer Cell 2011;20(1):104–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mahdavinezhad A, Mousavi-Bahar SH, Poorolajal J, Yadegarazari R, Jafari M, Shabab N, Saidijam M. Evaluation of miR-141, miR-200c, miR-30b expression and clinicopathological features of bladder cancer. Int J Mol Cell Med. 2015;4(1):32–9. [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu ED, Li N, Li BS, Li W, Zhang WJ, Mao XH, Guo G, Zou QM, Xiao B. miR-30b, down-regulated in gastric cancer, promotes apoptosis and suppresses tumor growth by targeting plasminogen activator inhibitor-1. PLoS One 2014;9(8):e106049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao H, Xu Z, Qin H, Gao Z, Gao L. miR-30b regulates migration and invasion of human colorectal cancer via SIX1. Biochem J. 2014;460(1):117–25. [DOI] [PubMed] [Google Scholar]
- 24. Xu G, Li JY. Differential expression of PDGFRB and EGFR in microvascular proliferation in glioblastoma. Tumour Biol. 2016;37(8):10577–86. [DOI] [PubMed] [Google Scholar]
- 25. Chen WJ, Lin Y, Xiong ZQ, Wei W, Ni W, Tan GH, Guo SL, He J, Chen YF, Zhang QJ, Li HF, Lin Y, Murong SX, Xu J, Wang N, Wu ZY. Exome sequencing identifies truncating mutations in PRRT2 that cause paroxysmal kinesigenic dyskinesia. Nat Genet. 2011;43(12):1252–5. [DOI] [PubMed] [Google Scholar]
- 26. Wang JL, Cao L, Li XH, Hu ZM, Li JD, Zhang JG, Liang Y, San A, Li N, Chen SQ, Guo JF, Jiang H, Shen L, Zheng L, Mao X, Yan WQ, Zhou Y, Shi YT, Ai SX, Dai MZ, Zhang P, Xia K, Chen SD, Tang BS. Identification of PRRT2 as the causative gene of paroxysmal kinesigenic dyskinesias. Brain 2011;134(Pt 12):3493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alves IT, Cano D, Bottcher R, van der Korput H, Dinjens W, Jenster G, Trapman J. A mononucleotide repeat in PRRT2 is an important, frequent target of mismatch repair deficiency in cancer. Oncotarget 2017;8(4):6043–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bi G, Yan J, Sun S, Qu X. PRRT2 inhibits the proliferation of glioma cells by modulating unfolded protein response pathway. Biochem Biophys Res Commun. 2017;485(2):454–60. [DOI] [PubMed] [Google Scholar]