Abstract
Long noncoding RNA CCAL has been reported to promote tumor progression in various human cancers, including hepatocellular carcinoma, osteosarcoma, and colorectal cancer. However, the role of CCAL in papillary thyroid cancer remains largely unknown. In the present study, we found that the expression of CCAL was upregulated in papillary thyroid tumor tissues compared to adjacent normal tissues. Moreover, the expression of CCAL was positively related with papillary thyroid cancer severity and TNM stage and predicated poor prognosis. Besides, we found that knockdown of CCAL significantly inhibited papillary thyroid cancer cell proliferation, migration, and invasion in vitro and reduced tumor growth and metastasis in vivo. We found that knockdown of CCAL dramatically decreased the expression of NOTCH1 and suppressed the activation of the NOTCH1 signaling pathway. Furthermore, overexpression of NOTCH1 rescued the proliferation, migration, and invasion in papillary thyroid cancer cells. Taken together, our data indicated that CCAL promoted papillary thyroid cancer development and progression by activation of the NOTCH1 pathway, which provided a new insight on the design of therapeutic targets.
Key words: Papillary thyroid cancer, Colorectal cancer-associated lncRNA (CCAL), Proliferation, Migration, NOTCH1
INTRODUCTION
Thyroid cancer is one of the most malignant and prevalent endocrine tumors around the world1. In recent years, the incidence of thyroid cancer has been rapidly increasing2. Nearly 80% of thyroid tumors are papillary thyroid carcinomas (PTCs)3. For the treatment of PTC, surgical resection combined with radioiodine and levothyroxine treatment is the main method4. Although most patients with PTC displayed a relatively good prognosis after surgical treatment, there are still many PTC-induced deaths every year worldwide. Until now, there have been no effective diagnostic biomarkers and therapeutic targets for PTC. Therefore, it is quite necessary to discover the molecular mechanisms in PTC for the development of novel therapies.
Long noncoding RNAs (lncRNAs) are a class of transcripts with a length longer than 200 nucleotides5. Many reports show that lncRNAs have no protein-coding potential6. Increasing evidence demonstrates that lncRNAs exerted very important functions in various biological processes, including cell development, immune regulation, and especially tumorigenesis7–9. More and more reports indicate that dysregulation of lncRNAs was closely related to tumor development and progression in various cancers10,11. lncRNAs could regulate cellular proliferation, apoptosis, migration, and invasion in human cancers12,13. For instance, overexpression of lncRNA small nucleolar RNA host gene 6 (SNHG6) is related to poor prognosis in gastric cancer and enhances the proliferation and epithelial–mesenchymal transition (EMT) of tumor cells14. lncRNA maternally expressed gene 3 (MEG3) could promote cisplatin-induced apoptosis in human glioma cells15. Some lncRNAs, such as metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), HIT000218960, and homeobox C transcript antisense intergenic RNA (HOTAIR), are reported to be involved in the progression of thyroid cancer16–18. However, most lncRNAs are waiting for identification, and their roles need to be investigated in thyroid cancer.
Previous studies indicate that colorectal cancer-associated lncRNA (CCAL) serves as an oncogene in hepatocellular carcinoma (HCC), osteosarcoma (OS), and colorectal cancer (CRC)19–21. However, whether CCAL plays a role in PTC remains to be explored. In our study, we found that CCAL was upregulated in PTC tissues compared with adjacent normal tissues. Furthermore, we found that overexpression of CCAL predicted PTC malignance, high TNM stage, and poor prognosis. Knockdown of CCAL significantly inhibited PTC cell proliferation, migration, and invasion in vitro and in vivo. To identify the mechanism of action, we found that knockdown of CCAL remarkably suppressed the expression of NOTCH1 and activation of NOTCH1 signaling pathway. Overexpression of NOTCH1 rescued the proliferation, migration, and invasion of PTC cells. Our study demonstrated that CCAL promoted PTC progression by activation of the NOTCH1 pathway.
MATERIALS AND METHODS
Patient Samples
Fifty-two PTC tissue specimens were obtained from the Shanghai University of Traditional Chinese Medicine (Shanghai, P.R. China). No patients received radiation therapy or chemotherapy prior to surgery. Tissue samples were classified according to the World Health Organization criteria and stored in liquid nitrogen or at −80°C. This study was approved by the Institutional Review Board of Shanghai University of Traditional Chinese Medicine. Written informed consent was obtained from all participating patients.
Cell Lines and Cell Culture
Human thyroid cancer epithelium cell lines (BCPAP, FTC133, 8505C, TPC-1, and HTH-83) and a normal human thyroid epithelium cell line (TEC) were commercially purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in RPMI-1640 medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a humidified atmosphere with 5% CO2.
Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA was extracted from tissue samples and cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer’s protocol. Total RNA was reverse transcribed into cDNA using the PrimeScript™ RT reagent kit with gDNA Eraser (Takara Biotechnology Co., Ltd., Dalian, P.R. China). RT reaction mixtures contained 1 μg of total RNA, 1 μl of RT Enzyme Mix 1, 1 μl of RT Primer Mix, 4 μl of 5× PrimeScript Buffer 2, and 4 μl of ddH2O. The temperature protocol was 37°C for 15 min and 85°C for 5 s. qPCR was performed on cDNA using the QuantiFast SYBR-Green PCR kit (Qiagen GmbH, Hilden, Germany) on a StepOnePlus™ Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). PCR mixtures contained 10 μl of 2× PCR Master Mix, 4 μl of sense and antisense primers, 2 μl of cDNA, and 4 μl of ddH2O. The specificity of each PCR was confirmed using a melting curve analysis. PCR primers were synthesized by Invitrogen, Thermo Fisher Scientific, Inc. The results were normalized to the expression of GAPDH. All samples were assessed in triplicate.
siRNA-Mediated Interference
Small interfering RNAs (siRNAs) against CCAL consisting of three target-specific siRNAs were designed and synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, P.R. China). BCPAP and TPC-1 cells at a density of 3 × 105 cells/ml were seeded into six-well plates and transfected with siRNAs using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) as the transfection reagent, according to the manufacturer’s protocol. After 48 h of transfection, cells were harvested for RT-qPCR to verify the silencing of CCAL expression.
In Vivo Tumor Growth and Metastasis Assays
For tumor growth assay, 6-week-old male mice with severe combined immune deficiency (SCID; Institute of Zoology, Chinese Academy of Sciences, Beijing, P.R. China) received subcutaneous injections of 1 × 106 cells infected with siCCAL or control (n = 6 mice/group). At 1, 3, 5, and 7 weeks after inoculation, tumor volume was monitored and calculated. All mice were sacrificed by euthanasia at 7 weeks postinoculation, and the tumors were removed. For tumor metastasis assay, 6-week-old male SCID mice were injected with 1 × 106 cells infected with siCCAL or control through the tail vein (n = 6 mice/group). The mice were sacrificed by euthanasia at 8 weeks postinoculation. Anatomized mice were examined for metastasis in the lung. Animal experiments were performed in accordance with relevant guidelines and regulations of the Institutional Animal Care and Use Committees at Shanghai University of Traditional Chinese Medicine, and protocols were approved by the Institutional Animal Care and Use Committees at Shanghai University of Traditional Chinese Medicine.
Western Blot
Protein was extracted by cell lysis using RIPA (Beyotime, P.R. China) containing protease inhibitors (Beyotime). Protein lysates were separated in 10% SDS-PAGE and transferred onto PVDF membrane (Millipore, USA). The membranes were blocked by 5% milk in TBST buffer and incubated with primary antibodies overnight at 4°C. PVDF membranes were washed in TBST and incubated with horseradish peroxidase-conjugated secondary antibodies (ProteinTech Group, USA). Proteins were visualized using ECL Western blotting substrate (Pierce, Rockford, IL, USA).
ChIP Assay
ChIP assay was performed according to a previous study22. In brief, cells were treated with 1% formaldehyde for 10 min for cross-linking, crashed with SDS lysis buffer, and followed by ultrasonication. Standard ChIP assay was performed using H3K4me3 antibody. Enriched DNA fragments were eluted and analyzed by qRT-PCR.
Cellular Proliferation Assays
Cells were seeded into 96-well plates at a density of 1 × 103 cells/well. After 24, 48, and 72 h of incubation at 37°C, cellular viability was evaluated using a cell counting kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) according to the manufacturer’s protocol. The absorbance was measured at a wavelength of 450 nm using a Multiskan™ GO Microplate Spectrophotometer (Thermo Fisher Scientific, Inc.).
A colony formation assay was also performed. Cells were seeded into six-well plates and cultured at 37°C with 5% CO2 for 14 days. Colonies were fixed with methanol at room temperature for 20 min and stained with 0.1% crystal violet (Sigma-Aldrich; Merck KGaA). The total number of visible colonies was determined under an optical microscope (Olympus Corporation, Tokyo, Japan). All experiments were repeated three times.
Cellular Migration and Invasion Assays
Cellular migration was assessed using 6.5-mm Transwell inserts with 8.0-μm-pore polycarbonate membranes (Costar; Corning Incorporated, Corning, NY, USA). A cell migration or invasion (coated with Matrigel) assay was performed using the Transwell inserts. Briefly, 2 × 105 transfected and nontransfected cells were suspended and seeded into the upper chambers of the inserts. Medium supplemented with 10% FBS was added into the lower chambers as a chemoattractant. Following 24 h of incubation at 37°C, cells on the upper surface of the membrane were removed; cells that had migrated to the lower membrane were fixed with 100% methanol at room temperature for 20 min and stained with crystal violet. Cells were observed using an optical microscope (Olympus Corporation). Cells were counted in five random fields from each well, and the average number of migrated or invaded cells was calculated. The assays were performed in triplicate.
Statistical Analysis
The statistical significance of the differences between groups was assessed using Student’s t-test for pairwise comparisons or one-way analysis of variance followed by Fisher’s least significant difference post hoc test for multiple comparisons. A value of p < 0.05 was considered to indicate a statistically significant difference. Data are expressed as the mean ± standard deviation of three independent experiments. Statistical analysis was performed using the SPSS software version 20.0 (IBM Corp., Armonk, NY, USA).
RESULTS
CCAL Was Highly Expressed in PTC Tissues
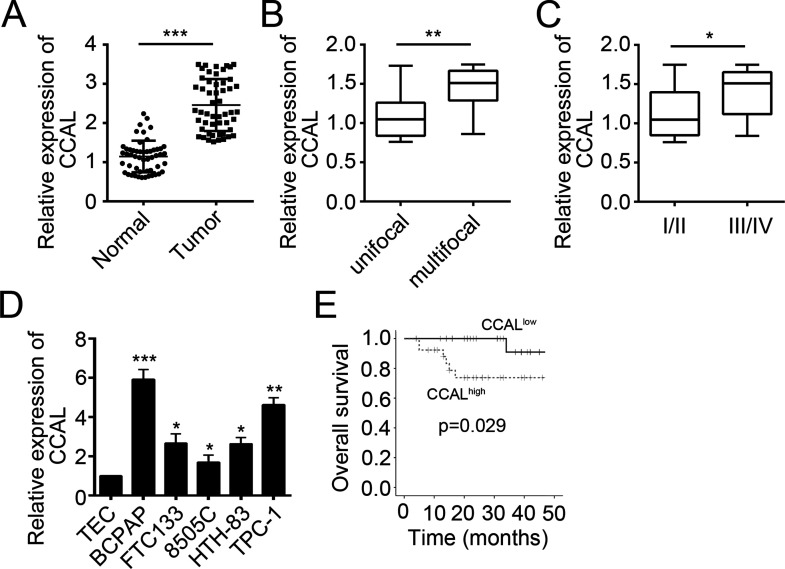
To determine the function of CCAL in PTC, we examined the expression of CCAL in PTC tissues (n = 52) and normal tissues (n = 52) by RT-qPCR. Results indicated that CCAL was upregulated in PTC tissues compared with adjacent normal tissues (p < 0.001) (Fig. 1A). Moreover, as shown in Figure 1B and C, upregulated CCAL expression was significantly correlated with multifocality (p < 0.01) and TNM stage (p < 0.05). Then we checked the expression level of CCAL in PTC cell lines (BCPAP, FTC133, 8505C, HTH-83, and TPC-1). Compared to the normal human thyroid epithelium cell line (TEC), CCAL was highly expressed in PTC cell lines as shown by RT-qPCR (Fig. 1D). We then divided the PTC tissues into two subgroups based on CCAL expression. We performed Kaplan–Meier analysis to determine the correlation between CCAL expression and clinical outcome in PTC patients. Results indicated that patients with a higher CCAL expression have worse overall survival (p = 0.029) (Fig. 1E).
Figure 1.
Colon cancer-associated long noncoding RNA (CCAL) was highly expressed in papillary thyroid carcinoma (PTC) tissues. (A) Comparing the different expression of CCAL between PTC tissues (n = 52) and normal thyroid tissues (n = 16) by reverse transcription quantitative polymerase chain reaction (RT-qPCR). (B) CCAL expression was dramatically upregulated in patients with multifocal PTC. (C) CCAL expression was dramatically upregulated in patients with advanced TNM stage. (D) CCAL expression between different types of thyroid carcinoma cell lines and a normal human thyroid epithelium cell line (TEC) by RT-qPCR. (E) Kaplan–Meier survival analysis based on CCAL expression in PTC tissues. *p < 0.05, **p < 0.01, and ***p < 0.001 by two-tailed Student’s t-test. All data presented are shown as means ± SD collected from three independent experiments.
CCAL Knockdown Suppressed Cell Proliferation
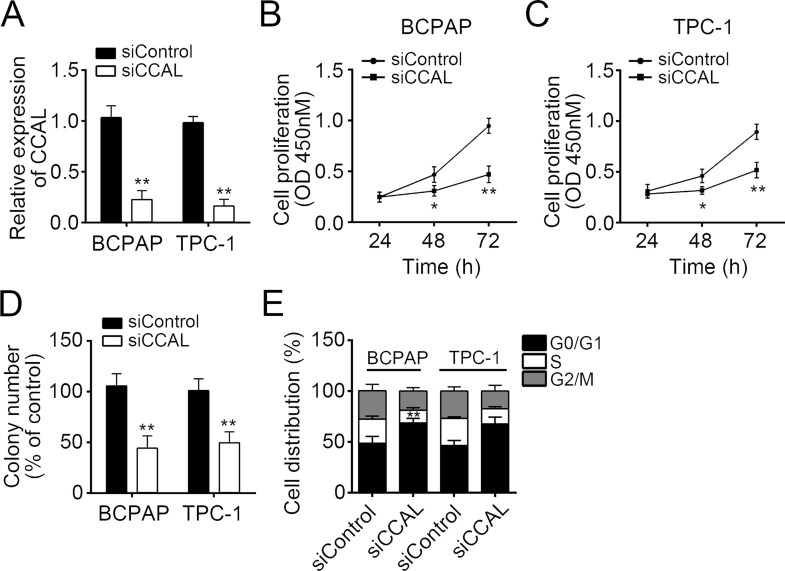
To further explore the effect of CCAL on PTC cells, we silenced CCAL in BCPAP and TPC-1 cells (Fig. 2A). To understand the impact of CCAL on the proliferation of thyroid carcinoma cell, CCK-8 assay was conducted after transfection. As shown in Figure 2B and C, the proliferation rate of BCPAP and TPC-1 cells was suppressed by transfecting with siCCAL at 48 and 72 h (p < 0.05). As shown in Figure 2D, the colony formation assay showed a significant reduction in colony numbers consistent with the CCK-8 assay. To further evaluate the effect of CCAL expression on cell proliferation, the cell cycle distribution was explored in BCPAP and TPC-1 cells transfected with siCCAL by flow cytometry, and the results showed that the cell cycle progression was stagnated at the G1–G0 phase compared with the control cells transfected with empty vector (Fig. 2E). Taken together, CCAL knockdown could inhibit PTC cell proliferation by blocking the cell cycle.
Figure 2.
CCAL knockdown suppressed cell proliferation. (A) RT-qPCR was used to determine the knockdown efficiency of CCAL in BCPAP and TPC-1 cells. (B–D) Cell counting kit-8 (CCK-8) and colony formation assays were used for the detection of cell proliferation potential in BCPAP and TPC-1 cells. (E) Cell cycle distribution of BCPAP and TPC-1 cells transfected with siCCAL relative to cells transfected with control was measured by propidium iodide staining using flow cytometry. *p < 0.05 and **p < 0.01 by two-tailed Student’s t-test. All data presented are shown as means ± SD collected from three independent experiments.
Knockdown of CCAL Inhibited Cell Migration and Invasion
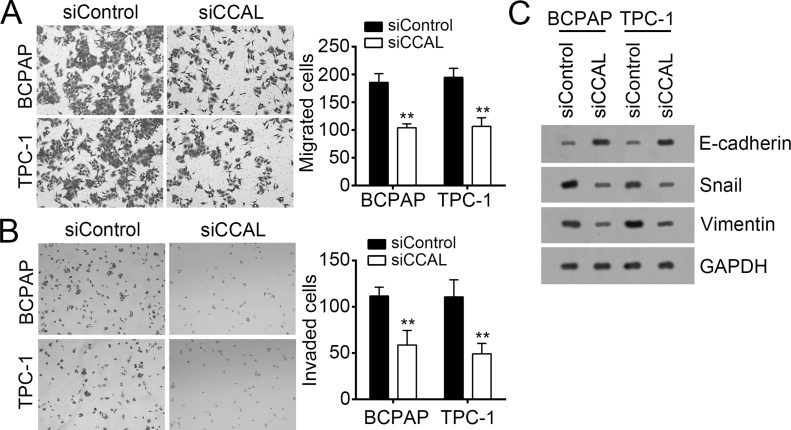
A previous study showed that CCAL could promote the metastasis of OS19. To further determine whether CCAL also regulates the metastasis in PTC, we performed Transwell assays with CCAL-depleted or control BCPAP and TPC-1 cells. As shown, CCAL knockdown significantly inhibited the numbers of migrated and invaded BCPAP and TPC-1 cells (Fig. 3A and B). EMT is a hallmark of metastatic neoplasms. As shown in Figure 3C, knockdown of CCAL upregulated the epithelial cell marker E-cadherin and downregulated vimentin and Snail, which are characteristic of mesenchymal cells. These data suggested that CCAL promoted PTC cell migration and invasion.
Figure 3.
Knockdown of CCAL inhibited cell migration and invasion. (A) Transwell migration and (B) invasion assays were used to assess the migration and invasion abilities of BCPAP and TPC-1 cells. (C) The protein levels of Snail, vimentin, and E-cadherin were checked by Western blot in BCPAP and TPC-1 cells. GAPDH acted as loading control. **p < 0.01 by two-tailed Student’s t-test. All data presented are shown as means ± SD collected from three independent experiments.
CCAL Knockdown Inhibited Tumor Growth and Metastasis In Vivo
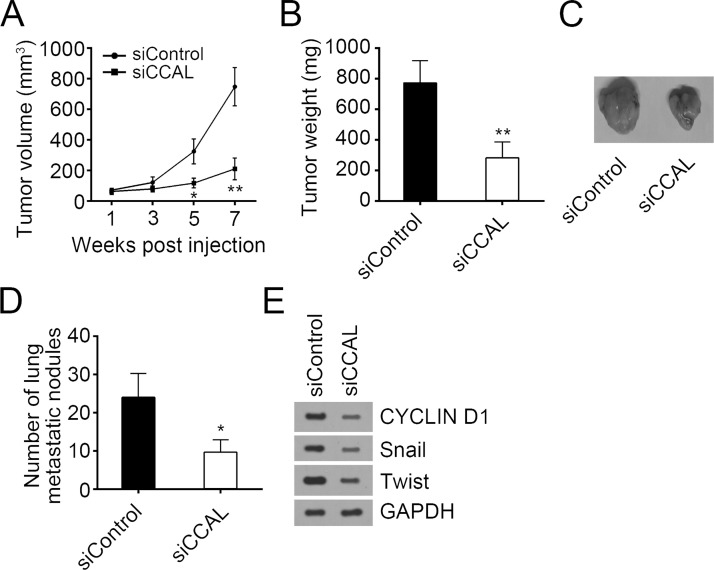
In the next step, to evaluate the effect of CCAL on PTC cells in vivo, we subcutaneously injected BCPAP cells into SCID mice. Tumor volumes were measured at weeks 1, 3, 5, and 7 postinjection, and the mice were sacrificed at 7 weeks. The volume and weight of the tumors derived from CCAL-depleted BCPAP cells were lower than those of the control group (Fig. 4A–C). Furthermore, the rate of lung metastasis was also lower in xenograft tumors expressing siCCAL than control group (Fig. 4D). Then Western blot was used to assess the proliferation and metastasis of formed tumor tissues. As shown in Figure 4E, knockdown of CCAL reduced the levels of cyclin D1, Snail, and Twist, which suggested that CCAL knockdown inhibited tumor proliferation and metastasis in vivo.
Figure 4.
CCAL knockdown inhibited tumor growth and metastasis in vivo. (A–C) BCPAP cells were injected into nude mice subcutaneously. Then tumor volumes (A) and tumor weight (B and C) were measured at indicative time points. (D) The numbers of metastatic foci in the lungs of mice from various groups at 8 weeks after tail vein injection were counted. (E) The protein levels of cyclin D1, Twist, and Snail were measured by Western blot. *p < 0.05 and **p < 0.01 by two-tailed Student’s t-test. All data presented are shown as means ± SD collected from three independent experiments.
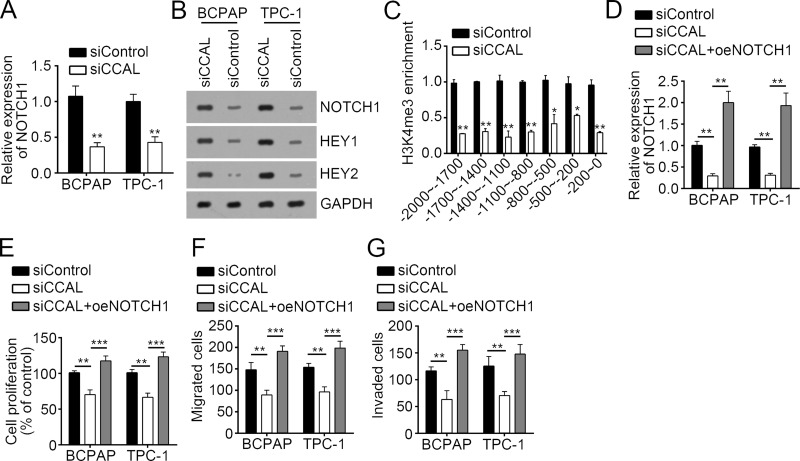
Knockdown of CCAL Inhibited the Activation of NOTCH1 Pathway in PTC Cells
Emerging evidence has shown that NOTCH1 signaling is important for tumor growth and metastasis in TPC22–24. In our study, we also found that knockdown of CCAL significantly inhibited the expression of NOTCH1, HEY1, and HEY2 in BCPAP and TPC-1 cells, which suggested that CCAL knockdown led to inactivation of NOTCH1 signaling (Fig. 5A and B). To determine how CCAL regulates NOTCH1 expression, we performed ChIP assay with CCAL-silenced or control BCPAP cells. We found that CCAL knockdown significantly inhibited the enrichment of histone H3 lysine 4 trimethylation (H3K4me3), an active histone modification, on NOTCH1 promoter (Fig. 5C), suggesting that CCAL regulates NOTCH1 transcription accessibility. To further determine whether CCAL-mediated regulation on PTC cell proliferation and metastasis relied on the NOTCH1 signaling pathway, we restored the activation of NOTCH1 pathway by overexpressing NOTCH1 in CCAL-silenced BCPAP and TPC-1 cells (Fig. 5D). As shown by the CCK-8 and Transwell assays, overexpression of NOTCH1 rescued the proliferation and invasion of CCAL-silenced BCPAP and TPC-1 cells (Fig. 5E–G). Taken together, CCAL promoted the proliferation, migration, and invasion of PTC cells through activating the NOTCH1 signaling pathway.
Figure 5.
Knockdown of CCAL inhibited the activation of NOTCH1 pathway in PTC cells. Knockdown of CCAL significantly inhibited the (A) mRNA and (B) protein levels of NOTCH1 and the protein levels of its target genes (HEY1 and HEY2) in BCPAP and TPC-1 cells. (C) CCAL knockdown significantly inhibited the enrichment of histone active modification H3K4me3 on NOTCH1 promoter. ChIP assay was conducted with CCAL-silenced or control BCPAP cells. (D) RT-qPCR analysis indicated that NOTCH1 was restored in CCAL-depleted BCPAP and TPC-1 cells. (E) CCK-8 assays showed that overexpression of NOTCH1 rescued the proliferation of CCAL-depleted BCPAP and TPC-1 cells. Transwell assays indicated that overexpression of NOTCH1 rescued the (F) migration and (G) invasion of CCAL-depleted BCPAP and TPC-1 cells. *p < 0.05, **p < 0.01 and ***p < 0.001 by two-tailed Student’s t-test. All data presented are shown as means ± SD collected from three independent experiments.
DISCUSSION
PTC patients have died mainly as a result of insufficient specific diagnostic biomarkers and therapeutic strategies25. Defining the molecular mechanism of PTC progression and identifying novel therapeutic targets for PTC intervention are of great importance. More and more evidence shows that lncRNAs have broad biological functions, especially in cell growth, differentiation, and tumorigenesis26. lncRNAs may be a good candidate for tumor diagnosis and prognosis. For example, lncRNA chromosome 17 open reading frame 91 (C17orf91) is a potential prognostic marker and serves as an oncogene in ovarian cancer27. A recently identified lncRNA named sex-determining region Y box 21 antisense 1 (SOX21-AS1) indicates poor prognosis in lung adenocarcinoma28. In this study, we found that CCAL was highly expressed in PTC tissues and aimed to determine its function in PTC.
Dysregulation of lncRNAs has been related to diverse human cancers including HCC, gastric cancer, and colon cancer11,29,30. Previous reports showed that a lncRNA was highly expressed in HCC tissues and associated with HCC metastasis and TNM stage19. They found that CCAL promoted HCC cellular invasion and proliferation and inhibited cell apoptosis by activating the Wnt/β-catenin pathway. Another report indicated that CCAL was significantly overexpressed in OS tissues compared with adjacent normal tissues20. Increased expression of CCAL was also correlated with advanced TNM stage and metastasis in OS. Additionally, Ma and colleagues demonstrated that CCAL was a key regulator of CRC progression. CCAL could activate Wnt/β-catenin signaling via suppression of activator protein 2 α (AP-2α) expression in CRC21. Until now, the function of CCAL is largely unknown. We found that the expression of CCAL was significantly upregulated in PTC tissues. Increased expression of CCAL was positively related with advanced TNM stage and predicted poor prognosis in PTC. By CCK-8 and Transwell assays, we found that knockdown of CCAL in BCPAP and TPC-1 cells remarkably suppressed cellular proliferation, migration, and invasion in vitro. Moreover, xenograft experiments indicated that knockdown of CCAL markedly inhibited tumor growth and lung metastasis in vivo. Thus, our data suggested that CCAL might be a good biomarker for PTC patients’ prognosis and be a potential therapeutic target.
Previous studies widely proved that NOTCH signaling is very important for tumor progression in various human cancers23,24,31–33. For instance, miR-199a-3p inhibits cell proliferation and induces apoptosis by suppressing Jagged1–Notch signaling in human HCC31. The NOTCH1 pathway promoted microRNA-151-5p expression and contributed to gastric cancer progression32. In PTC, researchers found that NOTCH1 signaling was activated and promoted the proliferation and cell cycle of PTC cells24. Another study indicates that activation of NOTCH1 signaling inhibits Prospero homeobox 1 (PROX1) activity and contributes to the malignant behavior of thyroid cancer cells23. A previous study also shows that the NOTCH1 receptor was upregulated and serves as a marker of lymph node metastases in PTC33. In our study, we showed that CCAL promoted the activation of NOTCH1 signaling pathway. By Western blot, we found that CCAL knockdown significantly inhibited the protein levels of NOTCH1 and its target genes (HEY1 and HEY2). Moreover, we also found that restoration of NOTCH1 promoted the proliferation, migration, and invasion of PTC cells. Our data revealed the relationship between CCAL expression and NOTCH1 pathway activation in PTC.
In summary, our research, for the first time, demonstrated the function of CCAL in PTC and explained its functional mechanism. We found that CCAL promoted the proliferation, migration, and invasion of PTC cells, at least in part, by activation of the NOTCH1 signaling pathway. Our findings highlight the importance of the CCAL/NOTCH1 axis in PTC progression.
ACKNOWLEDGMENTS
This study was supported by the Natural Science Foundation of China (No. 81571718), Key Disciplines Group Construction Project of Pudong Health Bureau of Shanghai (PWZxk2017-06), Science and Technology Development Fund (14DZ1940605), Science and Technology Development Fund of Shanghai Pudong New Area (PKJ2017-Y14), and Talents Training Program of the Seventh People’s Hospital of Shanghai University of TCM (Grant No. XX2017-04).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. [DOI] [PubMed] [Google Scholar]
- 2. Singh A, Butuc R, Lopez R. Metastatic papillary thyroid carcinoma with absence of tumor focus in thyroid gland. Am J Case Rep. 2013;14:73–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988-2005. Cancer 2009;115(16):3801–7. [DOI] [PubMed] [Google Scholar]
- 4. Voutilainen PE, Multanen MM, Leppaniemi AK, Haglund CH, Haapiainen RK, Franssila KO. Prognosis after lymph node recurrence in papillary thyroid carcinoma depends on age. Thyroid 2001;11(10):953–7. [DOI] [PubMed] [Google Scholar]
- 5. Fatica A, Bozzoni I. Long non-coding RNAs: New players in cell differentiation and development. Nat Rev Genet. 2014;15(1):7–21. [DOI] [PubMed] [Google Scholar]
- 6. Dai MY, Chen SY, Wei XM, Zhu X, Lan F, Dai SM, Qin X. Diagnosis, prognosis and bioinformatics analysis of lncRNAs in hepatocellular carcinoma. Oncotarget 2017;8(56):95799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li JY, Han W, Shen XL, Han SB, Ye H, Huang GN. DNA methylation signature of long noncoding RNA genes during human pre-implantation embryonic development. Oncotarget 2017;8(34):56829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu BY, Ye BQ, Yang LL, Zhu XX, Huang GL, Zhu PP, Du Y, Wu JY, Qin XW, Chen RS, Tian Y, Fan ZS. Long noncoding RNA lncKdm2b is required for ILC3 maintenance by initiation of Zfp292 expression. Nat Immunol. 2017;18(5):499–508. [DOI] [PubMed] [Google Scholar]
- 9. Li Y, Zhu G, Ma Y, Qu H. LncRNA CCAT1 contributes to the growth and invasion of gastric cancer via targeting miR-219-1. J Cell Biochem. 2017. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10. Hu J, Song C, Duan B, Zhang X, Li D, Zhu L, Gao H. LncRNA-SVUGP2 suppresses progression of hepatocellular carcinoma. Oncotarget 2017;8(58):97835–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin Z, Lai S, He X, Zhuo W, Wang L, Si J, Chen S. Decreased long non-coding RNA MTM contributes to gastric cancer cell migration and invasion via modulating MT1F. Oncotarget 2017;8(57):97371–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kong D, Wang Y. Knockdown of lncRNA HULC inhibits proliferation, migration, invasion, and promotes apoptosis by sponging miR-122 in osteosarcoma. J Cell Biochem. 2018;119(1):1050–61. [DOI] [PubMed] [Google Scholar]
- 13. Stephanova DI, Kossev A. Theoretical predication of temperature effects on accommodative processes in simulated amyotrophic lateral sclerosis during hypothermia and hyperthermia. J Integr Neurosci. 2016;15(4):553–69. [DOI] [PubMed] [Google Scholar]
- 14. Yan K, Tian J, Shi WZ, Xia H, Zhu YF. LncRNA SNHG6 is associated with poor prognosis of gastric cancer and promotes cell proliferation and EMT through epigenetically silencing p27 and sponging miR-101-3p. Cell Physiol Biochem. 2017;42(3):999–1012. [DOI] [PubMed] [Google Scholar]
- 15. Ma BB, Gao ZB, Lou JC, Zhang HQ, Yuan ZB, Wu Q, Li XY, Zhang B. Long non-coding RNA MEG3 contributes to cisplatin-induced apoptosis via inhibition of autophagy in human glioma cells. Mol Med Rep. 2017;16(3):2946–52. [DOI] [PubMed] [Google Scholar]
- 16. Li T, Yang XD, Ye CX, Shen ZL, Yang Y, Wang B, Guo P, Gao ZD, Ye YJ, Jiang KW, Wang S. Long noncoding RNA HIT000218960 promotes papillary thyroid cancer oncogenesis and tumor progression by upregulating the expression of high mobility group AT-hook 2 (HMGA2) gene. Cell Cycle 2017;16(2):224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang JK, Ma L, Song WH, Lu BY, Huang YB, Dong HM, Ma XK, Zhu ZZ, Zhou R. MALAT1 promotes the proliferation and invasion of thyroid cancer cells via regulating the expression of IQGAP1. Biomed Pharmacother. 2016;83:1–7. [DOI] [PubMed] [Google Scholar]
- 18. Zhu H, Lv Z, An CM, Shi M, Pan WT, Zhou LQ, Yang WJ, Yang M. Onco-lncRNA HOTAIR and its functional genetic variants in papillary thyroid carcinoma. Sci Rep. 2016;6:31969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Y, Yang Y, Wang T, Wang L, Wang X, Li T, Shi Y, Wang Y. Long non-coding RNA CCAL promotes hepatocellular carcinoma progression by regulating AP-2alpha and Wnt/beta-catenin pathway. Int J Biol Macromol. 2018;109:424–34. [DOI] [PubMed] [Google Scholar]
- 20. Zhou DK, Yang XW, Li H, Yang Y, Zhu ZJ, Wu N. Up-regulation of long noncoding RNA CCAL predicts poor patient prognosis and promotes tumor metastasis in osteosarcoma. Int J Biol Markers 2017;32(1):e108–12. [DOI] [PubMed] [Google Scholar]
- 21. Ma YL, Yang YZ, Wang F, Moyer MP, Wei Q, Zhang P, Yang Z, Liu WJ, Zhang HZ, Chen NW, Wang H, Wang HM, Qin HL. Long non-coding RNA CCAL regulates colorectal cancer progression by activating Wnt/beta-catenin signalling pathway via suppression of activator protein 2 alpha. Gut 2016;65(9):1494–504. [DOI] [PubMed] [Google Scholar]
- 22. Jung CW, Kong JS, Seol H, Park S, Koh JS, Lee SS, Kim MJ, Choi IJ, Myung JK. Expression of activated Notch1 and Hey1 in papillary thyroid carcinoma. Histopathology 2017;70(2):301–8. [DOI] [PubMed] [Google Scholar]
- 23. Choi D, Ramu S, Park E, Jung E, Yang S, Jung W, Choi I, Lee S, Kim KE, Seong YJ, Hong M, Daghlian G, Kim D, Shin E, Seo JI, Khatchadourian V, Zou M, Li W, De Filippo R, Kokorowski P, Chang A, Kim S, Bertoni A, Furlanetto TW, Shin S, Li M, Chen Y, Wong A, Koh C, Geliebter J, Hong YK. Aberrant activation of Notch signaling inhibits PROX1 activity to enhance the malignant behavior of thyroid cancer cells. Cancer Res. 2016;76(3):582–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamashita AS, Geraldo MV, Fuziwara CS, Kulcsar MAV, Friguglietti CUM, da Costa RB, Baia GS, Kimura ET. Notch pathway Is activated by MAPK signaling and influences papillary thyroid cancer proliferation. Transl Oncol. 2013;6(2):197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vriens MR, Suh I, Moses W, Kebebew E. Clinical features and genetic predisposition to hereditary nonmedullary thyroid cancer. Thyroid 2009;19(12):1343–9. [DOI] [PubMed] [Google Scholar]
- 26. Gutschner T, Diederichs S. The hallmarks of cancer: A long non-coding RNA point of view. RNA Biol. 2012;9(6):703–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li J, Yu HL, Xi ML, Lu X. Long noncoding RNA C17orf91 is a potential prognostic marker and functions as an oncogene in ovarian cancer. J Ovarian Res. 2016;9(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lu XY, Huang CJ, He XZ, Liu XY, Ji JM, Zhang EB, Wang W, Guo RH. A novel long non-coding RNA, SOX21-AS1, indicates a poor prognosis and promotes lung adenocarcinoma proliferation. Cell Physiol Biochem. 2017;42(5):1857–69. [DOI] [PubMed] [Google Scholar]
- 29. Xu YC, Liang CJ, Zhang DX, Li GQ, Gao X, Fu JZ, Xia F, Ji JJ, Zhang LJ, Li GM, Wu JX. LncSHRG promotes hepatocellular carcinoma progression by activating HES6. Oncotarget 2017;8(41):70630–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ouyang S, Zheng X, Zhou X, Chen Z, Yang X, Xie M. LncRNA BCAR4 promotes colon cancer progression via activating Wnt/beta-catenin signaling. Oncotarget 2017;8(54):92815–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ren K, Li T, Zhang W, Ren J, Li Z, Wu G. miR-199a-3p inhibits cell proliferation and induces apoptosis by targeting YAP1, suppressing Jagged1-Notch signaling in human hepatocellular carcinoma. J Biomed Sci. 2016;23(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hsu KW, Fang WL, Huang KH, Huang TT, Lee HC, Hsieh RH, Chi CW, Yeh TS. Notch1 pathway-mediated microRNA-151-5p promotes gastric cancer progression. Oncotarget 2016;7(25):38036–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Park HS, Jung CK, Lee SH, Chae BJ, Lim DJ, Park WC, Song BJ, Kim JS, Jung SS, Bae JS. Notch1 receptor as a marker of lymph node metastases in papillary thyroid cancer. Cancer Sci. 2012;103(2):305–9. [DOI] [PubMed] [Google Scholar]