Abstract
The urinary 8-hydroxy-2'-deoxyguanosine levels have been widely used as a biomarker of oxidative stress. The purpose of this study is to investigate the diurnal and day-to-day variations of urinary 8-hydroxy-2'-deoxyguanosine levels. For the diurnal variation, the urine samples were collected at the time of awakening and every 2 h, from 10:00 to 22:00, from 6 healthy participants. For the day-to-day variation, the urine samples were collected at the time of awakening for 35 consecutive days, from 27 healthy participants. As a result, no differences were observed in the diurnal urinary 8-hydroxy-2'-deoxyguanosine levels, and each subject had a characteristic 8-hydroxy-2'-deoxyguanosine level. On the other hand, the daily 8-hydroxy-2'-deoxyguanosine values showed a certain range of variation reflecting lifestyle factors, such as stress status, exercise, sleep time, drinking and diet. In conclusion, urinary 8-hydroxy-2'-deoxyguanosine may be a useful biomarker to control and prevent oxidative stress-related diseases, if the certain range of day-to-day variations of urinary 8-hydroxy-2'-deoxyguanosine is known. Even with only one measurement per year, the baseline urinary 8-hydroxy-2'-deoxyguanosine level could be achieved in a few years by incorporating the 8-hydroxy-2'-deoxyguanosine measurement as part of an annual health check. As the number of subjects was limited, further studies are needed for practical applications.
Keywords: urine, 8-hydroxy-2'-deoxyguanosine (8-OHdG), oxidative stress, diurnal variation, day-to-day variation
Introduction
Reactive oxygen species (ROS) are produced constantly as metabolic by-products, by either endogenous or exogenous factors in living cells. Oxidative stress is defined as a disturbance in the balance between the production of ROS and antioxidant defenses, and is considered to play a major part in the development of chronic and degenerative diseases.(1–6) 8-Hydroxy-2'-deoxyguanosine (8-OHdG), a DNA-adduct generated by ROS, is a useful biomarker for assessing oxidative stress.(7–9) The urinary 8-OHdG is frequently measured to evaluate the extent of oxidative DNA damage, because the collection is noninvasive and the sample is stable with no artefactual production.(10,11) Furthermore, the urinary 8-OHdG is considered as a good index to reflect whole body oxidative stress,(12) because the 8-OHdGTP hydrolysis by the sanitization enzyme MTH1 may contribute to the excretion of 8-OHdG into urine.(13,14) At the same time, the variation of the 8-OHdG levels due to differences in urine sample collection methods has long been discussed.(15–21) In those previous studies, the following three types of methods were mainly discussed: i) the 8-OHdG excretion in 24 h urine (ng/24 h/kg; nmol/24 h); ii) the levels of 8-OHdG/creatinine (ng/mg; µmol/mol) in a spot urine sample; and iii) the urinary excretion rate of 8-OHdG (ng/h/kg).(15,22) The 24-h excretion rate is currently the gold standard for the evaluation of 8-OHdG levels as an oxidative stress marker. However, it is impractical to collect 24 h urine samples for epidemiological studies or health management. It would be critically useful if the 8-OHdG levels in spot urine could be used in good conscience. In order to reappraise the usefulness of the spot urine 8-OHdG levels, we examined the diurnal and day-to-day variations of 8-OHdG in spot urine samples.
Materials and Methods
Chemicals and reagents
The 8-OHdG (≥98%) was purchased from Sigma–Aldrich (St. Louis, MO). The creatinine (99.0%) was purchased from Wako Pure Chemical Industrials (Osaka, Japan). HPLC-grade methanol and acetonitrile were procured from Wako Pure Chemical Industrials and Kanto Chemical (Tokyo, Japan), respectively.
Urine sample collection
In order to evaluate the diurnal variations of the urinary 8-OHdG levels, the urine samples were collected at the time of awakening and every 2 h, from 10:00 to 22:00, from 6 healthy participants (5 non-smokers: 3 males and 2 females, ages ranging from 28 to 68 years old; 1 smoker: male, age 27 years old). In addition, 24 h and every 2 h urine samples were collected from 3 healthy participants (2 males in their 30s and 1 female in their 40s), in order to compare the differences between the 24 h urine and spot urine samples. For the assessment of the day-to-day variations of urinary 8-OHdG, the urine samples were collected at the time of awakening for 35 consecutive days, from 27 healthy non-smoker subjects (22 males and 5 females, ages ranging from 22 to 25 years old). The participants’ diets were not controlled in this study. The urine samples were stored at –20°C until analysis. The study protocol was approved by the Ethics Committee of Medicine and Medical Care, University of Occupational and Environmental Health, Japan (No. H24-055 and No. H26-239). Written informed consent was obtained from all subjects for the publication of this manuscript.
Measurement of 8-OHdG in urine
The urinary 8-OHdG levels were determined according to our previous study.(23) Briefly, each urine sample was defrosted to room temperature and centrifuged at 8,500 × g for 5 min. A 50 µl portion of the urine supernatant was mixed with the same volume of a dilution solution, containing the ribonucleoside marker 8-hydroxyguanosine. Afterwards, a 20 µl portion of the prepared solution was fractionated and injected into the first HPLC column (MCI GEL CA08F, 1.5 × 150 mm; Mitsubishi Chemical, Tokyo, Japan). The chromatograph was recorded with a UV detector (Gilson UV/VIS-151, 235 nm). The fraction containing 8-OHdG was then automatically injected into the second HPLC column (InsertsilTM ODS-3, 3 µm, 4.6 × 250 mm; GL Sciences Inc., Tokyo, Japan). The chromatograph was recorded with an electrochemical (EC) detector (Coulochem II; ESA, Chelmsford, MA). The 8-OHdG levels were expressed as the ratios to the urinary creatinine contents (UV detector at 235 nm).
Statistical analysis
Statistical analysis was performed by a paired samples t test to determine individual differences. Data analysis was performed with GraphPad Prism ver. 7.04 (GraphPad Software, San Diego, CA).
Results
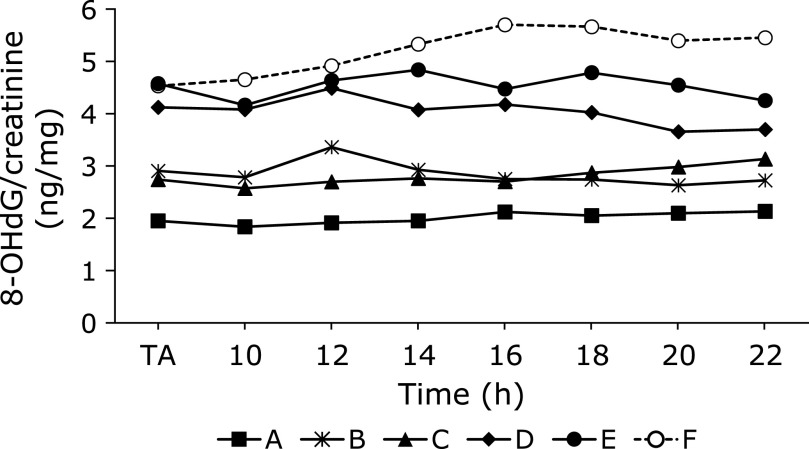
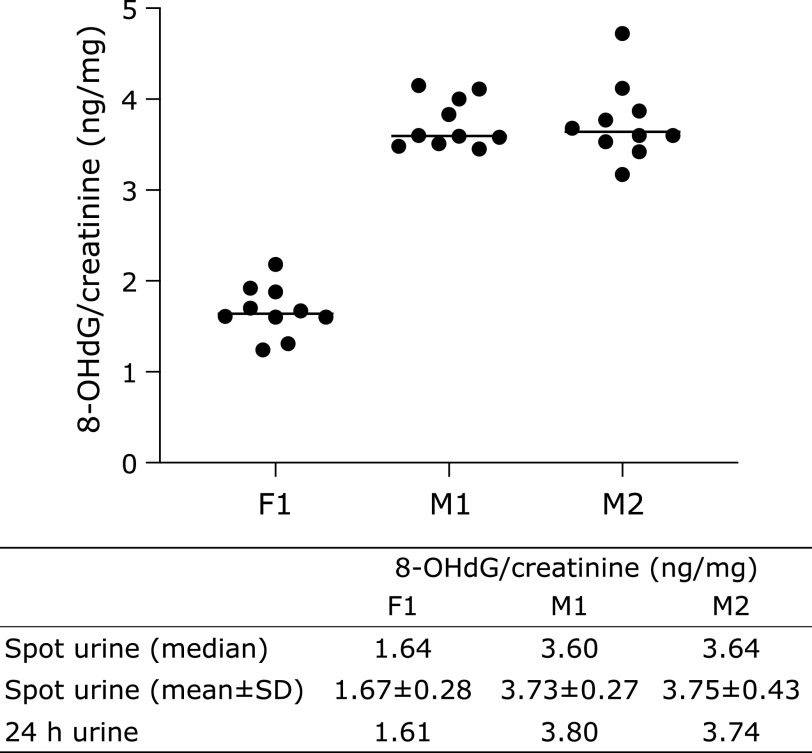
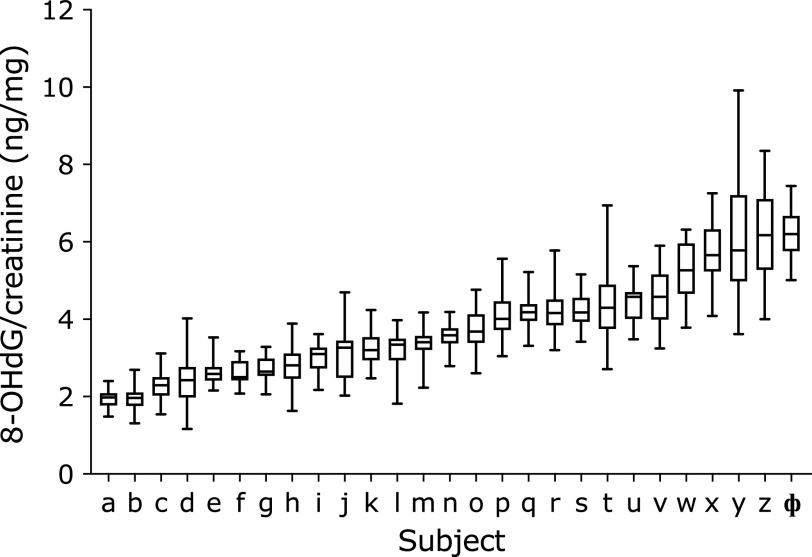
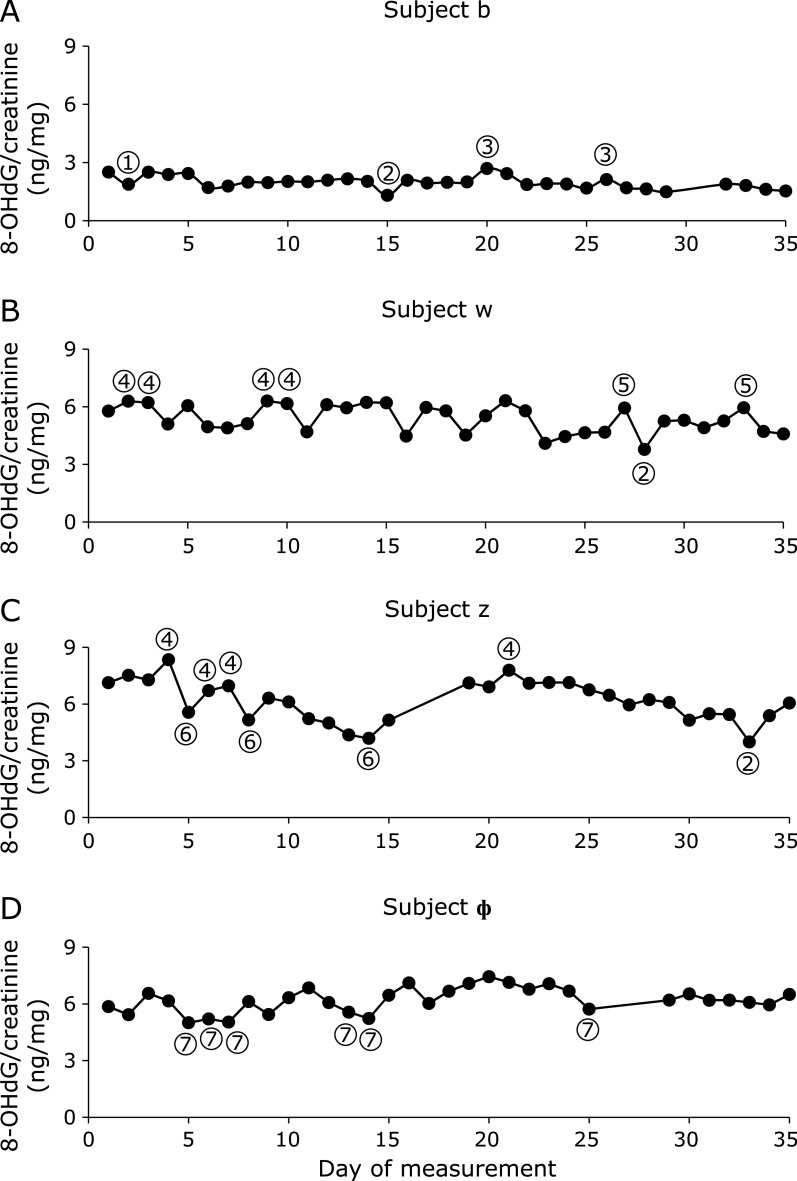
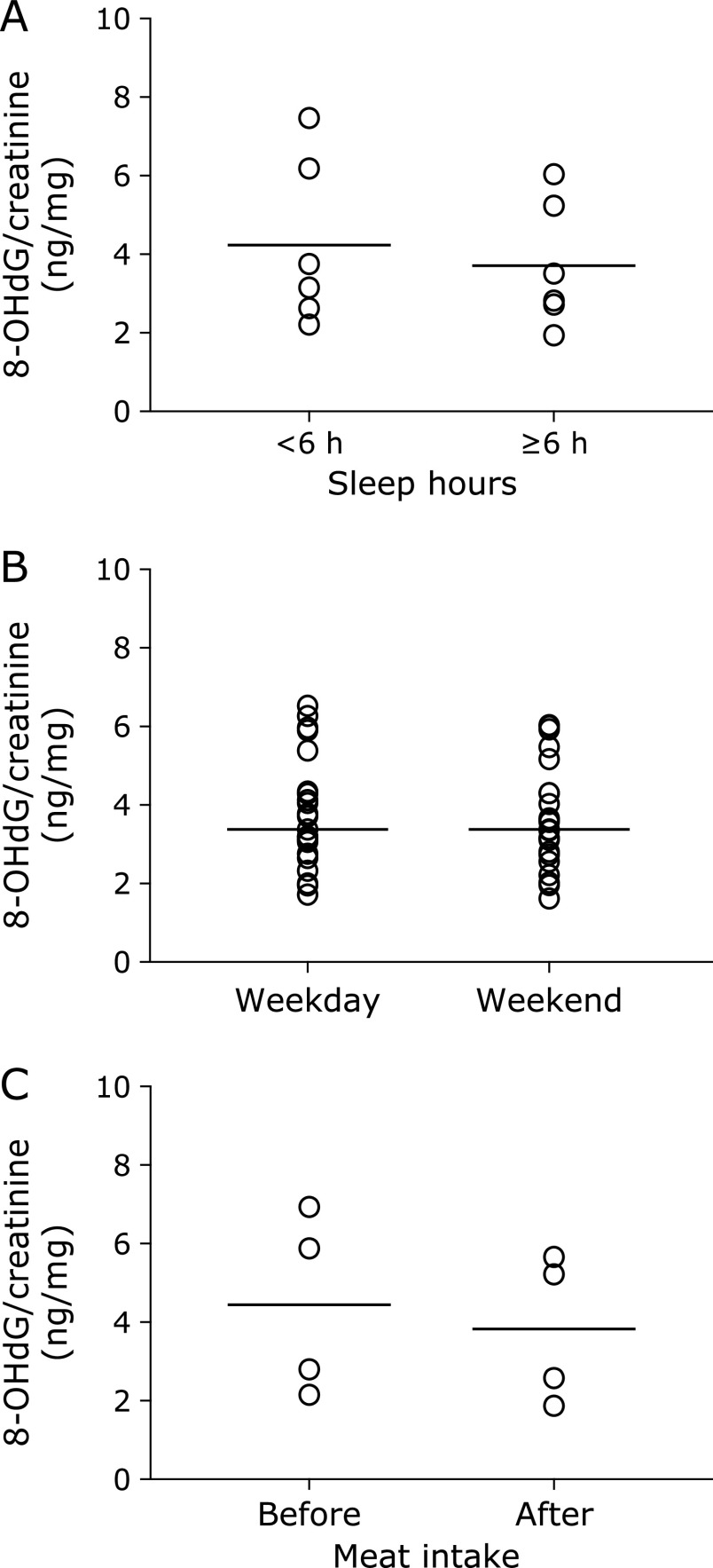
The diurnal changes of urinary 8-OHdG every 2 h are illustrated in Fig. 1. There were no significant differences among the urinary 8-OHdG levels at each time point in the non-smokers. In contrast, rather large changes were observed between the diurnal 8-OHdG levels in the smoker. The coefficients of variation (CV) of the diurnal changes were 5.2% to 7.9% for the 5 non-smokers. The CV for the smoker was 8.6%. The individual urinary 8-OHdG levels were relatively stable throughout the day, even though the urinary 8-OHdG levels varied more widely for the smokers (Fig. 1). In the other experiment, the amounts of 8-OHdG in the 24 h urine samples were similar to those in the every 2 h samples, on the same day (Fig. 2). Overall, each person had a characteristic value, even if there were some daily fluctuations. These results indicated that the 8-OHdG level of a spot urine sample can represent the characteristic value of one person on that day. For the other group, the day-to-day variations for 35 consecutive days of the 27 subjects are shown in Fig. 3. Each plot represents the individual mean level of the urinary 8-OHdG for 35 continuous days, along with the minimum and maximum values in those periods. The CV of the day-to-day variations for the 27 subjects were between 8.7–26.8%. The range between the minimum and maximum values varied from person to person. Given the availability of certain lifestyle records for the 35 days, the day-to-day changes of the 8-OHdG levels of 4 subjects (b, w, z, ɸ) are shown in Fig. 4. The individual 8-OHdG levels fluctuated within a certain range. From the results of the lifestyle records, several events on the day prior to the urine collection are shown in Fig. 4, such as 1: moderate workout, 2: party with friends, 3: mental strain, 4: sleep deprivation, 5: physical fatigue, 6: meat or fish intake, 7: weekend off. In the subjects who reported their sleep hours adequately, the average urinary 8-OHdG levels were higher with less than 6 h of sleep (Fig. 5A, n = 6, p = 0.071). The urinary 8-OHdG levels were lower on the weekend (Fig. 5B, n = 23, p = 0.011) and with meat intake (Fig. 5C, n = 4, p = 0.087).
Fig. 1.
The diurnal variation of urinary 8-OHdG levels, determined in 5 non-smoking subjects (A–E) and 1 smoking subject (F), at the time of awakening (TA) and every 2 h from 10:00 to 22:00. As typical examples, one day data of subjects (A–F) in April.
Fig. 2.
The 8-OHdG levels of the 24 h and every 2 h urine samples from 3 healthy participants (F1: female; M1 and M2: male) in one day. The upper graph shows the measurement results of the every 2 h urine samples.
Fig. 3.
The ranges of urinary 8-OHdG for 35 consecutive days. n = 27. The urine samples were collected at the time of awakening (TA).
Fig. 4.
The typical day-to-day variations of urinary 8-OHdG in 4 subjects over a period of 35 consecutive days. (A) subject b; (B) subject w; (C) subject z; (D) subject ɸ (subject codes correspond to the subjects in Fig. 3). ① moderate workout; ② party with friends; ③ mental strain; ④ sleep deprivation; ⑤ physical fatigue; ⑥ meat or fish intake; ⑦ weekend off. The urine samples were collected at the time of awakening (TA).
Fig. 5.
Urinary 8-OHdG levels and lifestyle factors. Each point shows the mean value of each subject with related lifestyle events. Lines in the figure represent mean values. The urine samples were collected at the time of awakening. (A) sleep hours (n = 6, p = 0.071, Paired t test); (B) weekdays and weekends (n = 23, p = 0.011); (C) the day before and the day after meat intake (n = 4, p = 0.087).
Discussion
The 8-OHdG levels in spot urine samples, as a biomarker of oxidative stress, have been widely measured for estimating the adverse health effects of occupational and environmental exposure.(24–26) However, there are concerns about judging an individual’s oxidative stress level with just one spot urine sample. Pilger et al.(15,16) reported that single spot monitoring is not representative for the individual base levels of urinary 8-OHdG, because of the high diurnal variability. A subsequent report found that the creatinine-adjusted first morning samples reduced the intra-individual variability.(19,27) Grew et al.(20) reported that the 8-OHdG levels did not show diurnal variation, and the creatinine-corrected 6-h samples can be used as a substitute for 24-h sampling. The 8-OHdG values of the 24 h urine samples were similar to those of the every 2 h samples in this study. A recent report concluded that the creatinine-corrected concentrations of 8-OHdG were less variable, with low CV.(21) In this study, no differences were observed in the individual diurnal urinary 8-OHdG levels, and each subject had a characteristic 8-OHdG level. If higher diurnal variation is noted in the results, then it may be a good opportunity for health maintenance, considering lifestyle changes such as quitting smoking and assessing the risk of oxidative stress-related diseases. A previous report indicated the relatively high day-to-day variations of even 8-OHdG/24 h.(15) Presumably, the diurnal and day-to-day variations do not influence the large population epidemiological studies. In contrast, when we consider the issue on an individual level, the day-to-day variations may be more or less affected. Although we studied a limited number of subjects, our results seem to imply that lifestyle factors, such as a moderate workout, mental strain, sleep deprivation, physical fatigue, adequate nutrition, and rest, are apparently causally related to the day-to-day variations. These results were supported by previous reports.(28) In Fig. 4, subject z consumed a high carbohydrate diet. This unbalanced diet might induce the high urinary 8-OHdG levels. Our previous study indicated that an unbalanced diet could increase the urinary 8-OHdG levels.(29) The urinary 8-OHdG levels of subject z were decreased with meat or fish intake. A previous epidemiological study revealed that low meat intake was one of the causes of higher urinary 8-OHdG levels.(30) In this study, we also found that meat intake could decrease the urinary 8-OHdG levels (Fig. 5C). In addition, Yahia et al.(31) reported that fish protein intake could improve the tissue antioxidant status in rats. With regard to the sleeping status, the urinary 8-OHdG levels were increased upon sleep deprivation (<6 h) (Fig. 4 and 5A). A previous study found that the catalase and SOD activities in the brain, spleen and liver were decreased by sleep deprivation in animal models.(32) Anan et al.(33) studied the shift work groups of female nurses and reported significantly high mental stress levels and high urinary 8-OHdG levels before night shift work, as compared to after shift work. The mental stress occurring before the night shift work was considered to be the reason for the high urinary 8-OHdG levels. Our previous study also reported that day-night shift work increased the urinary 8-OHdG levels.(30) In this study, relaxation or a blissful state (Fig. 4: moderate workout, party with friends, weekend off) decreased the urinary 8-OHdG levels. The low mental stress might lead to the lower urinary 8-OHdG levels.
In conclusion, the results of this study suggest that it is possible to estimate the baseline level of the individual oxidative stress status by measuring spot urine samples for several days. Even if the measurement is only once per year, a baseline could be achieved in a few years by incorporating the 8-OHdG measurement as part of an annual health check. Furthermore, the daily values may reflect the impact of lifestyle on oxidative stress, and thus could be quite useful for the prevention of lifestyle-related diseases and the risk assessment of occupational and environmental exposure. As the number of subjects was limited in this study, additional work is needed to confirm this conclusion.
Author Contributions
YL, YK, SW, YO, and KK collected the samples and data. YL statistically analyzed the data. KK, HK, and YL designed and critically discussed the study.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number JP17H01908 and Industrial Disease Clinical Research Grant 170701-01.
Abbreviations
- 8-OHdG
8-hydroxy-2'-deoxyguanosine
- ROS
reactive oxygen species
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Halliwell B. Free radicals, antioxidants and human disease: curiosity, cause or consequence. Lancet 1994; 344: 721–724. [DOI] [PubMed] [Google Scholar]
- 2.Lee D, Xu IM, Chiu DK, et al. Induction of oxidative stress via inhibition of thioredoxin reductase 1 is an effective therapeutic approach for hepatocellular carcinoma. Hepatology 2018; 69: 1768–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bullone M, Lavoie JP. The contribution of oxidative stress and inflamm-aging in human and equine asthma. Int J Mol Sci 2017; 18. pii: E2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y-S, Song MF, Kasai H, Kawai K. 8-hydroxyguanine in urine and serum as an oxidative stress marker: effects of diabetes and aging. J UOEH 2013; 35: 119–127. [DOI] [PubMed] [Google Scholar]
- 5.Nair N, Gongora E. Oxidative stress and cardiovascular aging: interaction between NRF-2 and ADMA. Curr Cardiol Rev 2017; 13: 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Z, Zhou T, Ziegler AC, Dimitrion P, Zuo L. Oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications. Oxid Med Cell Longev 2017; 2017: 2525967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooke MS, Evans MD, Burd RM, et al. Induction and excretion of ultraviolet-induced 8-oxo-2'-deoxyguanosine and thymine dimers in vivo: implications for PUVA. J Invest Dermatol 2001; 116: 281–285. [DOI] [PubMed] [Google Scholar]
- 8.Lodovici M, Casalini C, Cariaggi R, Michelucci L, Dolara P. Levels of 8-hydroxydeoxyguanosine as a marker of DNA damage in human leukocytes. Free Radic Biol Med 2000; 28: 13–17. [DOI] [PubMed] [Google Scholar]
- 9.Li YS, Ootsuyama Y, Kawasaki Y, Morimoto Y, Higashi T, Kawai K. Oxidative DNA damage in the rat lung induced by intratracheal instillation and inhalation of nanoparticles. J Clin Biochem Nutr 2018; 62: 238–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooke MS, Evans MD, Herbert KE, Lunec J. Urinary 8-oxo-2'-deoxyguanosine--source, significance and supplements. Free Radic Res 2000; 32: 381–397. [DOI] [PubMed] [Google Scholar]
- 11.Poulsen HE, Loft S, Prieme H, et al. Oxidative DNA damage in vivo: relationship to age, plasma antioxidants, drug metabolism, glutathione-S-transferase activity and urinary creatinine excretion. Free Radic Res 1998; 29: 565–571. [DOI] [PubMed] [Google Scholar]
- 12.Guo C, Li X, Wang R, et al. Association between oxidative DNA damage and risk of colorectal cancer: sensitive determination of urinary 8-hydroxy-2'-deoxyguanosine by UPLC-MS/MS analysis. Sci Rep 2016; 6: 32581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasai H, Kawai K, Li YS. Analysis of 8-OH-dG and 8-OH-Gua as biomarkers of oxidative stress. Genes and Environ 2008; 30: 33–40. [Google Scholar]
- 14.Evans MD, Saparbaev M, Cooke MS. DNA repair and the origins of urinary oxidized 2'-deoxyribonucleosides. Mutagenesis 2010; 25: 433–442. [DOI] [PubMed] [Google Scholar]
- 15.Pilger A, Ivancsits S, Germadnik D, Rüdiger HW. Urinary excretion of 8-hydroxy-2'-deoxyguanosine measured by high-performance liquid chromatography with electrochemical detection. J Chromatogr B Analyt Technol Biomed Life Sci 2002; 778: 393–401. [DOI] [PubMed] [Google Scholar]
- 16.Pilger A, Germadnik D, Riedel K, Meger-Kossien I, Scherer G, Rüdiger HW. Longitudinal study of urinary 8-hydroxy-2'-deoxyguanosine excretion in health adults. Free Radic Res 2001; 35: 273–280. [DOI] [PubMed] [Google Scholar]
- 17.Kanabrocki EL, Murray D, Hermida RC, et al. Circadian variation in oxidative stress markers in healthy and type II diabetic men. Chronobiol Int 2002; 19: 423–439. [DOI] [PubMed] [Google Scholar]
- 18.Andreoli R, Manini P, Palma GD, et al. Quantitative determination of urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine, 8-oxo-7,8-dihydroguanine, 8-oxo-7,8-dihydroguanosine, and their non-oxidized forms: inter-day concentration profile in healthy volunteers. Biomarkers 2010; 15: 221–231. [DOI] [PubMed] [Google Scholar]
- 19.Barregard L, Møller P, Henriksen T, et al. Human and methodological sources of variability in the measurement of urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine. Antioxid Redox Signal 2013; 18: 2377–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grew IS, Cejvanovic V, Broedbaek K, et al. Diurnal variation of urinary markers of nucleic acid oxidation. Scand J Clin Lab Invest 2014; 74: 336–343. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Moral MP, Kannan K. How stable is oxidative stress level? An observational study of intra- and inter-individual variability in urinary oxidative stress biomarkers of DNA, proteins, and lipids in healthy individuals. Environ Int 2019; 123: 382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murashima M, Watanabe S, Zhuo XG, Uehara M, Kurashige A. Phase 1 study of multiple biomarkers for metabolism and oxidative stress after one-week intake of broccoli sprouts. Biofactors 2004; 22: 271–275. [DOI] [PubMed] [Google Scholar]
- 23.Kim M, Na H, Kasai H, Kawai K, Li YS, Yang M. Comparison of blueberry (Vaccinium spp.) and vitamin C via antioxidative and epigenetic effects in human. J Cancer Prev 2017; 22: 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harri M, Svoboda P, Mori T, Mutanen P, Kasai H, Savela K. Analysis of 8-hydroxydeoxyguanosine among workers exposed to diesel particulate exhaust: comparison with urinary metabolites and PAH air monitoring. Free Radic Res 2005; 39: 963–972. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt MC, Askew EW, Roberts DE, Prior RL, Ensign WY Jr, Hesslink RE Jr. Oxidative stress in humans training in a cold, moderate altitude environment and their response to a phytochemical antioxidant supplement. Wilderness Environ Med 2002; 13: 94–105. [DOI] [PubMed] [Google Scholar]
- 26.Huang YK, Lin CW, Chang CC, et al. Heat acclimation decreased oxidative DNA damage resulting from exposure to high heat in an occupational setting. Eur J Appl Physiol 2012; 112: 4119–4126. [DOI] [PubMed] [Google Scholar]
- 27.Miwa M, Matsumaru H, Akimoto H, Naito S, Ochi H. Quantitative determination of urinary 8-hydroxy-2'-deoxyguanosine level in healthy Japanese volunteers. Biofactors 2004; 22: 249–253. [DOI] [PubMed] [Google Scholar]
- 28.Kasai H, Kawai K. 8-Hydroxyguanine, an oxidative DNA and RNA modification. In: Jurga S, Erdmann VA, and Barciszewski J, eds. Modified Nucleic Acids in Biology and Medicine. Switzerland: Springer International Publishing, 2016; 147–185. [Google Scholar]
- 29.Li YS, Kawai K, Kasai H. Increase of urinary 8-OH-dG levels after administration of a vitamin-deficient diet and a sweet beverage. Gene Environ 2007; 29: 128–132. [Google Scholar]
- 30.Kasai H, Iwamoto-Tanaka N, Miyamoto T, et al. Life style and urinary 8-hydroxydeoxyguanosine, a marker of oxidative DNA damage: effects of exercise, working conditions, meat intake, body mass index, and smoking. Jpn J Cancer Res 2001; 92: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yahia DA, Madani S, Prost E, Prost J, Bouchenak M, Belleville J. Tissue antioxidant status differs in spontaneously hypertensive rats fed fish protein or casein. J Nutr 2003; 133: 479–482. [DOI] [PubMed] [Google Scholar]
- 32.Villafuerte G, Miguel-Puga A, Rodríguez EM, Machado S, Manjarrez E, Arias-Carrión O. Sleep deprivation and oxidative stress in animal models: a systematic review. Oxid Med Cell Longev 2015; 2015: 234952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anan A, Li YS, Tsuji M, et al. Mental/Physical stress levels of female nurses’ working rotational shifts. JJOMT 2019; 67: 167–174. [Google Scholar]