Abstract
The ABC method combined with Helicobacter pylori antibody and serum pepsinogen is a useful predictive method for stomach cancer. Kyoto classification is a new grading system for endoscopic gastritis. However, the consistency of the Kyoto score with the ABC method remains unclear. The Kyoto classification score, which ranges from 0 to 8, is based on the following findings: atrophy, intestinal metaplasia, diffuse redness, nodularity, and enlarged folds. Furthermore, we defined a simplified Kyoto classification score as the sum of scores of just atrophy and intestinal metaplasia. The association between the Kyoto classification score and the ABC method was analyzed using the Kruskal-Wallis and Steel-Dwass tests. A total of 307 subjects were enrolled. Kyoto classification scores were similar in groups B, C, and D, while scores in group A were significantly lower than those of the other groups. The simplified Kyoto classification score showed the same stepwise increase as the classification of the ABC method. In conclusion, unlike the Kyoto classification score, the simplified Kyoto score showed the same significant stepwise increase as the classification of the ABC method.
Keywords: Helicobacter pylori, gastritis, stomach neoplasms, endoscopy
Introduction
Stomach cancer is one of the leading causes of cancer-related death in the world.(1–3) Helicobacter pylori (H. pylori) infection and subsequent atrophic gastritis are main causes of stomach cancer.(4,5) Serological evaluation with anti-H. pylori immunoglobulin G antibody testing is an inexpensive and noninvasive method that is used commonly throughout the world for the diagnosis of H. pylori-related conditions.(6) The combination of H. pylori antibody and serum pepsinogen has been reported to serve as a useful predictive method for stomach cancer.(7,8) The ABC method classification consists of the following four groups: group A [pepsinogen (–) H. pylori (–)], group B [pepsinogen (–) H. pylori (+)], group C [pepsinogen (+) H. pylori (+)], and group D [pepsinogen (+) H. pylori (–)]. The risk of stomach cancer is lowest in group A, followed by B, C and D. A population-based cohort study also reported the usefulness of the ABC method.(9)
On the other hand, the endoscopic Kyoto classification of gastritis was advocated when the 85th Congress of the Japan Gastroenterological Endoscopy Society was held in Kyoto in 2013. The Kyoto classification score was defined as the sum of atrophy score, intestinal metaplasia score, diffuse redness score, nodularity score, and enlarged folds score.(10,11) A high score is believed to reflect an increased risk of stomach cancer.(12)
Research related to the Kyoto classification score and gastric cancer risk is still scarce, and the consistency of the Kyoto score with the ABC method remains unclear.(13) The investigation of the association between the Kyoto score and the ABC method may provide a better understanding of the Kyoto classification score as a marker of stomach cancer risk. Therefore, we examined the relation between the Kyoto score and the ABC method.
Methods
Study design and subjects
The institutional review board at University of Tokyo approved our study project on September 21, 2013 (approved no. 25-34-0921). All participants provided written informed consent.
This cohort study consisted of participants who underwent upper gastrointestinal endoscopy at Toyoshima Endoscopy Clinic (from December 2013 to January 2016). Upper gastrointestinal endoscopy was performed for the evaluation of present symptoms, surveillance of previous upper gastrointestinal diseases or screening. Sedation with midazolam and/or pethidine was induced based on the patient’s willingness.(14,15) The inclusion criteria were adult (≥20 years) without a history of H. pylori eradication, gastric neoplasia, or surgical gastrectomy. The exclusion criterion was withdrawal from the agreement.
The following demographic characteristics were collected: age, sex, body mass index, smoking history, habitual drinking, and first-degree family history of gastric cancer.(16) Smoking history was defined as a score ≥400 on the Brinkman index. Habitual drinking was defined as consumption ≥ one alcoholic drink per day.
Endoscopy-based Kyoto classification score
As mentioned above, the endoscopic Kyoto classification score of gastritis is defined as the sum of scores of five endoscopic findings (atrophy, intestinal metaplasia, diffuse redness, nodularity, and enlarged folds) ranging from 0 to 8. In this study, we defined the simplified Kyoto classification score as the sum of scores of just atrophy and intestinal metaplasia.
Endoscopic atrophy was diagnosed based on the Kimura and Takemoto classification.(17–19) Non-atrophy and C1 were scored as Atrophy score 0, C2 and C3 as Atrophy score 1, and O1 to O3 as Atrophy score 2.
Intestinal metaplasia typically appears as scattered grayish-white and slightly elevated lesions.(20) The absence of intestinal metaplasia was scored as Intestinal metaplasia score 0, the presence of intestinal metaplasia within the antrum as Intestinal metaplasia score 1, and intestinal metaplasia extending into the corpus as Intestinal metaplasia score 2. The Intestinal metaplasia score was diagnosed by using white light imaging.
Diffuse redness refers to uniform redness with continuous expansion observed in non-atrophic mucosa, mainly in the gastric body.(17) Regular arrangement of collecting venules (RAC) is a condition in which the collecting venules are arranged in the corpus. From a distance, it appears like numerous dots. In a close-up, it has the appearance of a regular pattern of starfish-like shapes. The absence of diffuse redness was scored as Diffuse redness score 0, mild diffuse redness or diffuse redness with RAC as Diffuse redness score 1, and severe diffuse redness or diffuse redness without RAC as Diffuse redness score 2.
Nodular gastritis is an endoscopic finding resembling “goose flesh” mainly located in the antrum. The absence and presence of nodularity was scored as Nodularity score 0 and 1, respectively.
An enlarged fold was defined as a fold with a width of 5 mm or more that is not flattened or is only partially flattened by insufflation of the stomach. The absence and presence of enlarged folds was scored as Enlarged folds score 0 and 1, respectively.
ABC method
Blood samples were obtained on the day of the upper gastrointestinal endoscopy. Serum H. pylori antibody titer was measured by E-plate Eiken H. pylori antibody II kit (Eiken Chemical, Tokyo, Japan). The cut-off value of 10 U/ml was used for positivity of H. pylori infection based on the manufacturer’s recommendation.(21–23) Serum pepsinogen was measured by Pepsinogen CLEIA kit (Fuji Rebio Ltd., Tokyo, Japan). Serological atrophy is diagnosed by serum pepsinogen levels. Pepsinogen test-positive criteria consist of pepsinogen I/II ratio ≤3.0 and pepsinogen I ≤70 ng/ml.(8,24) The ABC method classifies gastric condition into 4 groups according to the serum pepsinogen and H. pylori antibody test. Group A consists of subjects with normal pepsinogen and H. pylori antibody (–); group B has normal pepsinogen and H. pylori antibody (+); group C has atrophic pepsinogen and H. pylori antibody (+); and group D has atrophic pepsinogen and H. pylori antibody (–). Thus, groups A, B, C, and D represent subjects without H. pylori infection, H. pylori-infected patients without gastric atrophy, patients with H. pylori-associated gastric atrophy, and patients with intestinal metaplasia and severe gastric atrophy, respectively.
The pepsinogen test-negative group is divided into three subgroups according to cancer risk and gastritis activity: α, with pepsinogen I/II ratio >3.0 and pepsinogen I ≤70 ng/ml; β, with pepsinogen I/II ratio >3.0 and pepsinogen I >70 ng/ml, and γ, with pepsinogen I/II ratio ≤3.0 and pepsinogen I >70 ng/ml. Subjects in group B are further classified into three subgroups as Bα, Bβ and Bγ.(25) Group Bγ has been reported to have the highest risk for stomach cancer, especially for diffuse-type cancer, among the three subgroups.(26)
Statistical analysis
The association between the Kyoto classification score and the ABC method was analyzed using the Kruskal-Wallis and Steel-Dwass tests. Differences between the groups (group Bα and Bβ vs group Bγ) were analyzed using the Welch’s t test. A p value <0.05 was defined as statistically significant. Calculations were carried out by the Stat Mate IV software (ATOMS, Tokyo, Japan) and the statistical software Ekuseru-Toukei 2015 (Social Survey Research Information Co., Ltd., Tokyo, Japan).
Results
Table 1 shows the demographic data of the 307 patients included in our study. Table 2 shows the association between the ABC method and each score of atrophy, intestinal metaplasia, diffuse redness, nodularity, and enlarged folds. The Kruskal-Wallis tests showed significant differences between four groups of ABC method. The peak of atrophy score and intestinal metaplasia score was observed in group D. The peak of enlarged folds score, nodularity score, and diffuse redness score was observed in groups B and C.
Table 1.
Baseline characteristics of the subjects
| No. | 307 |
| Female sex, % | 47.9 |
| Age, mean (SD), y | 49.9 (12.1) |
| Body mass index, mean (SD), kg/m2 | 22.4 (3.2) |
| Drinking, % | 26.7 |
| Smoking, % | 8.1 |
| Family history of gastric cancer, % | 16.6 |
| Positive H. pylori antibody, % | 83.1 |
| Kyoto score, mean (SD) | 4.52 (1.56) |
| Atrophy score, mean (SD) | 1.34 (0.50) |
| Intestinal metaplasia score, mean (SD) | 0.60 (0.73) |
| Diffuse redness score, mean (SD) | 1.71 (0.34) |
| Nodularity score, mean (SD) | 0.39 (0.38) |
| Enlarged folds score, mean (SD) | 0.49 (0.36) |
Table 2.
Association between ABC method and each score of atrophy, intestinal metaplasia, enlarged folds, nodularity, and diffuse redness
| Group | A | B | C | D | p value |
|---|---|---|---|---|---|
| Atrophy score, mean (SD) | 0.77 (0.60) | 1.24 (0.49) | 1.64 (0.46) | 1.69 (0.42) | <0.001 |
| Intestinal metaplasia score, mean (SD) | 0.42 (0.68) | 0.53 (0.72) | 0.68 (0.74) | 1.19 (0.60) | <0.01 |
| Diffuse redness score, mean (SD) | 0.67 (0.68) | 1.81 (0.27) | 1.94 (0.20) | 1.56 (0.51) | <0.001 |
| Nodularity score, mean (SD) | 0.14 (0.33) | 0.44 (0.37) | 0.44 (0.37) | 0.19 (0.36) | <0.01 |
| Enlarged folds score, mean (SD) | 0.19 (0.36) | 0.59 (0.31) | 0.45 (0.37) | 0.31 (0.40) | <0.001 |
The p value was calculated by Kruskal-Wallis test.
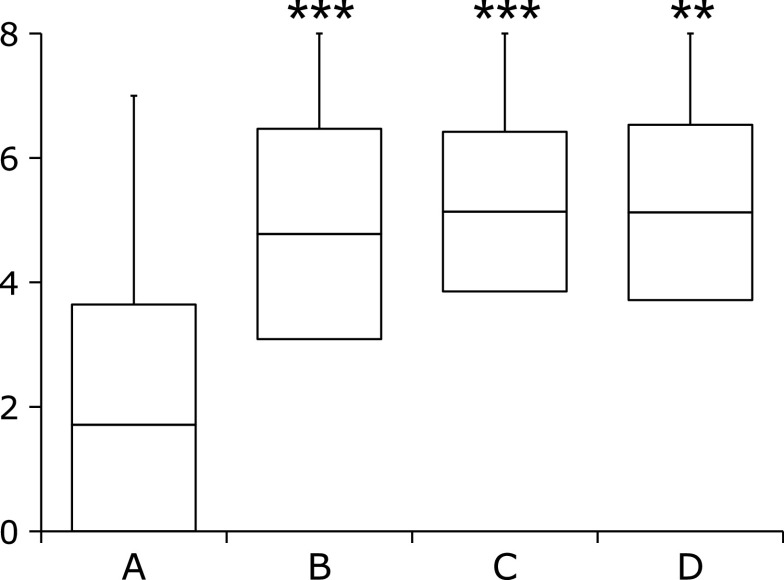
Figure 1 shows the association between the Kyoto classification score and the ABC method. The average Kyoto classification score of group A was significantly lower than those of groups B, C, and D. The Kyoto classification scores of groups B, C, and D were similar.
Fig. 1.
Kyoto score according to the ABC method. Box-plots depicting the average Kyoto score. The p value was calculated using the Steel-Dwass test. ** and *** indicate p<0.01 and p<0.001, respectively, compared with group A.
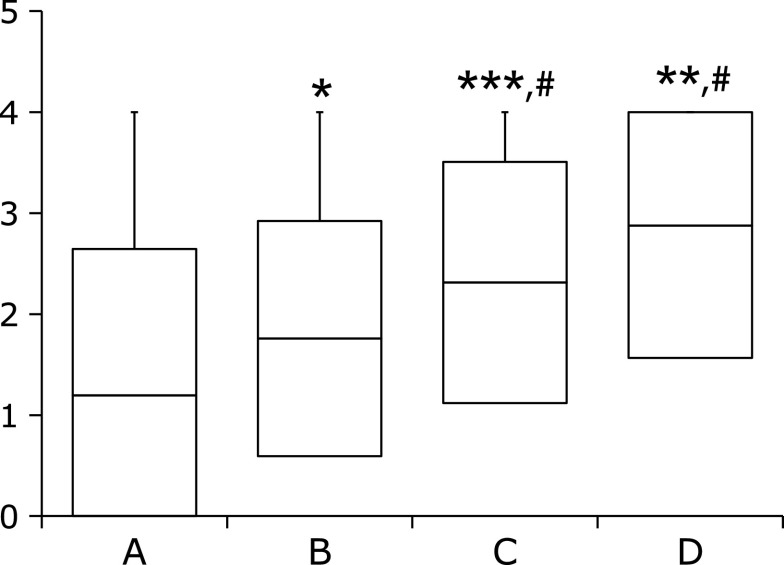
Figure 2 shows the association between the simplified Kyoto classification score and the ABC method. There was a stepwise increase in the simplified Kyoto classification score from group A to group D. The average simplified Kyoto classification score of group A was significantly lower than those of group B, C, and D. Furthermore, the average Kyoto classification score of group B was also significantly lower than those of group C, and D (p<0.01).
Fig. 2.
Simplified Kyoto score according to the ABC method. The simplified Kyoto classification score is based on the sum of scores of just atrophy and intestinal metaplasia. Box-plots depict the average Kyoto score. The p value was calculated using the Steel-Dwass test. *, **, and *** indicate p<0.05, p<0.01, and p<0.001, respectively, compared with group A. # indicates p<0.01 compared with group B.
In the subgroup analysis of group B, the average Kyoto classification score of group Bγ was 5.24 ± 1.47, and that of groups Bα and Bβ was 4.31 ± 1.71. There was a statistically significant difference (p<0.001). The average simplified Kyoto classification score of group Bγ was 2.02 ± 1.27 and that of group Bα and Bβ was 1.63 ± 1.10. There was no statistically significant difference (p = 0.07).
Discussion
Unlike the Kyoto classification score, the simplified Kyoto classification score is consistent with the classification of the ABC method. Since the ABC method is based on the presence of H. pylori infection and the degree of gastric atrophy with laboratory test, it is natural that the ABC method is highly related to the simplified Kyoto system, which includes only the degree of atrophy and intestinal metaplasia.
Intestinal-type gastric cancer mainly occurs following the gastritis-atrophy-metaplasia-cancer sequence due to H. pylori infection.(27) On the other hand, diffuse-type could occur from direct cancer development due to H. pylori-induced inflammation.(26) Therefore, intestinal metaplasia could induce intestinal-type cancer.(28) Enlarged folds and nodularity could induce diffuse-type gastric cancer.(29–31) The simplified Kyoto classification score is based only on the findings of atrophy and intestinal metaplasia scores. Therefore, the simplified Kyoto classification score mainly reflects the risk of intestinal-type cancer in relation to the gastritis-atrophy-metaplasia-cancer sequence. On the other hand, the Kyoto classification score considers atrophy, intestinal metaplasia, diffuse redness scores, nodularity, and enlarged folds, and thus reflects the risk of both intestinal-type cancer and diffuse-type cancer.
Especially, group Bγ indicates high risk of diffuse-type cancer. Group Bγ is characterized by highly active gastric inflammation owing to high pepsinogen II level, as indicated by the low serum pepsinogen I/II ratio. The hazard ratio of group Bγ for diffuse-type cancer was reported to be 7.1 compared with group Bα.(26) In our study, the Kyoto classification score of group Bγ was significantly higher.
In the ABC method, gastric cancer risk increases in the order of A, B, C and D in a stepwise manner. Ohata et al.(32) showed that the hazard ratios of B, C, and D were 7.1, 14.5, and 61.9, respectively. However, the Kyoto classification score was similar in B, C and D. This may be because the Kyoto classification score overestimates the risk of diffuse-type cancer. Diffuse-type cancer is less frequent than intestinal-type cancer;(32,33) hence, the Kyoto classification score might need correction.
This study has several limitations. First, the study was performed at a single institution. Second, it did not evaluate actual gastric cancer incidence. Further studies are warranted to clarify the association between Kyoto classification score and gastric cancer risk.
In conclusion, the simplified Kyoto classification score showed the same significant stepwise increase as the classification of the ABC method. The simplified Kyoto classification score might be promising because of its easy procedure, compared with the somewhat complicated scoring of the Kyoto classification.
Author Contributions
TN designed the study, analyzed data, and wrote the manuscript. OT designed the study, recruited patients, and analyzed data. RK and KS recruited patients, and performed endoscopy. YT HE, HS, CT, and KM critically revised the manuscript. KK revised and approved the final article.
Acknowledgments
This work was supported by Ministry of Education, Culture, Sports, Science and Technology of Japan (25134707 and 16H01566 to KM, 15K14377 to CT). This study was also supported by funding from the Tailor-Made Medical Treatment with the BBJ Project from Japan Agency for Medical Research and Development, AMED (from April 2015), and the Ministry of Education, Culture, Sports, Science, and Technology of Japan (from April 2003 to March 2015).
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Sakitani K, Nishizawa T, Arita M, et al. Early detection of gastric cancer after Helicobacter pylori eradication due to endoscopic surveillance. Helicobacter 2018; 23: e12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001; 345: 784–789. [DOI] [PubMed] [Google Scholar]
- 3.Nishizawa T, Suzuki H, Arano T, et al. Characteristics of gastric cancer detected within 1 year after successful eradication of Helicobacter pylori. J Clin Biochem Nutr 2016; 59: 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatakeyama M, Brzozowski T. Pathogenesis of Helicobacter pylori infection. Helicobacter 2006; 11 Suppl 1: 14–20. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki H, Nishizawa T, Tsugawa H, Mogami S, Hibi T. Roles of oxidative stress in stomach disorders. J Clin Biochem Nutr 2012; 50: 35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kodama M, Okimoto T, Mizukami K, et al. Differences in Helicobacter pylori and CagA antibody changes after eradication between subjects developing and not developing gastric cancer. J Clin Biochem Nutr 2019; 65: 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miki K. Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels—“ABC method”. Proc Jpn Acad Ser B Phys Biol Sci 2011; 87: 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kudo T, Kakizaki S, Sohara N, et al. Analysis of ABC (D) stratification for screening patients with gastric cancer. World J Gastroenterol 2011; 17: 4793–4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizuno S, Miki I, Ishida T, et al. Prescreening of a high-risk group for gastric cancer by serologically determined Helicobacter pylori infection and atrophic gastritis. Dig Dis Sci 2010; 55: 3132–3237. [DOI] [PubMed] [Google Scholar]
- 10.Toyoshima O, Nishizawa T, Koike K. Endoscopic Kyoto classification of Helicobacter pylori infection and gastric cancer risk diagnosis. World J Gastroenterol 2020; 26: 466–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agawa S, Futagami S, Yamawaki H, et al. Acylated ghrelin levels were associated with depressive status, physical quality of life, endoscopic findings based on Kyoto classification in Japan. J Clin Biochem Nutr 2019; 65: 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shichijo S, Hirata Y, Niikura R, Hayakawa Y, Yamada A, Koike K. Association between gastric cancer and the Kyoto classification of gastritis. J Gastroenterol Hepatol 2017; 32: 1581–1586. [DOI] [PubMed] [Google Scholar]
- 13.Sugimoto M, Ban H, Ichikawa H, et al. Efficacy of the Kyoto classification of gastritis in identifying patients at high risk for gastric cancer. Intern Med 2017; 56: 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishizawa T, Suzuki H, Arita M, et al. Pethidine dose and female sex as risk factors for nausea after esophagogastroduodenoscopy. J Clin Biochem Nutr 2018; 63: 230–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen LB, Delegge MH, Aisenberg J, et al. AGA Institute review of endoscopic sedation. Gastroenterology 2007; 133: 675–701. [DOI] [PubMed] [Google Scholar]
- 16.Nishizawa T, Suzuki H, Sakitani K, et al. Family history is an independent risk factor for the progression of gastric atrophy among patients with Helicobacter pylori infection. United European Gastroenterol J 2017; 5: 32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy 1969; 1: 87–97. [Google Scholar]
- 18.Toyoshima O, Yamaji Y, Yoshida S, et al. Endoscopic gastric atrophy is strongly associated with gastric cancer development after Helicobacter pylori eradication. Surg Endosc 2017; 31: 2140–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quach DT, Hiyama T. Assessment of endoscopic gastric atrophy according to the Kimura-Takemoto classification and its potential application in daily practice. Clin Endosc 2019; 52: 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuta N, Ida K, Kato T, et al.; Study Group for Investigating Endoscopic Diagnosis of Gastric Intestinal Metaplasia . Endoscopic diagnosis of gastric intestinal metaplasia: a prospective multicenter study. Dig Endosc 2013; 25: 526–534. [DOI] [PubMed] [Google Scholar]
- 21.Toyoshima O, Nishizawa T, Sakitani K, et al. Serum anti-Helicobacter pylori antibody titer and its association with gastric nodularity, atrophy, and age: a cross-sectional study. World J Gastroenterol 2018; 24: 4061–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kishikawa H, Kimura K, Takarabe S, Kaida S, Nishida J. Helicobacter pylori antibody titer and gastric cancer screening. Dis Markers 2015; 2015: 156719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishizawa T, Suzuki H, Fujimoto A, et al. Effects of patient age and choice of antisecretory agent on success of eradication therapy for Helicobacter pylori infection. J Clin Biochem Nutr 2017; 60: 208–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toyoshima O, Tanikawa C, Yamamoto R, et al. Decrease in PSCA expression caused by Helicobacter pylori infection may promote progression to severe gastritis. Oncotarget 2018; 9: 3936–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe M, Kato J, Inoue I, et al. Development of gastric cancer in nonatrophic stomach with highly active inflammation identified by serum levels of pepsinogen and Helicobacter pylori antibody together with endoscopic rugal hyperplastic gastritis. Int J Cancer 2012; 131: 2632–2642. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida T, Kato J, Inoue I, et al. Cancer development based on chronic active gastritis and resulting gastric atrophy as assessed by serum levels of pepsinogen and Helicobacter pylori antibody titer. Int J Cancer 2014; 134: 1445–1457. [DOI] [PubMed] [Google Scholar]
- 27.Yoon H, Kim N. Diagnosis and management of high risk group for gastric cancer. Gut Liver 2015; 9: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung SJ, Park MJ, Kang SJ, et al. Effect of annual endoscopic screening on clinicopathologic characteristics and treatment modality of gastric cancer in a high-incidence region of Korea. Int J Cancer 2012; 131: 2376–2384. [DOI] [PubMed] [Google Scholar]
- 29.Nishibayashi H, Kanayama S, Kiyohara T, et al. Helicobacter pylori-induced enlarged-fold gastritis is associated with increased mutagenicity of gastric juice, increased oxidative DNA damage, and an increased risk of gastric carcinoma. J Gastroenterol Hepatol 2003; 18: 1384–1391. [DOI] [PubMed] [Google Scholar]
- 30.Nishikawa I, Kato J, Terasoma S, et al. Nodular gastritis in association with gastric cancer development before and after Helicobacter pylori eradication. JGH Open 2018; 2: 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki M, Suzuki H, Minegishi Y, Ito K, Nishizawa T, Hibi T. H. pylori-eradication therapy increases RUNX3 expression in the glandular epithelial cells in enlarged-fold gastritis. J Clin Biochem Nutr 2010; 46: 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohata H, Kitauchi S, Yoshimura N, et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer 2004; 109: 138–143. [DOI] [PubMed] [Google Scholar]
- 33.Sakitani K, Hirata Y, Watabe H, et al. Gastric cancer risk according to the distribution of intestinal metaplasia and neutrophil infiltration. J Gastroenterol Hepatol 2011; 26: 1570–1575. [DOI] [PubMed] [Google Scholar]