Abstract
Tyrosine kinase inhibitors (TKIs) are very effective against non-small cell lung cancer (NSCLC) caused by epidermal growth factor receptor (EGFR) mutation. Before the approval of osimertinib in March 2016, there were only three available EGFR TKIs (gefitinib, erlotinib, and afatinib) for the therapy of NSCLC in Japan. Osimertinib can be indicated only against T790M+ lung cancer as a second-line therapy. However, whether gefitinib, erlotinib, or afatinib is most appropriate as a first-line therapy is still a controversial issue. The aim of this study was to compare the effectiveness of gefitinib, erlotinib, and afatinib. We retrospectively reviewed the records of 310 patients with the diagnosis of EGFR mutation-associated NSCLC including 147 patients treated with EGFR TKIs. Time to treatment failure and overall survival were evaluated. There were no significant differences in time to treatment failure (gefitinib: 9.2 months; erlotinib: 9.8 months; afatinib: 13.1 months) and overall survival (gefitinib: 27.3 months; erlotinib: 29.3 months; afatinib data not available) among NSCLC patients treated with the three different EGFR TKIs. Subgroup analysis showed that smoking status has a significant influence on both time to treatment failure and overall survival. In conclusion, this study showed comparable clinical efficacy of gefitinib, erlotinib, and afatinib in Japanese patients with NSCLC.
Key words: Gefitinib, Erlotinib, Afatinib, Non-small cell lung cancer (NSCLC), Epidermal growth factor receptor (EGFR) mutation, Adenocarcinoma, Japanese population
INTRODUCTION
Non-small cell lung cancer (NSCLC) is one of the major causes of death worldwide1. Therapy with cytotoxic drugs is associated with 20%–35% response rate and 10–12 months of median survival time among patients with advanced NSCLC2. Subsequent clinical trials have shown the significant efficacy of epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs) in NSCLC associated with EGFR mutation3,4. Before the approval of osimertinib in March 2016, there were only three available EGFR TKIs (gefitinib, erlotinib, and afatinib) for the therapy of NSCLC in Japan. Phase III clinical trials have clearly demonstrated the superior efficacy of EGFR TKIs over standard chemotherapy for improving progression-free survival (PFS)5–9. Therefore, EGFR TKIs are presently recommended as first-line therapy of lung tumors caused by EGFR mutation10. However, whether gefitinib, erlotinib, or afatinib should be used as the first-line therapy still remains as a controversial issue.
A few prospective trials have demonstrated similar effects of gefitinib and erlotinib on PFS and superiority of afatinib over gefitinib to improve PFS11–13. The LUX-Lung 7 trial has shown that afatinib significantly prolongs PFS but not overall survival (OS) compared to gefitinib12. In addition, while the combined analysis of both LUX-Lung 3 and 6 has shown that afatinib is effective in tumors with Ex19 deletion but not in those with L858R mutation, the results of the LUX-Lung 7 trial demonstrated no difference in efficacy between tumors with Ex19 deletion and L858R mutation8,12,14,15. These previous contradicting reports underscore the importance of determining which of the EGFR TKIs should be the best indication as a first-line therapy for EGFR mutant-positive NSCLC.
The aim of this retrospective study was to evaluate and compare the efficacy of gefitinib, erlotinib, and afatinib in NSCLC caused by EGFR mutation in a Japanese population.
MATERIALS AND METHODS
Patients
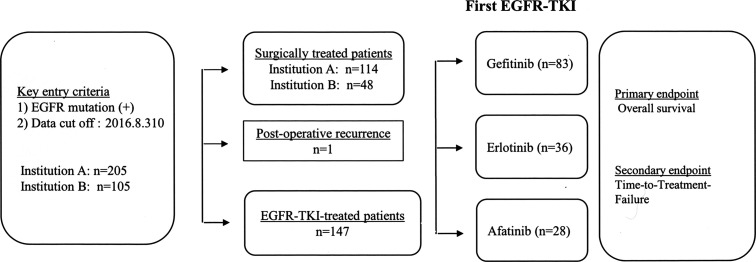
The electronic medical records of 310 EGFR mutation-related NSCLC patients who were diagnosed and treated at the Mie Prefectural General Medical Center and Matsusaka Municipal Hospital from January 2010 through April 2016 were evaluated. Among all patients, 162 received surgical treatment and 147 therapy with gefitinib (n = 83), erlotinib (n = 36), or afatinib (n = 28), and 1 patient surgically treated received TKI because of tumor recurrence. The dose of gefitinib was 250 mg/day, erlotinib 150 mg/day, and afatinib 40 mg/day. Time to treatment failure (TTF) was the primary endpoint and OS the secondary endpoint (Fig. 1). In the subgroup analysis, TTF and OS were calculated after categorizing the patients by sex, smoking habit, EGFR mutation (Ex 19 del or L858R), and by the presence or absence of brain metastasis regardless of the EGFR TKI used for the treatment. Data collection was terminated on February 28, 2017. The study was approved by the Institutional Ethical Committee for Clinical Investigation of Matsusaka Municipal Hospital and Mie Prefectural General Medical Center (Approval date: April 2016; Approval No. 150401-1).
Figure 1.
Flowchart of the patient selection process. Patients positive for EGFR mutation treated with tyrosine kinase inhibitors were included in the study.
Genetic Testing
Genetic analysis to determine EGFR mutation was performed at LSI Medience Corporation (Tokyo, Japan) using the PCR clamp method.
Statistical Analysis
TTF was defined as the period from the day of starting induction therapy with any EGFR TKI to the day of its discontinuation for any cause. OS was calculated from the date of induction therapy with EGFR TKI to the date of death for any cause. Patients alive on February 28, 2017, were considered as censored cases. Survival curves were drawn using the Kaplan–Meier method, and statistical differences were calculated by the log-rank test. Values of p < 0.05 were considered as statistically significant. All statistical analyses were performed using the SPSS software version 23.0 (IBM Japan, Ltd., Tokyo, Japan).
RESULTS
Characteristics of the Patients
Among 310 EGFR mutation-associated NSCLC patients, 147 were at clinical stage 3A/B or stage 4, and 1 with postsurgical recurrence received therapy with gefitinib, erlotinib, or afatinib. There was a significant difference in age between the treatment arms but not between other variables (Table 1).
Table 1.
Demographic Data of the Patients Treated With Tyrosine Kinase Inhibitors
| Variables | Gefitinib (n = 83) | Erlotinib (n = 36) | Afatinib (n = 28) | p Value |
|---|---|---|---|---|
| Median age [year (range)] | 75 (50–95) | 72 (52–87) | 68 (37–82) | 0.014 |
| Sex | 0.849 | |||
| Female | 54 | 22 | 19 | |
| Male | 29 | 14 | 9 | |
| Smoking status | 0.153 | |||
| Never | 58 | 18 | 22 | |
| Former | 14 | 13 | 5 | |
| Current | 9 | 2 | 1 | |
| Unknown | 2 | 3 | 0 | |
| Histological subtype | 0.120 | |||
| Adenocarcinoma | 78 | 33 | 23 | |
| Squamous cell carcinoma | 1 | 0 | 3 | |
| Other | 4 | 3 | 2 | |
| Clinical stage | 0.742 | |||
| 3A | 20 | 10 | 8 | |
| 3B | 5 | 3 | 3 | |
| 4 | 58 | 23 | 16 | |
| Recurrent | 0 | 0 | 1 |
TTF and OS in Patients Treated With Each TKI
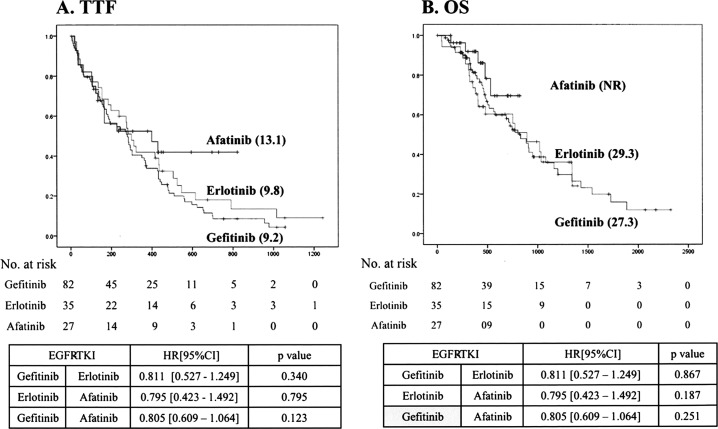
The median TTF was not significantly different between patients treated with gefitinib (9.2 months), erlotinib (9.8 months), or afatinib (13.1 months) (Fig. 2A). The median OS was not significantly different between patients treated with gefitinib (27.3 months) and erlotinib (29.3 months) (Fig. 2B). The survival of the group of patients treated with afatinib could not be recorded, and thus the data were unavailable.
Figure 2.
The time to treatment failure (TTF) and overall survival (OS) in patients treated with each tyrosine kinase inhibitor. The values of TTF (A) and OS (B) were not significantly different between the treatment groups.
Subgroup Analysis
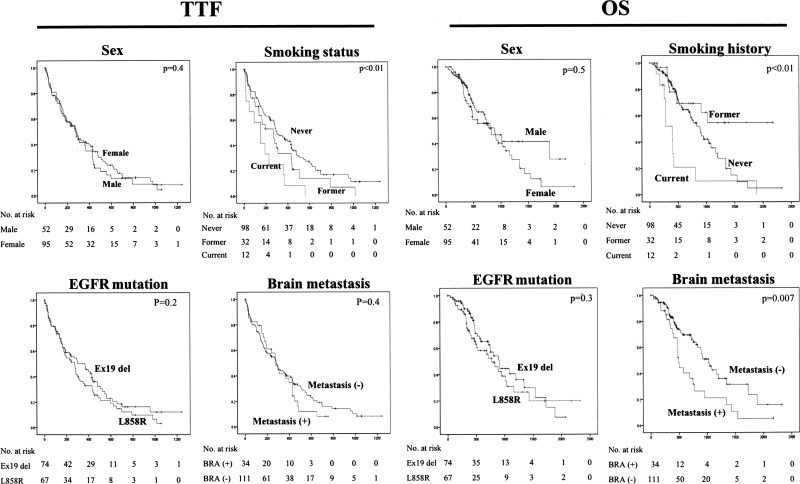
Smoking status significantly affected both TTF and OS, and the presence of brain metastasis was found to be significantly associated with worse survival (Fig. 3).
Figure 3.
Subgroup analysis. Patients who smoked had significantly worse TTF and OS than nonsmokers, and there was a significant difference in TTF and OS between patients with and without brain metastasis.
DISCUSSION
Gefitinib, a first-generation TKI, was approved in 2002 for use in patients with lung cancer in Japan. Early studies have shown cases with dramatic therapeutic response to gefitinib as well as cases without any response3,4. Subsequent large population clinical trials demonstrated that patients responsive to gefitinib with significant prolongation of PFS harbored a mutation in the EGFR gene6,16. Mutation of the EGFR gene occurs much more frequently in the Asian population, including Japan, than in Caucasians17. Among Japanese patients with lung adenocarcinoma, around 50% have the EGFR gene mutation17. Exon 19 deletion and L858R are the two major mutations. Takano et al. reported that therapy with gefitinib prolongs twice the medial survival time of patients with EGFR mutation18. The first-generation TKIs, gefitinib and erlotinib, and the second-generation TKI, afatinib, are currently available for use as first-line therapy in NSCLC patients10. Of the two first-generation TKIs, erlotinib can reach higher concentrations in blood and cerebrospinal fluid and shows more clinical efficacy in cases with leptomeningeal carcinomatosis than gefitinib19,20. Therapeutic effectiveness surpassing that of the first-generation TKIs has been expected from afatinib, the second-generation TKI, because afatinib has been reported to bind irreversibly to EGFR and to have strong antitumor activity in cancer cell lines positive for the T790M mutation21.
In the present study, to get more information on the comparative efficacy of gefitinib, erlotinib, and afatinib, we retrospectively evaluated the clinical response to each TKI in the real-world clinical practice in the Japanese population. The selection of TKI was done by the attending physician based on the age, sex, constitution, performance status of the patients, and presence of adverse effects. Compared to patients treated with erlotinib and afatinib, patients treated with gefitinib were aged subjects and showed significantly less frequent and milder grade of adverse effects. However, comparable values of TTF and OS were observed in patients treated with gefitinib, erlotinib, or afatinib. These observations suggest that gefitinib tends to be used more commonly in the aging population than erlotinib or afatinib.
TTF tended to be longer in patients treated with afatinib than in those treated with gefitinib or erlotinib. Dose reduction of afatinib from 40 mg/day to 30 and 20 mg/day was also possible in many cases, although we have not performed a strict evaluation of the TKI dose. OS was assessed in patients treated with gefitinib or erlotinib, but not in those treated with afatinib because of the short observation period in the afatinib-treated group. In agreement with previous studies, never smokers had more prolonged OS than smokers22. OS, but not TTF, was significantly different between patients with and without brain metastasis. This may be explained by the frequent recurrence of the brain metastatic disease in patients with EGFR mutation despite several cycles of radiotherapy23. It is expected that osimertinib, the third-generation TKI that has been recently approved in Japan for use against T790M mutation-associated lung tumors, will significantly improve OS in patients with EGFR mutation and brain metastasis24.
No definite conclusions can be drawn from the present study due to limitations such as the retrospective nature of the study, small sample size, selection bias, and the insufficient follow-up of the patients. However, consistent with the results of our present study, previous meta-analysis also reported comparable clinical efficacy of gefitinib, erlotinib, and afatinib (Table 2)13,25.
Table 2.
Comparison With Results of Previous Studies
| Variable | EGFR TKI | PFS (Months) | Reference |
|---|---|---|---|
| NEJ002 | Gefitinib | 10.8 | 15 |
| WJOG3405 | Gefitinib | 9.2 | 6 |
| LUX-Lung 7 | Gefitinib | 10.9 | 12 |
| EURTAC | Erlotinib | 9.7 | 7 |
| OPTIMAL | Erlotinib | 13.1 | 9 |
| JO22903 | Erlotinib | 11.8 | 11 |
| LUX-Lung 3 | Afatinib | 11.1 | 8 |
| LUX-Lung 6 | Afatinib | 11.0 | 14 |
| LUX-Lung 3 (Japanese) | Afatinib | 13.8 | 5 |
| LUX-Lung 7 | Afatinib | 11.0 | 12 |
| Present study | Gefitinib | 9.2 | |
| Present study | Erlotinib | 9.8 | |
| Present study | Afatinib | 13.1 |
EGFR TKI, epidermal growth factor receptor tyrosine kinase inhibitor; PFS, progression-free survival.
In brief, the results of the present retrospective study showed comparable clinical efficacy of gefitinib, erlotinib, and afatinib in Japanese patients with NSCLC.
ACKNOWLEDGMENTS
This work was in part financially supported by a grant-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Kakenhi No. 15K09170). E.C.G. has received research funding from Astellas, Aqua Therapeutics, Asahi Kassei, and Shionogi. C.N.D.-G. and E.C.G. have received travel fees from Aqua Therapeutics. T.K. and H.F. have received donations from Chugai Pharmaceutical. T.K. has received honoraria from AstraZeneca. O.H. and K.I. have received donations from Novaltis, GlaxoSmithKline, Dai-Ichi-Sankyo, Bayer, Kyorin, and Boehringer Ingelheim. O.H. has received honoraria from Novaltis and Boehringer Ingelheim.
REFERENCES
- 1. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3(4):524–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Azim HA Jr, Elattar I, Loberiza FR Jr., Azim H, Mok T, Ganti AK. Third generation triplet cytotoxic chemotherapy in advanced non-small cell lung cancer: A systematic overview. Lung Cancer 2009;64(2):194–8. [DOI] [PubMed] [Google Scholar]
- 3. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–39. [DOI] [PubMed] [Google Scholar]
- 4. Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004;304(5676):1497–500. [DOI] [PubMed] [Google Scholar]
- 5. Kato T, Yoshioka H, Okamoto I, Yokoyama A, Hida T, Seto T, Kiura K, Massey D, Seki Y, Yamamoto N. Afatinib versus cisplatin plus pemetrexed in Japanese patients with advanced non-small cell lung cancer harboring activating EGFR mutations: Subgroup analysis of LUX-Lung 3. Cancer Sci. 2015;106(9):1202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M, West Japan Oncology G. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–8. [DOI] [PubMed] [Google Scholar]
- 7. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, Longo F, Moran T, Insa A, De Marinis F, Corre R, Bover I, Illiano A, et al. on behalf of the Spanish Lung Cancer Group in collaboration with the Groupe Français de Pneumo-Cancérologie and the Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–46. [DOI] [PubMed] [Google Scholar]
- 8. Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, Su WC, Bennouna J, Kato T, Gorbunova V, Lee KH, Shah R, Massey D, Zazulina V, Shahidi M, Schuler M. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–34. [DOI] [PubMed] [Google Scholar]
- 9. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, Lu S, Zhang L, Hu C, Hu C, Luo Y, Chen L, Ye M, Huang J, Zhi X, Zhang Y, Xiu Q, Ma J, Zhang L, You C. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–42. [DOI] [PubMed] [Google Scholar]
- 10. Tan DS, Yom SS, Tsao MS, Pass HI, Kelly K, Peled N, Yung RC, Wistuba II, Yatabe Y, Unger M, Mack PC, Wynes MW, Mitsudomi T, Weder W, Yankelevitz D, Herbst RS, Gandara DR, Carbone DP, Bunn PA Jr, Mok TS, Hirsch FR. The International Association for the Study of Lung Cancer consensus statement on optimizing management of EGFR mutation-positive non-small cell lung cancer: Status in 2016. J Thorac Oncol. 2016;11(7):946–63. [DOI] [PubMed] [Google Scholar]
- 11. Goto K, Nishio M, Yamamoto N, Chikamori K, Hida T, Maemondo M, Katakami N, Kozuki T, Yoshioka H, Seto T, Fukuyama T, Tamura T. A prospective, phase II, open-label study (JO22903) of first-line erlotinib in Japanese patients with epidermal growth factor receptor (EGFR) mutation-positive advanced non-small-cell lung cancer (NSCLC). Lung Cancer 2013;82(1):109–14. [DOI] [PubMed] [Google Scholar]
- 12. Park K, Tan EH, O’Byrne K, Zhang L, Boyer M, Mok T, Hirsh V, Yang JC, Lee KH, Lu S, Shi Y, Kim SW, Laskin J, Kim DW, Arvis CD, Kolbeck K, Laurie SA, Tsai CM, Shahidi M, Kim M, Massey D, Zazulina V, Paz-Ares L. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17(5):577–89. [DOI] [PubMed] [Google Scholar]
- 13. Yang Z, Hackshaw A, Feng Q, Fu X, Zhang Y, Mao C, Tang J. Comparison of gefitinib, erlotinib and afatinib in non-small cell lung cancer: A meta-analysis. Int J Cancer 2017;140(12):2805–19. [DOI] [PubMed] [Google Scholar]
- 14. Mitsudomi T, Kobayashi Y. Afatinib in lung cancer harboring EGFR mutation in the LUX-Lung trials: Six plus three is greater than seven? Transl Lung Cancer Res. 2016;5(4):446–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, Li W, Hou M, Shi JH, Lee KY, Xu CR, Massey D, Kim M, Shi Y, Geater SL. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–22. [DOI] [PubMed] [Google Scholar]
- 16. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T, North-East Japan Study G. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–8. [DOI] [PubMed] [Google Scholar]
- 17. Fukui T, Mitsudomi T. Mutations in the epidermal growth factor receptor gene and effects of EGFR-tyrosine kinase inhibitors on lung cancers. Gen Thorac Cardiovasc Surg. 2008;56(3):97–103. [DOI] [PubMed] [Google Scholar]
- 18. Takano T, Fukui T, Ohe Y, Tsuta K, Yamamoto S, Nokihara H, Yamamoto N, Sekine I, Kunitoh H, Furuta K, Tamura T. EGFR mutations predict survival benefit from gefitinib in patients with advanced lung adenocarcinoma: A historical comparison of patients treated before and after gefitinib approval in Japan. J Clin Oncol. 2008;26(34):5589–95. [DOI] [PubMed] [Google Scholar]
- 19. Lee E, Keam B, Kim DW, Kim TM, Lee SH, Chung DH, Heo DS. Erlotinib versus gefitinib for control of leptomeningeal carcinomatosis in non-small-cell lung cancer. J Thorac Oncol. 2013;8(8):1069–74. [DOI] [PubMed] [Google Scholar]
- 20. Togashi Y, Masago K, Masuda S, Mizuno T, Fukudo M, Ikemi Y, Sakamori Y, Nagai H, Kim YH, Katsura T, Mishima M. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol. 2012;70(3):399–405. [DOI] [PubMed] [Google Scholar]
- 21. Li D, Ambrogio L, Shimamura T, Kubo S, Takahashi M, Chirieac LR, Padera RF, Shapiro GI, Baum A, Himmelsbach F, Rettig WJ, Meyerson M, Solca F, Greulich H, Wong KK. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008;27(34):4702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sohn HS, Kwon JW, Shin S, Kim HS, Kim H. Effect of smoking status on progression-free and overall survival in non-small cell lung cancer patients receiving erlotinib or gefitinib: A meta-analysis. J Clin Pharm Ther. 2015;40(6):661–71. [DOI] [PubMed] [Google Scholar]
- 23. Jiang T, Min W, Li Y, Yue Z, Wu C, Zhou C. Radiotherapy plus EGFR TKIs in non-small cell lung cancer patients with brain metastases: An update meta-analysis. Cancer Med. 2016;5(6):1055–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hanna N, Johnson D, Temin S, Baker S Jr, Brahmer J, Ellis PM, Giaccone G, Hesketh PJ, Jaiyesimi I, Leighl NB, Riely GJ, Schiller JH, Schneider BJ, Smith TJ, Tashbar J, Biermann WA, Masters G. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017:JCO2017746065. [DOI] [PubMed] [Google Scholar]
- 25. Krawczyk P, Kowalski DM, Ramlau R, Kalinka-Warzocha E, Winiarczyk K, Stencel K, Powrozek T, Reszka K, Wojas-Krawczyk K, Bryl M, Wojcik-Superczynska M, Glogowski M, Barinow-Wojewodzki A, Milanowski J, Krzakowski M. Comparison of the effectiveness of erlotinib, gefitinib, and afatinib for treatment of non-small cell lung cancer in patients with common and rare EGFR gene mutations. Oncol Lett. 2017;13(6):4433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]