Abstract
Bladder cancer (BC) is one of the leading causes of cancer-related deaths in the world. Long noncoding RNA (lncRNA) taurine-upregulated gene 1 (TUG1) plays an important role in the development and progression of numerous cancers, including BC. However, the exact role of TUG1 in modulating BC progression is still poorly known. In this study, we found that TUG1 was upregulated and microRNA-29c (miR-29c) was downregulated in BC tissues and cell lines. Overexpression of TUG1 promoted the cell proliferation of T24 and EJ cells, whereas TUG1 knockdown had the opposite effect. Upregulation of TUG1 obviously facilitated the migration and invasion of T24 and EJ cells. In contrast, TUG1 silencing repressed the migration and invasion of T24 and EJ cells. Furthermore, TUG1 knockdown markedly increased the expression of miR-29c in vitro. On the contrary, overexpression of TUG1 remarkably decreased the expression of miR-29c. Transfection with plasmids containing mutant TUG1 has no effect on the expression of miR-29c. There were direct interactions between miR-29c and the binding sites of TUG1. In addition, the inhibitory effects of small interfering RNA specific for TUG1 on BC cell proliferation, migration, and invasion were reversed by downregulation of miR-29c. Collectively, our study strongly demonstrates that TUG1 promotes BC cell proliferation, migration, and invasion by inhibiting miR-29c, suggesting that lncRNA TUG1 may be a promising target for BC gene therapy.
Key words: Long noncoding RNA, Taurine-upregulated gene 1 (TUG1), Bladder cancer (BC), MicroRNA 29c (miR-29c)
INTRODUCTION
Bladder cancer (BC) is considered as one of the most prevalent carcinomas worldwide and is hallmarked by high rates of relapse and metastasis1. BC is one of the leading causes of cancer-related deaths, resulting in 188,000 deaths in 20152. BC is classified into non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC)3. Compared with NMIBC, MIBC commonly progresses to metastasis4. No effective treatments are currently available for BC. A growing number of studies have shown that long noncoding RNAs (lncRNAs) were dysregulated in multiple cancers, such as BC, gastric cancer, and renal cell carcinoma5. However, the roles of lncRNAs in BC were not well known.
The independent transcriptional units without protein-coding potential, known as lncRNAs, have been shown to participate in the development and progression of tumors by regulation of cell proliferation, apoptosis, migration, and invasion6,7. lncRNAs are capable of regulating several signaling pathways, which play oncogenic or tumor-repressive roles during tumorigenesis8. Among all of the cancer-related lncRNAs, taurine-upregulated gene 1 (TUG1) is defined as an oncogenic lncRNA in cancers9. For instance, Ren et al. reported that TUG1 facilitated the proliferation and invasion of gastric cancer cells. Moreover, the inhibitory effect of miR-145 on the proliferation and invasion of gastric cancer cells could be overturned by overexpression of TUG1 in vitro10. According to Zhang et al., TUG1 expression was upregulated in renal cell carcinoma tissues and cells and was positively correlated with Fuhrman grade and tumor size. Downregulation of TUG1 could suppress the renal cell carcinoma cell proliferation, migration, and invasion, and induce apoptosis in vitro11. Wang et al. found that TUG1 promoted osteosarcoma cell migration and invasion and modulated the expression of Rho-associated coiled coil-containing protein kinase 1 in a competitive endogenous RNA manner via inhibition of miR-335-5p12. According to Xu et al., TUG1 knockdown induced repression of esophageal squamous cell carcinoma cell proliferation and migration and caused the inhibition of cell cycle progression13. TUG1 was aberrantly expressed in bladder urothelia carcinoma, and TUG1 knockdown inhibited the cell proliferation and induced cell apoptosis based on the studies of Han et al.14. However, the exact molecular mechanism by which TUG1 exerts a promotional effect on the progress of BC is still poorly understood.
In this study, we investigated the effect of TUG1 on the cell proliferation, migration, and invasion in BC and its underlying molecular mechanism. Our results suggested that TUG1 may be a promising target for BC gene therapy.
MATERIALS AND METHODS
Cell Culture and Tissue Samples
Normal human urothelial cells (control) and BC cell lines (T24, 5637, UMUC-2, and EJ) were obtained from the Chinese Academy of Sciences Cell Bank (Shanghai, P.R. China). T24, 5637, UMUC-2, and EJ cells were cultured in RPMI-1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Sijiqing, Hangzhou, P.R. China) in a humidified incubator containing 5% CO2 at 37°C.
BC tissues and adjacent normal tissues were obtained from 22 BC patients treated without chemotherapy or radiation therapy. Histopathological diagnosis was confirmed for all BC patients. Samples were collected and stored at −80°C. This study was reviewed and approved by the Ethics Committee of The First Affiliated Hospital of Anhui Medical University, and all patients’ permissions were obtained before surgery.
Cell Transfection
The wild-type (WT) and mutant (MUT) TUG1 fragments were amplified and cloned into pcDNA3.1 vector (pcDNA; Invitrogen, Carlsbad, CA, USA). Small interfering RNA specific for TUG1 (si-TUG1), negative control (si-NC), microRNA-29c (miR-29c) mimic, or miR-29c inhibitor was designed and synthesized by Sangon Biotech (Shanghai, P.R. China). Subsequently, si-TUG1, si-NC, miR-29c mimic, or miR-29c inhibitor was transfected into T24 or EJ cells by Lipofectamine 2000 (Invitrogen) according to the protocol of the manufacturer. After incubation for indicated times, cells were collected for further assay.
Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated from cells or tissues using TRIzol reagent (Invitrogen) in accordance with the manufacturer’s instructions. cDNA was synthesized with One Step PrimeScript cDNA kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. The expressions of TUG1 and miR-29c were evaluated with the ABI PRISM 7900 sequence detection system (Life Technologies, Carlsbad, CA, USA). The primers in this study were as follows: TUG1, 5′-TAG CAG TTC CCC AAT CCT TG-3′ (forward) and 5′-CAC AAA TTC CCA TCA TTC CC-3′ (reverse); β-actin, 5′-TCC CTG GAG AAG AGC TAC GA-3′ (forward) and 5′-AGC ACT GTG TTG GCG TAC AG-3′ (reverse); miR-29c, 5′-GCC TAG CAC CAT TTG AAA TCG-3′ (forward) and 5′-GTG CAG GGT CCG AGG T-3′ (reverse); U6, 5′-CTC GCT TCG GCA GCA CA-3′ (forward) and 5′-AAC GCT TCA CGA ATT TGC GT-3′ (reverse). β-Actin and U6 were selected as references for TUG1 and miR-29c expression levels, respectively. The 2−ΔΔCt method was used to quantify the relative expression of each gene.
Cell Viability Assay
Cells were seeded in 96-well plates and grown for 24 h. pcDNA, pcDNA-TUG1, si-TUG1, si-NC, or miR-29c was transfected into T24 or EJ cells, respectively. At 0, 24, 48, 72, and 96 h posttransfection, cell viability was evaluated using the Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan). Briefly, CCK-8 reagent (10 μl/well) was added, and plates were detected using a microplate reader set at 490 nm.
Colony Formation Assay
T24 or EJ cells (250 cells/well) were seeded into six-well plates. After transfection with different molecules, cells were cultured in RPMI-1640 medium (containing 10% FBS) for 14 days. The cells were fixed in methanol for 20 min, and subsequently stained with 0.1% crystal violet for 30 min at room temperature. The number of cell colonies larger than 0.1 mm in diameter was counted.
Transwell Migration and Invasion Assay
Cell migration was assayed using a Transwell chamber (Corning Inc., Corning, NY, USA). At 12 h posttransfection, cells were collected and resuspended in serum-free medium. Then the cell suspensions (1 × 104 cells/well) were seeded into the upper chambers, and the lower chambers were full of medium supplemented with 10% FBS. The cells were cultured for 24 h in a 5% CO2 incubator at 37°C. Cell invasion was assayed using Matrigel-precoated Transwell chambers (Corning). Nonmigrated and noninvaded cells were removed, and migrated and invaded cells were stained with 4′,6′-diamidino-2-phenylindole (DAPI; Sigma-Aldrich, St. Louis, MO, USA) for 15 min. The migrated and invaded cells were calculated in five random fields from each sample.
Dual-Luciferase Assay
The fragments of TUG1 containing the predicted WT or MUT miR-29c binding sites were chemically synthesized and cloned into the downstream of the luciferase reporter gene in pmirGLO dual-luciferase vector (Promega, Madison, WI, USA).
T24 or EJ cells were seeded in 12-well plates and cultured overnight. The next day, cells were cotransfected with pmirGLO-WT-TUG1 (0.5 μg) or pmirGLO-MUT-TUG1 (0.5 μg) and miR-29c mimic (50 nM), inhibitor (50 nM), or scramble control using Lipofectamine 2000 (Invitrogen). After 24 h, the cells were collected and lysed. The luciferase activities were detected using a Dual-Luciferase Reporter Assay System (Promega) in accordance with the manufacturer’s protocol. Firefly luciferase activity was normalized against Renilla luciferase activity.
Statistical Analysis
Statistical analyses were performed with the SPSS 18.0 statistical software package (SPSS, Chicago, IL, USA). Differences among different groups were tested by one-way analysis of variance followed by Newman–Keuls post hoc test. A value of p < 0.05 was considered to be significant.
RESULTS
The Expression of TUG1 and miR-29c in the BC Tissues and Cells
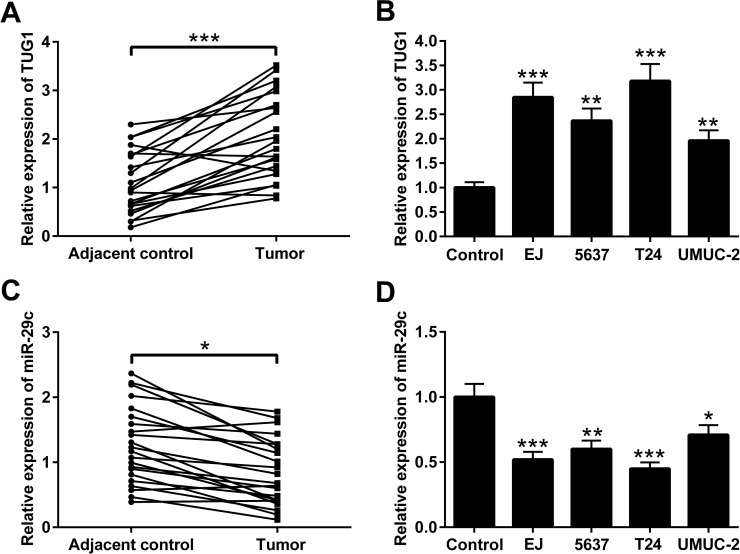
The expression levels of TUG1 and miR-29c were detected by qRT-PCR in BC tissues and adjacent normal tissues from 22 patients with BC. We found that the expression level of TUG1 in BC tissues was much higher than that in adjacent normal tissues (Fig. 1A). The TUG1 expression level in BC cell lines (EJ, 5637, T24, and UMUC-2) was also higher than that in the normal urothelial cells (Fig. 1B). The expression of miR-29c was remarkably decreased in BC tissues compared to that in adjacent normal tissues (Fig. 1C). In comparison with the normal urothelial cells, miR-29c expression was markedly decreased in BC cell lines including EJ, 5637, T24, and UMUC-2 (Fig. 1D). These results provide evidence that TUG1 and miR-29c may play a role in the course of BC.
Figure 1.
The expression of taurine-upregulated gene 1 (TUG1) and microRNA-29c (miR-29c) in bladder cancer (BC) tissues and cells. (A) TUG1 expression was analyzed and found to be increased in 22 pairs of BC tissues and adjacent normal tissues by qualitative real-time reverse transcription polymerase chain reaction (qRT-PCR). (B) TUG1 levels were also increased in BC cell lines including EJ, 5637, T24, and UMUC-2. Conversely miR-29c expression was decreased in (C) BC tissues and (D) BC cell lines. Normal urothelial cells served as the control. Data are presented as mean ± standard deviation (SD). *p < 0.05, **p < 0.01, ***p < 0.001.
TUG1 Promotes BC Cell Proliferation
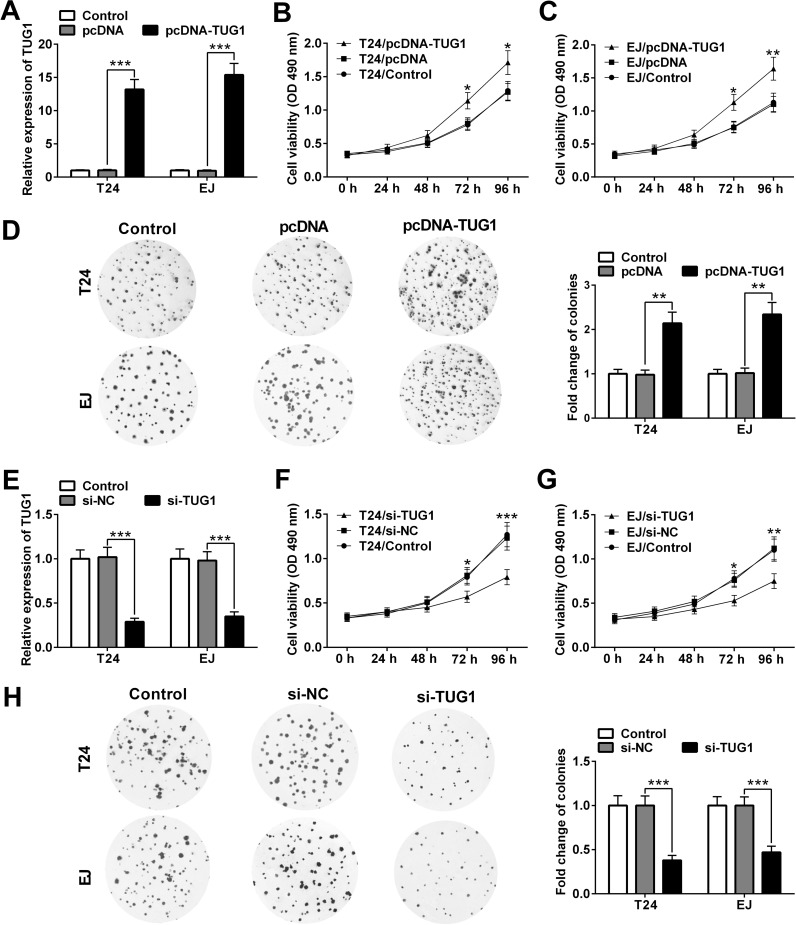
To explore the role of TUG1 on cell proliferation in BC cells, we altered the expression of TUG1 in T24 and EJ cells by transfecting pcDNA-TUG1 or si-TUG1 (Fig. 2). The transfection efficiency was confirmed by qRT-PCR. As expected, transfection of pcDNA-TUG1 elevated TUG1 level remarkably in T24 and EJ cells, whereas si-TUG1 reduced TUG1 expression (Fig. 2A and E). At 72 and 96 h posttransfection, compared with the pcDNA group, the viability of T24 and EJ cells in the pcDNA-TUG1 group was elevated (Fig. 2B and C). In contrast, the viability of T24 and EJ cells in the si-TUG1 group was reduced in comparison with the si-NC group (Fig. 2F and G). Consistently, colony formation assay also showed that overexpression of TUG1 could obviously promote the colony-forming ability of T24 and EJ cells (Fig. 2D), whereas TUG1 knockdown reduced the colony-forming ability of T24 and EJ cells (Fig. 2H). Taken together, these results indicated that upregulation of TUG1 can promote BC cell proliferation.
Figure 2.
TUG1 promotes cell proliferation in T24 and EJ cells. (A) qRT-PCR analysis of the level of TUG1 in T24 and EJ cells after transfection of pcDNA or pcDNA-TUG1. The viability of T24 (B) and EJ (C) cells transfected with pcDNA or pcDNA-TUG1 was investigated by the Cell Counting Kit-8 (CCK-8) assay. (D) T24 and EJ cells were transfected with pcDNA or pcDNA-TUG1 and then subjected to colony formation assay and stained with crystal violet. (E) qRT-PCR analysis of the level of TUG1 in T24 and EJ cells after transfection of si-TUG1 or si-NC. The viability of T24 (F) and EJ (G) cells transfected with si-TUG1 or si-NC was assessed by the CCK-8 assay. (H) T24 and EJ cells transfected with si-TUG1 or si-NC were subjected to the colony formation assay. Data of the qRT-PCR and colony formation assay were presented as mean ± SD. Data of the cell viability assay are presented as mean ± standard error (SE). *p < 0.05, **p < 0.01, ***p < 0.001.
TUG1 Promotes Migration and Invasion of BC Cell Lines In Vitro
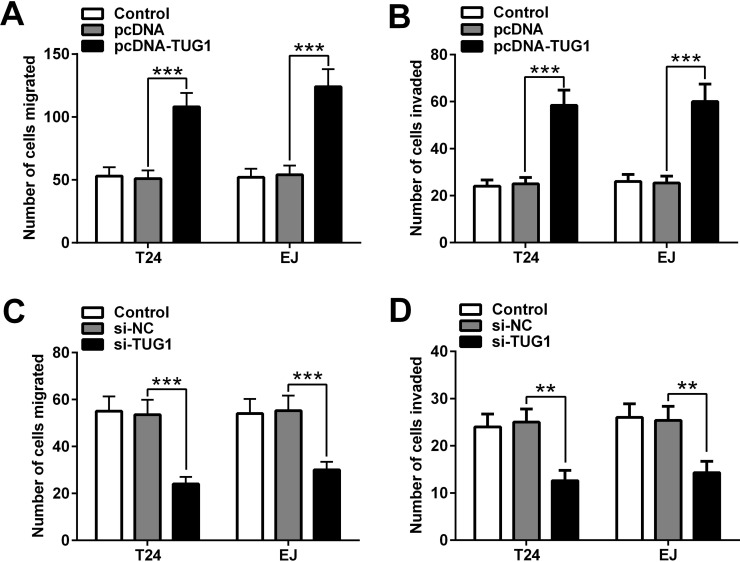
To confirm the function of TUG1 in BC cells, Transwell migration and invasion assays were performed. As shown in Figure 3A and B, compared with the pcDNA group, the migration and invasion capabilities of the T24 and EJ cells were markedly increased in the pcDNA-TUG1 group. On the contrary, the migration and invasion capabilities of T24 and EJ cells decreased upon transfection of si-TUG1 (Fig. 3C and D). Overall, these data demonstrated that upregulation of TUG1 promotes the migration and invasion capabilities of BC cell lines.
Figure 3.
Overexpression of TUG1 promotes migration and invasion of BC cell lines. (A) Cell migration and (B) invasion abilities of T24 and EJ cells transfected with pcDNA-TUG1 or pcDNA were examined by Transwell migration and invasion assays. (C) Cell migration and (D) invasion abilities of T24 and EJ cells transfected with si-TUG1 or si-NC were examined by Transwell migration and invasion assays. Data are presented as mean ± SD. **p < 0.01, ***p < 0.001.
TUG1 Suppresses the Expression of miR-29c
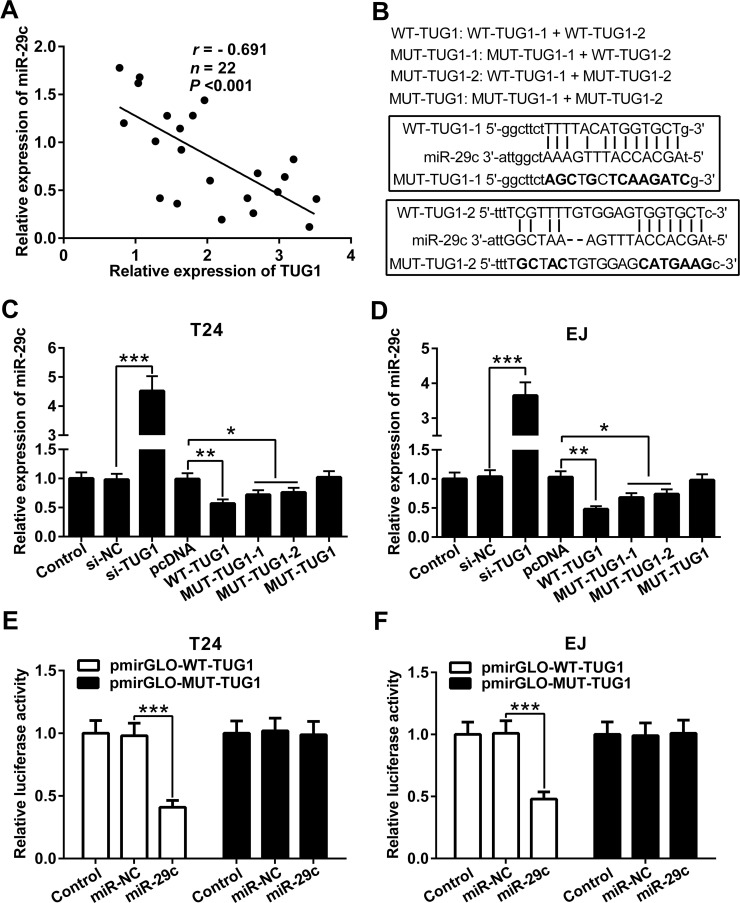
To assess the relationship of the mRNA expression levels between TUG1 and miR-29c in BC tissues, Pearson correlation analysis was performed. Our results showed that the expression of miR-29c was negatively correlated with TUG1 in BC tissues (Fig. 4A). The target binding sites of TUG1 and miR-29c are shown in Figure 4B. MUT-TUG1-1 and MUT-TUG1-2 are two mutated sequences of MUT-TUG1. The level of miR-29c was obviously increased in T24 and EJ cells transfected with si-TUG1 compared to that in T24 and EJ cells transfected with si-NC. In contrast, the level of miR-29c in T24 and EJ cells transfected with WT-TUG1 was much lower than that in T24 and EJ cells transfected with pcDNA. Transfection of MUT-TUG1-1 and MUT-TUG1-2 could also reduce the level of miR-29c in T24 and EJ cells (due to the presence of WT-TUG1-2 or WT-TUG1-1, respectively). Notably, MUT-TUG1 had no effect on the expression of miR-29c in T24 and EJ cells (Fig. 4C and D). To further verify the direct interaction between TUG1 and miR-29c, the luciferase assay was conducted in both T24 and EJ cells. Compared with the miR-NC, miR-29c mimic notably reduced the luciferase activity in pmirGLO-WT-TUG1. In contrast, the luciferase activity in pmirGLO-MUT-TUG1 was not changed in the miR-29c mimic group compared with the miR-NC group (Fig. 4E and F). These data suggested that TUG1 could directly interact with miR-29c and suppress the expression of miR-29c in BC cells.
Figure 4.
TUG1 represses the expression of miR-29c. (A) The negative correlation between the miR-29c level and the TUG1 level in BC tissues. (B) Putative miR-29c-binding sequences of TUG1 are shown. Mutation was generated on the TUG1 sequence in the complementary site for the seed region of miR-29c. qRT-PCR analysis of miR-29c expression in T24 (C) and EJ (D) cells after transfection with different molecules including WT-TUG1, WT-TUG1 and MUT-TUG1, or MUT-TUG1. Relative luciferase activities of luciferase reporters containing the WT or MUT TUG1 fragment were detected 24 h following transfection with miR-29c mimic or miR-NC in T24 (E) and EJ (F) cells. Data are presented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001.
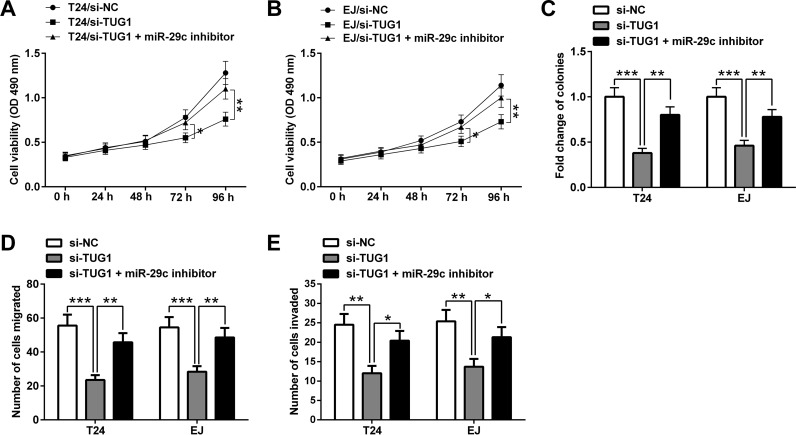
Downregulation of miR-29c Reverses the Effect of si-TUG1 on Bladder Cancer Cell Proliferation, Migration, and Invasion
To further validate the role of the TUG1/miR-29c axis in BC, miR-29c expression was downregulated in T24 and EJ cells by transfection with miR-29c inhibitor. Compared with the si-TUG1 group, the viability of T24 and EJ cells in the si-TUG1 + miR-29c inhibitor group was remarkably increased (Fig. 5A and B). The colony-forming ability of T24 and EJ cells in the si-TUG1 + miR-29c inhibitor group was obviously higher than that in the si-TUG1 group. In addition, the migration and invasion capabilities of T24 and EJ cells cotransfected with si-TUG1 and miR-29c inhibitor were markedly increased in comparison with those transfected with si-TUG1 alone. Thus, these results suggested that TUG1 promotes BC cell proliferation, migration, and invasion via inhibiting miR-29c.
Figure 5.
Downregulation of miR-29c reverses the effect of si-TUG1. miR-29c inhibitor reduced the inhibitory effect of si-TUG1 on the viability of T24 (A) and EJ (B) cells. (C) miR-29c inhibitor attenuated the inhibitory effect of si-TUG1 on the colony-forming ability of T24 and EJ cells. miR-29c inhibitor abrogated the inhibitory effect of si-TUG1 on the migration and invasion capabilities of T24 (D) and EJ (E) cells. Data of the cell viability assay are presented as mean ± SE. Data of the colony formation, migration, and invasion assays are presented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001.
DISCUSSION
Recently, much attention has been focused on identifying the roles of lncRNAs in cancer development and metastasis. Many studies have indicated that numerous lncRNAs remarkably changed in BC, which is tightly linked to the occurrence and development of BC15–18. For examples, Liu et al. showed that lncRNA Sprouty receptor tyrosine kinase (RTK) signaling antagonist 4 intronic transcript 1 (SPRY4-IT1) silencing suppressed cell proliferation, migration, and invasion and induced apoptosis in BC progression. Moreover, SPRY4-IT1 as an oncogene inhibited the expression of enhancer of zeste homolog 2 by sponging miR-101-3p16. Wang et al. indicated that lncRNA urothelial carcinoma-associated 1a (UCA1a) was upregulated in BC tissues. Forced expression of UCA1a induced the proliferation, migration, and invasion of BC cells in vitro, repressed cell apoptosis, and facilitated the tumorigenicity of BC cells17. Liu et al. noted that lncRNA growth arrest-specific 5 suppressed the cell proliferation and negatively modulated the expression of cyclin-dependent kinase 6 (CDK6) in BC cell lines. In addition, the inhibitory effect of growth arrest-specific 5 on cell proliferation could be reversed by overexpression of CDK618. Together, these studies showed that lncRNAs may exhibit crucial roles in the development and progression of BC.
Studies have shown that TUG1 is abnormally expressed and closely related with tumor progression and development of BC19–21. For instance, Liu et al. suggested that TUG1 silencing repressed proliferation and promoted apoptosis in BC cell lines via regulation of zinc finger E-box-binding homeobox 2 (ZEB2) by targeting miR-142. In addition, TUG1 knockdown also repressed the expression of ZEB2 to further induce the inactivation of the Wnt/β-catenin pathway, which plays a crucial role in BC cell growth and apoptosis19. Tan et al. found that the level of TUG1 was elevated in BC cell lines and tissues, and TUG1 knockdown could repress BC cell metastasis through inhibition of epithelial-to-mesenchymal transition via the miR-145/ZEB2 axis20. Iliev et al. demonstrated that TUG1 was obviously upregulated in metastatic tumors and negatively correlated with the overall survival of MIBC patients. Moreover, TUG1 knockdown resulted in a 34% decline in T24 cell proliferation and 23% reduction in migration capacity of T24 cells in vitro21. However, the exact molecular mechanism by which TUG1 promotes BC progression is still unclear. In this study, we investigated the role of TUG1 in BC cells. Our results showed that TUG1 expression was upregulated in BC tissues and cell lines. Upregulation of TUG1 could promote BC cell proliferation, migration, and invasion, whereas TUG1 knockdown repressed BC cell proliferation, migration, and invasion. These findings indicate that TUG1 is a potential oncogene in BC.
The small RNAs (20–22 nucleotides in length) without protein-coding capability, known as miRNAs, are considered as vital regulators of development and progression of numerous cancers, including BC22. miRNAs are capable of repressing the expression of key genes via binding the 3′-untranslated region of the target mRNAs23. miR-29c has been identified as a cancer-suppressor gene, which suppresses the cell proliferation, migration, and invasion in BC. For example, Fan et al. found that miR-29c was downregulated in BC tissues, whereas upregulation of miR29c in T24 cells could obviously repress cell proliferation, reduce motility, inhibit the G1/S cell cycle transition, and promote cell apoptosis24. Xu et al. reported that the expression of miR-29c was increased in T24 cells, and miR-29c knockdown could strongly repress T24 cell growth25. Zhao et al. demonstrated that miR-29c could also repress the proliferation, migration, and invasion of T24 cells by inhibiting CDK6 expression26. Here we found that silencing of TUG1 could obviously increase the expression of miR-29c, whereas upregulation of TUG1 had opposite effects. More importantly, transfection of MUT-TUG1-1 and MUT-TUG1-2 (which is composed of a mutated and WT TUG1 sequence) could also reduce the expression of miR-29c, but MUT-TUG1 (only mutated TUG1 sequences) did not affect the expression of miR-29c. Further studies suggested that the miR-29c inhibitor could dramatically reverse the inhibitory effect of si-TUG1 on cell proliferation, migration, and invasion in vitro. Taken together, these results demonstrated that TUG1 functioned as an oncogene in BC via suppression of miR-29c.
In summary, our study confirmed that TUG1 was markedly upregulated and miR-29c was remarkably downregulated in BC tissues and cell lines. Furthermore, we provide evidence that TUG1 as an oncogene promotes the proliferation, migration, and invasion of BC cells, which will help to develop efficient therapeutic strategies for BC.
ACKNOWLEDGMENTS
This study was supported by The Special Fund for Health Care of the General Logistics Department of Chinese People’s Liberation Army (Grant No. 11BJZ24).
REFERENCES
- 1. Knowles MA, Hurst CD. Molecular biology of bladder cancer: New insights into pathogenesis and clinical diversity. Nat Rev Cancer 2015;15(1):25–41. [DOI] [PubMed] [Google Scholar]
- 2. GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388(10053):1459–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tavora F, Epstein JI. Bladder cancer, pathological classification and staging. BJU Int. 2008;102(9):1216–20. [DOI] [PubMed] [Google Scholar]
- 4. Prout GR, Wesley MN, Greenberg RS, Chen VW, Brown CC, Miller AW, Weinstein RS, Robboy SJ, Haynes MA, Blacklow RS. Bladder cancer: Race differences in extent of disease at diagnosis. Cancer 2015;89(6):1349–58. [DOI] [PubMed] [Google Scholar]
- 5. Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21(11):1253–61. [DOI] [PubMed] [Google Scholar]
- 6. Xue M, Pang H, Li X, Li H, Pan J, Chen W. Long non-coding RNA urothelial cancer-associated 1 promotes bladder cancer cell migration and invasion by way of the hsa-miR-145–ZEB1/2–FSCN1 pathway. Cancer Sci. 2016;107(1):18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martensuzunova ES, Böttcher R, Croce CM, Jenster G, Visakorpi T, Calin GA. Long noncoding RNA in prostate, bladder, and kidney cancer. Eur Urol. 2014;65(6):1140–51. [DOI] [PubMed] [Google Scholar]
- 8. Hu X, Feng Y, Zhang D, Zhao SD, Hu Z, Greshock J, Zhang Y, Yang L, Zhong X, Wang LP. A Functional genomic approach identifies FAL1 as an oncogenic long noncoding RNA that associates with BMI1 and represses p21 expression in human cancer. Cancer Cell 2014;26(3):344–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li Z, Shen J, Chan MT, Wu WK. TUG1: A pivotal oncogenic long non-coding RNA of human cancers. Cell Prolif. 2016;49(4):471–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ren K, Li Z, Li Y, Zhang W, Han X. Long non-coding RNA taurine-upregulated gene 1 promotes cell proliferation and invasion in gastric cancer via negatively modulating miRNA-145-5p. Oncol Res. 2016;25(5):789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang M, Lu W, Huang Y, Shi J, Wu X, Zhang X, Jiang R, Cai Z, Wu S. Downregulation of the long noncoding RNA TUG1 inhibits the proliferation, migration, invasion and promotes apoptosis of renal cell carcinoma. J Mol Histol. 2016;47(4):421–8. [DOI] [PubMed] [Google Scholar]
- 12. Wang Y, Yang T, Zhang Z, Lu M, Zhao W, Zeng X, Zhang W. Long non-coding RNA TUG1 promotes migration and invasion by acting as a ceRNA of miR-335-5p in osteosarcoma cells. Cancer Sci. 2017;108(5):859–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu Y, Wang J, Qiu M, Xu L, Li M, Jiang F, Yin R, Xu L. Upregulation of the long noncoding RNA TUG1 promotes proliferation and migration of esophageal squamous cell carcinoma. Tumor Biol. 2015;36(3):1643–51. [DOI] [PubMed] [Google Scholar]
- 14. Han Y, Liu Y, Gui Y, Cai Z. Long intergenic non-coding RNA TUG1 is overexpressed in urothelial carcinoma of the bladder. J Surg Oncol. 2013;107(5):555–9. [DOI] [PubMed] [Google Scholar]
- 15. Zhang S, Zhong G, He W, Yu H, Huang J, Lin T. lncRNA up-regulated in nonmuscle invasive bladder cancer facilitates tumor growth and acts as a negative prognostic factor of recurrence. J Urol. 2016;196(4):1270–8. [DOI] [PubMed] [Google Scholar]
- 16. Liu D, Li Y, Luo G, Xiao X, Tao D, Wu X, Wang M, Huang C, Wang L, Zeng F. LncRNA SPRY4-IT1 sponges miR-101-3p to promote proliferation and metastasis of bladder cancer cells through up-regulating EZH2. Cancer Lett. 2017;388:281–91. [DOI] [PubMed] [Google Scholar]
- 17. Wang Y, Chen W, Yang C, Wu W, Wu S, Qin X, Li X. Long non-coding RNA UCA1a(CUDR) promotes proliferation and tumorigenesis of bladder cancer. Int J Oncol. 2012;41(1):276–84. [DOI] [PubMed] [Google Scholar]
- 18. Liu Z, Wang W, Jiang J, Bao E, Xu D, Zeng Y, Tao L, Qiu J. Downregulation of GAS5 promotes bladder cancer cell proliferation, partly by regulating CDK6. PLoS One 2013;8(9):e73991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Q, Liu H, Cheng H, Li Y, Li X, Zhu C. Downregulation of long noncoding RNA TUG1 inhibits proliferation and induces apoptosis through the TUG1/miR-142/ZEB2 axis in bladder cancer cells. Onco Targets Ther. 2017;10:2461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tan J, Qiu K, Li M, Liang Y. Double-negative feedback loop between long non-coding RNA TUG1 and miR-145 promotes epithelial to mesenchymal transition and radioresistance in human bladder cancer cells. FEBS Lett. 2015;589(20):3175–81. [DOI] [PubMed] [Google Scholar]
- 21. Iliev R, Kleinova R, Juracek J, Dolezel J, Ozanova Z, Fedorko M, Pacik D, Svoboda M, Stanik M, Slaby O. Overexpression of long non-coding RNA TUG1 predicts poor prognosis and promotes cancer cell proliferation and migration in high-grade muscle-invasive bladder cancer. Tumour Biol. 2016;37(10):13385–90. [DOI] [PubMed] [Google Scholar]
- 22. Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer 2015;15(6):321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Braicu C, Cojocneanupetric R, Chira S, Truta A, Floares A, Petrut B, Achimascadariu P, Berindanneagoe I. Clinical and pathological implications of miRNA in bladder cancer. Int J Nanomedicine 2015;10:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fan Y, Song X, Du H, Luo C, Wang X, Yang X, Wang Y, Wu X. Down-regulation of miR-29c in human bladder cancer and the inhibition of proliferation in T24 cell via PI3K-AKT pathway. Med Oncol. 2014;31(7):65. [DOI] [PubMed] [Google Scholar]
- 25. Xu F, Zhang Q, Cheng W, Zhang Z, Wang J, Ge J. Effect of miR-29b-1* and miR-29c knockdown on cell growth of the bladder cancer cell line T24. J Int Med Res. 2013;41(6):1803–10. [DOI] [PubMed] [Google Scholar]
- 26. Zhao X, Li J, Huang S, Wan X, Luo H, Wu D. MiRNA-29c regulates cell growth and invasion by targeting CDK6 in bladder cancer. Am J Transl Res. 2015;7(8):1382–9. [PMC free article] [PubMed] [Google Scholar]