Abstract
Baicalein, a flavonoid derived from the root of Scutellaria baicalensis, has been reported to possess multiple pharmacological activities, such as anticancer and anti-inflammatory properties. This study investigated the effect of baicalein in cervical cancer cells. Cell growth curve and MTT assay were performed and revealed that baicalein inhibited the proliferation of SiHa and HeLa cells in a dose-dependent manner. We further found that baicalein arrested the cell cycle of SiHa and HeLa cells at the G0/G1 phase by suppressing the expression of cyclin D1 through the downregulation of phosphorylated protein kinase B (p-AKT) and phosphorylated glycogen synthase kinase 3β (p-GSK3β) according to FACS assays and Western blotting. Moreover, when CHIR-99021, a GSK3β inhibitor, was added to baicalein-treated SiHa cells, the expression of cyclin D1 was recovered, and cell proliferation was promoted. In conclusion, these data indicated that baicalein suspended the cell cycle at the G0/G1 phase via the downregulation of cyclin D1 through the AKT–GSK3β signaling pathway and further inhibited the proliferation of SiHa and HeLa cervical cancer cells.
Key words: Baicalein, Cyclin D1, Glycogen synthase kinase 3β (GSK3β), Cervical cancer, Proliferation
INTRODUCTION
Cervical cancer (CC) is one of the most common malignant cancers among females throughout the world1. In developing countries, CC is the second most common gynecological cancer and is the second leading cause of cancer-related deaths among women2. Scutellariae radix is the root of Scutellariae baicalensis, containing three primary components, including baicalin, baicalein, and wogonin3. Scutellariae radix is a medicinal plant that has been traditionally used in Chinese herbal medicine since ancient times to treat diseases such as allergic and inflammatory ailments.
Baicalein (5,6,7-trihydroxyflavone) is an effective component of Scutellariae baicalensis and shows many pharmacological effects, such as anti-inflammatory4, antioxidative5,6, antibacterial7, antiviral8, and antiallergy properties9. Recently, cellular and animal experiments have demonstrated that baicalein possesses strong antitumor activity; therefore, attention from researchers has rapidly increased. For example, in human breast cancer, baicalein significantly inhibited cell proliferation, migration, and invasiveness10,11. In a combined treatment, baicalein also induced the apoptosis of human breast cancer cells by activating caspase 3/caspase 9, downregulating B-cell lymphoma 2 (Bcl-2), and upregulating Bcl-2-associated X protein (BAX), or tumor protein 53 (p53) through the extracellular signal-regulated kinase/p38 mitogen-activated protein kinase (ERK/p38 MAPK) pathway12. Furthermore, baicalein induced apoptosis in HeLa CC cells via the caspase 3 pathway13. In ovarian cancer cells, baicalein inhibited the proliferation14 and invasion of cells by reducing the p38 content via the MAPK-dependent nuclear factor κ-light chain-enhancer of activated B cell (NF-κB) signaling pathway15. In gastric cancer, baicalein inhibited gastric cancer cell growth in vitro, inhibited tumor formation in vivo, and induced the apoptosis of gastric cancer cells through the downregulation of Bcl-2 and upregulation of BAX via the mitochondrial pathway16. Moreover, baicalein inhibited the migration and invasion of gastric cancer cells by suppressing the transforming growth factor-β (TGF-β) signaling pathway17 and p38 signaling pathway18. Under hypoxic conditions, baicalein increased the sensitivity of AGS cells to 5-fluorouracil (5-FU) treatment by inhibiting the phosphorylation of protein kinase B (AKT) through the accumulation of phosphatase and tensin homolog (PTEN)19. In colorectal cancer cells, baicalein inhibited cell migration and invasion via the downregulation of the AKT signaling pathway20. Specifically, baicalein induced apoptosis in a p53-dependent manner via the AKT signaling pathway in HT-29 colon cancer cells21. Baicalein also inhibited the growth of colorectal cancer cells (CRCs) and attenuated the generation of reactive oxygen species (ROS) by upregulating the expression of peroxiredoxin 6 (PRDX6)22. In prostate cancer, baicalein exhibited dose-dependent growth inhibitory effects23, inhibiting cell metastasis and inducing apoptosis through the suppression of the caveolin-1/AKT/mammalian target of rapamycin (mTOR) pathway24. In human non-small cell lung cancer cells, baicalein induced cell apoptosis and inhibited cell proliferation through a MAPK- and MAPK kinase (MEK)/ERK1/2-mediated increase and the interaction of forkhead box protein O3a (FOXO3a) and runt-related transcription factor 3 (RUNX3) protein25.
Although the dose-dependent growth inhibitory effects of baicalein have been reported in various human cancers, the molecular mechanism of the inhibitory effect of baicalein on cell proliferation in CC cells is poorly understood. In the present study, the human CC cell lines SiHa and HeLa were treated with baicalein, and the effect of baicalein on the proliferation of SiHa and HeLa cells has been explored in this study.
MATERIALS AND METHODS
Cell Culture and Reagents
Human cervical carcinoma cell lines SiHa and HeLa were purchased from the American Type Culture Collection (Manassas, VA, USA). SiHa and HeLa human cervical carcinoma cell lines were cultured in high-glucose Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA), which contained 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA). The cell lines were incubated at 37°C in a humidified atmosphere containing 5% CO2. The culture medium was changed every 3 days until confluence was achieved. The cells were then subcultured by trypsinization every 5 days. Baicalein (Sigma-Aldrich) was dissolved in dimethyl sulfoxide (DMSO) and stored at −20°C until use. CHIR-99201 (Selleck, Houston, TX, USA) was dissolved in DMSO and stored at −20°C until use. The concentration of DMSO was less than 0.1% in both the control and experimental groups.
Western Blotting
SiHa and HeLa cells were treated with different concentrations of baicalein (0, 20, 40, and 80 μg/ml) for 48 h, lysed in the sample buffer solution, and subsequently denatured.
The total protein concentration of the cell extracts was determined via the bicinchoninic acid (BCA) assay system (Beyotime, Shanghai, P.R. China) using bovine serum albumin (BSA) as a standard. Equal quantities of total proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. The proteins were transferred to nitrocellulose membranes, which were blocked with 5% skimmed milk, and were incubated with β-actin (1:1,000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA), p-AKT (1:1,000 dilution; Santa Cruz), p-GSKβ (1:1,000 dilution; Santa Cruz), GSKβ (1:1,000 dilution; Santa Cruz), and cyclin D1 (1:500 dilution, Santa Cruz) at 4°C overnight, followed by a secondary incubation using a horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG (Thermo Fisher Scientific, New York, NY, USA). The proteins were visualized with an enhanced chemiluminescence reagent (Millipore, Billerica, MA, USA). Photographs were taken, and the optical densities of the bands were scanned and quantified using Gel Doc 2000 (Bio-Rad, Hercules, CA, USA).
Cell Growth and Cell Viability Assays
Cell growth curves were generated to assess cell proliferation. Briefly, SiHa or HeLa cells (5 × 104) were seeded into 2 ml of media in six-well plates containing medium with different concentrations of baicalein. The experiment was performed in triplicate, and the cells were counted every 2 days for 1 week using a hemocytometer. The cell viability was assessed using 3-(4,5-dimethylthiazole-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma-Aldrich) dye according to the standard protocol. Briefly, SiHa or HeLa cells (1 × 103 cells/well) were seeded into 96-well plates, and the media were replaced with medium containing different concentrations of baicalein after 12 h. The cultured cells were subsequently treated with MTT and were incubated at 37°C for an additional 4 h. The absorbance values were detected by measuring the absorbance at 490 nm. All of the experiments were repeated at least three times.
Flow Cytometry Analysis
SiHa and HeLa cells were treated with baicalein (0, 20, 40, and 80 μg/ml) for 48 h. Subsequently, the cells were collected and fixed with cold ethanol (70%) stored at −20°C. Prior to fluorescence-activated cell sorting (FACS) analysis, 1 × 106 cells were harvested, washed twice with cold phosphate-buffered saline (PBS), and treated with 10 mg/ml RNase and 1 mg/ml propidium iodide (Sigma-Aldrich) for 30 min at 37°C. Next, the cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA) using CellQuest software. The percentage of cells in different cell cycle phases was determined using the Cell Quest acquisition software (BD Biosciences). All of the experiments were repeated at least three times.
Statistical Analysis
Statistical analysis was performed with SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). The significance of the differences between covariates was determined using the two-tailed chi-square test. To examine the differences among the three groups, an ANOVA analysis was performed. Student’s t-test was used to determine the statistical significance of two-group analyses. In all tests, statistical significance was defined as p < 0.05.
RESULTS
Baicalein Inhibits the Proliferation of SiHa and HeLa Cells In Vitro
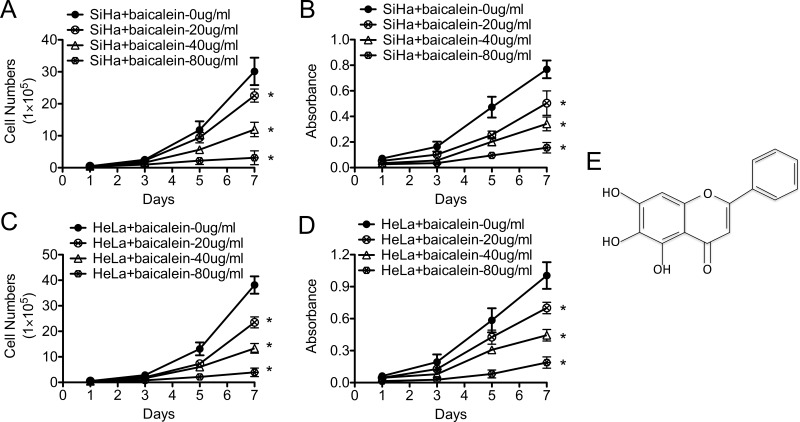
SiHa and HeLa CC cells were individually treated with different concentrations of baicalein, and the effect of baicalein on the growth of CC cells was determined using cell growth curves and MTT assays. As shown in Figure 1, after treatment with 20, 40, and 80 μg/ml of baicalein, the growth of SiHa cells was slower than that of the control cells (baicalein: 0 μg/ml; p < 0.05) (Fig. 1A). The viability of SiHa cells treated with baicalein at 20, 40, and 80 μg/ml was also lower than that of the control cells (baicalein: 0 μg/ml; p < 0.05) (Fig. 1B). In addition, similar results were observed in HeLa cells after treatment with 20, 40, and 80 μg/ml of baicalein. The growth of HeLa cells was slower than that of the control cells (baicalein: 0 μg/ml; p < 0.05) (Fig. 1C), and the viability of HeLa cells treated with 20, 40, and 80 μg/ml of baicalein was also decreased (baicalein: 0 μg/ml; p < 0.05) (Fig. 1D). These results suggested that baicalein inhibited the proliferation of SiHa and HeLa cells in vitro, and the inhibitory effect of baicalein on cell proliferation was dose dependent.
Figure 1.
Baicalein inhibits the proliferation of SiHa and HeLa human cervical cancer cells in vitro. The proliferation of SiHa (A) and HeLa (C) cells treated with baicalein at 0, 20, 40, and 80 μg/ml was detected using growth curves. MTT (3-(4,5-dimethylthiazole-yl)-2,5-diphenyl tetrazolium bromide) assay detected the viability of SiHa (B) and HeLa cells (D) treated with baicalein at 0, 20, 40, and 80 μg/ml. (E) The chemical structure of baicalein. The data are shown as the mean ± SD of three independent experiments (*p < 0.05 vs. control using one-way ANOVA).
Baicalein Arrests Cells at the G0/G1 Phase by Suppressing the Expression of Cyclin D1 in SiHa Cells
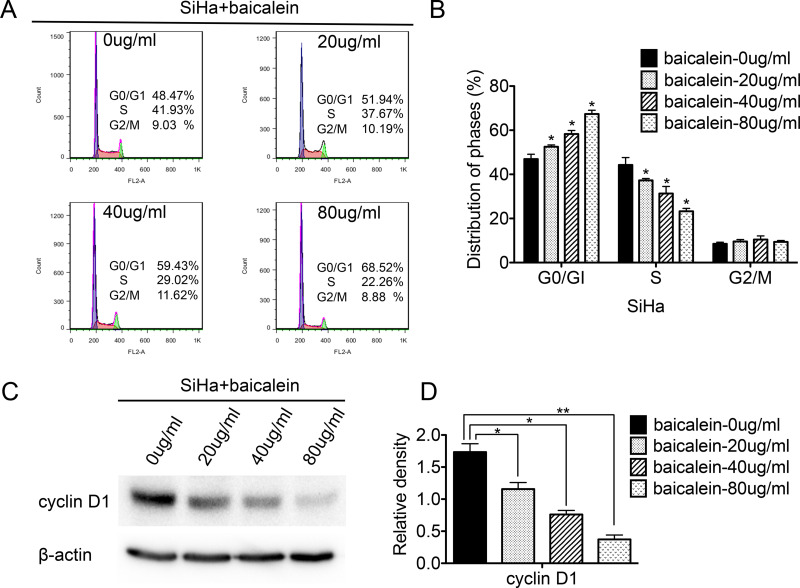
To investigate how baicalein affected the cell cycle of CC cells, FACS was used to analyze the cell cycle between baicalein-treated SiHa cells and control cells. As shown in Figure 2, after treatment with 20, 40, and 80 μg/ml of baicalein, the percentage of SiHa cells in the G0/G1 phase was 51.94%, 59.43%, and 68.52%, which was higher than that of untreated SiHa cells, respectively (48.47%; p < 0.05) (Fig. 2A and B). The percentage of SiHa cells in the S phase after treatment with 20, 40, and 80 μg/ml of baicalein was 37.67%, 29.02%, and 22.26%, which was significantly lower than that of untreated SiHa cells, respectively (41.93%; p < 0.05) (Fig. 2A and B). The ratio of G1/S after SiHa cells were treated with 20, 40, and 80 μg/ml of baicalein was 1.38 (51.94%/37.67%), 2.05 (59.43%/29.02%), and 3.08 (68.52%/22.26%), which was higher than that of untreated SiHa cells (1.15, 48.47%/41.93%). These results suggested that baicalein arrested the cell cycle at the G0/G1 phase in baicalein-treated SiHa cells.
Figure 2.
Baicalein suppresses the expression of cyclin D1 in SiHa cells and arrests cells at the G0/G1 phase. Fluorescence-activated cell sorting (FACS) was used to analyze the cell cycle in baicalein-treated SiHa cells, and the corresponding quantitative analysis of the cell cycle is shown. (A) Cell cycles of SiHa cells treated with baicalein at 0, 20, 40, and 80 μg/ml. (B) Quantitative analysis of (A). (C) Expression of cyclin D1 in SiHa cells treated with baicalein at 0, 20, 40, and 80 μg/ml, which was detected using Western blot. (D) Quantitative analysis of (C). The data are shown as the mean ± SD of three independent experiments (*p < 0.05, **p < 0.01 vs. control using one-way ANOVA).
Furthermore, Western blot analysis was performed to detect the expression of cell cycle regulatory proteins associated with the progression from the G0/G1 phase to the S phase in SiHa cells after treatment with 20, 40, and 80 μg/ml of baicalein for 48 h. As shown in Figure 2C, the expression of cyclin D1 decreased in baicalein-treated SiHa cells compared to that of untreated cells (p < 0.05) (Fig. 2C and D). These results suggested that baicalein arrested the cell cycle at the G0/G1 phase and suppressed the cell proliferation of SiHa cells by downregulating the expression of cyclin D1.
Baicalein Arrests Cells at the G0/G1 Phase by Suppressing the Expression of Cyclin D1 in HeLa Cells
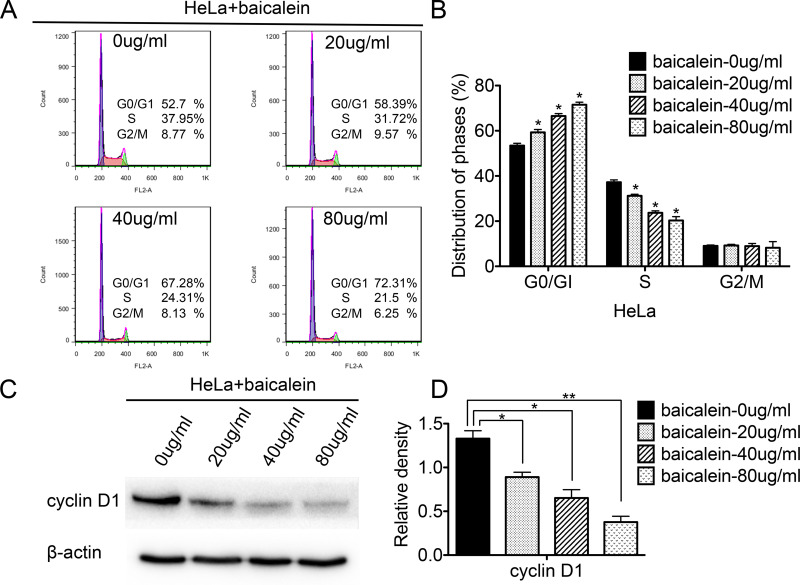
FACS was also used to analyze the cell cycle of baicalein-treated HeLa cells and control cells. As shown in Figure 3, the percentage of HeLa cells in the G0/G1 phase was 58.39%, 67.28%, and 72.31% after treatment with 20, 40, and 80 μg/ml of baicalein, respectively, which was higher than that of the control cells (52.7%; p < 0.05) (Fig. 3A and B). The percentage of cells in the S phase was 31.72%, 24.31%, and 21.5% after treatment with 20, 40, and 80 μg/ml of baicalein, respectively, which was lower than that of the control cells (37.95%; p < 0.05) (Fig. 3A and B). The ratio of G1/S in HeLa cells after treatment with baicalein at 20, 40, and 80 μg/ml was 1.84 (58.39%/31.72%), 2.76 (67.28%/24.31%), and 3.36 (72.31%/21.5%), respectively, which was higher than that of the control cells (1.38, 52.7%/37.95%). These results suggested that baicalein arrested the cell cycle at the G0/G1 phase in baicalein-treated HeLa cells. Furthermore, compared to untreated cells, the expression of cyclin D1 also decreased in HeLa cells treated with 20, 40, and 80 μg/ml of baicalein (p < 0.05) (Fig. 3C and D). All of these results suggested that baicalein could arrest the cell cycle at the G0/G1 phase and suppress the proliferation of HeLa cells by downregulating the expression of cyclin D1.
Figure 3.
Baicalein suppresses the expression of cyclin D1 in HeLa cells and arrests cells at the G0/G1 phase. FACS was used to analyze the cell cycle in baicalein-treated HeLa cells, and a quantitative analysis of the cell cycle is shown. (A) The cell cycles of HeLa cells treated with baicalein at 0, 20, 40, and 80 μg/ml. (B) The quantitative analysis of (A). (C) The expression of cyclin D1 in HeLa cells treated with baicalein at 0, 20, 40, and 80 μg/ml, which was detected by Western blot. (D) The quantitative analysis of (C). The data are shown as the mean ± SD of three independent experiments (*p < 0.05, **p < 0.01 vs. control using one-way ANOVA).
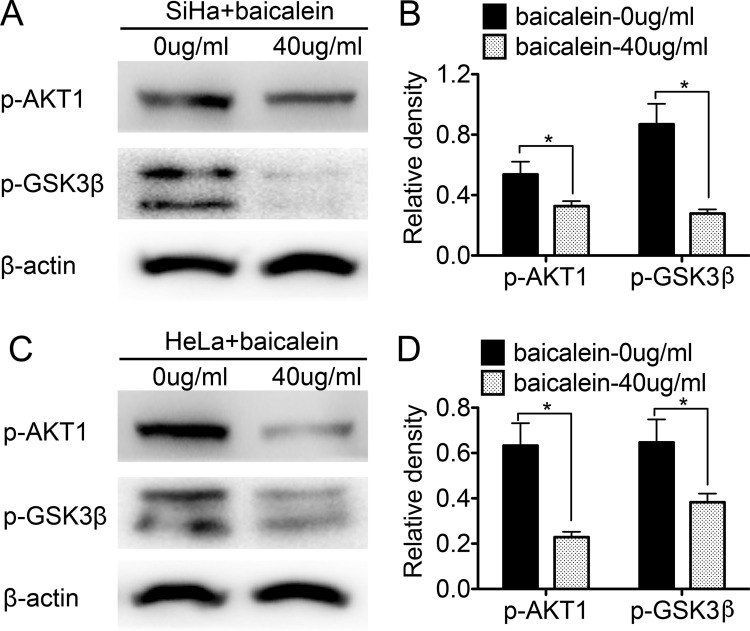
Baicalein Promotes the Activity of GSK3β by Suppressing the Expression of AKT1 in SiHa and HeLa Cells
Previous studies have shown that cyclin D1 can be phosphorylated by GSK3β and subsequently degraded. Additionally, the activity of GSK3β decreases when GSK3β is phosphorylated through the phosphoinositide 3-kinase (PI3K)/AKT pathway26. To investigate whether the baicalein-regulated expression of cyclin D1 occurred through the PI3K/AKT–GSK3β pathway in SiHa and HeLa cells, Western blot analysis was performed to detect the expression of p-AKT and p-GSK3β in baicalein-treated SiHa and HeLa cells. After treatment with 40 μg/ml of baicalein for 24 h, both p-AKT and p-GSK3β were downregulated in SiHa cells compared to untreated cells (p < 0.05) (Fig. 4A and B). Furthermore, similar results were also observed in HeLa cells treated with 40 μg/ml of baicalein for 24 h: the expression of p-AKT and p-GSK3β was lower in the baicalein-treated HeLa cells than in the untreated cells (p < 0.05) (Fig. 4C and D). These results suggested that baicalein could suppress the expression of p-AKT and p-GSK3β in SiHa and HeLa cells.
Figure 4.
Baicalein suppresses the expression of phosphorylated protein kinase B (p-AKT) and phosphorylated glycogen synthase kinase 3β (p-GSK3β) in SiHa and HeLa cells. (A) The expression of p-AKT and p-GSK3β in SiHa cells treated with baicalein at 0 and 40 μg/ml was detected by Western blot. (B) The quantitative analysis of the Western blotting. (C) The expression of p-AKT and p-GSK3β in HeLa cells treated with baicalein at 0 and 40 μg/ml was detected by Western blotting. (D) Corresponding quantitative analysis of the Western blotting. The data are shown as the mean ± SD of three independent experiments (*p < 0.05 vs. control using the t-test).
To further confirm that the inhibitory effect of baicalein on the proliferation of CC cells was mediated through the AKT/GSK3β/cyclin D1 pathway, CHIR-99021, an inhibitor of GSK3β, was used to block the activity of GSK3β in baicalein-treated SiHa cells. When CHIR-99021 was added to SiHa cells treated with 40 μg/ml of baicalein for 24 h, the expression of overall GSK3β was lower than that of baicalein-treated and baicalein-untreated SiHa cells (p = 0.019) (Fig. 5A and B). Furthermore, the expression of cyclin D1 was upregulated in CHIR-99021-treated SiHa cells (p = 0.023) (Fig. 5A and B). When CHIR-99021 was added to SiHa cells treated with 40 μg/ml of baicalein, cell growth was faster than that of baicalein-treated cells (p < 0.05) (Fig. 5C), and the viability of SiHa cells treated with 40 μg/ml of baicalein and CHIR-99021 was also higher than that of baicalein-treated cells (p < 0.05) (Fig. 5D). These results further confirmed that baicalein could inhibit the proliferation of human CC cells via the downregulation of cyclin D1 through the PI3K/AKT–GSK3β signaling pathway.
Figure 5.
Blockage of the GSK3β by CHIR-99201 promotes cell proliferation in baicalein-treated SiHa cells. (A) The expression of GSK3β, p-GSK3β, and cyclin D1 in SiHa cells treated with baicalein at 0 and 40 μg/ml and CHIR-99201 was detected by Western blotting. (B) Corresponding quantitative analysis of the Western blotting. (C) The proliferation of SiHa cells treated with baicalein at 0 and 40 μg/ml and CHIR-99201 was detected using growth curves. (D) The viability of SiHa cells treated with baicalein at 0 and 40 μg/ml and CHIR-99201 was detected using the MTT assay. The data are shown as the mean ± SD of three independent experiments. *p < 0.05, versus control using one-way ANOVA.
DISCUSSION
Baicalein is an effective component of Scutellariae baicalensis, and cellular and animal experiments have demonstrated that baicalein shows strong antitumor activity toward various human cancers23. In the present study, baicalein inhibited the proliferation and viability of SiHa cells treated with 20, 40, and 80 μg/ml of baicalein (p < 0.05) (Fig. 1A and B), and similar trends were also observed in HeLa cells (p < 0.05) (Fig. 1C and D). These results suggested that baicalein could inhibit the proliferation of SiHa and HeLa cells in a dose-dependent manner in vitro.
Normally, changes in cell proliferation are related to cell cycle modulations27. The FACS results obtained herein showed that the percentage of cells in the G0/G1 phase was higher in baicalein-treated SiHa and HeLa cells than untreated cells, and the percentage of cells in the S phase was lower in baicalein-treated SiHa (p < 0.05) (Fig. 2A and B) and HeLa cells (p < 0.05) (Fig. 3A and B) than in untreated cells. These results suggested that the inhibitory effect of baicalein on the proliferation of SiHa and HeLa cells was due to an arrest in the transition from the G0/G1 phase to the S phase during the progression of the cell cycle.
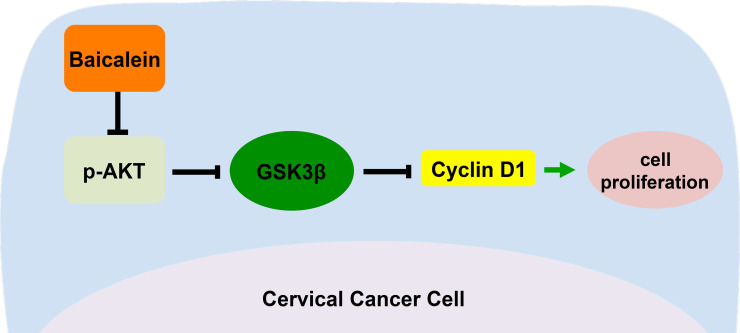
Cyclin D1 is an important cell cycle regulator associated with the progression from the G0/G1 to S phase transition. In oral cancer cells, baicalein inhibited cell proliferation by enhancing the degradation of cyclin D126. As shown in Figures 2C and 3C, the content of cyclin D1 decreased in baicalein-treated SiHa and HeLa cells compared to untreated cells, respectively. These results suggested that baicalein could arrest the cell cycle at the G0/G1 phase and suppress the proliferation of SiHa and HeLa cells by downregulating the protein level of cyclin D1. Furthermore, the expression of p-AKT and p-GSK3β was also lower in baicalein-treated SiHa and HeLa cells than in untreated cells (p < 0.05) (Fig. 4A and B). To confirm whether the observed reduction in cyclin D1 in baicalein-treated SiHa and HeLa cells was mediated through the PI3K/AKT–GSK3β pathway, CHIR-99021, an inhibitor of GSK3β, was used to block the activity of GSK3β in baicalein-treated SiHa cells. When CHIR-99021 was added to SiHa cells treated with 40 μg/ml of baicalein for 24 h, the expression of GSK3β was downregulated, and the original expression level of cyclin D1 was recovered (p = 0.019; p = 0.023) (Fig. 5A and B). In addition, cell growth was faster compared to baicalein-treated cells (p < 0.05) (Fig. 5C), and higher cell viability was observed (p < 0.05) (Fig. 5D). These results suggested that baicalein could reduce the expression of p-GSK3β by suppressing the protein level of p-AKT in baicalein-treated SiHa and HeLa cells. The reduced levels of p-GSK3β enhanced the activity of GSK3β, enabling the phosphorylation and degradation of cyclin D1 by GSK3β, which further inhibited the proliferation of human CC cells (Fig. 6).
Figure 6.
Proposed model for baicalein as an inhibitor to suppress the cell proliferations in SiHa and HeLa cells. Baicalein decreased the expression of p-GSK3β via the downregulation of p-AKT, thereby enhancing the activity of GSK3β. GSK3β phosphorylated cyclin D1, promoting the degradation of cyclin D1, which further arrested cells at the G0/G1 phase, inhibiting the proliferation of SiHa and HeLa cervical cancer cells.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (81471670), the Fundamental Research Funds for the Central Universities of China (2014qngz-04), and the Science and Technology Plan of Innovation Project of Shaanxi Province in China (2015KTCL03-06). Huixia Peng and Zhijun Dai conceived and designed the study; Xiao-Ling Wu and Hui-Min Dang carried out the study; Xiao-Ling Wu, Hui-Min Dang, and Zhi-Qin Yang analyzed the data and composed the manuscript.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Meng XY, Liao Y, Liu XP, Li S, Shi MJ, Zeng XT. Concurrent cisplatin-based chemoradiotherapy versus exclusive radiotherapy in high-risk cervical cancer: A meta-analysis. Onco Targets Ther. 2016;9:1875–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu X, Yang WT, Zheng PS. Msi1 promotes tumor growth and cell proliferation by targeting cell cycle checkpoint proteins p21, p27 and p53 in cervical carcinomas. Oncotarget 2014;5(21):10870–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ikemoto S, Sugimura K, Yoshida N, Yasumoto R, Wada S, Yamamoto K, Kishimoto T. Antitumor effects of Scutellariae radix and its components baicalein, baicalin, and wogonin on bladder cancer cell lines. Urology 2000;55(6):951–5. [DOI] [PubMed] [Google Scholar]
- 4. Lin CC, Shieh DE. The anti-inflammatory activity of Scutellaria rivularis extracts and its active components, baicalin, baicalein and wogonin. Am J Chin Med. 1996;24(1):31–6. [DOI] [PubMed] [Google Scholar]
- 5. Hamada H, Hiramatsu M, Edamatsu R, Mori A. Free radical scavenging action of baicalein. Arch Biochem Biophys. 1993;306(1):261–6. [DOI] [PubMed] [Google Scholar]
- 6. Shieh DE, Liu LT, Lin CC. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res. 2000;20(5a):2861–5. [PubMed] [Google Scholar]
- 7. Tsai TH, Liu SC, Tsai PL, Ho LK, Shum AY, Chen CF. The effects of the cyclosporin A, a P-glycoprotein inhibitor, on the pharmacokinetics of baicalein in the rat: A microdialysis study. Br J Pharmacol. 2002;137(8):1314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hour MJ, Huang SH, Chang CY, Lin YK, Wang CY, Chang YS, Lin CW. Baicalein, ethyl acetate, and chloroform extracts of Scutellaria baicalensis inhibit the neuraminidase activity of pandemic 2009 H1N1 and seasonal influenza A viruses. Evid Based Complement Alternat Med. 2013;2013:750803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chi DS, Lin TC, Hall K, Ha T, Li C, Wu ZD, Soike T, Krishnaswamy G. Enhanced effects of cigarette smoke extract on inflammatory cytokine expression in IL-1beta-activated human mast cells were inhibited by baicalein via regulation of the NF-kappaB pathway. Clin Mol Allergy 2012;10:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao XY, Xue XH, Ma YN, Zhang SQ. Effect of baicalein on the expression of SATB1 in human breast cancer cells. Exp Ther Med. 2015;9(5):1665–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang N, Ren D, Deng S, Yang X. Differential effects of baicalein and its sulfated derivatives in inhibiting proliferation of human breast cancer MCF-7 cells. Chem Biol Interact. 2014;221:99–108. [DOI] [PubMed] [Google Scholar]
- 12. Zhou QM, Wang S, Zhang H, Lu YY, Wang XF, Motoo Y, Su SB. The combination of baicalin and baicalein enhances apoptosis via the ERK/p38 MAPK pathway in human breast cancer cells. Acta Pharmacol Sin. 2009;30(12):1648–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peng Y, Guo C, Yang Y, Li F, Zhang Y, Jiang B, Li Q. Baicalein induces apoptosis of human cervical cancer HeLa cells in vitro. Mol Med Rep. 2015;11(3):2129–34. [DOI] [PubMed] [Google Scholar]
- 14. Chen J, Li Z, Chen AY, Ye X, Luo H, Rankin GO, Chen YC. Inhibitory effect of baicalin and baicalein on ovarian cancer cells. Int J Mol Sci. 2013;14(3):6012–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yan H, Xin S, Wang H, Ma J, Zhang H, Wei H. Baicalein inhibits MMP-2 expression in human ovarian cancer cells by suppressing the p38 MAPK-dependent NF-kappaB signaling pathway. Anticancer Drugs 2015;26(6):649–56. [DOI] [PubMed] [Google Scholar]
- 16. Mu J, Liu T, Jiang L, Wu X, Cao Y, Li M, Dong Q, Liu Y, Xu H. The traditional Chinese medicine Baicalein potently inhibits gastric cancer cells. J Cancer 2016;7(4):453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen F, Zhuang M, Peng J, Wang X, Huang T, Li S, Lin M, Lin H, Xu Y, Li J, Chen Z, Huang Y. Baicalein inhibits migration and invasion of gastric cancer cells through suppression of the TGF-beta signaling pathway. Mol Med Rep. 2014;10(4):1999–2003. [DOI] [PubMed] [Google Scholar]
- 18. Yan X, Rui X, Zhang K. Baicalein inhibits the invasion of gastric cancer cells by suppressing the activity of the p38 signaling pathway. Oncol Rep. 2015;33(2):737–43. [DOI] [PubMed] [Google Scholar]
- 19. Chen F, Zhuang M, Zhong C, Peng J, Wang X, Li J, Chen Z, Huang Y. Baicalein reverses hypoxia-induced 5-FU resistance in gastric cancer AGS cells through suppression of glycolysis and the PTEN/Akt/HIF-1alpha signaling pathway. Oncol Rep. 2015;33(1):457–63. [DOI] [PubMed] [Google Scholar]
- 20. Rui X, Yan XI, Zhang K. Baicalein inhibits the migration and invasion of colorectal cancer cells via suppression of the AKT signaling pathway. Oncol Lett. 2016;11(1):685–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim SJ, Kim HJ, Kim HR, Lee SH, Cho SD, Choi CS, Nam JS, Jung JY. Antitumor actions of baicalein and wogonin in HT-29 human colorectal cancer cells. Mol Med Rep. 2012;6(6):1443–9. [DOI] [PubMed] [Google Scholar]
- 22. Huang WS, Kuo YH, Chin CC, Wang JY, Yu HR, Sheen JM, Tung SY, Shen CH, Chen TC, Sung ML, Liang HF, Kuo HC. Proteomic analysis of the effects of baicalein on colorectal cancer cells. Proteomics 2012;12(6):810–9. [DOI] [PubMed] [Google Scholar]
- 23. Miocinovic R, McCabe NP, Keck RW, Jankun J, Hampton JA, Selman SH. In vivo and in vitro effect of baicalein on human prostate cancer cells. Int J Oncol. 2005;26(1):241–6. [PubMed] [Google Scholar]
- 24. Guo Z, Hu X, Xing Z, Xing R, Lv R, Cheng X, Su J, Zhou Z, Xu Z, Nilsson S, Liu Z. Baicalein inhibits prostate cancer cell growth and metastasis via the caveolin-1/AKT/mTOR pathway. Mol Cell Biochem. 2015;406(1–2):111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zheng F, Wu J, Zhao S, Luo Q, Tang Q, Yang L, Li L, Wu W, Hann SS. Baicalein increases the expression and reciprocal interplay of RUNX3 and FOXO3a through crosstalk of AMPKalpha and MEK/ERK1/2 signaling pathways in human non-small cell lung cancer cells. J Exp Clin Cancer Res. 2015;34:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng YH, Li LA, Lin P, Cheng LC, Hung CH, Chang NW, Lin C. Baicalein induces G1 arrest in oral cancer cells by enhancing the degradation of cyclin D1 and activating AhR to decrease Rb phosphorylation. Toxicol Appl Pharmacol. 2012;263(3):360–7. [DOI] [PubMed] [Google Scholar]
- 27. Teng H, Sui X, Zhou C, Shen C, Yang Y, Zhang P, Guo X, Huo R. Fatty acid degradation plays an essential role in proliferation of mouse female primordial germ cells via the p53-dependent cell cycle regulation. Cell Cycle 2016;15(3):425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]