Abstract
Long noncoding RNAs (lncRNAs), a new class of functional regulators involved in human tumorigenesis, have been attracting the increasing attention of researchers. The lncRNA colorectal neoplasia differentially expressed (CRNDE) gene, transcribed from chromosome 16 on the strand opposite the adjacent IRX5 gene, was originally found to be increased in CRC and was reported to be abnormally expressed in many cancers. However, its potential role and the molecular mechanism underlying the radioresistant phenotype formation of lung adenocarcinoma (LAD) remain unclear. In our present study, we identified that CRNDE was significantly upregulated in LAD tissue and radioresistant LAD cell lines. A high level of CRNDE expression was significantly correlated with poor differentiation, TNM stage, lymph node metastasis, radiotherapy response, and a significantly shorter overall survival. Gain- and loss-of-function tests revealed that CRNDE could influence the radiosensitivity of LAD cells by affecting the G1/S transition and causing apoptosis of LAD cells in vitro. Additionally, the mechanistic investigations showed that CRNDE could interact with PRC2 and recruit its core component EZH2 to p21 (CDKN1A) promoter regions and repress its transcription. Furthermore, rescue experiments were performed to confirm that CRNDE oncogenic function was partly through regulating p21. In conclusion, our data suggest that CRNDE may function as an oncogene by modulating p21, finally contributing to the radioresistant phenotype formation of LAD cells.
Key words: Long noncoding RNA colorectal neoplasia differentially expressed (lncRNA CRNDE), Polycomb-repressive complex 2 (PRC2), p21, Lung adenocarcinoma (LAD), Radioresistant
INTRODUCTION
Non-small cell lung cancer (NSCLC) accounts for 85% of lung cancers, among which lung adenocarcinoma (LAD) is the predominant histologic subtype1. Despite many theoretical and clinical efforts that have been attempted, the survival rate of LAD patients is still unsatisfactory. A sophisticated understanding of the pathogenetic mechanism underlying LAD is critical for exploration of novel treatment methods. Radiotherapy, as one of the main methods for the treatment of LAD, is widely used in clinical practice. However, clinical observation found that many patients with LAD are not sensitive to radiotherapy2. Therefore, it is of great significance to seek a potential radioresistant mechanism in LAD.
Long noncoding RNAs (lncRNAs) are a class of RNA molecules with little protein-coding ability that have attracted the attention of many researchers3,4. Accumulating evidence has revealed that lncRNAs play a critical role in human carcinogenesis, including LAD. For instance, Chen et al. reported that the lncRNA colon cancer-associated transcript 1 (CCAT1) promoted chemoresistance in LAD5; Liu et al. demonstrated that the lncRNA maternally expressed 3 (MEG3) contributes to cisplatin resistance in human LAD6. However, the biological function of lncRNAs in LAD radioresistance needs to be further investigated.
It has been identified that lncRNAs participate in intertwined gene regulatory networks at various levels including the epigenetic, transcriptional, and posttranscriptional levels7–12. Recently, lncRNAs are reported to act as modular guided scaffolds recruiting polycomb-repressive complex 2 (PRC2) to the targeting sites and mediate histone modification. PRC2, consisting of enhancer of zeste homolog 2 (EZH2), suppressor of zeste 12 (SUZ12), and embryonic ectoderm development (EED), is a methyltransferase for histone H3 lysine 27 trimethylation (H3K27me3)13. The lncRNA colorectal neoplasia differentially expressed (CRNDE) is transcribed from chromosome 16 on the strand opposite the adjacent Iroquois homeobox 5 (IRX5) gene, which was originally found to be increased in colorectal cancer (CRC)14 and was also upregulated in a series of other cancer types, including hepatocellular carcinoma (HCC), glioma, renal cell carcinoma, gallbladder carcinoma, and ovarian cancer15–20. Despite some preliminary evidence that CRNDE could interact with chromatin-modifying complexes to affect epigenetic regulation of gene expression21, the mechanism by which CRNDE plays a role in LAD radioresistance has not been fully elucidated.
In the present study, we sought to assess the function and molecular mechanism of CRNDE in LAD. We found that CRNDE expression is upregulated in LAD tissues, radioresistant LAD tissues, and radioresistant LAD cell lines. Moreover, a high level of CRNDE is closely associated with an advanced pathological stage and radiotherapy response in LAD patients. Gain- and loss-of-function tests revealed that the forced expression of CRNDE in LAD cells significantly decreased the sensitivity to radiotherapy, whereas silencing CRNDE in radioresistant LAD cells obviously increased their sensitivity to radiotherapy. Furthermore, we discovered that CRNDE exerted its function in LAD mainly through acting as a modular scaffold of histone medication complexes via binding with EZH2 to the promoter of p21 and thereby regulating the expression of p21. Our findings revealed that CRNDE contributed to the radioresistant phenotype of LAD cells and provided novel insights for exploring clinical treatment targets.
MATERIALS AND METHODS
Patients
A total of 66 pairs of fresh LAD specimens and adjacent nontumor specimens were collected from patients who underwent surgery at the Department of Thoracic Surgery, The Third Affiliated Hospital of Kunming Medical University, Tumor Hospital of Yunnan Province, Kunming, P.R. China. The pathological stage, grade, and nodal status were appraised by an experienced pathologist. All experiments were approved by the Research Ethics Committee of The Third Affiliated Hospital of Kunming Medical University. Written informed consent was obtained from all patients.
Cell Lines
Human lung cancer cell lines A549 and H1299 were purchased from Keygen Biotech (Nanjing, P.R. China). The radioresistant cells derived from A549 and H1299 were generated by dose-gradient irradiation and named A549R and H1299R. All cells were maintained in RPMI-1640 medium (Gibco, New York, NY, USA) containing 10% fetal bovine serum (FBS) at 37°C with 5% CO2 in a humidified incubator.
Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA was extracted from LAD cell lines and patient specimens using TRIzol reagent (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s manual. To measure the expression levels of mRNA, RT-qPCR was performed according to a previous study22. The SuperScript III Platinum SYBR Green One-Step qRT-PCR kit (Invitrogen, Carlsbad, CA, USA) was used to quantify the levels of the indicated genes. The primer sequences were as follows: CRNDE, 5′-TGAAGGAAGGAAGTGGTGCA-3′ (forward) and 5′-TCCAGTGGCATCCTACAAGA-3′ (reverse); p15, 5′-GGACTAGTGGAGAAGGTGCG-3′ (forward) and 5′-GGGC GCTGCCCATCATCATG-3′ (reverse); p16, 5′-CACCGAATAGTTACGGTCGG-3′ (forward) and 5′-GCACGGGTCGGGTGAGAGTG-3′ (reverse); p21, 5′-AAGTCAGTTCCTTGTGGAGCC-3′ (forward) and 5′-GGTTCTGACGGACATCCCCA-3′ (reverse); p27, 5′-TGCAACCGACGATTCTTCTACTCAA-3′ (forward) and 5′- CAAGCAGTGATGTATCTGATAAACAAGG -3′ (reverse); EZH2, 5′-TGCACATCCTGACTTCTGTG-3′ (forward) and 5′-AAGGGCATTCACCAACTCC-3′ (reverse) (149 bp); U6, 5′-CTCGCTTCGGCAGCACA-3′ (forward) and 5′-AACGCTTCACGAATTTGCGT-3′ (reverse); and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), 5′-TGCACCACCAACTGCTTAG-3′ (forward) and 5′-AGTAGAGGCAGGGATGATGTTC-3′ (reverse). Amplification was performed using an ABI Prism 7000 (ABI; Applied Biosystems, Foster City, CA, USA) sequence analyzer using the following conditions: 30 s at 95°C, 45 cycles of 10 s 95°C; 10 s at 62°C; and 10 s at 72°C. Expression levels of genes were normalized to that of the housekeeping gene GAPDH. The 2−ΔΔCt method was applied for calculation of relative levels of gene expression.
Cell Transfection
To overexpress CRNDE, the full-length coding sequence for CRNDE was subcloned into the pcDNA 3.1(+) vector (Realgene, Shanghai, P.R. China) and amplified according to the manufacturer’s instructions. CRNDE, p21, EZH2, and scrambled negative control (NC) small interfering RNAs (siRNAs) were purchased from Invitrogen and transfected into cells using Lipofectamine 2000 (Invitrogen), as per the manufacturer’s protocol.
Western Blot Analysis
Cells were harvested in lysis buffer containing protease inhibitor cocktail (Roche Applied Science, Penzberg, Germany). Proteins were separated with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA) and then incubated with primary antibodies including anti-rabbit p21 (1:1,000 dilution; Santa Cruz, Santa Cruz, CA, USA), anti-rabbit cyclin-dependent kinase 2 (CDK2; 1:1,000 dilution; Cell Signaling Technology, Beverly, MA, USA), anti-rabbit cleaved caspase 3 (1:1,000 dilution; Cell Signaling Technology), anti-rabbit cleaved caspase 9 (1:1,000 dilution; Cell Signaling Technology), and anti-rabbit GAPDH (1:5,000 dilution; Cell Signaling Technology). The secondary antibody was horseradish peroxidase-conjugated goat anti-rabbit IgG. An enhanced chemiluminescence (ECL) chromogenic substrate was used to visualize the bands, and the intensity of the bands was quantified by densitometry (Quantity One software; Bio-Rad, Hercules, CA, USA).
Cell Cytoplasm/Nucleus Fraction Isolation
The separation of nuclear and cytosolic fractions was performed using the PARIS Kit (Life Technologies) according to the manufacturer’s instructions. RNAs extracted from each of the fractions were subjected to RT-qPCR analysis to demonstrate the levels of nuclear control transcript (U6), cytoplasmic control transcript (GAPDH), and lncRNA CRNDE.
RNA Immunoprecipitation (RIP)
RIP was performed using a Thermo Fisher RIP Kit (Thermo Fisher, Waltham MA, USA) based on the manufacturer’s protocol. Cells at 80%–90% confluency were scraped off and lysed in complete RIP lysis buffer. One hundred microliters of whole-cell extract was then incubated with RIP buffer containing magnetic beads conjugated with human anti-EZH2 antibody (Cell Signaling Technology), anti-SUZ12 antibody (Cell Signaling Technology), and NC normal mouse IgG (Millipore).
Chromatin Immunoprecipitation (ChIP) Assay
The radioresistant LAD cells were treated with formaldehyde and incubated for 10 min to generate DNA–protein cross-links. The cell lysates were then sonicated to generate chromatin fragments of 200 to 300 bp and immunoprecipitated with H3K27me3, EZH2, and H3K4me2-specific antibody (Cell Signaling Technology) or IgG as the control. Precipitated chromatin DNA was recovered and analyzed by RT-qPCR. The following are the primer sequences: p21, 5′-GGTGTCTAGGTGCTCCAGGT-3′ (forward) and 5′- GCACTCTCCAGGAGGACACA-3′ (reverse).
MTT Assay
The sensitivity of cells to radiotherapy was assessed via 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) assay. Cells were seeded into a 96-well flat-bottomed plate at a density of 5 × 103 cell/well for 24 h, then transfected with the indicated vectors and cultured in normal medium. Cells were then treated with 0, 2, 4, 6, 8, and 10 Gy of ionizing radiation (X-ray) after transfection. MTT solution (5 mg/ml, 20 μl) was added to each well. Following incubation for 4 h, the medium was removed, and 100 μl of dimethyl sulfoxide (DMSO) was added to each well. The relative number of surviving cells was assessed by measuring the optical density (OD) of cell lysates at 560 nm. All assays were performed in triplicate.
Colony Formation Assay
Cells (500 cells/well) were plated onto six-well plates and incubated in normal medium at 37°C. After 2 weeks, the cells were fixed and stained with 0.1% crystal violet. The number of visible colonies was counted manually.
Flow Cytometric Analysis of Apoptosis
Apoptosis was performed using flow cytometric analysis with Annexin-V/Fluorescein Isothiocyanate (FITC) Apoptosis Detection Kits (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instructions. All samples were assayed in triplicate.
Flow Cytometric Analysis of Cell Cycle Distribution
Cells were collected directly or 48 h after transfection and washed with ice-cold phosphate-buffered saline (PBS), and then fixed with 70% ethanol overnight at −20°C. Fixed cells were rehydrated in PBS for 10 min and incubated in RNase A (1 mg/ml) for 30 min at 37°C. The cells were then subjected to propidium iodide (PI)/RNase staining followed by flow cytometric analysis using a FACScan instrument (Becton Dickinson, Mountain View, CA, USA) and CellQuest software (BD Biosciences) as described previously23.
Statistical Analysis
All data were expressed as means ± SD. Student’s t-test and one-way ANOVA were used to determine the significance of differences between two groups or among multiple groups, respectively, using the SPSS 17.0 software program (IBM, Armonk, NY, USA). Log-rank test was for Kaplan–Meier survival analysis. A value of p < 0.05 was considered to be statistically significant. All experiments were repeated at least three times with each sample run in triplicate.
RESULTS
lncRNA CRNDE Is Upregulated in LAD Tissues, Radioinsensitive LAD Tissues, and Radioresistant Cell Lines
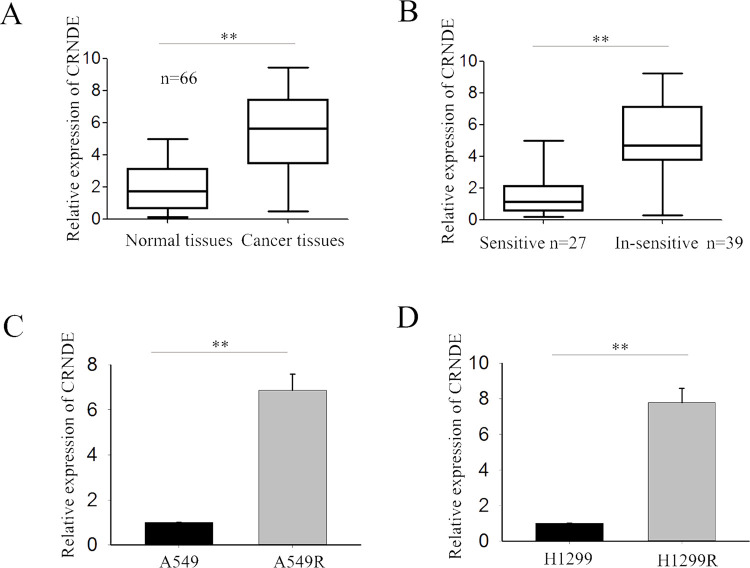
To explore the potential role of CRNDE in regulating the radioresistance of LAD, we first measured the level of CRNDE in 66 LAD tissues obtained from patients at an advanced stage and their corresponding normal tissues. CRNDE expression was significantly upregulated in cancer tissues compared with normal tissues (p < 0.01) (Fig. 1A). Additionally, according to radiologic Response Evaluation Criteria in Solid Tumors (RECIST), the 66 cases of clinical LAD tissues were divided into “sensitive” (complete or partial response) and “insensitive” (stable or progressive disease) groups in response to radiotherapy. CRNDE was significantly upregulated in the radioinsensitive groups (n = 39) compared with the radiosensitive groups (n = 27) as assessed by RT-qPCR (p < 0.01) (Fig. 1B). Furthermore, we examined the level of CRNDE in two radioresistant LAD cell lines (A549R and H1299R) and the corresponding parental cell lines (A549 and H1299). Increased expression of CRNDE was observed in radioresistant LAD cell lines compared with the corresponding parental cell lines (Fig. 1C and D). These results indicated that CRNDE might be involved in the radioresistance of LAD.
Figure 1.
Long noncoding RNA colorectal neoplasia differentially expressed (lncRNA CRNDE) is upregulated in lung adenocarcinoma (LAD) tissues, radioinsensitive LAD tissues, and radioresistant cell lines. (A) The level of CRNDE in 66 LAD tissues and corresponding normal tissues was measured by reverse transcription quantitative polymerase chain reaction (RT-qPCR). (B) The level of CRNDE in the radioinsensitive (n = 39) and radiosensitive groups (n = 27) was assessed by RT-qPCR. (C, D) The level of CRNDE in two radioresistant LAD cells and corresponding parental cells was detected by RT-qPCR. Data are represented as the mean ± SD from three independent experiments. The p value represents the comparison between groups (**p < 0.01).
CRNDE Is Correlated With Clinicopathological Features and Radiotherapy Response
We then evaluated the correlation of CRNDE expression with the clinicopathological parameters of 66 patients. The median value of CRNDE in all LAD tissues was used as a cutoff value, and all samples were divided into two groups [high expression group (n = 36) vs. low expression group (n = 30)]. A high level of CRNDE expression was significantly correlated with poor differentiation (p = 0.013) (Table 1), TNM stage (p ≤ 0.001), and lymph node metastasis (p = 0.000), but had no significant correlation with age, gender, or smoking history (p > 0.05). According to RECIST, about 41% of patients responded to radiotherapy with complete response or partial response, whereas 59% of patients were not responsive and had either stable disease or disease progression. The results showed that CRNDE is significantly associated with therapeutic response, exhibiting a higher expression level in nonresponsive patients (p = 0.000) (Table 1). Furthermore, Kaplan–Meier analysis (log-rank test) was performed to determine the association between CRNDE expression and overall survival of patients. Patients with a high level of CRNDE expression had a significantly shorter overall survival than those having a low level of CRNDE (p = 0.000) (Fig. 2).
Table 1.
Correlation Between lncRNA CRNDE Expression and Clinical Features (n = 66)
| Variable | lncRNA2 CRNDE Expression | p Value | |
|---|---|---|---|
| Low | High | ||
| Age | 0.807 | ||
| <60 | 13 | 17 | |
| ≥60 | 17 | 19 | |
| Gender | 0.794 | ||
| Male | 21 | 23 | |
| Female | 9 | 13 | |
| Smoking | 0.353 | ||
| Smoking | 26 | 27 | |
| No smoking | 4 | 9 | |
| Differentiation | 0.013 | ||
| Poor | 20 | 12 | |
| Well/moderate | 10 | 24 | |
| TNM stage | 0.001 | ||
| I–II | 23 | 13 | |
| IIIa | 7 | 23 | |
| Lymph metastasis | 0.000 | ||
| Absent | 23 | 8 | |
| Present | 7 | 28 | |
| Treatment response | 0.000 | ||
| Complete response + partial response | 22 | 5 | |
| Stable disease + progressive disease | 8 | 31 | |
Low/high by the sample median. Pearson chi-square test. A value of p < 0.05 was considered statistically significant.
Figure 2.
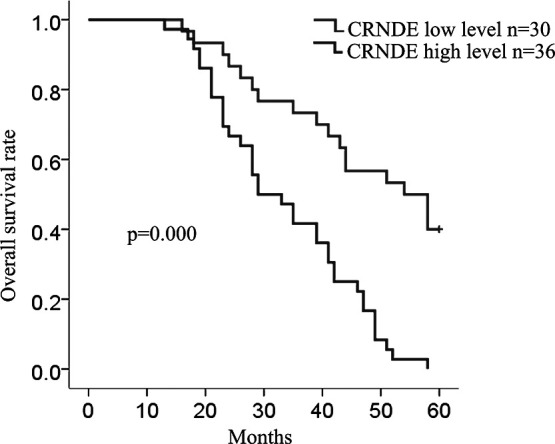
CRNDE is correlated with clinicopathological features and radiotherapy response. The overall survival in 66 LAD patients is represented by Kaplan–Meier curves.
Forced Expression of lncRNA CRNDE Increased the Radioresistance of LAD Cells
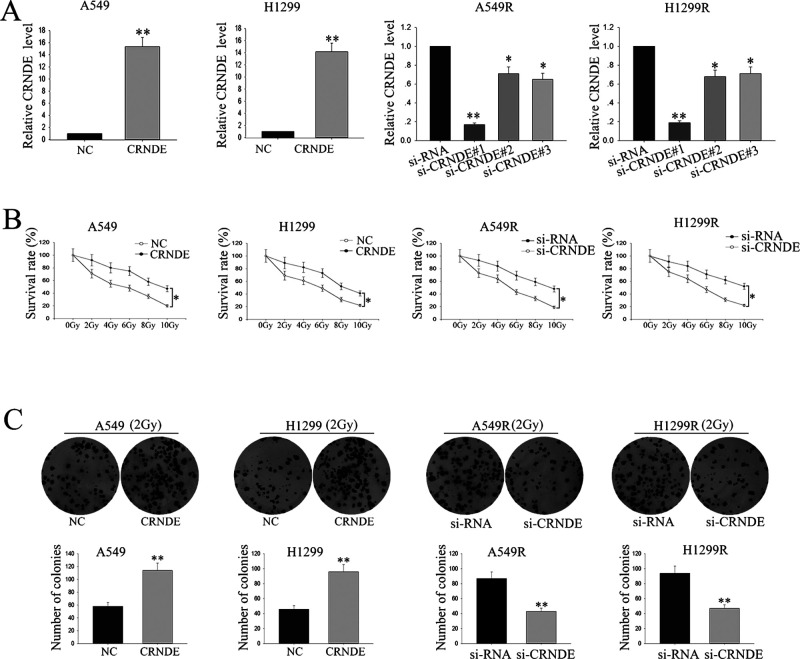
To investigate the biological function of CRNDE in the radioresistance of LAD, A549 (or H1299), and A549R (or H1299R), cells were transfected with a CRNDE expression vector or CRNDE-specific siRNA (si-CRNDE#1, si-CRNDE#1, and si-CRNDE#3), respectively, using the empty vector or control siRNA as a NC. Satisfactory transfection efficiency was obtained at 48 h posttransfection (Fig. 3A), and of the three siRNAs, si-CRNDE#1 (as the si-CRNDE in Fig. 3A) demonstrated the best silencing capacity. An MTT assay was performed to assess the biological function of CRNDE on the sensitivity of LAD cells to radiation. A549 and H1299 cells transfected with the CRNDE expression vector were treated with different doses of ionizing radiation, from 0 to 10 Gy. Ionizing radiation treatment exhibited a dose-dependent inhibitory effect on the growth of A549 and H1299 cells, whereas overexpression of CRNDE weakened this effect, and vice versa in A549R and H1299R cells transfected with si-CRNDE (Fig. 3B). Furthermore, colony formation assays were performed to measure the effect of CRNDE on cell proliferation exposed to 2 Gy of X-ray ionizing radiation. Overexpression of CRNDE significantly enhanced the colony formation rate, whereas knockdown of CRNDE weakened the A549R or H1299R cells’ colony formation rate (Fig. 3C). The findings revealed that CRNDE may function as an oncogene and is associated with the radioresistance of LAD cells.
Figure 3.
Forced expression of lncRNA CRNDE increased the radioresistance of LAD cells. (A) A549 (or H1299) and A549R (or H1299R) cells were stably transfected with the CRNDE expression vector or CRNDE-specific small interfering RNA (siRNA) (si-CRNDE#1, si-CRNDE#1, and si-CRNDE#3), respectively, using the empty vector or control siRNA as a negative control (NC). (B) MTT assay was performed to assess the biological function of CRNDE on the sensitivity of LAD cells to radiation. (C) Colony formation assays were performed to measure the effect of CRNDE on cell proliferation exposed to 2 Gy by X-ray ionizing radiation. Data are represented as the mean ± SD from three independent experiments. The p value represents the comparison between groups (*p < 0.05, **p < 0.01).
Knockdown of lncRNA CRNDE Promotes Cell Cycle Arrest at the G1 Phase and Causes Cell Apoptosis
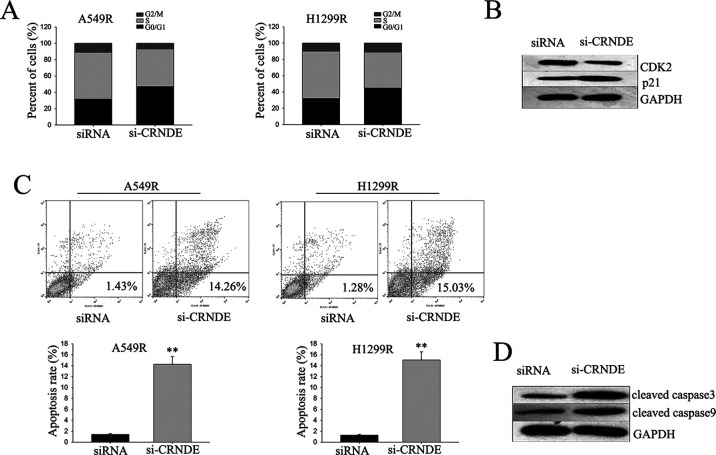
To investigate the mechanism of CRNDE-mediated radioresistance in LAD cells, flow cytometric analysis of apoptosis and cell cycle distribution was utilized. Knockdown of CRNDE in A549R cells and H1299R cells significantly caused cell cycle arrest at the G1 phase, with an obvious reduction in the number of cells in the S phase (Fig. 4A). Consistent with this result, Western blot revealed that reduction of CRNDE changed the level of CDK2 and p21 (Fig. 4B). Flow cytometric analysis of apoptosis was then performed to detect the function of CRNDE on apoptosis in radioresistant LAD cells. Deletion of CRNDE significantly increased the apoptosis rate of A549R and H1299R cells (Fig. 4C). Additionally, Western blot assay revealed that cleaved caspase 3 and cleaved caspase 9 were significantly increased in CRNDE knockdown A549R and H1299R cells (Fig. 4D). The data indicate that CRNDE contributed to the radioresistance of radioresistant LAD cells, which might be attributed to its influence on cell cycle and apoptosis.
Figure 4.
Knockdown of lncRNA CRNDE promotes cell cycle arrest at the G1 phase and causes cell apoptosis. (A, C) Flow cytometric analysis of cell cycle distribution (A) and apoptosis (C) was utilized to determine the effect of CRNDE on the cell cycle and apoptosis rate. (B, D) Western blot was performed to assess the function of CRNDE on p21 and CDK2 (B), and cleaved caspase 3 and cleaved caspase 9 (D). Data are represented as the mean ± SD from three independent experiments. The p value represents the comparison between groups (**p < 0.01).
lncRNA CRNDE Recruited EZH2 and Suppressed the Expression of p21
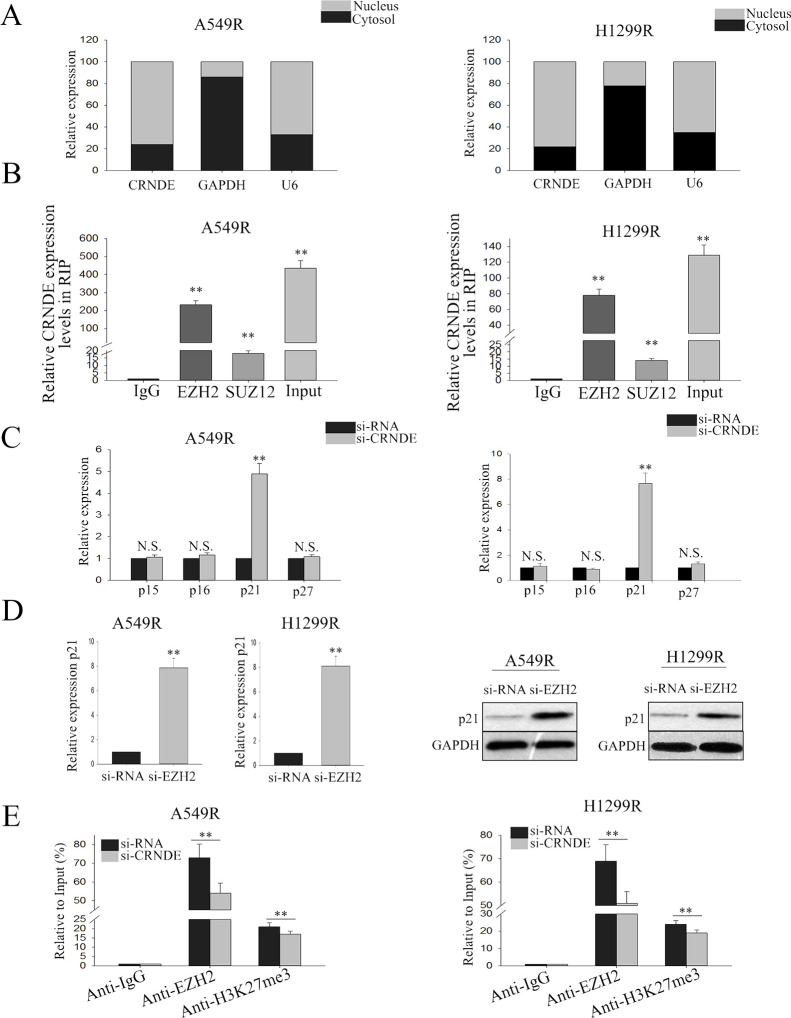
To examine the mechanism by which CRNDE exerted its function in radioresistant LAD cells, we first measured the percentage of CRNDE in the cytoplasmic and nuclear fractions of A549R and H1299R cells. RT-qPCR of nuclear and cytoplasmic fractions of A549R and H1299R cells shows that CRNDE was mainly located in the nucleus, indicating that CRNDE might be involved in transcriptional regulation (Fig. 5A). It has been described that lncRNA could interact with PRC224. Therefore, we detected the relationship between CRNDE and PRC2. RIP assay confirmed the combination of CRNDE and EZH2 (p < 0.01) (Fig. 5B). We then focused on the cell cycle-related target of EZH2, which might be involved in the contributions of CRNDE to the radioresistance of LAD cells. The RT-qPCR results showed that inhibition of CRNDE expression significantly increased the level of p21, and there was no significant difference in other genes (Fig. 5C). Furthermore, silencing EZH2 could increase the level of p21 at both the mRNA and protein levels (Fig. 5D). Additionally, ChIP assay revealed that CRNDE could recruit EZH2 to the promoter region of p21 and induce H3K27me3 modification, therefore suppressing the transcription of p21 (Fig. 5E). These findings indicate that CRNDE regulates its target through interacting with PRC2.
Figure 5.
lncRNA CRNDE recruits enhancer of zeste homolog 2 (EZH2) and suppresses the expression of p21. (A) RT-qPCR was performed to measure the level of CRNDE in nuclear and cytoplasmic fractions of A549R and H1299R. (B) RIP assay was performed to confirm the combination of CRNDE and EZH2. (C) The cell cycle-related target of EZH2 was measured in LAD radioresistant cells in response to CRNDE knockdown. (D) The level of p21 both in mRNA and protein was detected in response to EZH2 silencing. (E) Chromatin Immunoprecipitation (ChIP) assay revealed that CRNDE could recruit EZH2 to the promoter region of p21 and induces histone H3 lysine 27 trimethylation (H3K27me3) modification. All data are represented as the mean ± SD from three independent experiments. The p value represents the comparison between groups (**p < 0.01).
Oncogenic Function of CRNDE in Radioresistant LAD Cells Is in a p21-Dependent Manner
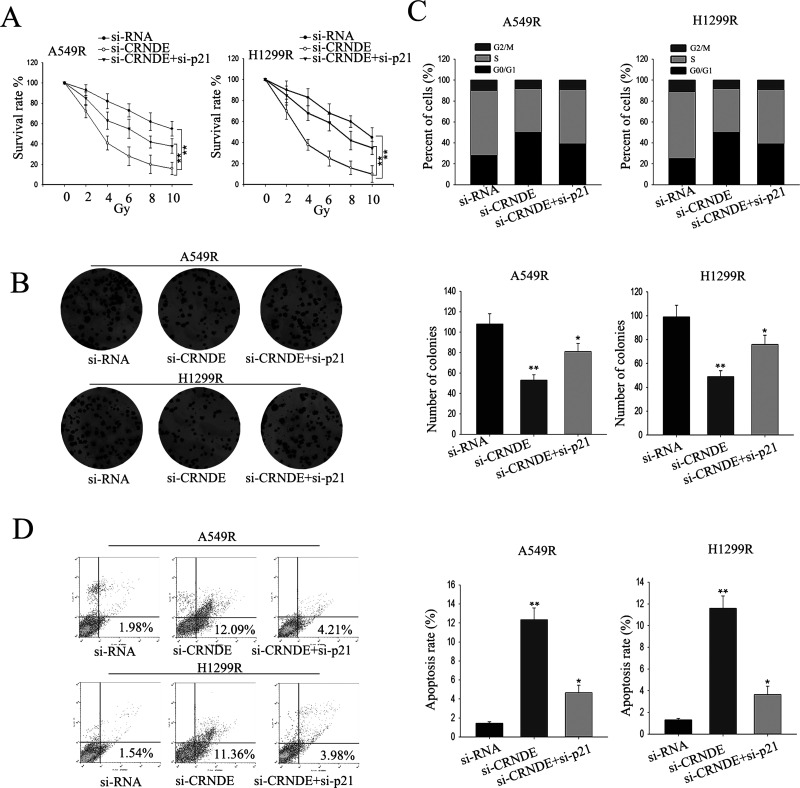
To further explore whether CRNDE exerted its function in radioresistant LAD cells in a p21-dependent manner, rescue assays were performed. A549R or H1299R cells were cotransfected with si-CRNDE and si-p21 and treated with different doses of ionizing radiation, from 0 to 10 Gy. Knockdown of CRNDE exhibited a dose-dependent inhibitory effect on growth, and such inhibition could be rescued by silencing p21 (Fig. 6A). Results from the colony formation assay indicated that cotransfection partially rescued the si-CRNDE-damaged proliferation ability (p < 0.05) (Fig. 6B). Moreover, flow cytometric analysis indicated that deletion of CRNDE caused G0/G1 phase arrest, and apoptosis increase could be abolished by silencing p21 (Fig. 6C and D). These results indicate that the effect of CRNDE on radioresistant LAD cells is partially through targeting p21.
Figure 6.
The oncogenic function of CRNDE in radioresistant LAD cells is in a p21-dependent manner. (A) MTT assay was performed to assess the sensitivity of LAD cells cotransfected with siRNA, si-CRNDE, and si-p21 to radiation. (B) Colony formation assays were performed to measure the proliferation ability of LAD cells cotransfected with siRNA, si-CRNDE, or si-p21. Flow cytometry analysis was performed to assess the cell cycle percentages (C) and apoptosis rate (D) of LAD cells cotransfected with siRNA, si-CRNDE, or si-p21. All data are represented as the mean ± SD from three independent experiments. The p value represents the comparison between groups (*p < 0.05, **p < 0.01).
DISCUSSION
It has been identified that intrinsic and/or acquired resistance to radiotherapy has been recognized as an important impediment for clinical cancer treatment25. Radioresistant cancer cells tend to obtain malignant behaviors and are partly responsible for the recurrence and metastasis of lung cancer after radiotherapy2,26–28. Currently, accumulating evidence has identified that lncRNAs play a critical role in tumorigenesis. Nonetheless, the precise underlying molecular mechanisms of LAD radiotherapy resistance still remain largely unclear. Herein, we uncovered a novel carcinogenic role for CRNDE in LAD.
In our present study, we demonstrated that CRNDE was significantly increased in LAD tissues and radioresistant LAD cells. In addition, increased expression of CRNDE in LAD patients is associated with poor differentiation, TNM stage, and lymph node metastasis, and also was significantly associated with therapeutic response. Gain- and loss-of-function tests demonstrated that CRNDE knockdown significantly increased the sensitivity of radioresistant LAD cells to ionizing radiation and caused a dramatic decrease in LAD cell colony formation, whereas forced CRNDE expression has the opposite result. Furthermore, deletion of CRNDE caused cell arrest in the G0/G1 phase and facilitated apoptosis rate. Our findings indicated that CRNDE acted as an oncogene in LAD radiotherapy resistance.
It has been documented that lncRNAs could serve as molecular guiding scaffolds in recruiting DNA, histone protein modification enzymes, or transcription factors to specific genomic loci, leading to inactivation of tumor suppressors or activation of oncogenes24,29–32, such as interacting with PRC233,34. To investigate whether CRNDE exerted its function through interacting with PRC2, we first measured the level of CRNDE in the nucleus and cytoplasm and found that CRNDE was mainly distributed in the nucleus, indicating that CRNDE may regulate transcriptional function. We then performed RIP and found that CRNDE could bind with EZH2, the critical component of PRC2. p21 is a tumor suppressor and has been reported to be related to cell cycle arrest and is downregulated in certain cancers35–37. In our study, we identified that p21 could be silenced by CRNDE, indicating that p21 may be the possible target of CRNDE. Furthermore, the results of the ChIP analysis demonstrated that EZH2 could directly bind to p21 promoter regions and induce H3K27me3 modification. Our findings reveal that CRNDE contributes to the radioresistant phenotype formation of LAD cells through regulating p21 expression by binding to EZH2.
In general, we first described that CRNDE expression was significantly increased in the LAD tissues and radioresistant LAD cell lines. CRNDE functioned as an oncogene through decreasing the sensitivity of LAD cells to ionizing radiation and causing a dramatic decrease in LAD cell colony formation through affecting the G1/S transition and suppressing cell apoptosis. Additionally, we proved that CRNDE exerts its function in LAD cells through epigenetically silencing p21 transcription by binding to EZH2. Our study provides new insights into the underlying mechanism of the radioresistant phenotype formation of LAD cells, which may be targeted for therapeutic benefits.
ACKNOWLEDGMENTS
The study was supported by the Technology and Applied Basic Research Program of Yunnan Province (Grant No. 2012FB165), the Health and Technology Program of Yunnan Province (Grant No. 2016NS010), and the Yunnan Provincial Doctoral Graduate Student Award.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Bae JM, Jeong JY, Lee HY, Sohn I, Kim HS, Son JY, Kwon OJ, Choi JY, Lee KS, Shim YM. Pathologic stratification of operable lung adenocarcinoma using radiomics features extracted from dual energy CT images. Oncotarget 2017;8:523–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Y, Li H, Peng W, He XY, Huang M, Qiu D, Xue YB, Lu L. DNA-dependent protein kinase catalytic subunit inhibitor reverses acquired radioresistance in lung adenocarcinoma by suppressing DNA repair. Mol Med Rep. 2015;12:1328–34. [DOI] [PubMed] [Google Scholar]
- 3. Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: Insights into functions. Nat Rev Genet 2009;10:155–9. [DOI] [PubMed] [Google Scholar]
- 4. Orom UA, Derrien T, Beringer M, Gumireddy K, Gardini A, Bussotti G, Lai F, Zytnicki M, Notredame C, Huang Q, Guigo R, Shiekhattar R. Long noncoding RNAs with enhancer-like function in human cells. Cell 2010;143:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen J, Zhang K, Song H, Wang R, Chu X, Chen L. Long noncoding RNA CCAT1 acts as an oncogene and promotes chemoresistance in docetaxel-resistant lung adenocarcinoma cells. Oncotarget 2016;7:62474–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu J, Wan L, Lu K, Sun M, Pan X, Zhang P, Lu B, Liu G, Wang Z. The long noncoding RNA MEG3 contributes to cisplatin resistance of human lung adenocarcinoma. PLoS One 2015;10:e0114586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sun CC, Li SJ, Li G, Hua RX, Zhou XH, Li DJ. Long intergenic noncoding RNA 00511 acts as an oncogene in non-small-cell lung cancer by binding to EZH2 and suppressing p57. Mol Ther Nucleic Acids 2016;5:e385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Terashima M, Tange S, Ishimura A, Suzuki T. MEG3 long noncoding RNA contributes to the epigenetic regulation of epithelial-mesenchymal transition in lung cancer cell lines. J Biol Chem. 2017;292:82–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu CZ, Jiang C, Wu Q, Liu L, Yan X, Shi R. A feed-forward regulatory loop between HuR and the long noncoding RNA HOTAIR promotes head and neck squamous cell carcinoma progression and metastasis. Cell Physiol Biochem. 2016;40:1039–51. [DOI] [PubMed] [Google Scholar]
- 10. Xu J, Zhang R, Zhao J. The novel long noncoding RNA TUSC7 inhibits proliferation by sponging MiR-211 in colorectal cancer. Cell Physiol Biochem. 2017;41:635–44. [DOI] [PubMed] [Google Scholar]
- 11. Zhou M, Ding WJ, Chen YW, Shen F, Zeng JY, Qu CY, Wei YF, Xu LM. Expression changes of long noncoding RNA in the process of endothelial cell activation. Cell Physiol Biochem. 2017;41:115–23. [DOI] [PubMed] [Google Scholar]
- 12. Zhang E, Han L, Yin D, He X, Hong L, Si X, Qiu M, Xu T, De W, Xu L, Shu Y, Chen J. H3K27 acetylation activated-long non-coding RNA CCAT1 affects cell proliferation and migration by regulating SPRY4 and HOXB13 expression in esophageal squamous cell carcinoma. Nucleic Acids Res. 2017;45:3086–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Conway E, Healy E, Bracken AP. PRC2 mediated H3K27 methylations in cellular identity and cancer. Curr Opin Cell Biol. 2015;37:42–8. [DOI] [PubMed] [Google Scholar]
- 14. Graham LD, Pedersen SK, Brown GS, Ho T, Kassir Z, Moynihan AT, Vizgoft EK, Dunne R, Pimlott L, Young GP, Lapointe LC, Molloy PL. Colorectal neoplasia differentially expressed (CRNDE), a novel gene with elevated expression in colorectal adenomas and adenocarcinomas. Genes Cancer 2011;2:829–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen Z, Yu C, Zhan L, Pan Y, Chen L, Sun C. LncRNA CRNDE promotes hepatic carcinoma cell proliferation, migration and invasion by suppressing miR-384. Am J Cancer Res. 2016;6:2299–309. [PMC free article] [PubMed] [Google Scholar]
- 16. Jing SY, Lu YY, Yang JK, Deng WY, Zhou Q, Jiao BH. Expression of long non-coding RNA CRNDE in glioma and its correlation with tumor progression and patient survival. Eur Rev Med Pharmacol Sci. 2016;20:3992–6. [PubMed] [Google Scholar]
- 17. Shao K, Shi T, Yang Y, Wang X, Xu D, Zhou P. Highly expressed lncRNA CRNDE promotes cell proliferation through Wnt/beta-catenin signaling in renal cell carcinoma. Tumour Biol. 2016. DOI: 10.1007/s13277-016-5440-0 [DOI] [PubMed] [Google Scholar]
- 18. Shen S, Liu H, Wang Y, Wang J, Ni X, Ai Z, Pan H, Liu H, Shao Y. Long non-coding RNA CRNDE promotes gallbladder carcinoma carcinogenesis and as a scaffold of DMBT1 and C-IAP1 complexes to activating PI3K-AKT pathway. Oncotarget 2016;7:72833–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Szafron LM, Balcerak A, Grzybowska EA, Pienkowska-Grela B, Podgorska A, Zub R, Olbryt M, Pamula-Pilat J, Lisowska KM, Grzybowska E, Rubel T, Dansonka-Mieszkowska A, Konopka B, Kulesza M, Lukasik M, Kupryjanczyk J. The putative oncogene, CRNDE, is a negative prognostic factor in ovarian cancer patients. Oncotarget 2015;6:43897–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dong R, Liu XQ, Zhang BB, Liu BH, Zheng S, Dong KR. Long non-coding RNA-CRNDE: A novel regulator of tumor growth and angiogenesis in hepatoblastoma. Oncotarget 2017;8:42087–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, van Oudenaarden A, Regev A, Lander ES, Rinn JL. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci USA 2009;106:11667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guo W, Xie L, Zhao L, Zhao Y. mRNA and microRNA expression profiles of radioresistant NCI-H520 non-small cell lung cancer cells. Mol Med Rep. 2015;12:1857–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang SZ, Pan FY, Xu JF, Yuan J, Guo SY, Dai G, Xue B, Shen WG, Wen CJ, Zhao DH, Li CJ. Knockdown of c-Met by adenovirus-delivered small interfering RNA inhibits hepatocellular carcinoma growth in vitro and in vivo. Mol Cancer Ther. 2005;4:1577–84. [DOI] [PubMed] [Google Scholar]
- 24. Sun M, Nie F, Wang Y, Zhang Z, Hou J, He D, Xie M, Xu L, De W, Wang Z, Wang J. LncRNA HOXA11-AS promotes proliferation and invasion of gastric cancer by scaffolding the chromatin modification factors PRC2, LSD1, and DNMT1. Cancer Res. 2016;76:6299–310. [DOI] [PubMed] [Google Scholar]
- 25. Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer 2008;8:545–54. [DOI] [PubMed] [Google Scholar]
- 26. Keta O, Bulat T, Golic I, Incerti S, Korac A, Petrovic I, Ristic-Fira A. The impact of autophagy on cell death modalities in CRL-5876 lung adenocarcinoma cells after their exposure to gamma-rays and/or erlotinib. Cell Biol Toxicol. 2016;32:83–101. [DOI] [PubMed] [Google Scholar]
- 27. Su YL, Rau KM. Adding bevacizumab to chemotherapy effectively control radioresistant brain metastases in ALK-positive lung adenocarcinoma. J Thorac Oncol. 2015;10:e21–22. [DOI] [PubMed] [Google Scholar]
- 28. Gomez-Casal R, Epperly MW, Wang H, Proia DA, Greenberger JS, Levina V. Radioresistant human lung adenocarcinoma cells that survived multiple fractions of ionizing radiation are sensitive to HSP90 inhibition. Oncotarget 2015;6:44306–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang M, Hou J, Wang Y, Xie M, Wei C, Nie F, Wang Z, Sun M. Long noncoding RNA LINC00673 is activated by SP1 and exerts oncogenic properties by interacting with LSD1 and EZH2 in gastric cancer. Mol Ther. 2017;25:1014–26. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30. Zhang Y, He Q, Hu Z, Feng Y, Fan L, Tang Z, Yuan J, Shan W, Li C, Hu X, Tanyi JL, Fan Y, Huang Q, Montone K, Dang CV, Zhang L. Long noncoding RNA LINP1 regulates repair of DNA double-strand breaks in triple-negative breast cancer. Nat Struct Mol Biol. 2016;23:522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun TT, He J, Liang Q, Ren LL, Yan TT, Yu TC, Tang JY, Bao YJ, Hu Y, Lin Y, Sun D, Chen YX, Hong J, Chen H, Zou W, Fang JY. LncRNA GClnc1 promotes gastric carcinogenesis and may act as a modular scaffold of WDR5 and KAT2A complexes to specify the histone modification pattern. Cancer Discov. 2016;6:784–801. [DOI] [PubMed] [Google Scholar]
- 32. Li Y, Wang Z, Shi H, Li H, Li L, Fang R, Cai X, Liu B, Zhang X, Ye L. HBXIP and LSD1 scaffolded by lncRNA hotair mediate transcriptional activation by c-Myc. Cancer Res. 2016;76:293–304. [DOI] [PubMed] [Google Scholar]
- 33. Lin PC, Huang HD, Chang CC, Chang YS, Yen JC, Lee CC, Chang WH, Liu TC, Chang JG. Long noncoding RNA TUG1 is downregulated in non-small cell lung cancer and can regulate CELF1 on binding to PRC2. BMC Cancer 2016;16:583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Portoso M, Ragazzini R, Brencic Z, Moiani A, Michaud A, Vassilev I, Wassef M, Servant N, Sargueil B, Margueron R. PRC2 is dispensable for HOTAIR-mediated transcriptional repression. EMBO J. 2017;36:981–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang ST, Ho HJ, Lin JT, Shieh JJ, Wu CY. Simvastatin-induced cell cycle arrest through inhibition of STAT3/SKP2 axis and activation of AMPK to promote p27 and p21 accumulation in hepatocellular carcinoma cells. Cell Death Dis. 2017;8:e2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Biswas K, Sarkar S, Du K, Brautigan DL, Abbas T, Larner JM. The E3 ligase CHIP mediates p21 degradation to maintain radioresistance. Mol Cancer Res. 2017;15:651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fragou A, Tzimagiorgis G, Karageorgopoulos C, Barbetakis N, Lazopoulos A, Papaioannou M, Haitoglou C, Kouidou S. Increased delta133p53 mRNA in lung carcinoma corresponds with reduction of p21 expression. Mol Med Rep. 2017;15:1455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]