Abstract
HIF-2α knockdown inhibits proliferation, arrests the cell cycle, and promotes apoptosis and autophagy under hypoxic conditions in cervical cancer. However, the upstream regulatory mechanism of HIF-2α expression is unclear. MicroRNAs (miRNAs) degrade target mRNAs by binding to the 3′-untranslated region of mRNAs. In this study, we investigated the role of miRNAs in the regulation of HIF-2α expression in cervical cancer under hypoxic conditions. miRNAs regulating HIF-2α expression were predicted using TargetScan and miRanda and were determined in cervical cancer under hypoxic conditions by qRT-PCR. Additionally, the targeted regulation of HIF-2α by miR-519d-3p was evaluated by Western blot and luciferase reporter assays. Effects of miR-519d-3p and HIF-2α on cell proliferation, cell cycle, and apoptosis were analyzed by CCK-8 and flow cytometry assays, respectively. miR-106a-5p, miR-17-5p, miR-519d-3p, miR-526b-3p, and miR-20b-5p are potentially regulatory miRNAs that bound to the HIF-2α 3′-untranslated region as per TargetScan and miRanda predictions. Expression of the five miRNAs was inhibited in HeLa cells under hypoxic conditions compared to normoxic conditions, and the expression of miR-519d-3p was lower than that of other miRNAs. Luciferase reporter assays showed that HIF-2α was a target of miR-519d-3p. Additionally, miR-519d-3p overexpression inhibited cell proliferation, arrested the cell cycle transition from the G1 stage to the S stage, and promoted cell apoptosis under hypoxic conditions in cervical cancer. HIF-2α overexpression partially reversed the effect of miR-519d-3p. In conclusion, miR-519d-3p overexpression suppressed proliferation, inhibited the cell cycle, and promoted apoptosis of HeLa cells by targeting HIF-2α under hypoxic conditions.
Key words: MicroRNAs (miRNAs), Hypoxia-inducible factor-2α (HIF-2α), Cervical cancer, Cell hypoxia
INTRODUCTION
Cervical cancer is the second most commonly diagnosed cancer and third leading cause of mortality with an estimated 527,600 new cervical cancer cases and 265,700 mortalities worldwide in 20121. Although most patients can be cured with treatments involving surgery and radiotherapy, other patients show recurrence, leading to death. Therefore, studies of the mechanism of the development of cervical cancer contribute to the discovery of novel targets. Hypoxia, a reduction in tissue oxygen tension due to inadequate oxygen supply, is a common phenomenon that occurs in most solid tumors in humans2. The presence of hypoxia is associated with aggressive tumor progression, resistance to chemotherapy and radiation, and poor prognosis3,4.
Hypoxia-inducible factor (HIF) is one of the principal response factors under hypoxic conditions. It is a heterodimeric basic helix–loop–helix PAS domain-containing transcription factor that consists of a constitutively expressed β-subunit (HIF-β/ARNT) and one of the three oxygen-regulated α-subunits (HIF-1α, HIF-2α, and HIF-3α)5. HIF-2α is highly related to HIF-1α, but HIF-2α can activate different target genes such as Cited-2, EPO, and PAI-1 compared with HIF-1α6,7. In our previous study, we found that HIF-2α expression was significantly higher under hypoxic conditions than under normoxic conditions and that HIF-2α knockdown inhibited cervical cancer proliferation, arrested cell cycle progression, and promoted apoptosis and autophagy under hypoxic conditions8. These results suggest that HIF-2α has as an oncogene role. However, the potentially upstream regulatory mechanism of HIF-2α expression is unclear.
MicroRNAs (miRNAs) degrade their target mRNA sequences or inhibit translation by binding to the 3′-untranslated region (3′-UTR) of mRNAs9,10. In cervical cancer, previous studies demonstrated that miRNAs regulate growth, cycle, apoptosis, migration, invasion, angiopoiesis, and cell adhesion and play an important role in cancer occurrence and progression11–13. Thus, we speculated that a special miRNA can regulate the expression of HIF-2α in cervical cancer under hypoxic conditions by binding the HIF-2α 3′-UTR.
In this study, we predicted the miRNAs that bind the HIF-2α 3′-UTR using TargetScan and miRanda. Additionally, we analyzed the effect of the miRNAs on the proliferation and apoptosis of cervical cancer cells and targeting regulation of miRNA to HIF-2α in cervical cancer under hypoxic conditions.
MATERIALS AND METHODS
Cell Culture and Hypoxic Exposure
The HeLa human cervical cancer cell line was purchased from the American Type Culture Collection (Manassas, VA, USA). HeLa cells were cultured in RPMI-1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco), 100 U/ml penicillin G, and 100 μg/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA). The cells were maintained at 37°C in a humidified 5% CO2 incubator. To expose cells to hypoxic conditions, the cells were cultured in a Billups-Rothenburg chamber with 94% N2, 1% O2, and 5% CO2 at 37°C.
RNA Extraction and Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and reverse transcribed into cDNA using a miRcute miRNA first-strand cDNA synthesis kit (TIANGEN Biotech, Beijing, P.R. China) and PrimeScript RT Reagent Kit with gDNA Eraser (TIANGEN). miRNA expression was detected by qPCR using a miRcute miRNA qPCR detection kit (SYBR® Green; TIANGEN) with U6 as an internal reference. HIF-2α expression was evaluated by qPCR using Power SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. qPCR was performed on a 7500 Real-Time PCR system (Applied Biosystems) and conducted under the following conditions: 95°C for 5 min followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. Gene expression was measured in triplicate, quantified using the 2−ΔΔCT method, and normalized to an internal control. Primer sequences used for RT-qPCR are presented in Table 1.
Table 1.
Primer Sequences Used for RT-qPCR
| miRNA | 5′–3′ |
| miR-106a-5p-F | ACACTCCAGCTGGGAAAAGTGCTTACAGTGCAGG |
| miR-17-5p-F | ACACTCCAGCTGGGCAAAGTGCTTACAGTGCAGG |
| miR-519d-3p-F | ACACTCCAGCTGGGCAAAGTGCCTCCCTTTAGAG |
| miR-526b-3p-F | ACACTCCAGCTGGGGAAAGTGCTTCCTTTTAGAG |
| miR-20b-5p-F | ACACTCCAGCTGGGCAAAGTGCTCATAGTGCAG |
| miRNA-R* | CTCAACTGGTGTCGTGGA |
| U6-F | CTCGCTTCGGCAGCACA |
| U6-R | AACGCTTCACGAATTTGCGT |
General downstream primer.
Transfection With miR-519d-3p Mimic and HIF-2α
miR-519d-3p mimic (5′-UUU GUU CGU UCG GCU CGC GUG A-3′) and negative control (NC; 5′-UCA CAA CCU CCU AGA AAG AGU AGA-3′) were purchased from GenePharma Co., Ltd. (Shanghai, P.R. China). Cells were plated at 50% confluence and transfected with 300 nM NC or miR-519d-3p mimic using Lipofectamine® 2000 reagent (Invitrogen) according to the manufacturer’s protocol. To examine the relationship between miR-519d-3p and HIF-2α, full-length HIF-2α was cloned and inserted into the pcDNA3.1 expression plasmid (pcDNA-HIF-2α; Promega, Madison, WI, USA). Cells were plated at 50% confluence and cotransfected with 300 nM miR-519d-3p mimic and 10 μM pcDNA3.1 (miR-519d-3p mimic + pcDNA3.1) or pcDNA-HIF-2α (miR-519d-3p mimic + pcDNA-HIF-2α) using Lipofectamine® 2000 reagent (Invitrogen) according to the manufacturer’s protocol.
Proliferation, Apoptosis, and Cell Cycle Assays
For the proliferation assay, a CCK-8 assay kit reagent (Beyotime, Shanghai, P.R. China) was used, and the assay was performed according to the manufacturer’s instructions. Absorbance was measured at 450 nm with a microplate reader. For the cell apoptosis assay, an Annexin-V-allophycocyanin apoptosis detection kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing, P.R. China) was used to detect the apoptotic rate according to the manufacturer’s instructions. For cell cycle analysis, cell cycle detection kits (Nanjing KeyGen Biotech Co., Ltd.) were used to evaluate the cell cycle according to the manufacturer’s instructions, and the cell cycle distribution was determined using the ModFit LT 3.0 program (BD Biosciences, Franklin Lakes, NJ, USA). Cell apoptosis and cell cycle were detected by flow cytometry (BD Biosciences). Each experiment was repeated three times.
Western Blot Assay
HeLa cells were lysed in ice-cold RIPA buffer (Beyotime Institute of Biotechnology, Nantong, P.R. China) containing 1 mM phenylmethylsulfonyl fluoride and a cocktail of protease inhibitors (1:100; Beyotime Institute of Biotechnology) and then centrifuged at 4°C for 15 min at 800 × g. The total protein in supernatants was quantified using a BCA Protein Assay Kit (Pierce Biotechnology, Waltham, MA, USA). Next, 30 μg of protein was loaded and separated by 12% SDS-PAGE and transferred to polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). The membranes were blocked for 1 h at room temperature with 5% milk in Tris-buffered saline containing Tween 20 (TBST) and incubated at 37°C for 1 h with anti-HIF-2α mouse monoclonal antibody (dilution, 1:2,000; Cat. No. ab157249) and anti-GAPDH mouse monoclonal antibody (dilution, 1:5,000; Cat. No. ab8245) (both from Abcam, Cambridge, UK). The membranes were washed three times with TBST and incubated with horseradish peroxidase-conjugated mouse anti-rabbit IgG (1:2,000; Cat. No. BM2006) or horseradish peroxidase-conjugated goat anti-mouse IgG (1:3,000; Cat. No. BA1051) (both from Wuhan Boster Biological Technology, Ltd., Wuhan, P.R. China) for 40 min at 37°C. The membranes were then washed three times with TBST and visualized using Immobilon Western Chemilum horseradish peroxidase substrate (EMD Millipore). GAPDH served as an internal loading control. Blot images were scanned, and densitometric analysis was performed using Image Pro-Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA). Each experiment was repeated three times.
Reporter Vector Construction and Luciferase Reporter Assay
TargetScan and miRanda were used to predict regulated miRNAs of HIF-2α. To determine whether the 3′-UTR of HIF-2α mRNA was a direct target of miR-519d-3p, the full-length wild-type (WT) 3′-UTR and mutant (Mut) 3′-UTR of HIF-2α were amplified and cloned into the psi-CHECK-2 vector (Promega). HeLa cells were cotransfected with 100 ng of plasmid (WT-3′-UTR-psi-CHECK-2 or Mut-3′-UTR-psi-CHECK-2) and 200 nM miR-519d-3p mimic or NC in 24-well plates. Cell lysates were harvested 48 h after transfection. The firefly and Renilla luciferase fluorescence intensities were measured using the Dual-Luciferase Reporter Assay System (Promega), and experiments were performed in triplicate. The luciferase activity was normalized to Renilla luciferase activity.
Statistical Analyses
All statistical analyses were performed using SPSS software, version 19.0 (SPSS, Inc., Chicago, IL, USA). Data are expressed as the mean ± standard deviation. Statistical comparisons were performed using one-way analysis of variance, followed by Scheffe’s test. Differences between two groups were determined using unpaired Student’s t-test. A value of p < 0.05 was considered to indicate a statistically significant difference.
RESULTS
Expression of miRNA Targeting HIF-2α Under Hypoxic Exposure
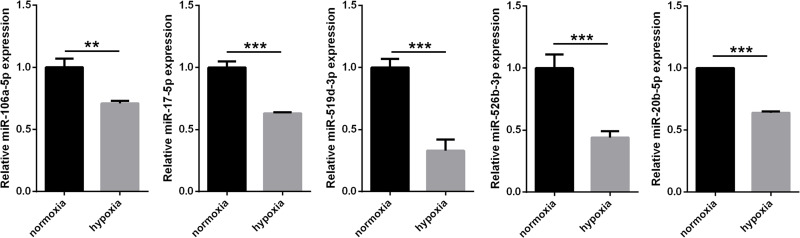
To evaluate the upstream mechanism underlying HIF-2α-mediated proliferation and apoptosis of HeLa cells, the potential regulation of miRNAs was identified using the prediction algorithms of TargetScan and miRanda. The results showed that miR-106a-5p, miR-17-5p, miR-519d-3p, miR-526b-3p, and miR-20b-5p potentially regulate miRNAs by binding to the HIF-2α 3′-UTR. Additionally, the expressions of miR-106a-5p, miR-17-5p, miR-519d-3p, miR-526b-3p, and miR-20b-5p in HeLa cells under hypoxic or normoxic conditions were measured by RT-qPCR. The results showed that the expressions of miR-106a-5p, miR-17-5p, miR-519d-3p, miR-526b-3p, and miR-20b-5p in HeLa cells were significantly decreased under hypoxic conditions compared to normoxic conditions (Fig. 1). The expression of miR-519d-3p was lower than that of miR-106a-5p, miR-17-5p, miR-526b-3p, and miR-20b-5p (Fig. 1). Based on these results, miR-519d-3p was selected for further analysis.
Figure 1.
Expression of miR-106a-5p, miR-17-5p, miR-519d-3p, miR-526b-3p, and miR-20b-5p in HeLa cells under hypoxic or normoxic conditions as measured by reverse transcription quantitative polymerase chain reaction (RT-qPCR). Data are presented as the mean ± standard deviation. **p < 0.01 and ***p < 0.001 versus normoxic conditions.
miR-519d-3p Overexpression Inhibits Proliferation and Cell Cycle and Promotes Apoptosis in HeLa Cells Under Hypoxic Conditions
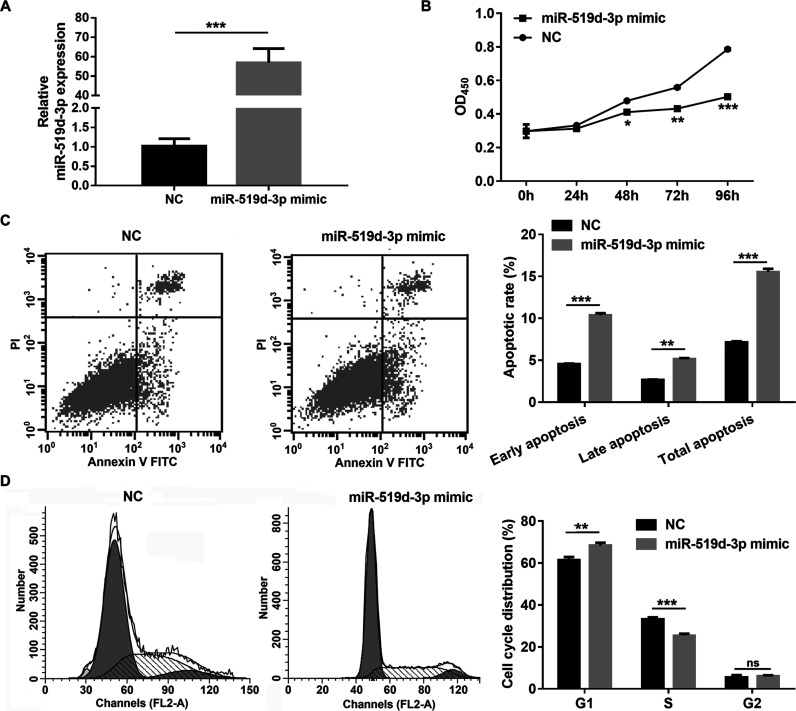
To assess the role of miR-519d-3p in regulating cell proliferation and apoptosis under hypoxic conditions, miR-519d-3p mimic or NC was transfected into HeLa cells. The RT-qPCR results showed that miR-519d-3p expression was significantly enhanced in HeLa cells transfected with miR-519d-3p mimic compared to HeLa cells transfected with NC at 48 h posttransfection under hypoxic conditions (Fig. 2A). Additionally, cell proliferation and the cell cycle were significantly inhibited, and apoptosis was promoted in HeLa cells transfected with the miR-519d-3p mimic compared to HeLa cells transfected with NC under hypoxic conditions (Fig. 2B–D).
Figure 2.
miR-519d-3p overexpression inhibits proliferation and cell cycle and promotes apoptosis in HeLa cells under hypoxic conditions. (A) Expression levels of miR-519d-3p were measured by RT-qPCR following transfection with a miR-519d-3p mimic or negative control (NC) for 48 h under hypoxic conditions. (B) Inhibited proliferation of HeLa cells by miR-519d-3p overexpression by CCK-8 analyzed under hypoxic conditions. (C) Promoted apoptosis of HeLa cells by miR-519d-3p overexpression by flow cytometry analyzed under hypoxic conditions. (D) Inhibition of HeLa cell cycle by miR-519d-3p overexpression by flow cytometry analyzed under hypoxic conditions. Data are presented as the mean ± standard deviation. *p < 0.05, **p < 0.01, ***p < 0.001, and ns (not significant) versus NC.
miR-519d-3p Regulates HIF-2α Expression Levels by Targeting its 3′-UTR
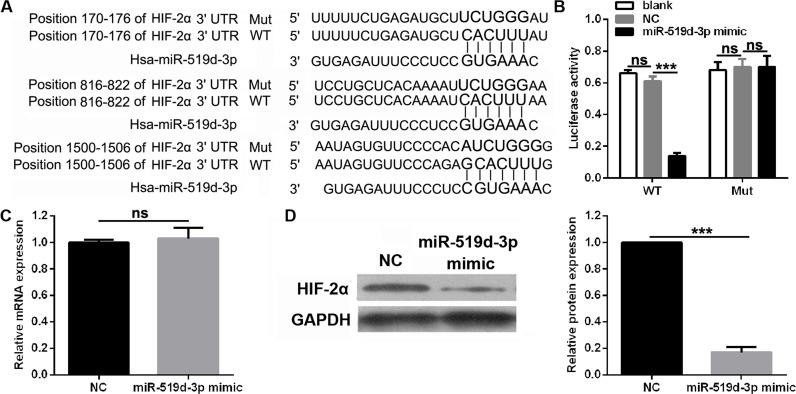
HIF-2α was a potential target of miR-519d-3p based on the putative binding site at positions 170–176, 816–822, and 1,500–1,506 of the HIF-2α 3′-UTR according to the TargetScan and miRanda analyses (Fig. 3A). Results of the luciferase reporter assays revealed a significantly decreased level of luciferase activity in cells transfected with the HIF-2α-WT vector and miR-519d-3p, while no significant change was observed in cells transfected with HIF-2α-Mut vector and miR-519d-3p (Fig. 3B). The effects of miR-519d-3p overexpression on the HIF-2α mRNA and protein levels under hypoxic conditions were examined. As expected, miR-519d-3p overexpression did not change the mRNA level of HIF-2α (Fig. 3C) but significantly inhibited the protein level of HIF-2α under hypoxic conditions (Fig. 3D).
Figure 3.
miR-519d-3p regulates HIF-2α expression levels via targeting of its 3′-untranslated region (3′-UTR). (A) The putative binding site between HIF-2α 3′-UTR and miR-519d-3p was analyzed by TargetScan and miRanda. (B) Luciferase activity of wild-type (WT) or mutant (Mut) HIF-2α 3′-UTR in 293T cells transfected with miR-519d-3p mimic or NC (negative control). (C) HIF-2α mRNA in HeLa cells transfected with miR-519d-3p mimic or NC under hypoxic conditions was measured by RT-qPCR. (D) HIF-2α mRNA in HeLa cells transfected with miR-519d-3p mimic or NC under hypoxic conditions was measured by Western blotting. Data are presented as the mean ± standard deviation. ***p < 0.001 and ns versus NC.
HIF-2α Overexpression Partially Reverses the miR-519d-3p-Induced Effects on the Proliferation and Apoptosis of HeLa Cells Under Hypoxic Conditions
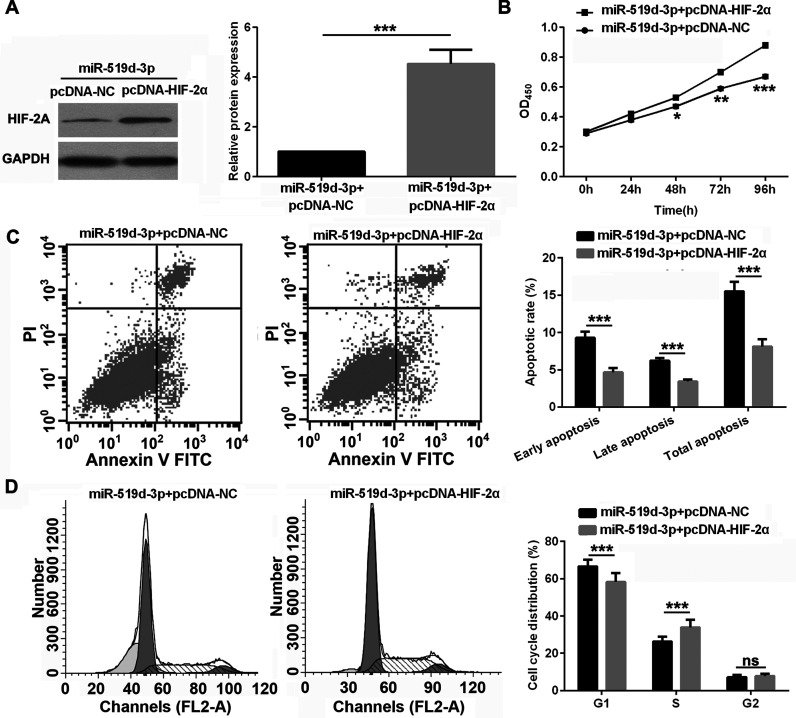
To determine whether miR-519d-3p contributes to the proliferation and apoptosis of HeLa cells via HIF-2α, HeLa cells were cotransfected with the miR-519d-3p mimic and pcDNA-HIF-2α vector. The expression of HIF-2α was significantly increased in HeLa cells cotransfected with the miR-519d-3p mimic and pcDNA-HIF-2α vector compared to HeLa cells cotransfected with the miR-519d-3p mimic and pcDNA vector under hypoxic conditions (Fig. 4A). HIF-2α overexpression promoted proliferation and the cell cycle from G1 to S and inhibited apoptosis of HeLa cells under hypoxic conditions (Fig. 4B–D), partially reversing the effect of miR-519d-3p on the proliferation, cell cycle, and apoptosis of HeLa cells under hypoxic conditions.
Figure 4.
HIF-2α overexpression partially reverses the miR-519d-3p-induced effects on the proliferation and apoptosis of HeLa cells under hypoxic conditions. (A) The expression of HIF-2α was measured by Western blot assay after cotransfection with miR-519d-3p mimic and pcDNA-NC or pcDNA-HIF-2α under hypoxic conditions. (B–D) Proliferation was significantly enhanced (B), apoptosis was significantly decreased (C), and cell cycle from G1 to S was significantly promoted (D) in HeLa cells cotransfected with miR-519d-3p mimic and pcDNA-HIF-2α compared to Hela cells cotransfected with miR-519d-3p mimic and pcDNA-NC under hypoxic conditions. Data are presented as the mean ± standard deviation. *p < 0.05, **p < 0.01, ***p < 0.001, and ns versus NC.
DISCUSSION
HIF-2α plays an important role in cancer occurrence and progression. Previous studies showed that HIF-2a expression is significantly increased in colon cancer, oral squamous cell carcinoma, pancreatic ductal adenocarcinoma, and other cancers14–16. High expression of HIF-2α promotes cancer proliferation, metastasis, and angiogenesis, as well as regulating glutamine metabolism16–18. However, the potential upstream regulatory mechanism of HIF-2α expression remains unclear. In this study, we found that miR-519d-3p was downregulated in cervical cancer cell under hypoxic conditions compared to normoxic conditions, and inhibited proliferation and promoted apoptosis under hypoxic conditions by targeting HIF-2α in cervical cancer.
miRNAs degrade their target mRNA sequences by binding to the 3′-UTR of mRNA and play an important role in the occurrence and progression of cervical cancer11–13. In this study, miR-106a-5p, miR-17-5p, miR-519d-3p, miR-526b-3p, and miR-20b-5p were implicated as potential regulator miRNAs that bind the HIF-2α 3′-UTR based on the prediction algorithms of TargetScan and miRanda. The expression of the five miRNAs was inhibited in HeLa cells under hypoxic conditions compared to normoxic conditions, and the expression of miR-519d-3p was lower than the expressions of the other miRNAs. A previous study demonstrated the obvious downregulation of miR-519d-3p in human hepatocellular carcinoma cells19, ovarian cancer cells20, and gastric cancer21, similar to the results of our study.
In hepatocellular carcinoma cells, miR-519d-3p suppresses cell growth by targeting MKi6719. In colorectal cancer, miR-519d-3p inhibits oncogenicity and promotes apoptosis by targeting C14orf2822. In lung adenocarcinoma, overexpression of miR-519d-3p inhibits cell proliferation by targeting eIF4H23. A previous study demonstrated that miR-519d-3p is associated with cancer cell growth and apoptosis. Our results suggest that overexpression of miR-519d-3p inhibits cell proliferation, arrests the cell cycle from the G1 to S stage, and promotes cell apoptosis under hypoxic conditions in cervical cancer, which are similar to the results of previous studies. Additionally, luciferase reporter assays showed that HIF-2α was a target of miR-519d-3p. Furthermore, miR-519d-3p overexpression did not change the mRNA level of HIF-2α but significantly inhibited the protein level of HIF-2α under hypoxic conditions. The results suggest that miR-519d-3p does not degrade the mRNA of HIF-2α but inhibits the translation of HIF-2α by binding to the 3′-UTR of mRNA under hypoxic conditions. Finally, HIF-2α overexpression promoted proliferation and cell cycle progression from G1 to S, as well as inhibited apoptosis in transfected miR-519d-3p mimic HeLa cells under hypoxic conditions, partially reversing the effects of miR-519d-3p on the proliferation, cell cycle, and apoptosis of HeLa cells under hypoxic conditions. These results indicate that miR-519d-3p suppresses cell proliferation and the cell cycle and promotes the apoptosis of HeLa cells by targeting HIF-2α under hypoxic conditions.
In conclusion, miR-519d-3p is expressed at a low level in HeLa cells under hypoxic conditions. Its overexpression suppresses cell proliferation and the cell cycle and promotes the apoptosis of HeLa cells by targeting HIF-2α under hypoxic conditions. These results implicate miR-519d-3p as a potential target for the treatment of cervical cancer.
ACKNOWLEDGMENTS
This work is supported by the National Natural Science Foundation of China (No. 81760472) and the Natural Science Foundation of Jiangxi, P.R. China (No. 20171BAB205066).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 2. Li SH, Shin DH, Chun YS, Lee MK, Kim MS, Park JW. A novel mode of action of YC-1 in HIF inhibition: Stimulation of FIH-dependent p300 dissociation from HIF-1{alpha}. Mol Cancer Ther. 2008;7(12):3729–38. [DOI] [PubMed] [Google Scholar]
- 3. Lou JJ, Chua YL, Chew EH, Gao J, Bushell M, Hagen T. Inhibition of hypoxia-inducible factor-1alpha (HIF-1alpha) protein synthesis by DNA damage inducing agents. PLoS One 2010;5(5):e10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang J, Cao J, Weng Q, Wu R, Yan Y, Jing H, Zhu H, He Q, Yang B. Suppression of hypoxia-inducible factor 1alpha (HIF-1alpha) by tirapazamine is dependent on eIF2alpha phosphorylation rather than the mTORC1/4E-BP1 pathway. PLoS One 2010;5(11):e13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pugh CW. Modulation of the hypoxic response. Adv Exp Med Biol. 2016;903:259–71. [DOI] [PubMed] [Google Scholar]
- 6. Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11(1):72–82. [DOI] [PubMed] [Google Scholar]
- 7. Hu CJ, Sataur A, Wang L, Chen H, Simon MC. The N-terminal transactivation domain confers target gene specificity of hypoxia-inducible factors HIF-1alpha and HIF-2alpha. Mol Biol Cell 2007;18(11):4528–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jiang LX, Shi SH, Shi QF, Zhang HJ, Hu R, Wang MZ. Similarity in the functions of HIF 1α and HIF 2α proteins in cervical cancer cells. Oncol Lett. 2017;17:5643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004;116(2):281–97. [DOI] [PubMed] [Google Scholar]
- 10. Graves P, Zeng Y. Biogenesis of mammalian microRNAs: A global view. Genomics Proteomics Bioinformatics 2012;10(5):239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li S, Yang F, Wang M, Cao W, Yang Z. miR-378 functions as an onco-miRNA by targeting the ST7L/Wnt/beta-catenin pathway in cervical cancer. Int J Mol Med. 2017;40(4):1047–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang X, Xie Y, Wang J. Overexpression of microRNA-34a-5p inhibits proliferation and promotes apoptosis of human cervical cancer cells by downregulation of Bcl-2. Oncol Res. 2017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liang H, Luo R, Chen X, Zhao Y, Tan A. miR-187 inhibits the growth of cervical cancer cells by targeting FGF9. Oncol Rep. 2017;38(4):1977–84. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14. Lim E, Kuo CC, Tu HF, Yang CC. The prognosis outcome of oral squamous cell carcinoma using HIF-2alpha. J Chin Med Assoc. 2017;80(10):651–6. [DOI] [PubMed] [Google Scholar]
- 15. Vandyke K, Zeissig MN, Hewett DR, Martin SK, Mrozik KM, Cheong CM, Diamond P, To LB, Gronthos S, Peet DJ, Croucher PI, Zannettino ACW. HIF-2alpha promotes dissemination of plasma cells in multiple myeloma by regulating CXCL12/CXCR4 and CCR1. Cancer Res. 2017;30(10):5452–63. [DOI] [PubMed] [Google Scholar]
- 16. Gao ZJ, Wang Y, Yuan WD, Yuan JQ, Yuan K. HIF-2alpha not HIF-1alpha overexpression confers poor prognosis in non-small cell lung cancer. Tumour Biol. 2017;39(6):1010428317709637. [DOI] [PubMed] [Google Scholar]
- 17. Ma X, Zhang H, Xue X, Shah YM. Hypoxia-inducible factor 2alpha (HIF-2alpha) promotes colon cancer growth by potentiating Yes-associated protein 1 (YAP1) activity. J Biol Chem. 2017;292(41):17046–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lai XM, Liu SY, Tsai YT, Sun GH, Chang SY, Huang SM, Cha TL. HAF mediates the evasive resistance of anti-angiogenesis TKI through disrupting HIF-1alpha and HIF-2alpha balance in renal cell carcinoma. Oncotarget 2017;8(30):49713–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hou YY, Cao WW, Li L, Li SP, Liu T, Wan HY, Liu M, Li X, Tang H. MicroRNA-519d targets MKi67 and suppresses cell growth in the hepatocellular carcinoma cell line QGY-7703. Cancer Lett. 2011;307(2):182–90. [DOI] [PubMed] [Google Scholar]
- 20. Pang Y, Mao H, Shen L, Zhao Z, Liu R, Liu P. MiR-519d represses ovarian cancer cell proliferation and enhances cisplatin-mediated cytotoxicity in vitro by targeting XIAP. Onco Targets Ther. 2014;7:587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yue H, Tang B, Zhao Y, Niu Y, Yin P, Yang W, Zhang Z, Yu P. MIR-519d suppresses the gastric cancer epithelial-mesenchymal transition via Twist1 and inhibits Wnt/beta-catenin signaling pathway. Am J Transl Res. 2017;9(8):3654–64. [PMC free article] [PubMed] [Google Scholar]
- 22. Yang X, Hu Y, Liu Y, Liu W, Zhao X, Liu M, Tang H. C14orf28 downregulated by miR-519d contributes to oncogenicity and regulates apoptosis and EMT in colorectal cancer. Mol Cell Biochem. 2017;434(1–2):197–208. [DOI] [PubMed] [Google Scholar]
- 23. Bai Y, Lu C, Zhang G, Hou Y, Guo Y, Zhou H, Ma X, Zhao G. Overexpression of miR-519d in lung adenocarcinoma inhibits cell proliferation and invasion via the association of eIF4H. Tumour Biol. 2017;39(3):1010428317694566. [DOI] [PubMed] [Google Scholar]