Abstract
B7-homolog 4 (B7-H4), a member of the B7 family of costimulatory molecules, has been reported to be upregulated in urothelial cell carcinoma. This study was conducted to explore the biological role of B7-H4 in the aggressiveness of bladder cancer and the associated molecular mechanism. We found that the mRNA and protein levels of B7-H4 were significantly greater in bladder cancer cell lines than in SV-HUC-1 (normal human urothelial cells). Overexpression of B7-H4 significantly promoted bladder cancer cell migration and invasion, whereas knockdown of B7-H4 exerted an opposite effect. However, the growth of bladder cancer cells was not altered by B7-H4 overexpression or knockdown. Overexpression of B7-H4 promoted epithelial–mesenchymal transition (EMT), as evidenced by decreased E-cadherin and increased vimentin expression. The EMT inducers Twist1 and Snail were upregulated by B7-H4 overexpression and downregulated by B7-H4 silencing. Mechanistically, overexpression of B7-H4 induced the activation of NF-κB signaling. Pharmacological inhibition of NF-κB partially prevented B7-H4-mediated bladder cancer cell invasion. Taken together, B7-H4/NF-κB signaling is involved in the EMT and invasion of bladder cancer cells and represents a new candidate target for the treatment of bladder cancer.
Key words: B7-H4, Bladder cancer, Invasion, NF-κB signaling
INTRODUCTION
B7-homolog 4 (B7-H4) is a member of the B7 family of costimulatory molecules and has the ability to induce cell cycle arrest and apoptosis, and suppress cytokine secretion in activated T cells1. Accumulating evidence indicates that B7-H4 exerts an immunosuppressive effect in malignant diseases2. It has been documented that B7-H4 upregulation is associated with impaired CD8+ T-cell responses in cervical cancer3. In vitro studies demonstrated that overexpression of B7-H4 on the surface of tumor cells renders them resistant to T-cell-mediated lysis4. In vivo studies showed that administration of therapeutic monoclonal antibodies against B7-H4 significantly impaired the growth of B7-H4-expressing tumors1. These studies suggest that B7-H4 serves as a promising target for cancer immunotherapy.
In addition to modulation of the anticancer immune response, B7-H4 also shows direct effects on the aggressive phenotype of cancer cells5,6. For instance, knockdown of B7-H4 leads to reduced cellular proliferation and colony formation in esophageal squamous cell carcinoma cells in vitro5. Similarly, targeted reduction of B7-H4 significantly suppressed cell proliferation, invasion, and migration in lung cancer cells6. B7-H4 upregulation has a poor prognostic impact on multiple cancer types such as breast cancer7, gastric cancer8, and oral squamous cell carcinoma9. A previous study has reported that B7-H4 expression is upregulated in urothelial cell carcinoma (UCC) relative to adjacent normal tissues and correlates with reduced overall survival of patients with UCC10. However, the biological function of B7-H4 in bladder cancer has not yet been clarified.
In the present work, we performed B7-H4 overexpression and knockdown experiments to determine its role in the regulation of bladder cancer cell growth and invasion. The signaling pathway(s) involved was explored.
MATERIALS AND METHODS
Cell Culture
Human bladder cancer cell lines (T24, TCCSUP, and J82) and normal urothelial SV-HUC-1 cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). Bladder cancer cells were cultured in minimum essential medium (MEM) supplemented with 10% fetal bovine serum (FBS; Invitrogen, Grand Island, NY, USA). SV-HUC-1 cells were cultured in F-12K medium (ATCC) supplemented with 10% FBS.
RNA Isolation and Quantitative Real-Time PCR Analysis
Total RNA isolation from cells was performed using TRIzol (Invitrogen) following the manufacturer’s protocol. cDNA was synthesized using PrimeScript RT Master Mix (TaKaRa Biotechnology Co., Ltd., Dalian, P.R. China) with random hexamers. Real-time PCR was performed with SYBR Green PCR Mix (Applied Biosystems, Foster City, CA, USA) with the following primers11: B7-H4, 5′-AGGGAGTGGAGGAGGATACAG-3′ (forward) and 5′-GCAGCAGCCAAAGAGACAG-3′ (reverse); β-actin, 5′-AGAAAATCTGGCACCACACC-3′ (forward) and 5′-AGAGG CGTACAGGGATAGCA-3′ (reverse). β-Actin was used as a normalization control.
Subcellular Fractionation
Nuclear and cytoplasmic fractions of cells were harvested using the NE-PER Nuclear and Cytoplasmic Extraction Reagents Kit (Thermo Scientific, Waltham, MA, USA) as per the manufacturer’s recommendations.
Western Blot Analysis
Cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (Abcam, Cambridge, UK) containing protease inhibitors (Roche Applied Science, Mannheim, Germany). Lysates (50 μg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes. After blocking nonspecific binding sites, the membranes were probed overnight at 4°C with the primary antibodies: anti-B7-H4 (1:300 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-E-cadherin (1:300 dilution; Abcam), anti-vimentin (1:500 dilution; Abcam), anti-Twist1 (1:500 dilution; Abcam), anti-Snail (1:300 dilution; Abcam), anti-phospho-IκBα (1:300 dilution; Cell Signaling Technology, Beverly, MA, USA), anti-IκBα (1:300 dilution; Cell Signaling Technology), anti-p65 (1:300 dilution; Cell Signaling Technology), anti-Akt (1:500 dilution; Cell Signaling Technology), anti-phospho-Akt (1:300 dilution; Cell Signaling Technology), anti-ERK (1:500 dilution; Cell Signaling Technology), anti-phospho-ERK (1:500 dilution; Cell Signaling Technology), anti-p38 (1:500 dilution; Cell Signaling Technology), anti-phospho-p38 (1:500 dilution; Cell Signaling Technology), anti-STAT3 (1:500 dilution; Cell Signaling Technology), anti-phospho-STAT3 (1:500 dilution; Cell Signaling Technology), anti-tubulin (1:2,000 dilution; Santa Cruz Biotechnology), and anti-lamin B1 (1:2,000 dilution; Santa Cruz Biotechnology). The membranes were then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5,000 dilution) for 1 h at room temperature. Proteins were visualized by enhanced chemiluminescence (ECL; Cell Signaling Technology) and quantitated by densitometric analysis.
Plasmids, Small Interfering RNAs (siRNAs), and Transfection
Full-length human B7-H4 cDNA was obtained from Origene Technologies (Rockville, MD, USA) and cloned into the pcDNA3.1(+) expression vector. B7-H4-targeting siRNA and scrambled control siRNA were purchased from Santa Cruz Biotechnology. The pcDNA3.1/B7-H4 plasmid or empty vector was transfected into bladder cancer cells 24 h after seeding using Lipofectamine 3000 transfection reagent (Invitrogen) according to the manufacturer’s protocol. Transfections of individual siRNAs (40 nM) were performed using DharmaFECT transfection reagents (Dharmacon, Lafayette, CO, USA). In some experiments, T24 cells were pretreated with SN50 (10 μM; Calbiochem, San Diego, CA, USA)12 for 30 min before transfection with the pcDNA3.1/B7-H4 plasmid. For generation of stable cell clones, cells transfected with the pcDNA3.1/B7-H4 plasmid or empty vector were selected in the presence of 800 μg/ml G418 (Sigma-Aldrich, St. Louis, MO, USA).
Cell Proliferation and Colony Formation Assay
Cells were seeded in 96-well plates (3,000 cells/well) and cultured for 24, 48, and 72 h. Cell viability was measured at different time points using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay. MTT (Sigma-Aldrich) was added to each well and incubated for 4 h at 37°C. Dimethyl sulfoxide (DMSO) was added, and absorbance was measured at 570 nm.
For the colony formation assay, cells were plated at a low density in 12-well plates (400 cells/well) and allowed to grow at 37°C for 10 days. The cells were washed and stained with 0.5% crystal violet. The number of clones formed was counted.
In Vitro Wound Healing Assay
For evaluation of cell migration capacity, in vitro wound healing assay was performed as described previously13. Cells were plated on six-well plates, and after reaching confluence, the cell culture was scratched using a sterile pipette tip. The cell debris was removed, and the wounded layers were cultured for 24 h in the presence of mitomycin C (10 ng/ml; Sigma-Aldrich). The percentage of wound closure was evaluated under a microscope.
Transwell Invasion Assay
Cells suspended in serum-free medium were plated on the upper chamber (8 μm in pore size) of a 24-well Transwell plate (5 × 104 cells/well). The chamber was coated with Matrigel (Sigma-Aldrich) for assessment of cell invasion. The lower chamber was filled with MEM supplemented with 10% FBS. After incubation for 24 h, the invaded cells were stained with 0.5% crystal violet and counted under a microscope.
Dual-Luciferase Reporter Assay
Nuclear factor (NF)-κB-dependent transcriptional activity was assessed using luciferase reporter assay, as described previously13. Bladder cancer cells were seeded in triplicate in 24-well plates and cotransfected with the pNF-κB-Luc luciferase reporter (Stratagene, La Jolla, CA, USA), pcDNA3.1/B7-H4 plasmid or empty vector, together with pRL-TK Renilla luciferase reporter (Promega, Mannheim, Germany) using Lipofectamine 3000 reagent. The luciferase activities were measured 48 h after transfection using the Dual-Luciferase Reporter Assay Kit (Promega).
Statistical Analysis
Data are presented as the mean ± standard deviation and analyzed by the Student’s t-test or one-way analysis of variance (ANOVA) with Dunnett’s posttest. A value of p < 0.05 was considered statistically significant.
RESULTS
B7-H4 Is Upregulated in Bladder Cancer Cells
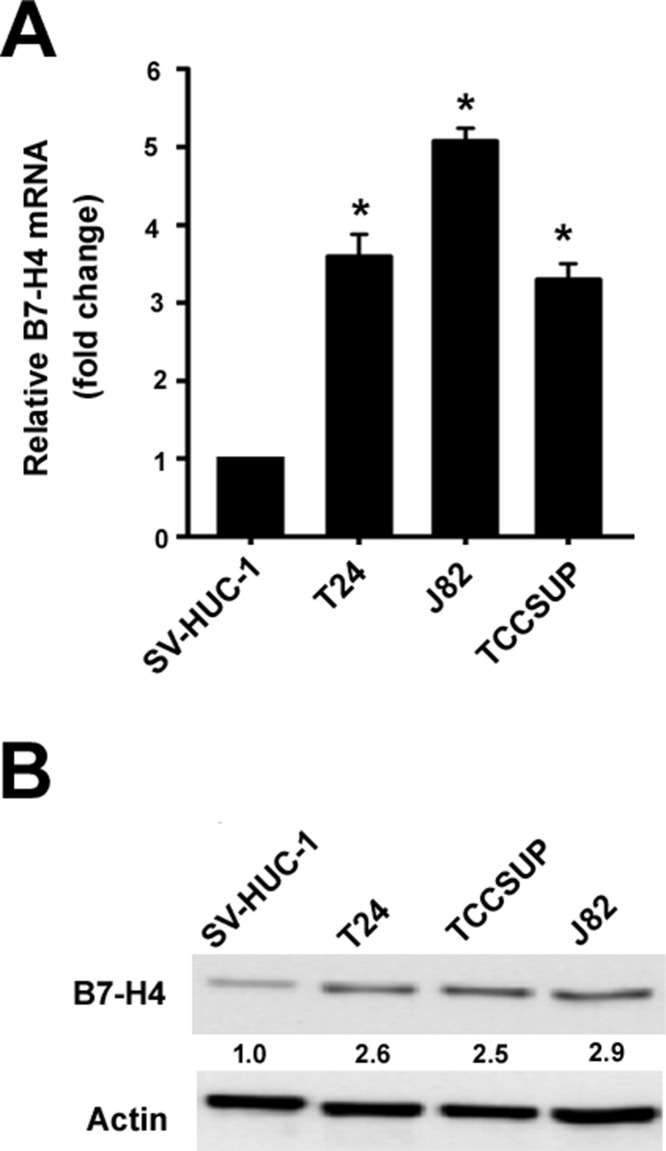
Real-time PCR analysis revealed that B7-H4 mRNA levels were significantly higher in the bladder cancer cell lines tested (i.e., T24, TCCSUP, and J82) than in the SV-HUC-1 normal human urothelial cells (p < 0.05) (Fig. 1A). Similarly, the protein levels of B7-H4 were markedly increased in bladder cancer cells, compared to SV-HUC-1 cells (Fig. 1B).
Figure 1.
B7 homolog 4 (B7-H4) is upregulated in bladder cancer cells. (A) Real-time PCR and (B) Western blot analysis of B7-H4 expression in bladder cancer cell lines and normal human urothelial (SV-HUC-1) cells. Numbers below the Western blots represent fold change in B7-H4 protein levels after normalization to β-actin. *p < 0.05 compared to SV-HUC-1 cells.
B7-H4 Has no Significant Impact on the Growth of Bladder Cancer Cells
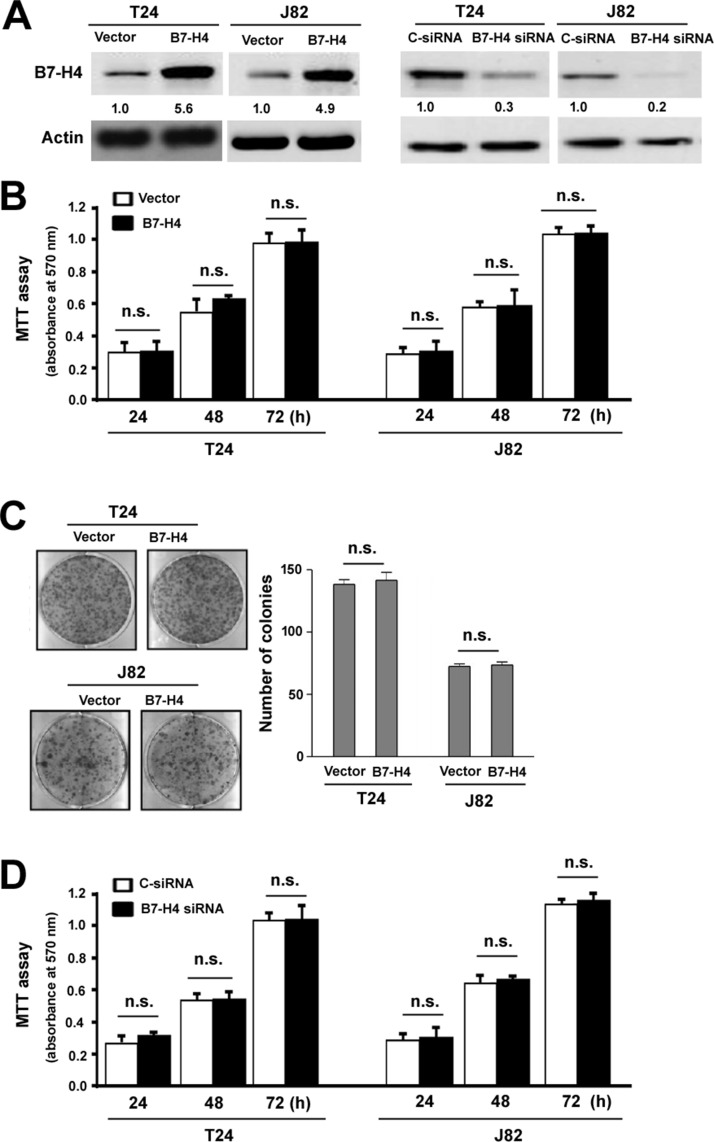
To examine the biological role of B7-H4 in bladder cancer, gain- and loss-of-function experiments were carried out. Western blot analysis confirmed the overexpression of B7-H4 in T24 and J82 cells transfected with the B7-H4-expressing plasmid (Fig. 2A). Compared to vector-transfected control cells, B7-H4-overexpressing bladder cells had a comparable proliferation rate within a 72-h culture period (Fig. 2B). Colony formation assay also showed that B7-H4 overexpression did not alter the capacity of bladder cancer cells to form colonies after culturing for 14 days (Fig. 2C). For downregulation of B7-H4, specific B7-H4-targeting siRNAs were employed. Knockdown efficiency was confirmed by Western blot analysis (Fig. 2A). The growth of T24 and J82 cells was not affected when B7-H4 was depleted (Fig. 2D).
Figure 2.
B7-H4 has no significant impact on the growth of bladder cancer cells. (A) Left: Western blot analysis of B7-H4 protein levels in T24 and J82 cells transfected with the B7-H4-expressing plasmid or empty vector. Right: Western blot analysis of B7-H4 protein levels in T24 and J82 cells transfected with control small interfering RNA (C-siRNA) or B7-H4-targeting siRNA. (B) Cell proliferation determined by MTT assays in T24 and J82 cells transfected with the B7-H4-expressing plasmid or empty vector. (C) Colony formation assay. T24 and J82 cells stably transfected with the B7-H4-expressing plasmid or empty vector were cultured for 14 days to form colonies. (D) Cell proliferation determined by MTT assays in T24 and J82 cells transfected with C-siRNA or B7-H4-targeting siRNA. n.s., no significance.
B7-H4 Promotes Bladder Cancer Cell Migration and Invasion
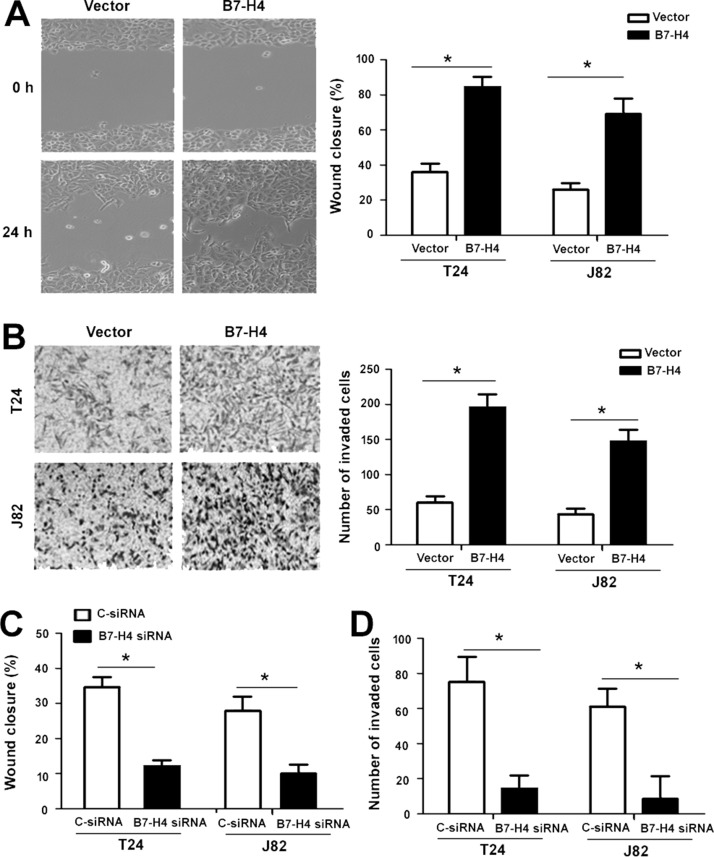
Next, we explored whether B7-H4 modulates the migration and invasion of bladder cancer cells. In vitro wound healing assay showed that ectopic expression of B7-H4 accelerated cell migration compared to empty vector-transfected cells (Fig. 3A). Moreover, B7-H4-overexpressing bladder cancer cells displayed a significant increase in invasion capacity in comparison to control cells, as determined by Transwell invasion assay (p < 0.05) (Fig. 3B). Next, we examined the impact of silencing of B7-H4 on bladder cancer cell migration and invasion. As shown in Figure 3C and D, depletion of B7-H4 suppressed the migration and invasion of bladder cancer cells.
Figure 3.
B7-H4 promotes bladder cancer cell migration and invasion. (A) Wound healing assays. Left: Representative images showing the migration of T24 cells transfected with the B7-H4-expressing plasmid or empty vector after 24-h incubation. Right: Mean percentage of wound closure determined from three independent experiments. (B) Transwell invasion assay performed in T24 and J82 cells transfected with the B7-H4-expressing plasmid or empty vector. (C, D) T24 and J82 cells transfected with C-siRNA or B7-H4-targeting siRNA were subjected to (C) wound healing and (D) Transwell invasion assays. *p < 0.05.
Overexpression of B7-H4 Induces Epithelial–Mesenchymal Transition (EMT) in Bladder Cancer Cells
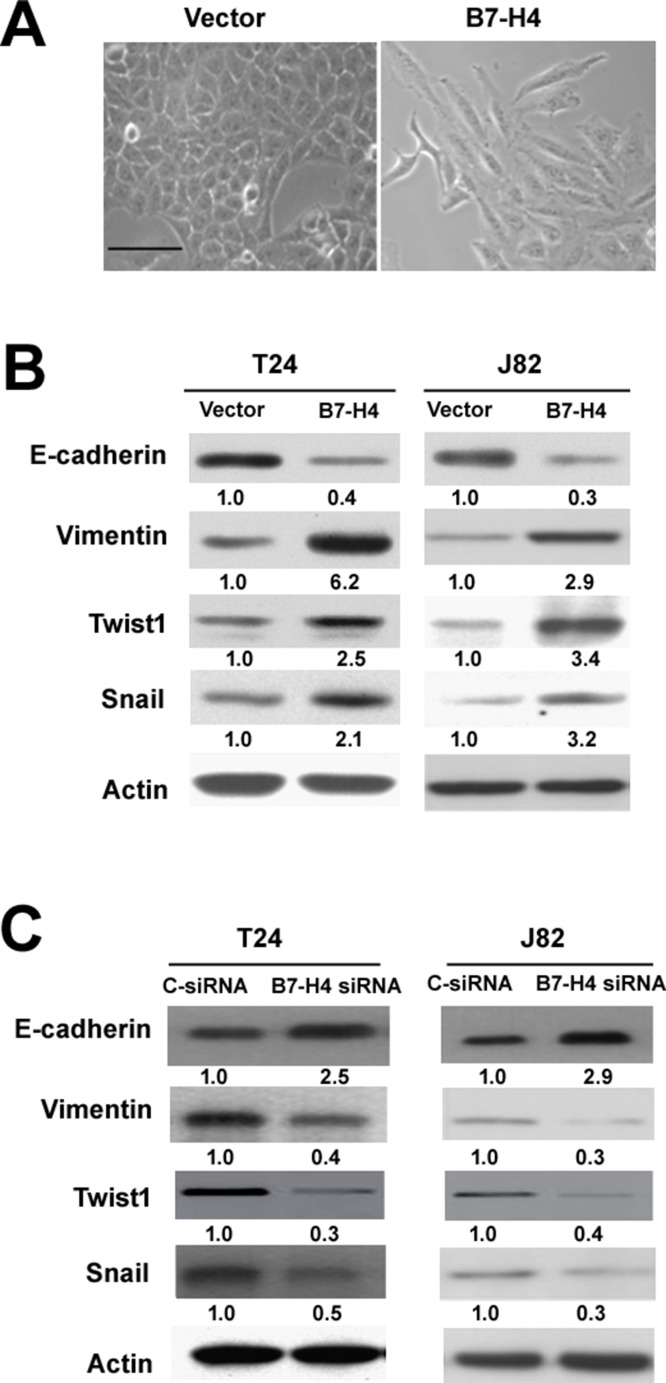
Next, we checked whether the proinvasive activity of B7-H4 is linked to induction of EMT in bladder cancer cells. While empty vector-transfected T24 cells showed a typical morphology of epithelial cells, B7-H4-overexpressing equivalents acquired a spindle-like phenotype (Fig. 4A). Western blot analysis of EMT markers (E-cadherin and vimentin) demonstrated that E-cadherin expression was decreased and vimentin expression was increased in both B7-H4-overexpressing T24 and J82 cells (Fig. 4B). In addition, the expression of EMT inducers Twist1 and Snail was elevated by B7-H4 overexpression (Fig. 4B). We also knocked down B7-H4 to investigate its effect on the expression of EMT markers. It was found that B7-H4 silencing remarkably upregulated E-cadherin and downregulated vimentin, Twist1, and Snail in T24 and J82 cells (Fig. 4C). Collectively, these data highlight the role of B7-H4 in EMT induction.
Figure 4.
Overexpression of B7-H4 induces epithelial–mesenchymal transition (EMT) in bladder cancer cells. (A) Morphological changes in T24 cells with overexpression of B7-H4. Scale bar: 60 μm. (B) Western blot analysis of indicated proteins in T24 and J82 cells transfected with the B7-H4-expressing plasmid or empty vector. (C) Western blot analysis of indicated proteins in T24 and J82 cells transfected with C-siRNA or B7-H4-targeting siRNA. Numbers below Western blots represent fold change in protein levels after normalization to β-actin.
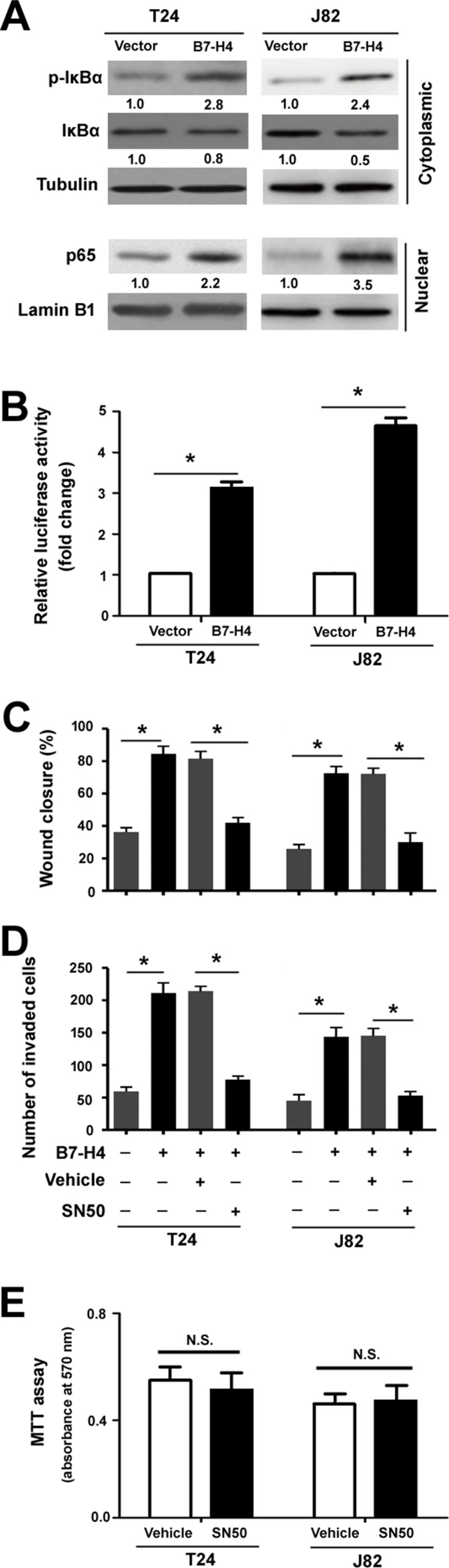
Activation of NF-κB Signaling Contributes to B7-H4-Mediated Aggressive Phenotype
To gain insights into the mechanism by which B7-H4 promotes aggressive phenotype in bladder cancer cells, we examined the effect of B7-H4 overexpression on multiple signaling pathways involved in cancer progression. Western blot analysis revealed that B7-H4 overexpression enhanced the phosphorylation of IκBα and induced nuclear accumulation of NF-κB p65 (Fig. 5A). However, the phosphorylation of Akt, ERK, p38, and STAT3 was not altered by B7-H4 overexpression (data not shown). Luciferase reporter assay confirmed that overexpression of B7-H4 causes a 3-5-fold increase in NF-κB-dependent reporter activity relative to control cells (p < 0.05) (Fig. 5B). These results indicate the activation of NF-κB signaling by B7-H4.
Figure 5.
Activation of NF-κB signaling contributes to B7-H4-mediated aggressive phenotype. (A) Western blot analysis of total and phosphorylated IκBα levels in cytosolic fractions and NF-κB p65 levels in nuclear fractions. Numbers below Western blots represent fold change in normalized protein levels. (B) NF-κB-dependent luciferase reporter assay. T24 and J82 cells were cotransfected with the pNF-κB-Luc luciferase reporter, pcDNA3.1/B7-H4 plasmid, or empty vector, together with pRL-TK Renilla luciferase reporter. The luciferase activities were measured 48 h after transfection. (C, D) T24 and J82 cells were pretreated with 10 μM SN50, before transfection with the pcDNA3.1/B7-H4 plasmid, and subjected to (C) wound healing and (D) Transwell invasion assays. *p < 0.05. (E) Measurement of cell viability by MTT assays in T24 and J82 cells treated with 10 μM SN50 or vehicle for 24 h. N.S., no significance.
To validate the role of NF-κB signaling in the activity of B7-H4, we pretreated bladder cancer cells with a NF-κB inhibitor before B7-H4 overexpression. Of note, B7-H4-mediated cancer cell migration (Fig. 5C) and invasion (Fig. 5D) were partially inhibited by SN50 pretreatment. MTT assay revealed that SN50 treatment for 24 h did not alter the viability of bladder cancer cells (Fig. 5E), suggesting that the inhibition of migration and invasion was not due to decreased cell viability.
DISCUSSION
B7-H4 is frequently deregulated in human cancers including breast cancer7, gastric cancer8, gallbladder carcinoma14, and esophageal cancer15. In this study, we showed that B7-H4 expression was raised in bladder cancer cells relative to normal human urothelial cells, which was consistent with clinical data that showed an upregulation of B7-H4 in UCC10. To determine the biological relevance of B7-H4 upregulation in bladder cancer cells, we performed B7-H4 overexpression and knockdown experiments. It was found that the growth of bladder cancer cells was not altered by either overexpression or knockdown of B7-H4. However, ectopic expression of B7-H4 displayed the ability to facilitate the migration and invasion of bladder cancer cells. Depletion of B7-H4 exerted an opposite effect on bladder cancer cell migration and invasion. These data point toward the proinvasive activity of B7-H4 in bladder cancer cells. In agreement with our findings, B7-H4 overexpression can also promote the invasion of lung cancer6, cervical cancer16, and ovarian cancer17 cells.
Induction of EMT is causally linked to enhanced aggressiveness of cancer18,19. A previous study demonstrated that Krüppel-like factor 4-mediated EMT facilitates cell migration and invasion in urothelial carcinoma cells20. Pharmacological inhibition of EMT led to reduced migration and invasion in bladder cancer cells21. Our data indicated that enforced expression of B7-H4 promoted EMT in both T24 and J82 cells, as evidenced by decreased E-cadherin and increased vimentin expression. B7-H4 knockdown led to an increase in E-cadherin expression and decrease in vimentin expression. Moreover, the expression of Twist1 and Snail, two important EMT inducers22, was markedly regulated by overexpression or knockdown of B7-H4. To the best of our knowledge, this is the first report of induction of EMT by B7-H4. However, several other members of the B7 family (e.g., B7-H123 and B7-H324), have exhibited the capacity to trigger EMT in cancer cells.
We found that B7-H4 overexpression induced nuclear accumulation of NF-κB and enhanced NF-κB-dependent reporter activity in bladder cancer cells, indicating the activation of NF-κB by B7-H4. NF-κB is a well-defined transcription factor, which undergoes nuclear translocation and transactivates a lot of target genes after degradation of the cytoplasmic inhibitor IκB25. Twist has been identified to be a conserved target of NF-κB26. Upregulation of snail accounts for NF-κB activation-mediated EMT in hepatocellular carcinoma cells27. Another study reported that the NF-κB/snail axis is involved in the EMT of glioma cells after interleukin-8 stimulation28. Thus, the activation of NF-κB signaling may provide a mechanistic explanation for the upregulation of Twist1 and Snail and induction of EMT by B7-H4.
In conclusion, our results demonstrate that B7-H4 upregulation promotes EMT and cell invasion in bladder cancer cells through activation of NF-κB. The B7-H4/NF-κB signaling may represent a potential target for the treatment of bladder cancer.
ACKNOWLEDGMENT
This work was supported by grants from Changzhou Sci and Tech Program (Grant WS201423) and the Wujin Hospital, Affiliated to Jiangsu University (Changzhou, P.R. China).
REFERENCES
- 1. Jeon H, Vigdorovich V, Garrett-Thomson SC, Janakiram M, Ramagopal UA, Abadi YM, Lee JS, Scandiuzzi L, Ohaegbulam KC, Chinai JM, Zhao R, Yao Y, Mao Y, Sparano JA, Almo SC, Zang X. Structure and cancer immunotherapy of the B7 family member B7x. Cell Rep. 2014;9(3):1089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Podojil JR, Miller SD. Potential targeting of B7-H4 for the treatment of cancer. Immunol Rev. 2017;276(1):40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang X, Wang T, Xu M, Xiao L, Luo Y, Huang W, Zhang Y, Geng W. B7-H4 overexpression impairs the immune response of T cells in human cervical carcinomas. Hum Immunol. 2014;75(12):1203–9. [DOI] [PubMed] [Google Scholar]
- 4. Chen C, Qu QX, Shen Y, Mu CY, Zhu YB, Zhang XG, Huang JA. Induced expression of B7-H4 on the surface of lung cancer cell by the tumor-associated macrophages: A potential mechanism of immune escape. Cancer Lett. 2012;317(1):99–105. [DOI] [PubMed] [Google Scholar]
- 5. Chen X, Wang L, Wang W, Zhao L, Shan B. B7-H4 facilitates proliferation of esophageal squamous cell carcinoma cells through promoting interleukin-6/signal transducer and activator of transcription 3 pathway activation. Cancer Sci. 2016;107(7):944–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang X, Cai L, Zhang G, Shen Y, Huang J. B7-H4 promotes tumor growth and metastatic progression in lung cancer by impacting cell proliferation and survival. Oncotarget 2017;8(12):18861–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang H, Li C, Ren G. Clinical significance of the B7-H4 as a novel prognostic marker in breast cancer. Gene 2017;623:24–8. [DOI] [PubMed] [Google Scholar]
- 8. Cui Y, Li Z. B7-H4 is predictive of poor prognosis in patients with gastric cancer. Med Sci Monit. 2016;22:4233–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu L, Deng WW, Yu GT, Mao L, Bu LL, Ma SR, Liu B, Zhang WF, Sun ZJ. B7-H4 expression indicates poor prognosis of oral squamous cell carcinoma. Cancer Immunol Immunother. 2016;65(9):1035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fan M, Zhuang Q, Chen Y, Ding T, Yao H, Chen L, He X, Xu X. B7-H4 expression is correlated with tumor progression and clinical outcome in urothelial cell carcinoma. Int J Clin Exp Pathol. 2014;7(10):6768–75. [PMC free article] [PubMed] [Google Scholar]
- 11. Cui L, Gao B, Cao Z, Chen X, Zhang S, Zhang W. Downregulation of B7-H4 in the MHCC97-H hepatocellular carcinoma cell line by arsenic trioxide. Mol Med Rep. 2016;13(3):2032–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wei C, Li L, Gupta S. NF-κB-mediated miR-30b regulation in cardiomyocytes cell death by targeting Bcl-2. Mol Cell Biochem. 2014;387(1–2):135–41. [DOI] [PubMed] [Google Scholar]
- 13. Cai QY, Liang GY, Zheng YF, Tan QY, Wang RW, Li K. CCR7 enhances the angiogenic capacity of esophageal squamous carcinoma cells in vitro via activation of the NF-κB/VEGF signaling pathway. Am J Transl Res. 2017;9(7):3282–92. [PMC free article] [PubMed] [Google Scholar]
- 14. Liu CL, Zang XX, Huang H, Zhang H, Wang C, Kong YL, Zhang HY. The expression of B7-H3 and B7-H4 in human gallbladder carcinoma and their clinical implications. Eur Rev Med Pharmacol Sci. 2016;20(21):4466–73. [PubMed] [Google Scholar]
- 15. Chen L, Xie Q, Wang Z, Shi L, Wu C, Jiang J. Assessment of combined expression of B7-H3 and B7-H4 as prognostic marker in esophageal cancer patients. Oncotarget 2016;7(47):77237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Han S, Li Y, Zhang J, Liu L, Chen Q, Qian Q, Li S, Zhang Y. Roles of immune inhibitory molecule B7-H4 in cervical cancer. Oncol Rep. 2017;37(4):2308–16. [DOI] [PubMed] [Google Scholar]
- 17. Cheng L, Jiang J, Gao R, Wei S, Nan F, Li S, Kong B. B7-H4 expression promotes tumorigenesis in ovarian cancer. Int J Gynecol Cancer 2009;19(9):1481–6. [DOI] [PubMed] [Google Scholar]
- 18. Yang S, Liu Y, Li MY, Ng CSH, Yang SL, Wang S, Zou C, Dong Y, Du J, Long X, Liu LZ, Wan IYP, Mok T, Underwood MJ, Chen GG. FOXP3 promotes tumor growth and metastasis by activating Wnt/β-catenin signaling pathway and EMT in non-small cell lung cancer. Mol Cancer 2017;16(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pan T, Chen W, Yuan X, Shen J, Qin C, Wang L. miR-944 inhibits metastasis of gastric cancer by preventing the epithelial-mesenchymal transition via MACC1/Met/AKT signaling. FEBS Open Bio. 2017;7(7):905–14. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20. Tseng WC, Chuang CW, Yang MH, Pan CC, Tarng DC. Krüppel-like factor 4 is a novel prognostic predictor for urothelial carcinoma of bladder and it regulates TWIST1-mediated epithelial-mesenchymal transition. Urol Oncol. 2016;34(11):485.e15–e24. [DOI] [PubMed] [Google Scholar]
- 21. Zhang Y, Liu W, He W, Zhang Y, Deng X, Ma Y, Zeng J, Kou B. Tetrandrine reverses epithelial-mesenchymal transition in bladder cancer by downregulating Gli-1. Int J Oncol. 2016;48(5):2035–42. [DOI] [PubMed] [Google Scholar]
- 22. Lovisa S, LeBleu VS, Tampe B, Sugimoto H, Vadnagara K, Carstens JL, Wu CC, Hagos Y, Burckhardt BC, Pentcheva-Hoang T, Nischal H, Allison JP, Zeisberg M, Kalluri R. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med. 2015;21(9):998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cao Y, Zhang L, Kamimura Y, Ritprajak P, Hashiguchi M, Hirose S, Azuma M. B7-H1 overexpression regulates epithelial-mesenchymal transition and accelerates carcinogenesis in skin. Cancer Res. 2011;71(4):1235–43. [DOI] [PubMed] [Google Scholar]
- 24. Kang FB, Wang L, Jia HC, Li D, Li HJ, Zhang YG, Sun DX. B7-H3 promotes aggression and invasion of hepatocellular carcinoma by targeting epithelial-to-mesenchymal transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int. 2015;15:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Q, Lenardo MJ, Baltimore D. 30 Years of NF-κB: A blossoming of relevance to human pathobiology. Cell 2017;168(1–2):37–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pham CG, Bubici C, Zazzeroni F, Knabb JR, Papa S, Kuntzen C, Franzoso G. Upregulation of Twist-1 by NF-kappaB blocks cytotoxicity induced by chemotherapeutic drugs. Mol Cell Biol. 2007;27(11):3920–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu TJ, Chang SS, Li CW, Hsu YH, Chen TC, Lee WC, Yeh CT, Hung MC. Severe hepatitis promotes hepatocellular carcinoma recurrence via NF-κB pathway-mediated epithelial-mesenchymal transition after resection. Clin Cancer Res. 2016;22(7):1800–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang B, Shi L, Lu S, Sun X, Liu Y, Li H, Wang X, Zhao C, Zhang H, Wang Y. Autocrine IL-8 promotes F-actin polymerization and mediate mesenchymal transition via ELMO1-NF-κB-Snail signaling in glioma. Cancer Biol Ther. 2015;16(6):898–911. [DOI] [PMC free article] [PubMed] [Google Scholar]