Abstract
Carbonic anhydrase (CA) IX has emerged as a promising target for cancer therapy. It is highly upregulated in hypoxic regions and mediates pH regulation critical for tumor cell survival as well as extracellular acidification of the tumor microenvironment, which promotes tumor aggressiveness via various mechanisms, such as augmenting metastatic potential. Therefore, the aim of this study was to analyze the complex interdependency between CA IX and the tumor microenvironment in prostate tumor cells with regard to potential therapeutic implications. CA IX was upregulated by hypoxia as well as acidosis in prostate cancer cells. This induction did not modulate intracellular pH but led to extracellular acidification. Pharmacological inhibition of CA IX activity by U104 (SLC-0111) resulted in a reduction in tumor cell growth and an increase in apoptotic cell death. Intracellular pH was reduced under normoxic and even more so under hypoxic conditions when CA IX level was high. However, although intracellular pH regulation was disturbed, targeting CA IX in combination with daunorubicin or cisplatin did not intensify apoptotic tumor cell death. Hence, targeting CA IX in prostate cancer cells can lead to intracellular pH dysregulation and, consequently, can reduce cellular growth and elevate apoptotic cell death. Attenuation of extracellular acidification by blocking CA IX might additionally impede tumor progression and metastasis. However, no beneficial effect was seen when targeting CA IX in combination with chemotherapeutic drugs.
Key words: Carbonic anhydrase IX (CA IX), Acidosis, Intracellular pH, Cytotoxicity
INTRODUCTION
Tumor cells need to be able to cope with strongly varying conditions both spatially and temporally during the development of solid tumors. These conditions include a supply of oxygen, nutrients, and soluble factors involved in signaling and growth1. Deviations result from impaired blood flow due to the aberrant vasculature, elevated intestinal pressure, accumulation of metabolic products, and spatial constraints by the surrounding normal tissue2. Together with the genetic background, these factors define tumor cell behavior.
In line with this, hypoxia has been shown to foster tumor progression and is linked to poor prognosis and treatment resistance3–5. Additionally, hypoxia induces a shift to glycolytic metabolism, which results in an excess of metabolic acid products like protons, lactic acid, and carbon dioxide and thereby in tumor microenvironmental acidosis6. This can even occur in the presence of oxygen, known as the Warburg effect. Acidosis can facilitate resistance to nonsurgical therapy and support local invasion and the formation of metastases7–11. To maintain a slightly alkaline to neutral intracellular pH (pHi) that facilitates cellular proliferation and survival, tumor cells activate certain transporters and ion exchangers involved in pH regulation12. Basically, to stabilize pHi, acidic products need to be extruded (for instance, through Na+/H+ exchanger, vacuolar H+-ATPase, and monocarboxylate transporters) while bicarbonate is taken up by the tumor cells (through Na+-HCO3 − cotransporters and anion exchangers)13. However, solid tumors often display a lack of bicarbonate compared to normal tissue, and formation of bicarbonate ions is hampered by extracellular acidosis14. To feed the pH regulatory machinery, carbonic anhydrases (CAs) catalyze the reversible conversion of carbon dioxide to a proton and bicarbonate ion; the latter is readily taken up by closely located bicarbonate transporters.
CAs are a family of zinc metalloenzymes. The transmembrane isoform CA IX is highly expressed in many solid tumors and protects the tumor cells from both hypoxic and acidic conditions15. CA IX activity leads to extracellular acidification and is implicated in facilitating transmembrane bicarbonate transport and the extrusion of protons and carbon dioxide out of the cell16. At the cellular level, CA IX is involved in pH regulation as well as signaling, and modulates survival, proliferation, adhesion, migration, and invasion17–19. CA IX expression depends on hypoxia-inducible factor (HIF) elements and correlates with an aggressive phenotype and poor prognosis; thus, CA IX is discussed as a therapeutic target for anticancer therapy16,20.
Knockdown of CA IX expression seems to depress primary tumor growth and reduce tumor cell resistance to conventional anticancer therapies21. Moreover, chemoresistant cancer cells display an upregulation of CA IX and of multidrug efflux transporter P-glycoprotein (Pgp; responsible for a multidrug-resistant phenotype of tumors) at the cell surface, and pHi regulation by CA IX is critical for the ATPase activity of Pgp22. Therefore, we analyzed the impact of CA IX inhibition on pHi regulation and survival of Pgp+ AT-1 prostate tumor cells. As a possible treatment modality, the sensitivity of AT-1 cells against daunorubicin, which is a Pgp substrate, and cisplatin, which is not transported by Pgp, was studied with and without the blocking of CA IX activity.
MATERIALS AND METHODS
Cell Culture
The subline AT-1 of the rat R-3327 Dunning prostate carcinoma (CLS, Eppelheim, Germany) was grown in RPMI medium supplemented with 10% fetal calf serum (FCS) at 37°C under a humidified 5% CO2 atmosphere and subcultivated twice a week. For short-term incubation (3 h), cells were transferred into medium without additional FCS supplementation for 24 h under normoxic or hypoxic conditions (0.2% O2, 5% CO2; hypoxia incubator; HypoxyLab; Oxford Optronix, Abingdon, UK) and subsequently incubated in bicarbonate-HEPES or -MES-buffered Ringer solution adjusted to pH 7.4 or pH 6.6 as described previously8. For incubation periods longer than 3 h, cells were transferred to an RPMI medium with 10 mM HEPES and 10 mM MES (lacking FCS) under normoxic or hypoxic conditions. The pH was adjusted to pH 7.4 or pH 6.6 using 1 M HCl.
Extracellular pH and Lactate Formation
pH was measured with a blood gas analyzer (ABL5; Radiometer, Copenhagen, Denmark). Lactate was determined using lactate reagent (Trinity Biotech, Bray, Ireland) according to the manufacturer’s instructions (10 μl of sample plus 100 μl of reagent). Each experiment was performed at least in triplicate.
Cytosolic pH and Intracellular Calcium
Cytosolic pH of single cells was determined using the pH-sensitive dye BCECF/AM [2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethyl ester; Invitrogen, Paisley, UK] as previously described23. In brief, cells were grown on coverslips and incubated with 5 μM BCECF-AM for 15 min. The coverslips were then rinsed two times to remove the excess dye and transferred to the stage of an inverted Axiovert 100 TV microscope (Zeiss, Oberkochen, Germany). The excitation wavelengths were 450 nm/490 nm, and emitted light was measured through a bandpass filter (515–565 nm). The data acquisition rate was one fluorescence intensity ratio every 10 s. After background subtraction, fluorescence intensity ratios were calculated, and pH calibration was performed using a two-point calibration (pH 6.8 and 7.5). The calibration solutions contained 132 mM KCl and 1 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, and 10 μM nigericin (Sigma-Aldrich, St. Louis, MO, USA).
Free cytosolic calcium of single cells was determined using Ca2+-sensitive dye Fura-2/AM (Merck, Darmstadt, Germany) as previously described24. Cells on the coverslips were incubated for 15 min with 5 μM Fura-2, rinsed twice, and analyzed with an Axio Observer.A1 microscope (Zeiss) at excitation wavelengths of 340 and 380 nm. The fluorescence intensity ratio was calculated every 5 s, and two-point calibration was performed using solutions containing 141 mM NaCl, 4 mM KCl, 1 mM MgCl2, 3.2 mM Na2HPO4, 0.8 mM NaH2PO4, 1 μM ionomycin, and either 1 mM CaCl2 or 1 mM EGTA. Intracellular Ca2+ was calculated according to Grynkiewicz et al.25
LDH Release
LDH activity in media and in cell lysates was measured using a standard protocol22 adapted to lower scale (200 μl) in a multiwell reader (Infinite; Tecan, Berlin, Germany).
Caspase 3 Activity
Cells were washed once with PBS buffer (4°C) and incubated with 100 μl of cell lysis buffer [10 mM TRIS, 100 mM NaCl, 1 mM EDTA, 0.01% Triton X-100 (pH 7.5)] for 10 min on ice, harvested, and centrifuged at 16,000 × g for 10 min at 4°C. Sixty microliters of the supernatant was incubated with 65 μl of reaction buffer [20 mM PIPES, 4 mM EDTA, 0.2% CHAPS, 10 mM DTT (pH 7.4)] containing 42 μM DEVD-AFC at 37°C, and fluorescence of the cleaved product, 7-amino-4-trifluoromethylcoumarin (AFC), was measured at 400-nm excitation and 505-nm emission wavelength using a multiwell counter (Infinite; Tecan). Cleaved AFC was quantified by a calibration curve using known AFC concentrations (0–8 μM). Protein content was determined with Pierce BCA protein assay (Thermo Scientific, Waltham, MA, USA) using bovine serum albumin as standard.
SRB Assay
SRB assay was performed as described previously26. AT-1 cells were seeded into 96-well plates (TPP, Trasadingen, Switzerland), and 72 h after treatment with U104 (0–250 μM) under normoxic and hypoxic conditions, cells were fixed, washed, and dyed with 0.4% sulforhodamine. Absorbance was measured at 540 nm using a Genios plate reader (Tecan, Männedorf, Switzerland).
Quantitative PCR
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). RNA (1 μg) was subjected to reverse transcription with SuperScript II reverse transcriptase (Invitrogen) and analyzed by qPCR using the Platinum SYBR Green qPCR Supermix (Invitrogen), each step according to the manufacturer’s instructions. The obtained data were normalized against Rn18S and Hprt1. The following primers were used: Car9, CGG ACC TCA GTC GCT ACT AC (forward) and AAA GGT TGT GTG GCT CGG AA (reverse); Hprt1, ACC AGT CAA CGG GGG ACA TA (forward) and TTG GGG CTG TAC TGC TTG AC (reverse); Rn18S, CTG AGA AAC GGC TAC CAC ATC (forward) and CCC AAG ATC CAA CTA CGA GC (reverse).
Determination of CA IX Expression and ERK1/2 as Well as p38 Phosphorylation
Overall protein and phosphorylation were analyzed by Western blotting using mouse CA IX antibody (1:2,000; BioScience, Bratislava, Slovakia) and mouse β-actin, ERK1/2, p38, or phospho-specific antibodies (1:1,000; Cell Signaling Technology, Danvers, MA, USA). In brief, cells were washed in PBS and lysed with Laemmli buffer [60 mM Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 5% β-mercaptoethanol, 0.005% bromophenol blue], separated by SDS-PAGE, and transferred to nitrocellulose membranes. Membranes were probed for rabbit phospho-ERK1/2 (Thr202/Tyr204) and overall mouse ERK1/2 or mouse phospho-p38 (Thr180/Tyr182) and rabbit p38 antibody simultaneously using LI-COR multiplex detection with secondary antibodies labeled with distinct near-infrared fluorescent dyes (1:40,000; IRDye 680/800; LI-COR Bioscience, Lincoln, NE, USA). Quantitative analysis was performed with the Image Studio Lite software (LI-COR Bioscience). Expression of CA IX was normalized to β-actin.
Chemicals
Unless stated otherwise, chemicals were obtained from Sigma-Aldrich (Munich, Germany). For CA IX inhibition, 50 μM U104 (SLC-0111) dissolved in DMSO was used (final concentration of DMSO: 1:2,000).
Data Analysis
Data are presented as mean ± SEM. All experiments were performed with at least three passages of cells, and n equals the number of culture plates to perform the measurements. Statistical significance was determined by unpaired Student’s t-test or ANOVA, as appropriate. Differences were considered statistically significant with a value of p < 0.05.
RESULTS
AT-1 Cells Express Carbonic Anhydrase (CA IX) Under Hypoxic Conditions
Because the expression of CA IX can vary profoundly in different tumor cells, CA IX expression at the mRNA and protein level was analyzed in the AT-1 rat prostate carcinoma cell line (Fig. 1). CA IX was barely detectable under normoxic cell culture conditions, whereas hypoxia strongly induced CA IX expression (Fig. 1B and C). Extracellular acidosis also led to an induction of CA IX expression, which is in good accordance with the literature27. However, when compared to hypoxia, acidosis-induced expression of CA IX was weak. Simultaneous incubation under hypoxic and acidic conditions did not raise the level of CA IX mRNA and protein further (no additive effect). Interestingly, it rather seemed to extenuate the induction of CA IX at both the mRNA and protein levels, when compared to hypoxia alone.
Figure 1.
Carbonic anhydrase 9 (CA IX) expression in AT-1 cells depends on the microenvironment. (A) CA IX mRNA level was weakly increased under acidic conditions (24 h, pH 6.6) and distinctly elevated under hypoxia (24 h, 0.2% O2). No additive effect was seen with simultaneous incubation (24 h, 0.2% O2, pH 6.6) when compared to control (24 h, 20% O2, pH 7.4) (n = 10, triplicates each). (B) Semiquantitative analysis of the impact of acidosis, hypoxia, or both on CA IX protein (normalized to β-actin, relative changes are shown, 24-h normoxia, pH 7.4 = 100%, n = 13–20). (C) Representative Western blot for CA IX and β-actin after 24-h normoxia (20% O2, pH 7.4), acidosis (pH 6.6), and hypoxia (0.2% O2, pH 7.4). *p < 0.05.
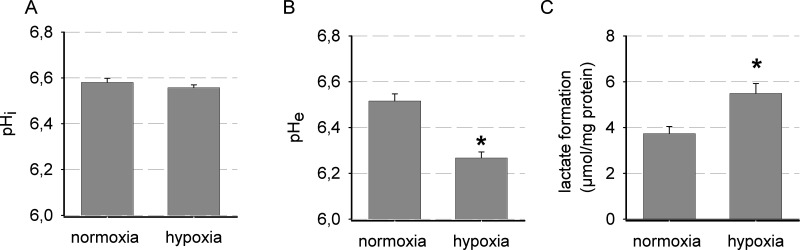
CA IX is critical for pH regulation of tumor cells16. Therefore, pH under normoxia (low CA IX expression) and under hypoxic conditions with a high level of CA IX was studied (Fig. 2). Cells were analyzed in an acidic Ringer solution with low concentration of bicarbonate (4.5 mM HCO3 −, pH 6.6) because bicarbonate can inhibit CA IX function28, and the contribution of CA IX to cellular pH regulation is not detectable when working with neutral buffers with relatively high bicarbonate concentrations (25 mM HCO3 −, pH 7.4)29. Increased CA IX expression after hypoxia treatment led to an acidification of the extracellular medium (Fig. 2B). Given that hypoxia-induced acidification of extracellular medium was shown to be a measure of biological activity of CA IX in cultured cells15,30, these results indicate increased CA IX activity. Resting pHi was not changed under hypoxic conditions when compared to normoxia (Fig. 2A). However, hypoxia also led to an elevated formation of lactic acid, which might be involved in the reduction of extracellular pH (Fig. 2C).
Figure 2.
Impact of hypoxia (24 h, 0.2 % O2, pH 6.6) on (A) intracellular pH (n = 3, 14 cells each), (B) extracellular pH (n = 19), as well as (C) lactate formation (n = 6, duplicates each) in AT-1 cells compared to control (24 h, 20% O2, pH 6.6). *p < 0.05.
Inhibition of CA IX
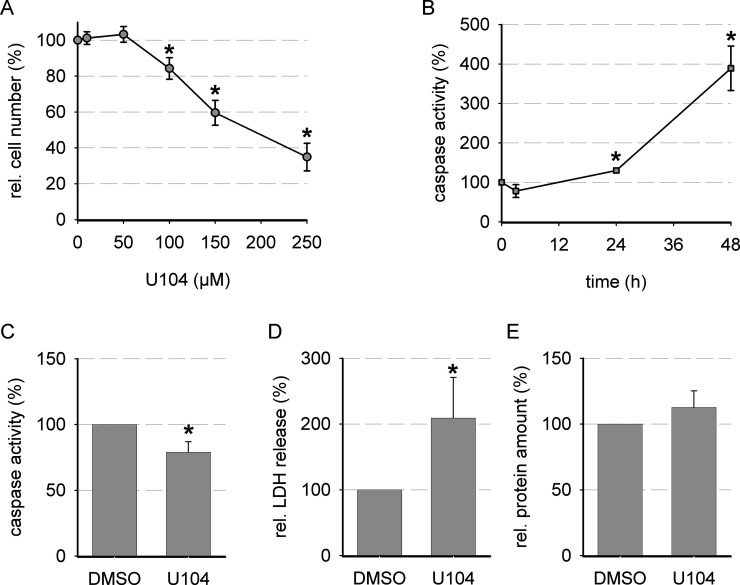
To elucidate the role of CA IX in cytotoxicity and signaling, CA IX activity had to be functionally blocked. For this purpose, U104, a ureido-sulfonamide inhibitor of CA IX (and CA XII), was used. Cellular cytotoxicity of U104 was analyzed by an SRB assay (Fig. 3A). The IC50 values for U104 under normoxic and hypoxic conditions are given in Table 1 and displayed no statistically relevant difference. High concentrations of U104 led to a distinct reduction in the growth of AT-1 cells (Fig. 3A). Cellular growth was not affected by U104 concentrations of 50 μM; thus, this concentration was used to analyze time-dependent apoptosis induction by U104 (Fig. 3B). Inhibition of CA IX with 50 μM U104 induced only minor changes in apoptotic cell death at time points 3 and 24 h, whereas 48 h led to pronounced caspase 3 activity (Fig. 3B). For further experiments, an incubation period of 3 h was chosen. During this short incubation period, the overall protein amount remained unchanged; however, a weak induction of necrosis by U104 was observed (Fig. 3C–E).
Figure 3.
Effect of CA IX inhibition on cell biological effects under normoxic and hypoxic conditions. (A) Cytotoxicity when CA IX was blocked with increasing concentrations of U104 (SRB assay, 72 h, 20% O2; n = 8–9). (B) Relative caspase 3 activity in the presence of 50 μM U104 compared to vehicle under hypoxic conditions (0.2% O2; n = 4–8). Relative changes in (C) caspase 3 activity, (D) LDH release, and (E) cellular protein after 3 h of incubation with 50 μM U104 under normoxic conditions (20% O2; vehicle = 100%, n = 4–8). *p < 0.05.
Table 1.
IC50 Values for U104 Under Normoxic and Hypoxic Conditions (72 h 20%/0.2% O2) Measured by SRB Assay in AT-1 Cells (n = 8–9)
| Incubation | IC50 |
|---|---|
| Normoxia | 139 ± 3 |
| Hypoxia | 122 ± 9 |
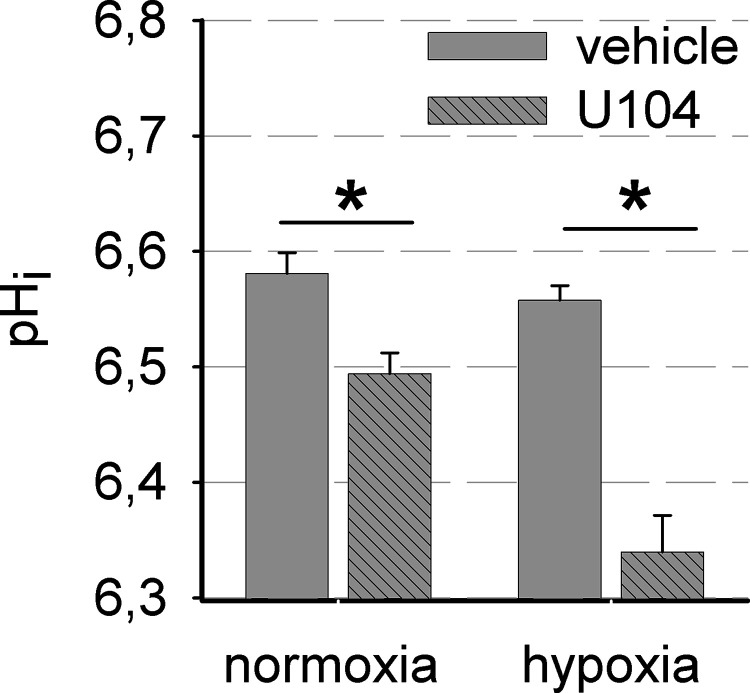
To determine whether functional inhibition of CA IX by U104 was effective, it was analyzed by assessing the changes in intracellular pH (pHi) of AT-1 cells. As CA IX activity provides bicarbonate for intracellular buffering, reduced CA IX activity is expected to cause a reduced pHi. Blocking CA IX activity led to a decrease in pHi by −0.09 ± 0.02 at normoxia and a stronger reduction of −0.21 ± 0.04 after hypoxia, when CA IX expression was high, proving that inhibition of CA IX was effective under the experimental settings (Fig. 4).
Figure 4.
Blocking CA IX activity reduced intracellular pH. Intracellular pH (pHi) after 3 h in acidic Ringer solution (4.5 mM HCO3 −, pH 6.6) in the presence of 50 μM U104 or DMSO is shown after AT-1 cells were pretreated for 24 h in normoxia (20% O2) or hypoxia (0.2 %O2) (n = 3, 14 cells each). *p < 0.05.
CA IX and Chemotherapy
To study the impact of CA IX function on the cytotoxicity of chemotherapeutic drugs, AT-1 cells were treated with cisplatin or daunorubicin alone or in the presence of CA IX inhibitor U104. Subsequently, the impact on apoptosis and necrosis was analyzed (Fig. 5A–C). Cisplatin and daunorubicin led to an increase in apoptotic cell death but had little or no effect on necrosis. Interestingly, in the presence of U104, when tumoral pHi regulation by CA IX was impaired, apoptosis induction was extenuated. Instead, necrotic cell death was induced by U104.
Figure 5.
Combinatory treatment of AT-1 cells with daunorubicin or cisplatin and U104. Cells were incubated for 3 h with 10 μM daunorubicin or 150 μM cisplatin with or without 50 μM U104 to block CA IX activity. Relative changes in (A) caspase 3 activity, (B) LDH release, (C) protein amount, and (D) intracellular fluorescence of daunorubicin (relative fluorescence units) are shown (n = 4–9, duplicates each). *p < 0.05.
Changes in pHi, such as the reduction induced by CA IX inhibition, can affect the activity of certain drug transporters like Pgp7,10. Therefore, changes in apoptosis and necrosis might be the result of varying intracellular concentrations of chemotherapeutics. The cellular uptake of daunorubicin, which is a Pgp substrate, was analyzed fluorometrically according to a previous study31. Functional inhibition of CA IX did not affect the intracellular level of daunorubicin and therefore Pgp activity seemed unaltered (Fig. 5D). Furthermore, the same reduction in apoptosis and induction of necrosis was observed with U104 and cisplatin, which is no substrate for Pgp. The cytotoxic mechanism of acute CA IX inhibition relies on necrotic cell death, in contrast to daunorubicin and cisplatin, which induce apoptosis.
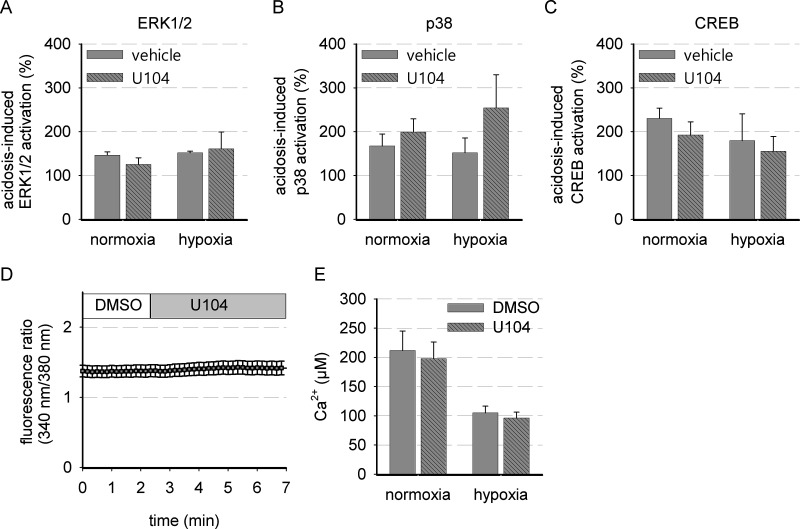
Apoptosis seemed slightly reduced during simultaneous incubation with cisplatin/daunorubicin and U104. This might be caused by intracellular acidification induced by acute CA IX inhibition, but also other mechanisms by CA IX have to be taken into account. In the literature, CA IX has been described to affect cell signaling13,16,20. In addition, reduction of intracellular pH can lead to signaling events transduced by intracellular pH sensors32. We analyzed whether CA IX inhibition can influence ERK1/2 and p38 MAP kinase signaling, which is typically induced by growth or stress signals, respectively (Fig. 6A and B). Phosphorylated ERK1/2 or p38 is shown in relation to total ERK1/2 or p38, respectively. MAPK signaling was induced by acidosis after 3 h in the presence of either DMSO as a control or U104 to block CA IX activity. No significant changes in the phosphorylation of ERK1/2 and p38 were observed in the presence of U104. However, a tendency for p38 activation by CA IX inhibition was seen, especially during hypoxic conditions (p = 0.20) (Fig. 6B), possibly due to a forced intracellular acidification (Fig. 4). A correlation of p38 phosphorylation with intracellular pH has been shown previously during strong artificial intracellular acidification33. Possibly, the reduction in intracellular pH induced by blocking CA IX was not strong enough to trigger p38 activation significantly. Whether CA IX might be able to influence cellular behavior by cAMP–PKA signaling was studied by analyzing phosphorylation state of PKA substrate cAMP response element-binding protein (CREB). However, inhibition of CA IX did not influence CREB phosphorylation related to total CREB (Fig. 6C). Also, calcium signaling was unaltered (Fig. 6D and E). Cytosolic free Ca2+ concentration was reduced by hypoxia per se, but not influenced by U104 incubation, either under normoxic or under hypoxic conditions when CA IX expression was high (Fig. 6E).
Figure 6.
MAP kinase, cAMP response element-binding protein (CREB), and Ca2+ signaling are independent of CA IX inhibition. Semiquantitative analysis of Western blots for phosphorylated (A) ERK1/2, (B) p38, and (C) CREB related to the respective total protein after 3 h of incubation at pH 6.6 in the presence of 50 μM U104 or DMSO (control: DMSO, 20% O2, pH 7.4; n = 3–4). (D) Representative time course showing dynamic changes in intracellular free Ca2+ when 50 μM U104 is added directly to the cells (14 cells). (E) Total amount of intracellular free Ca2+ when cells were pretreated for 24 h under normoxic (20% O2) or hypoxic conditions (0.2% O2) (n = 4–6, 14 cells each).
DISCUSSION
In the present study, we demonstrated that CA IX is present in AT-1 prostate carcinoma cells under normoxic conditions and is upregulated strongly by hypoxia and, to a lesser extent, by acidosis. A combination of hypoxia and acidosis, as present in the microenvironment of solid tumors, did not further enhance this increase. Thus, hypoxia and acidosis had no additive effect on CA IX expression. Because CA IX expression correlates with the risk of developing metastasis34, low levels of CA IX under normoxic conditions fit well to the basal low metastatic abilities of this cell line35. Hypoxia-induced CA IX expression augmented the acidification of the extracellular space and could thereby foster invasiveness and metastases formation.
To selectively block CA IX activity, the ureidosulfonamide U104 (or SLC-0111) was used. U104 is a highly selective “next-generation” small-molecule inhibitor, which has shown antitumor effects, both in vitro and in vivo, and is currently under investigation in an ongoing phase I clinical trial20,30,36,37. CA IX inhibition with U104 reduced cell growth and elevated apoptosis in AT-1 prostate tumor cells. This is most likely based on hampered cellular bicarbonate uptake, which can lead to intracellular acidification, reduced cell growth, and increased apoptosis38,39. Because the aim of the study was to analyze a combination of CA IX inhibition with the use of anticancer drugs, as discussed in the literature21, experimental settings had to be optimized to have minimal side effects for CA IX inhibition alone. Blocking CA IX activity for no longer than 3 h with 50 μM U104 was sufficient to impede pHi regulation, leading to intracellular acidification, while apoptosis was not induced. Additionally, CA IX expression is most likely not affected by the inhibitor after 3 h, as has been observed, for example, in breast cancer cells (M. Bache, unpublished results) and others39.
The cytotoxic efficiency of chemotherapeutic drugs in AT-1 prostate cancer cells was shown to be affected by the tumor microenvironment. Extracellular acidosis especially reduced the cytotoxicity of daunorubicin and cisplatin in vitro and in vivo, at least in part through activation of Pgp, thus leading to multidrug resistance7,10,40. CA IX promotes extracellular acidification and stabilizes pHi in an acidic environment, and therefore the inhibition of CA IX in combination with the use of daunorubicin or cisplatin should increase the efficiency of chemotherapeutic drugs and restore chemosensitivity. Increased efficiency of chemotherapeutics in vitro, when administered together with inhibitors of CA IX, was recently described41,42. Furthermore, a cotreatment of HT-29 tumor-bearing mice with doxorubicin (which is a 14-hydroxylated version of daunorubicin) and CA IX inhibitors led to augmented chemosensitization and reduced tumor growth43.
In AT-1 cells, daunorubicin and cisplatin induced apoptotic cell death, but simultaneous incubation with the CA IX inhibitor resulted in a reduction in apoptosis. This decrease in apoptosis was not caused by changes in the activity of active drug transporters because no changes in intracellular daunorubicin levels were observed, because changes were independent of the type of chemotherapeutic agent used, and because they were also seen in the absence of daunorubicin and cisplatin. In line with our results, the group of van Kuijk et al. also found no enhanced doxorubicin efficiency in xenografts when CA IX activity was simultaneously blocked41. To further complicate matters, some chemotherapeutic agents, including cisplatin, seem to be able to block CA IX activity as well44. The impact of chemotherapeutic agents in combination with CA IX inhibitors might be cell type specific or depend on the experimental settings chosen. Additionally, the choice of the CA IX inhibitor might be critical. In the work of Meehan et al., a beneficial effect in combination with irradiation was only observed with one of two tested CA IX inhibitors39. U104 blocks CA IX as well as CA XII, and as CA XII can also affect Pgp-mediated chemoresistance45, a differentiation between these isoforms would be necessary if effects on chemoresistance are observed. Therefore, our results show the need of further studies to increase the understanding of the role of CA IX inhibitors in tumor treatment, especially in combination with chemo- or radiation therapy.
To elucidate the role of different signaling pathways in the apoptosis (and necrosis) induction following CA IX inhibition, we analyzed different pathways known to be critical for cell survival. However, although pHi regulation was impaired, no change in the activity of ERK1/2, p38, or CREB, which is a PKA substrate, was seen. Additionally, the level of free intracellular Ca2+ remained unchanged. Hence, our recent results provide no evidence that stress-induced MAP kinases, PKA, or Ca2+ signaling pathways are involved in the observed changes in apoptosis or necrosis.
This study analyzed the complex interaction between tumor microenvironment, CA IX, and chemoresistance. Upregulation of CA IX was induced by hypoxia and acidosis and led to a reduction in extracellular pH. Thus, CA IX inhibition might be beneficial for tumor therapy by reducing acidosis-induced tumor cell aggressiveness and metastatic potential. Blocking CA IX activity with U104 impaired intracellular pH regulation and induced cell death but did not provoke chemosensitization in combination with daunorubicin or cisplatin. Therefore, additional preclinical and clinical data are needed to elucidate the mechanisms by which CA IX inhibition affects tumor cell growth and apoptosis.
ACKNOWLEDGMENT
This study was supported by the Wilhelm Sander-Stiftung für Krebsforschung (Grant No. FKZ: 2013.090.1).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Laconi E. The evolving concept of tumor microenvironments. BioEssays 2007;29(8):738–44. [DOI] [PubMed] [Google Scholar]
- 2. Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 1989;49(23):6449–65. [PubMed] [Google Scholar]
- 3. Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 2006;441(7092):437–43. [DOI] [PubMed] [Google Scholar]
- 4. Patel A, Sant S. Hypoxic tumor microenvironment: Opportunities to develop targeted therapies. Biotechnol Adv. 2016;34(5):803–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Michiels C, Tellier C, Feron O. Cycling hypoxia: A key feature of the tumor microenvironment. Biochim Biophys Acta 2016;1866(1):76–86. [DOI] [PubMed] [Google Scholar]
- 6. Chiche J, Brahimi-Horn MC, Pouysségur J. Tumour hypoxia induces a metabolic shift causing acidosis: A common feature in cancer. J Cell Mol Med. 2010;14(4):771–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sauvant C, Nowak M, Wirth C, Schneider B, Riemann A, Gekle M, Thews O. Acidosis induces multi-drug resistance in rat prostate cancer cells (AT1) in vitro and in vivo by increasing the activity of the p-glycoprotein via activation of p38. Int J Cancer 2008;123(11):2532–42. [DOI] [PubMed] [Google Scholar]
- 8. Riemann A, Schneider B, Gündel D, Stock C, Thews O, Gekle M. Acidic priming enhances metastatic potential of cancer cells. Pflugers Arch. 2014;466(11):2127–38. [DOI] [PubMed] [Google Scholar]
- 9. Stock C, Schwab A. Protons make tumor cells move like clockwork. Pflugers Arch. 2009;458(5):981–92. [DOI] [PubMed] [Google Scholar]
- 10. Thews O, Gassner B, Kelleher DK, Schwerdt G, Gekle M. Impact of extracellular acidity on the activity of P-glycoprotein and the cytotoxicity of chemotherapeutic drugs. Neoplasia 2006;8(2):143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fais S, Venturi G, Gatenby B. Microenvironmental acidosis in carcinogenesis and metastases: New strategies in prevention and therapy. Cancer Metast Rev. 2014;33(4):1095–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spugnini EP, Sonveaux P, Stock C, Perez-Sayans M, De Milito A, Avnet S, Garcìa AG, Harguindey S, Fais S. Proton channels and exchangers in cancer. Biochim Biophys Acta 2015;1848(10):2715–26. [DOI] [PubMed] [Google Scholar]
- 13. Sedlakova O, Svastova E, Takacova M, Kopacek J, Pastorek J, Pastorekova S. Carbonic anhydrase IX, a hypoxia-induced catalytic component of the pH regulating machinery in tumors. Front Physiol. 2014;4:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stubbs M, Rodrigues L, Howe FA, Wang J, Jeong KS, Veech RL, Griffiths JR. Metabolic consequences of a reversed pH gradient in rat tumors. Cancer Res. 1994;54(15):4011–6. [PubMed] [Google Scholar]
- 15. Ditte P, Dequiedt F, Svastova E, Hulikova A, Ohradanova-Repic A, Zatovicova M, Csaderova L, Kopacek J, Supuran CT, Pastorekova S, Pastorek J. Phosphorylation of carbonic anhydrase IX controls its ability to mediate extracellular acidification in hypoxic tumors. Cancer Res. 2011;71(24):7558–67. [DOI] [PubMed] [Google Scholar]
- 16. Swietach P, Vaughan-Jones RD, Harris AL. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metast Rev. 2007;26(2):299–310. [DOI] [PubMed] [Google Scholar]
- 17. Robertson N, Potter C, Harris AL. Role of carbonic anhydrase IX in human tumor cell growth, survival, and invasion. Cancer Res. 2004;64(17):6160–5. [DOI] [PubMed] [Google Scholar]
- 18. Shin H-J, Rho SB, Jung DC, Han I-O, Oh E-S, Kim J-Y. Carbonic anhydrase IX (CA9) modulates tumor-associated cell migration and invasion. J Cell Sci. 2011;124(Pt 7):1077–87. [DOI] [PubMed] [Google Scholar]
- 19. Svastová E, Zilka N, Zat’ovicová M, Gibadulinová A, Ciampor F, Pastorek J, Pastoreková S. Carbonic anhydrase IX reduces E-cadherin-mediated adhesion of MDCK cells via interaction with beta-catenin. Exp Cell Res. 2003;290(2):332–45. [DOI] [PubMed] [Google Scholar]
- 20. Lock FE, McDonald PC, Lou Y, Serrano I, Chafe SC, Ostlund C, Aparicio S, Winum J-Y, Supuran CT, Dedhar S. Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene 201;32(44):5210–9. [DOI] [PubMed] [Google Scholar]
- 21. Mahon B, Pinard M, McKenna R. Targeting carbonic anhydrase IX activity and expression. Molecules 2015;20(2):2323–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kopecka J, Campia I, Jacobs A, Frei AP, Ghigo D, Wollscheid B, Riganti C. Carbonic anhydrase XII is a new therapeutic target to overcome chemoresistance in cancer cells. Oncotarget 2015;6(9):6776–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bergman JA, McAteer JA, Evan AP, Soleimani M. Use of the pH-sensitive dye BCECF to study pH regulation in cultured human kidney proximal tubule cells. J Tissue Cult Methods 1991;13(3):205–9. [Google Scholar]
- 24. Malgaroli A, Milani D, Meldolesi J, Pozzan T. Fura-2 measurement of cytosolic free Ca2+ in monolayers and suspensions of various types of animal cells. J Cell Biol. 1987;105(5):2145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260(6):3440–50. [PubMed] [Google Scholar]
- 26. Bache M, Bernhardt S, Passin S, Wichmann H, Hein A, Zschornak M, Kappler M, Taubert H, Paschke R, Vordermark D. Betulinic acid derivatives NVX-207 and B10 for treatment of glioblastoma—An in vitro study of cytotoxicity and radiosensitization. Int J Mol Sci. 2014;15(11):19777–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ihnatko R, Kubes M, Takacova M, Sedlakova O, Sedlak J, Pastorek J, Kopacek J, Pastorekova S. Extracellular acidosis elevates carbonic anhydrase IX in human glioblastoma cells via transcriptional modulation that does not depend on hypoxia. Int J Oncol. 2006;29(4):1025–33. [PubMed] [Google Scholar]
- 28. Innocenti A, Vullo D, Scozzafava A, Casey JR, Supuran C. Carbonic anhydrase inhibitors. Interaction of isozymes I, II, IV, V, and IX with carboxylates. Bioorg Med Chem Lett. 2005;15(3):573–8. [DOI] [PubMed] [Google Scholar]
- 29. Chiche J, Ilc K, Laferrière J, Trottier E, Dayan F, Mazure NM, Brahimi-Horn MC, Pouysségur J. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009;69(1):358–68. [DOI] [PubMed] [Google Scholar]
- 30. Lou Y, McDonald PC, Oloumi A, Chia S, Ostlund C, Ahmadi A, Kyle A, Auf dem Keller U, Leung S, Huntsman D, Clarke B, Sutherland BW, Waterhouse D, Bally M, Roskelley C, Overall CM, Minchinton A, Pacchiano F, Carta F, Scozzafava A, Touisni N, Winum JY, Supuran CT, Dedhar S. Targeting tumor hypoxia: Suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. 2011;71(9):3364–76. [DOI] [PubMed] [Google Scholar]
- 31. Sauvant C, Thews O, Wirth C, Gekle M. Direct determination of intracellular daunorubicin in intact confluent monolayers of AT1 prostate carcinoma cells using a multiwell-multilabel counter. Anal Biochem. 2008;381(1):81–5. [DOI] [PubMed] [Google Scholar]
- 32. Srivastava J, Barber DL, Jacobson MP. Intracellular pH sensors: Design principles and functional significance. Physiology 2007;22:30–9. [DOI] [PubMed] [Google Scholar]
- 33. Riemann A, Schneider B, Ihling A, Nowak M, Sauvant C, Thews O, Gekle M. Acidic environment leads to ROS-induced MAPK signaling in cancer cells. PLoS One 2011;6(7):e22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Kuijk SJA, Yaromina A, Houben R, Niemans R, Lambin P, Dubois LJ. Prognostic significance of carbonic anhydrase IX expression in cancer patients: A meta-analysis. Front Oncol. 2016;6:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Isaacs JT, Isaacs WB, Feitz WF, Scheres J. Establishment and characterization of seven Dunning rat prostatic cancer cell lines and their use in developing methods for predicting metastatic abilities of prostatic cancers. Prostate 1986;9(3):261–81. [DOI] [PubMed] [Google Scholar]
- 36. McDonald PC, Chafe SC, Dedhar S. Overcoming hypoxia-mediated tumor progression: Combinatorial approaches targeting pH regulation, angiogenesis and immune dysfunction. Front Cell Dev Biol. 2016;4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Supuran CT, Winum J-Y. Designing carbonic anhydrase inhibitors for the treatment of breast cancer. Expert Opin Drug Discov. 2015;10(6):591–7. [DOI] [PubMed] [Google Scholar]
- 38. McIntyre A, Hulikova A, Ledaki I, Snell C, Singleton D, Steers G, Seden P, Jones D, Bridges E, Wigfield S, Li JL, Russell A, Swietach P, Harris AL. Disrupting hypoxia-induced bicarbonate transport acidifies tumor cells and suppresses tumor growth. Cancer Res. 2016;76(13):3744–55. [DOI] [PubMed] [Google Scholar]
- 39. Meehan J, Ward C, Turnbull A, Bukowski-Wills J, Finch AJ, Jarman EJ, Xintaropoulou C, Martinez-Perez C, Gray M, Pearson M, Mullen P, Supuran CT, Carta F, Harrison DJ, Kunkler IH, Langdon SP. Inhibition of pH regulation as a therapeutic strategy in hypoxic human breast cancer cells. Oncotarget 2017;8(26):42857–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li Y, Xiang J, Zhang S, Liu B, Gong F, Peng M. Analysis of the impact of extracellular acidity on the expression and activity of P-glycoprotein and on the P-glycoprotein-mediated cytotoxicity of daunorubicin in cancer cell by microfluidic chip technology. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2015;37(1):75–81. [DOI] [PubMed] [Google Scholar]
- 41. van Kuijk SJA, Gieling RG, Niemans R, Lieuwes NG, Biemans R, Telfer BA, Haenen GRMM, Yaromina A, Lambin P, Dubois LJ, Williams KJ. The sulfamate small molecule CAIX inhibitor S4 modulates doxorubicin efficacy. PLoS One 2016;11(8):e0161040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Amiri A, Le PU, Moquin A, Machkalyan G, Petrecca K, Gillard JW, Yoganathan N, Maysinger D. Inhibition of carbonic anhydrase IX in glioblastoma multiforme. Eur J Pharm Biopharm. 2016;109:81–92. [DOI] [PubMed] [Google Scholar]
- 43. Rami M, Dubois L, Parvathaneni N-K, Alterio V, van Kuijk SJA, Monti SM, Lambin P, De Simone G, Supuran CT, Winum J-Y. Hypoxia-targeting carbonic anhydrase IX inhibitors by a new series of nitroimidazole-sulfonamides/sulfamides/sulfamates. J Med Chem. 2013;56(21):8512–20. [DOI] [PubMed] [Google Scholar]
- 44. Ozensoy Guler O, Arslan O, Kockar F. Differential in vitro inhibitory effects of anticancer drugs on tumor-associated carbonic anhydrase isozymes CA IX and CA XII. Methods Find Exp Clin Pharmacol. 2008;30(5):335–40. [DOI] [PubMed] [Google Scholar]
- 45. Kopecka J, Rankin GM, Salaroglio IC, Poulsen S-A, Riganti C. P-glycoprotein-mediated chemoresistance is reversed by carbonic anhydrase XII inhibitors. Oncotarget 2016;7(52):85861–75. [DOI] [PMC free article] [PubMed] [Google Scholar]