Abstract
Pancreatic ductal adenocarcinoma (PDAC) is one of the leading causes of cancer deaths due to its highly aggressive biological nature and resistance to chemotherapy. Previous studies indicate that miR-21 is an important regulator in the activation of cancer-associated fibroblasts (CAFs). However, whether miR-21 in CAFs would regulate PDAC’s tumor microenvironment and lead to drug resistance remain unknown. In this study, we evaluated the relationship between CAF activation, miR-21 expression, and drug resistance using tumor samples from PDAC patients. We changed the miR-21 expression level in CAFs and tested its roles in regulating the function of CAFs. In addition, we explored the roles of miR-21 in CAFs in the development of PDAC using an animal model. We found that PDAC patients who were resistant to gemcitabine treatment tended to have higher miR-21 expression and more activated CAFs. An in vitro study showed that CAFs with high miR-21 expression had elevated MMP-3, MMP-9, PDGF, and CCL-7 expression and promoted the invasion of PDAC cell lines. miR-21 overexpression also contributed to the activation of CAFs by regulating the PDCD4 gene. The in vivo study showed that upregulating miR-21 in CAFs promoted PDAC desmoplasia and increased its drug resistance to gemcitabine treatment, but downregulating miR-21 in CAFs suppressed desmoplasia and enhanced the effect of gemcitabine. We concluded that miR-21 promoted the activation of CAFs and contributed to the drug resistance of PDAC.
Key words: Pancreatic cancer, Micro-RNA-21, Cancer-associated fibroblasts (CAFs), Drug resistance
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is one of the leading causes of cancer deaths due to its highly aggressive biological nature and resistance to chemotherapy1–3. Surgery is still the only way to cure PDAC; however, only a small proportion of patients are eligible for surgery2,4. The current treatment of PDAC largely relies on comprehensive strategies using gemcitabine-based chemotherapy3–5. However, a large number of PDAC patients are not sensitive to the current chemotherapy. Therefore, overcoming drug resistance is critical to improving the outcome for PDAC patients.
It is becoming clear that the stromal tissue in PDAC is not a bystander in disease progression6. Cancer–stroma interactions affect tumorigenesis, angiogenesis, therapy resistance, and, possibly, the metastatic spread of PDAC tumor cells6–8. Fibroblasts and myofibroblasts [also known as cancer-associated fibroblasts (CAFs) in tumors] are the major cell types in various human tumor stroma9. CAFs modulate the stroma via direct cell–cell contacts or secretion of a range of phenotypic and functional proteins9,10. Studies have shown that chemotherapies that could disrupt the tumor stroma of PDAC have a better response in PDAC preclinical models11. However, the mechanisms by which CAFs are activated in PDAC tumor tissues, and therefore induce drug resistance, are not clear. Micro-RNAs (miRs) are a class of endogenous, 20- to 22-nucleotide-long, noncoding single-stranded RNA molecules. miRs negatively regulate gene expression through mRNA degradation and/or translational inhibition of their targeted genes. The role of multiple miRs in PDAC tumor cells has been well studied; however, their function in regulating stromal cell functions is unclear12–14. miR-21 in PDAC has been investigated and identified as a predictor of poor survival12. Previous studies indicate that miR-21 in fibroblasts is an important regulator of their activation in transforming growth factor-β (TGF-β) induction15. However, whether miR-21 in CAFs would regulate PDAC’s tumor microenvironment and lead to drug resistance remains unknown.
In the current study, we focused on the function of miR-21 in the CAFs of PDAC. Our study identified one activation mechanism of CAFs in PDAC that is mediated by miR-21 expression in CAFs. More importantly, our results further contribute to the current knowledge on drug resistance of PDAC, which might not only be involved in mutations and phenotypes of cancer cells but also related to abnormal pathways in CAFs, which will stimulate tumor-promoting tumor–stroma interactions.
MATERIALS AND METHODS
Cell Isolation and Culture
Human CAFs were isolated from the pancreatic cancer tissue of patients, and mouse CAFs were isolated from the tumor tissue of a subcutaneous Panc02 mouse model according to the method of Sharon and colleagues16. Briefly, the tissues were cut into 2- to 3-mm pieces within a small amount of phosphate-buffered saline (PBS) on ice. The small fragments were then dissociated with a collagenase solution. The reaction was stopped by adding cold DMEM + 10% fetal bovine serum (FBS). The single-cell suspension was then used to isolate CAFs by fluorescence-activated cell sorting (FACS). MIA Paca-2 cells were purchased from the ATCC (Manassas, VA, USA). Panc02 cell lines were purchased from the Cell Resource Center of the Chinese Academy of Sciences (Shanghai, P.R. China). MIA Paca-2 cells and CAFs were cultured in DMEM, and Panc02 cells were cultured in RPMI-1640 medium. Both kinds of medium were supplemented with 100 U/ml of penicillin, 100 μg/ml of streptomycin, 2 mM l-glutamine, and 10% heat-inactivated FBS. All cells were cultured within a humidified incubator at 37°C with 5% CO2. Cells were subcultured when they reached 80%–100% confluence.
Cell Invasion Assay
Costar Transwell 24-well plates (Sigma-Aldrich, St. Louis, MO, USA) were used to perform the cell invasion assay following the manufacturer’s instructions. Briefly, human CAFs or murine CAFs (1 × 105/well) were seeded into the lower chambers, and Transwell inserts were placed into the upper chambers. A suspension of MIA Paca-2 or Panc02 tumor cells (1 × 105/well) was then added. The cells were cultured in complete medium for 12 h in a humidified incubator at 37°C with 5% CO2. Apical inserts were then washed with a wash buffer, and crystal violet stain solution was added to the inserts and incubated for 10 min. The inserts were then observed under a microscope.
Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA was performed to measure the protein levels of matrix metalloproteinase-3 (MMP-3), MMP-9, chemokine (C-C motif) ligand 7 (CCL-7), and platelet-derived growth factor (PDGF) of the CAFs after various transfections. The ELISA kit was purchased from Abcam (Cambridge, MA, USA), and the manufacturer’s instructions were followed.
Patient Samples
A total of 40 PDAC patients were included in this study. We collected the formalin-fixed, paraffin-embedded (FFPE) PDAC tumor tissues from these patients to evaluate the miR-21 level and the number of CAFs. These patients were diagnosed between January 2008 and December 2011 at The First Affiliated Hospital of Henan University of Science and Technology. All tissues were obtained during surgery before the patients had either chemotherapy or radiotherapy. This study was approved by the local ethics committee of The First Affiliated Hospital of Henan University of Science and Technology. Written informed consent was signed by each patient.
Histology and Immunostaining
Immunofluorescence (IF) staining was performed to evaluate the α-SMA expression of the tumor tissues of PDAC patients and the tumor tissue from a PDAC xenograft mouse model. Standard procedures for IF were followed. A primary antibody of α-SMA (Abcam) was diluted 1:100 and incubated with the tissue samples at 4°C overnight. A secondary antibody was added to the samples and incubated for 1 h at room temperature. The sections were then stained with DAPI for 1 min. Sections were observed under a fluorescence microscope.
Sirius red staining was conducted to evaluate the collagen expression of the tumor tissue from the PDAC xenograft mouse model. Sections were deparaffinized and rehydrated, followed by staining with Picrosirius red for 1 h and washing with acidified water twice. The sections were then dehydrated in three changes of 100% ethanol and cleared with xylene. Finally, the sections were mounted in resinous medium and observed under a microscope with a bright field. The scoring for both α-SMA and collagen was based on the principle: 4 for >80% positive cells, 3 for 55%–80% positive cells, 2 for 30%–55% positive cells, and 1 for <30% positive cells.
qRT-PCR
Total RNA was extracted from cells using a mirVana miRNA Isolation Kit. RNA was reverse transcribed using a miScript Reverse Transcription Kit (Qiagen, Hilden, Germany). Quantification was performed with SYBR Green (Qiagen, Valencia, CA, USA). Each sample was analyzed in triplicate using the LightCycler® 480 System (Roche Life Science, Indianapolis, IN, USA). The comparative threshold cycle (CT) method was used to determine relative gene expression. 18S rRNA was chosen as an internal control for both mRNA and miRNA.
Animal Model
A PDAC xenograft mouse model was established using the Panc02 cell line, murine CAFs, and C57BL/6 mice (Panc02/CAFs = 1:5). A total of 50 female C57BL/6 mice (19–21 g; 6 weeks old; Beijing Weitong Lihua Experimental Animal Technology Co. Ltd., P.R. China) were randomly divided into five groups (10 per group) to accept subcutaneous injections with Panc02 cells and CAFs (the ratio is 1:5, the total cell number was 3 × 106). Five different types of CAFs were used: CAFs transfected by 1) miR-21 mimic, 2) miR-21 inhibitor, 3) miR-21 mimic scramble, 4) miR-21 inhibitor scramble, and 5) wide-type CAFs that did not accept RNA transfection. Gemcitabine treatment started 1 week after the injections. All mice were raised in a clean environment at 37°C with free access to standard food and water. Survival status and tumor volume (length × width2 × π/6) were recorded.
Transfection of the miRNA Mimic, miRNA Inhibitor, and siRNA
The miR-21 inhibitor, miR-21 mimic, miR-21-inhibitor scramble, miR-21 mimic scramble RNA, and siRNA of PDCD4 were designed and purchased from Thermo Fisher Scientific (Waltham, MA, USA). CAFs were transfected with 50 nM miR-21 inhibitor or 5 nM miR-21 mimic using a Lipofectamine 3000 transfection reagent (Thermo Fisher Scientific). Twenty-four hours after successful transfection, the CAFs were incubated in complete medium with or without TGF or gemcitabine (1 μM for 24 h).
Statistical Analysis
Statistical analysis and data visualization were performed using the GraphPad Prism software (GraphPad Software, La Jolla, CA, USA) and the Tableau software (Seattle, WA, USA). One-way ANOVA and Tukey’s multiple comparisons test were used to test the mean difference among experimental groups. The Kaplan–Meier method was used to determine survival analysis, and the differences between survival curves were tested by the log-rank test. All data in the plots were shown as means and standard deviations. A two-tailed value of p < 0.05 was considered to be a significant difference.
RESULTS
High Expression of miR-21 Correlates With the Accumulation of CAFs and Gemcitabine Resistance in PDAC
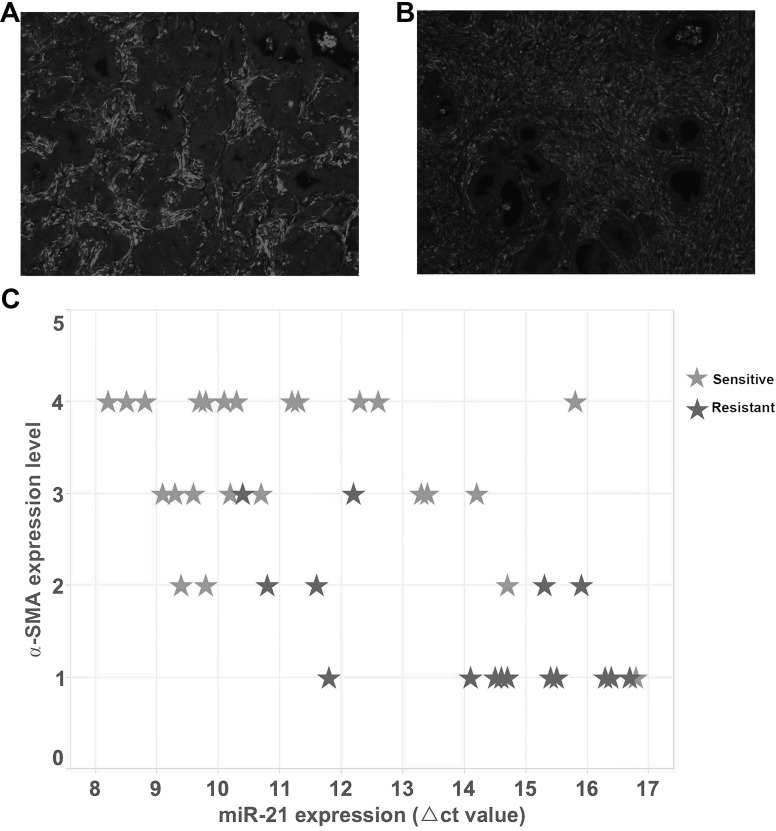
To investigate whether miR-21 actively regulates the activation of fibroblasts in PDAC and leads to gemcitabine resistance, we first correlated the miR-21 expression level with the amount of CAFs and drug resistance in clinical samples. Representative high and low CAF cases are shown in Figure 1A and B. We evaluated PDAC patients based on miR-21 expression level, amount of CAFs, and response to gemcitabine. Our data clearly show that patients having a high miR-21 expression also have a higher CAF number and a worse response to gemcitabine (Fig. 1C). These data established the phenotype that high miR-21 expression may lead to gemcitabine resistance by regulating the function of CAFs.
Figure 1.
The relationship between the number of cancer-associated fibroblasts (CAFs), miR-21 expression, and gemcitabine resistance in pancreatic ductal adenocarcinoma (PDAC). (A, B) Representative pictures of high α-SMA expression and low α-SMA expression in human PDAC tissues, respectively [stained by immunofluorescence (IF)]. (C) Each case of human PDAC tissue sample was evaluated by α-SMA, miR-21, and drug resistance to gemcitabine and plotted. Each star represents a PDAC patient. The scatterplot shows that patients who were resistant to gemcitabine treatment tended to have a higher miR-21 expression and a lower α-SMA expression compared to patients who were sensitive to gemcitabine treatment (higher ΔCT value indicates lower miR-21 expression).
miR-21 in CAFs, But Not in Cancer Cell Response to Gemcitabine Treatment
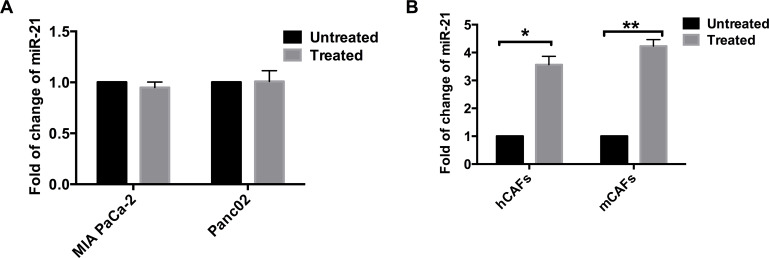
As desmoplasia is a feature of PDAC, an effective treatment should be able to deplete both cancer cells and CAFs effectively. We further tested whether miR-21 in tumor cells or CAFs can be regulated by gemcitabine treatment. Both tumor cells and CAFs were treated with gradient concentrations of gemcitabine to get half-maximal inhibitory concentration (data not shown). The cells were then treated with this concentration for 48 h. The expression of miR-21 in gemcitabine-treated cells and control group cells (cells not treated) was measured. We found that, in tumor cells, the expression of miR-21 did not change much with gemcitabine treatment (Fig. 2A). However, in CAFs, the gemcitabine-treated cells showed about four times the amount of miR-21 expression as the control group cells (Fig. 2B).
Figure 2.
miR-21 in CAFs was increased by gemcitabine treatment. (A) Two PDAC cell lines, MIA Paca-2 and Panc02, were treated with gemcitabine, and the miR-21 within these cells was measured. There was no apparent difference in the amount of miR-21 between the groups, whether they accepted gemcitabine or not. (B) Two kinds of CAFs, human CAFs (hCAFs) and murine CAFs (mCAFs), were treated with gemcitabine. The CAFs treated with gemcitabine had over three times the amount of miR-21 as the untreated CAFs. *p < 0.05; **p < 0.01.
miR-21 Regulates the Function of CAFs and Thus Influences Cancer Cells
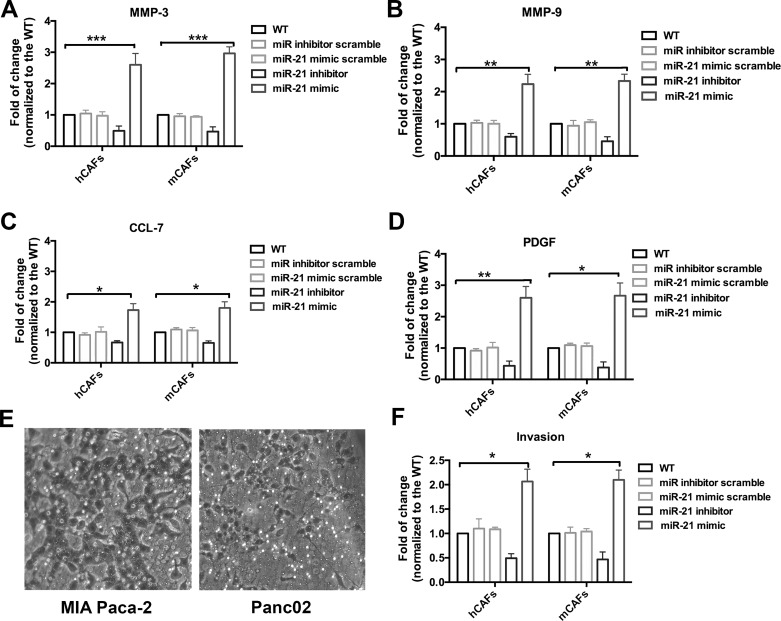
As our data indicated that miR-21 was upregulated in gemcitabine-treated CAFs and might regulate drug resistance, we further explored the potential mechanisms by which miR-21 might regulate CAFs’ drug resistance and thus promote tumor development. We found that CAFs treated with the miR-21 mimic showed elevated MMP-3, MMP-9, PDGF, and CCL-7 secretion (Fig. 3A–D). However, CAFs treated with the miR-21 inhibitor showed decreased MMP-3, MMP-9, PDGF, and CCL-7 (Fig. 3A–D). The ability of tumor cells to invade was measured by the Transwell chamber assay. The representative pictures are shown in Figure 3E. Quantitative data of cells that invaded indicated that CAFs treated with the miR-21 mimic significantly promoted tumor cell invasion (Fig. 3F). Taken together, our data support the hypothesis that upregulation of miR-21 positively regulates the tumor-promoting role of CAFs.
Figure 3.
miR-21 regulates the function of CAFs and promotes PDAC invasion. In the different experiment groups, hCAFs and mCAFs were transfected with various micro-RNAs (miR-21 mimic, miR-21miR inhibitor, scramble, or miR-21 mimic scramble). Expression of matrix metalloproteinase-3 (MMP-3), MMP-9, chemokine (C-C motif) ligand 7 (CCL-7), and platelet-derived growth factor (PDGF) was evaluated by enzyme-linked immunosorbent assay (ELISA). All data were normalized to the wide-type CAFs. (A–D) MMP-3, MMP-9, CCL-7, and PDGF were significantly increased in the CAFs with increased miR-21, while their expression was decreased in the CAFs with suppressed miR-21. (E) Representative pictures of the Transwell assay using the MIA Paca-2 and Panc02 cell lines and CAFs with different levels of miR-21. (F) Quantified data of invaded tumor cells in the Transwell assay. The groups with high miR-21 expression CAFs showed a highly increased number of invaded tumor cells, while the groups with low miR-21 expression CAFs had a decreased number of invaded tumor cells. *p < 0.05; **p < 0.01; ***p < 0.001.
miR-21 Mediates Activation of CAFs via PDCD4
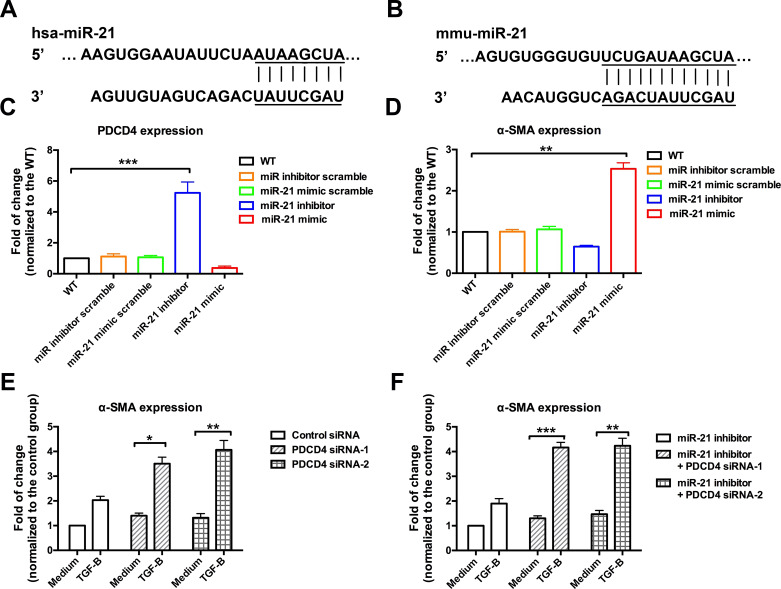
We further investigated the molecular mechanisms by which miR-21 mediates the activation of CAFs by focusing on the targets of miR-21. We found that PDCD4, one target of miR-21, was significantly downregulated by miR-21 mimic transfection (Fig. 4C). TGF-β was an important activator of CAFs and was commonly used to induce the activation CAFs in vitro. We treated the transfected CAFs with TGF-β and then evaluated α-SMA expression. We found that CAFs transfected with the miR-21 mimic had a highly increased level of α-SMA (more than 2.5 times that of the wide-type CAFs), while CAFs transfected with the miR-21 inhibitor had a significantly decreased α-SMA (Fig. 4D). In the PDCD4-deficient CAFs, TGF-β treatment highly promoted α-SMA expression (Fig. 4E). In addition, CAFs that were transfected with both miR-21 inhibitor and PDCD4 siRNA showed an increased level of α-SMA (Fig. 4F). In summary, these data indicate that miR-21 promotes the activation of CAFs by inhibiting the expression of PDCD4.
Figure 4.
miR-21 promoted the activation of CAFs by targeting PDCD4. PCDC4 and α-SMA expressions were measured in the murine CAFs transfected with different micro-RNAs and treated with TGF-β. (A, B) Sequences of miR-21 used to transfect human CAFs and murine CAFs, respectively. (C) PDCD4 expression was decreased in the CAFs with increased miR-21, but it was increased in the CAFs with miR-21 inhibitors. (D) In contrast to PDCD4, α-SMA expression was decreased in the murine CAFs with low miR-21 but was enhanced in the CAFs with high miR-21. (E) Murine CAFs transfected with PDCD4 siRNAs were treated with TGF-β to detect their activation by measuring α-SMA expression. The CAFs of the control groups were transfected with scrambled siRNA and treated with complete medium. The CAFs with PDCD4 deficiency had an increased α-SMA expression. (F) CAFs with the miR-21 inhibitor and PDCD4 deficiency showed an increased α-SMA compared with the CAFs with the miR-21 inhibitor alone. *p < 0.05; **p < 0.01; ***p < 0.001.
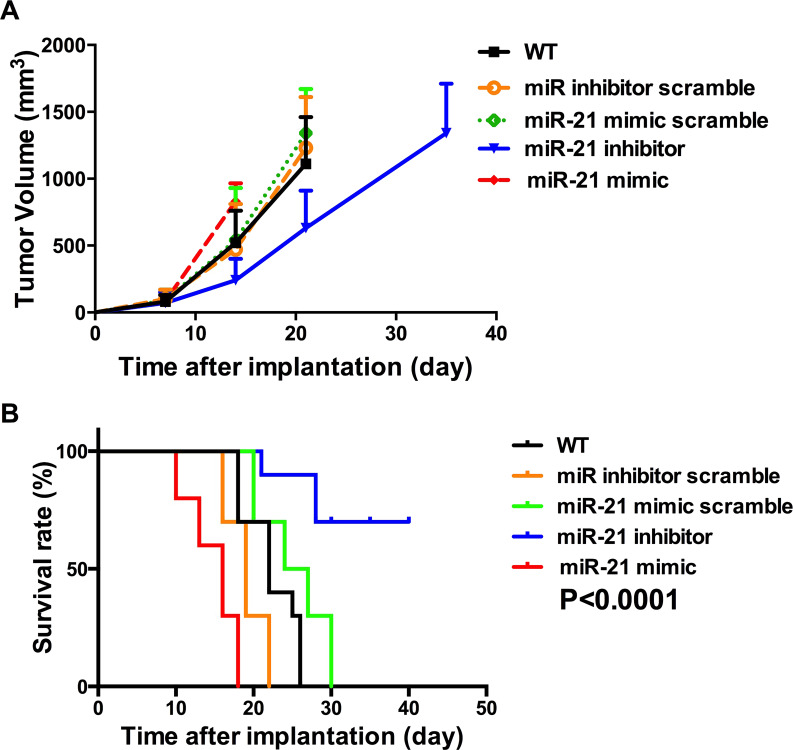
CAFs With a High Expression of miR-21 Promote Drug Resistance in the PDAC Mouse Model
The role that CAFs with a high expression of miR-21 play in promoting drug resistance was also tested in a PDAC xenograft mouse model. The mice with wide-type CAFs and CAFs transfected with RNS scrambles did not respond effectively to gemcitabine treatment, showing a rapid increase in tumor volume (Fig. 5A). However, the tumor volume growth of mice with CAFs transfected with the miR-21 inhibitor was significantly decreased (Fig. 5A). Mice with CAFs transfected with the miR-21 mimic showed very aggressive growth (Fig. 5A). Consistently, the survival analysis of these mice showed that mice with the miR-21 inhibitor-transfected CAFs had the longest survival time compared with the control groups and the mice with CAFs having a high miR-21 expression (Fig. 5B). These data confirm the role of CAFs with a high expression of miR-21 in promoting drug resistance in an in vivo model.
Figure 5.
miR-21 in CAFs regulates drug resistance to gemcitabine in a PDAC mouse model. A PDAC mouse model was established with Panc02 cell lines and CAFs with various levels of miR-21 and accepted gemcitabine treatment after 1 week of implantation. (A) Increase in tumor volume over time after implantation. The mice with CAFs having the miR-21 mimic grew the fastest, while the mice with CAFs having the miR-21 inhibitor grew the slowest. (B) Survival curves of the mice with CAFS having different levels of miR-21. Consistent with tumor volume growth, the mice with high miR-21 expression CAFs had the shortest survival time, and the mice with low miR-21 expression CAFs had the longest survival time.
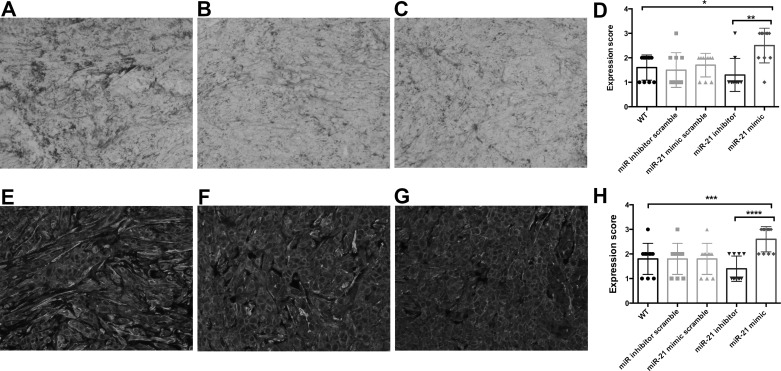
CAFs With a High Expression of miR-21 Promote Desmoplasia in the PDAC Mouse Model
The in vitro data clearly indicated that miR-21 positively regulates the activation of CAFs and promotes tumor cell invasion. Therefore, we analyzed the degree of desmoplasia in tumor tissues derived from our xenograft model. We detected the amount of collagen within tumor tissues (Fig. 6A–C). The tumors with CAFs having a high expression of miR-21 tended to have a higher degree of desmoplasia than tumors with CAFs having a low miR-21 expression. The quantified data also confirmed that the mice injected with the miR-21 inhibitor-transfected CAFs had a decreased level of collagen, and mice injected with miR-21 mimic-transfected CAFs had an increased level of collagen compared to the control groups (Fig. 6D). We observed various levels of α-SMA in these tumor tissues (Fig. 6E–G). Tumors with high-miR-21 CAFs had a much higher level of α-SMA than tumors with low-miR-21 CAFs (Fig. 6H). These data further confirmed that miR-21 could promote the activation of CAFs and desmoplasia of PDAC.
Figure 6.
Desmoplasia in the PDAC mouse model. Collagen amount and α-SMA expression of the tumor tissues from the PDAC mouse model were detected by Sirius red staining and IF staining, respectively. (A–C) Representative pictures showing high, medium, and low levels of collagen within the tumor tissues, respectively. (D) Quantified data showing the amount of collagen in the tumor tissues. Tumor tissues derived from mice with high miR-21 expression CAFs showed the highest amount of collagen, and the tumor tissue of mice with low miR-21 expression CAFs had the lowest amount of collagen. (E–G) Representative pictures showing high, medium, and low levels of α-SMA within the tumor tissues, respectively. (H) Tumor tissues from the mice with high miR-21 expression CAFs had the highest α-SMA expression, and tumor tissues from the mice with low miR-21 expression CAFs had the lowest α-SMA expression. *p < 0.05; **p < 0.01; ***p < 0.001; ****p <0.0001.
DISCUSSION
Interactions between tumor cells and the surrounding tumor stroma cells are critical in determining tumor aggressiveness. Features of the tumor stroma are prognostic markers for various cancers, including pancreatic cancer8,17,18. Previous studies indicate that targeting the tumor-promoting stroma of PDAC significantly enhanced the efficacy of chemotherapy in preclinical mouse models19,20. However, mechanisms that lead to tumor-promoting stroma remain poorly understood in PDAC. In the current study, we found that miR-21, a micro-RNA that is highly expressed in certain PDAC patients, drove the activation of CAFs, thus promoting chemoresistance in PDAC.
miR-21 is the most consistently upregulated micro-RNA in many cancer types including PDAC, in which it predicts poor survival12. The tumor-promoting function of miR-21 lies in its ability to regulate multiple cancer-associated pathways21,22. It was shown that miR-21 can inhibit the expression of significant tumorigenic or tumor suppressor genes, including phosphatase and tensin homolog (PTEN), PDCD4, tropomyosin 1 (TPM1), and maspin23–25. Interestingly, previous studies also reported that miR-21 was abnormally regulated in stroma cells, especially fibroblasts22,26. This feature arouses special interest in PDAC as the tumor stroma has a critical role in promoting PDAC development. In our study, we found that a high number of CAFs in PDAC tissues is correlated with drug resistance. More importantly, we showed that miR-21 was abnormally expressed in response to gemcitabine treatment in CAFs, but not in tumor cells. Together with the previous reports, our findings further confirm the tumor-promoting role of miR-21 in PDAC, and this may be due to its ability to regulate drug resistance.
We further investigated the mechanisms that lead to the miR-21-regulated, CAF-mediated drug resistance in PDAC. By modulating the expression level of miR-21 in both human and mouse CAFs, we found that a high expression of miR-21 in CAFs significantly upregulated invasion and tumor cell growth-related factor secretion in CAFs. This significantly increased the ability of tumor cells to invade when cocultured together. Targeting prediction analysis revealed that PDCD4 is an miR-21 target gene. Previous studies found that PDCD4 was also expressed in vascular smooth muscle cells, where it acts as a negative regulator of smooth muscle contractile genes27,28. Here we found that miR-21 suppresses the expression of PDCD4 in CAFs; therefore, it increases the expression of α-SMA in response to TGF-β treatment. These findings are also correlated with a previous report using a different line of CAFs.
To enhance the translational significance of our findings, we further verified the role of CAFs’ miR-21 in promoting drug resistance in a PDAC preclinical animal model. Our results clearly show that gemcitabine treatment could control the growth of tumors with CAFs having a low expression of miR-21. However, it failed to slow down growth of tumors having CAFs with a high expression of miR-21. In the following histological analyses, we found that CAFs with high miR-21 expression produced heavy desmoplasia in tumor tissues. Taken together, the in vivo data were in line with the in vitro findings, confirming that downregulation of miR-21 in CAFs could be a promising target to overcome PDAC drug resistance.
PDAC is a lethal disease due to its aggressive nature and heavy drug resistance. To overcome these issues, our study identified that a number of activated CAFs were associated with PDAC drug resistance, and miR-21 overexpression contributed to the activation of CAFs by regulating the PDCD4 gene, and downregulation of miR-21 in CAFs would enhance the response of the PDAC model to chemotherapy. On the basis of these findings, miR-21 in CAFs could be a promising candidate in overcoming the drug resistance of PDAC and thus additional studies are urgently needed.
ACKNOWLEDGMENT
This study was supported by the Internal Fund from The First Affiliated Hospital, Henan University of Science and Technology.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, DePinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20(10):1218–49. [DOI] [PubMed] [Google Scholar]
- 2. Ishiwata T. Pancreatic ductal adenocarcinoma: Basic and clinical challenges for better prognosis. J Carcinog Mutagen. 2013;S9:005. [Google Scholar]
- 3. Shah AN, Summy JM, Zhang J, Park SI, Parikh NU, Gallick GE. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol. 2007;14(12):3629–37. [DOI] [PubMed] [Google Scholar]
- 4. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(11):1039–49. [DOI] [PubMed] [Google Scholar]
- 5. Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA 2007;297(3):267–77. [DOI] [PubMed] [Google Scholar]
- 6. Farrow B, Albo D, Berger DH. The role of the tumor microenvironment in the progression of pancreatic cancer. J Surg Res. 2008;149(2):319–28. [DOI] [PubMed] [Google Scholar]
- 7. Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18(16):4266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Banerjee S, Modi S, McGinn O, Zhao X, Dudeja V, Ramakrishnan S, Saluja AK. Impaired synthesis of stromal components in response to Minnelide improves vascular function, drug delivery and survival in pancreatic cancer. Clin Cancer Res. 2016;22(2):415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Östman A, Augsten M. Cancer-associated fibroblasts and tumor growth—Bystanders turning into key players. Curr Opin Genet Dev. 2009;19(1):67–73. [DOI] [PubMed] [Google Scholar]
- 10. Zhao X, He Y, Chen H. Autophagic tumor stroma: Mechanisms and roles in tumor growth and progression. Int J Cancer 2013;132(1):1–8. [DOI] [PubMed] [Google Scholar]
- 11. Miyamoto H, Murakami T, Tsuchida K, Sugino H, Miyake H, Tashiro S. Tumor-stroma interaction of human pancreatic cancer: Acquired resistance to anticancer drugs and proliferation regulation is dependent on extracellular matrix proteins. Pancreas 2004;28(1):38–44. [DOI] [PubMed] [Google Scholar]
- 12. Dillhoff M, Liu J, Frankel W, Croce C, Bloomston M. MicroRNA-21 is overexpressed in pancreatic cancer and a potential predictor of survival. J Gastrointest Surg. 2008;12(12):2171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moriyama T, Ohuchida K, Mizumoto K, Yu J, Sato N, Nabae T, Takahata S, Toma H, Nagai E, Tanaka M. MicroRNA-21 modulates biological functions of pancreatic cancer cells including their proliferation, invasion, and chemoresistance. Mol Cancer Ther. 2009;8(5):1067–74. [DOI] [PubMed] [Google Scholar]
- 14. Giovannetti E, Funel N, Peters GJ, Del Chiaro M, Erozenci LA, Vasile E, Leon LG, Pollina LE, Groen A, Falcone A. MicroRNA-21 in pancreatic cancer: Correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res. 2010;70(11):4528–38. [DOI] [PubMed] [Google Scholar]
- 15. Yao Q, Cao S, Li C, Mengesha A, Kong B, Wei M. Micro-RNA-21 regulates TGF-β-induced myofibroblast differentiation by targeting PDCD4 in tumor-stroma interaction. Int J Cancer 2011;128(8):1783–92. [DOI] [PubMed] [Google Scholar]
- 16. Sharon Y, Alon L, Glanz S, Servais C, Erez N. Isolation of normal and cancer-associated fibroblasts from fresh tissues by fluorescence activated cell sorting (FACS). J Vis Exp. 2013;(71):e4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Erkan M, Michalski CW, Rieder S, Reiser–Erkan C, Abiatari I, Kolb A, Giese NA, Esposito I, Friess H, Kleeff J. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2008;6(10):1155–61. [DOI] [PubMed] [Google Scholar]
- 18. Stotz M, Gerger A, Eisner F, Szkandera J, Loibner H, Ress A, Kornprat P, Zoughbi W, Seggewies F, Lackner C. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer 2013;109(2):416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carapuça EF, Gemenetzidis E, Feig C, Bapiro TE, Williams MD, Wilson AS, Delvecchio FR, Arumugam P, Grose RP, Lemoine NR. Anti-stromal treatment together with chemotherapy targets multiple signalling pathways in pancreatic adenocarcinoma. J Pathol. 2016;239(3):286–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee JJ, Perera RM, Wang H, Wu D-C, Liu XS, Han S, Fitamant J, Jones PD, Ghanta KS, Kawano S. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proc Natl Acad Sci USA 2014;111(30):E3091–E100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chan JK, Blansit K, Kiet T, Sherman A, Wong G, Earle C, Bourguignon LY. The inhibition of miR-21 promotes apoptosis and chemosensitivity in ovarian cancer. Gynecol Oncol. 2014;132(3):739–44. [DOI] [PubMed] [Google Scholar]
- 22. Gong C, Nie Y, Qu S, Liao J-Y, Cui X, Yao H, Zeng Y, Su F, Song E, Liu Q. miR-21 induces myofibroblast differentiation and promotes the malignant progression of breast phyllodes tumors. Cancer Res. 2014;74(16):4341–52. [DOI] [PubMed] [Google Scholar]
- 23. Xiong B, Cheng Y, Ma L, Zhang C. MiR-21 regulates biological behavior through the PTEN/PI-3 K/Akt signaling pathway in human colorectal cancer cells. Int J Oncol. 2013;42(1):219–28. [DOI] [PubMed] [Google Scholar]
- 24. Nagao Y, Hisaoka M, Matsuyama A, Kanemitsu S, Hamada T, Fukuyama T, Nakano R, Uchiyama A, Kawamoto M, Yamaguchi K. Association of microRNA-21 expression with its targets, PDCD4 and TIMP3, in pancreatic ductal adenocarcinoma. Mod Pathol. 2012;25(1):112–21. [DOI] [PubMed] [Google Scholar]
- 25. Yu Y, Kanwar SS, Patel BB, Oh P-S, Nautiyal J, Sarkar FH, Majumdar AP. MicroRNA-21 induces stemness by downregulating transforming growth factor beta receptor 2 (TGFβR2) in colon cancer cells. Carcinogenesis 2012;33(1):68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. MacKenzie TA, Schwartz GN, Calderone HM, Graveel CR, Winn ME, Hostetter G, Wells WA, Sempere LF. Stromal expression of miR-21 identifies high-risk group in triple-negative breast cancer. Am J Pathol. 2014;184(12):3217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kang H, Davis-Dusenbery BN, Nguyen PH, Lal A, Lieberman J, Van Aelst L, Lagna G, Hata A. Bone morphogenetic protein 4 promotes vascular smooth muscle contractility by activating microRNA-21 (miR-21), which down-regulates expression of family of dedicator of cytokinesis (DOCK) proteins. J Biol Chem. 2012;287(6):3976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kang H, Hata A. MicroRNA regulation of smooth muscle gene expression and phenotype. Curr Opin Hematol. 2012;19(3):224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]